Abstract
Response inhibition is an essential control function necessary to adapt one’s behavior. This key cognitive capacity is assumed to be dependent on the prefrontal cortex and basal ganglia. It is unresolved whether varying inhibitory demands engage different control mechanisms or whether a single motor inhibitory mechanism is involved in any situation. We addressed this question by comparing electrophysiological activity in conditions that require stopping a response to conditions that require switching to an alternate response. Analyses of electrophysiological data obtained from stop-signal tasks are complicated by overlapping stimulus-related activity that is distributed over frontal and parietal cortical recording sites. Here, we applied Laplacian transformation and independent component analysis (ICA) to overcome these difficulties. Participants were faster in switching compared to stopping a response, but we did not observe differences in neural activity between these conditions. Both stop- and change-trials Laplacian transformed ERPs revealed a comparable bilateral parieto-occipital negativity around 180 ms and a frontocentral negativity around 220 ms. ICA results suggested an inhibition-related frontocentral component which was characterized by a negativity around 200 ms with a likely source in anterior cingulate cortex. The data provide support for the importance of posterior mediofrontal areas in inhibitory response control and are consistent with a common neural pathway underlying stopping and changing a motor response. The methodological approach proved useful to distinguish frontal and parietal sources despite similar timing and the ICA approach allowed assessment of single-trial data with respect to behavioral data.
Keywords: stop-signal task, cognitive control, Laplacian, independent component analysis
1. Introduction
Controlling behavior and cognition to avoid undesirable responses is essential for goal-directed behavior. Response inhibition, i.e. aborting planned behavior, is one such control function and is assumed to rely on prefrontal cortex and basal ganglia (Chambers et al., 2009; Simmonds et al., 2008). Everyday life, however, often requires a more selective type of inhibition that aborts one motor action but not another or that allows switching to another response. It is unresolved whether a single inhibitory mechanism is recruited for any situation calling for motor inhibition or whether varying inhibitory demands engage different neural mechanisms and brain structures (Aron et al., 2014; Boecker et al., 2012). In the present study, we addressed this question by comparing electrophysiological activity in conditions that require stopping a response to conditions that require switching to an alternate response.
Response inhibition has traditionally been studied using the Stop Signal Paradigm (SSP). The SSP involves a primary choice reaction time task, in which an occasional stop-signal is presented shortly after the go stimulus, signaling to inhibit the already prepared response (Logan and Cowan, 1984). Behavior in the SSP can be explained by the influential horse race model that assumes two stochastically independent processes racing against each other, the stop process and the go process. The winner of the race determines whether the response is inhibited or not (Logan et al., 1984). The model allows estimating the latency of the stop process, tagged the Stop Signal Reaction Time (SSRT), as a behavioral index of inhibitory control.
The Change Signal Paradigm (CSP) is a variation of the SSP, in which change-signal trials, also comprising two stimuli presented in rapid succession, are presented. In this case, however, the second stimulus indicates to not only inhibit the response to the first stimulus, but to switch to another response (Logan and Burkell, 1986; Verbruggen et al., 2008). Behavioral data in this paradigm can best be explained with a serial model in which change-trials require stopping first before instantiating the alternative go-process (Verbruggen et al., 2008). Comparisons of the change-signal reaction time (CSRT) with the SSRT has yielded inconclusive results with most studies reporting slower CSRTs, but others reporting no difference or a faster CSRT (reviewed in Boecker et al., 2012). These studies differed also in whether change- and stop-trials were presented in the same or alternate blocks and whether change-trials required switching to a new response or to a response that was part of the task-set of the primary go-task.
Regarding the underlying neural networks, an influential model relates inhibitory control to a cortico-basal ganglia-thalamocortical circuit (Aron et al., 2007). According to this view, the prefrontal cortex, particularly the right inferior frontal gyrus (rIFG), suppresses the thalamocortical output through the subthalamic nucleus (STN) (Aron and Poldrack, 2006). Inhibition via this hyperdirect pathway is proposed to be nonselective and globally suppresses basal ganglia output (Nambu et al., 2002). More integrative accounts of inhibitory control argue that signals of uncertainty, response conflict or difficulty (including stop- or change-signals) drive posterior frontal midline structures such as pre-SMA and anterior cingulate cortex (ACC) which then activate STN and globally inhibit motor responses until response selection is resolved (Wiecki and Frank, 2013). In this framework, the function of the IFG is to monitor the environment for task-relevant stimuli that indicate that a response has to be initiated or withheld.
Different suggestions have been made as to how inhibiting a response is different from or similar to switching to another response. Band and van Boxtel (1999) propose that CSP and SSP are performed by the same mechanism, which is refined in the change condition in order to execute a secondary response. Evidence from functional imaging supports this view, indicating that the same structures are active during stop-all and stop-change tasks (Boecker et al., 2011; Coxon et al., 2009; Kenner et al., 2010), with the main difference being stronger activation in the pre-SMA during change-trials, supposedly reflecting the conflict resolution needed in the change-signal task. In contrast, data from a patient with a unilateral pre-SMA lesion provided support for two different mechanisms as the patient was impaired in switching from contra- to ipsilesional responses but did not differ from healthy controls in her SSRT (Nachev et al., 2007).
Data from an EEG-study also pointed to different mechanisms employed when stopping or changing a motor response. Using an Eriksen Flanker Task featuring embedded stop- and change-signal trials, Krämer et al. (2011) observed differences between stop- and change-trials both on the behavioral and electrophysiological level. Specifically, the change-signal reaction time (CSRT) was faster than the SSRT, and stop-compared to change-trials elicited an enhanced stop-N2 and showed a reduced mu power decrease. The stop-N2 is a fronto-central negativity peaking around 200 – 250 ms after stimulus onset. It is reliably observed in SST or Go/Nogo-studies and is suggested to emanate from ACC or pre-SMA (reviewed in Huster et al., 2012). There is ongoing debate about what exact function the stop-N2 reflects. As the stop-N2 is observed in conditions requiring response inhibition and with a latency that is in the range of the SSRT, it has been suggested to reflect prefrontal inhibition of downstream motor regions. This is supported by studies reporting an enhanced N2 associated with lower false alarm rates (Falkenstein et al., 1999; Schmajuk et al., 2006) and with faster SSRTs (Ramautar et al., 2006; van Boxtel et al., 2001). Other reports relate this component to conflict monitoring (Azizian et al., 2006; Broere et al., 2009; Donkers and van Boxtel, 2004; Nieuwenhuis et al., 2003; Pfefferbaum et al., 1985). These studies, all employing the Go/Nogo paradigm, argue that the N2 reflects monitoring functions of ACC or pre-SMA which are sensitive to the response conflict between giving a motor response or not. However, this interpretation typically assumes compatibility between SST and Go/Nogo-tasks, although recent studies stress the differences between stopping an already prepared response as in the SST and restraining motor output as in the Go/Nogo-task (Krämer et al., 2013; Swick et al., 2011).
An enhanced stop-N2 in stop-compared to change-trials can therefore be interpreted as differences in inhibitory control or, within the conflict monitoring account, as differences in response conflict. The latter seems surprising as the above-mentioned fMRI study on the change-signal paradigm argued that enhanced conflict resolution in change-trials caused increased pre-SMA activity. How can the discrepancies between these findings be resolved? Krämer et al. (2011) used a modified Eriksen Flanker task with strong stimulus-response association (arrows pointing right and left), and stop- and change-trials were presented in the same blocks. One might argue that change-trials were similar to incongruent go-trials, i.e. trials in which the direction of the flanking arrows was incongruent to the central arrow’s direction. This would result in an overall higher probability of change-events compared to stop-trials and possibly alter neurophysiological processes. Finally, EEG- and fMRI differ substantially in their spatio-temporal resolution and in underlying mechanisms of the measured signal making results only partially comparable. Nevertheless, a reduced N2 in change-trials seem difficult to reconcile with increased pre-SMA activity.
The present study aimed to address some of these differences to provide a better understanding of the mechanisms enabling us to stop or switch a motor response. To accomplish this, we applied a combined stop- and change-signal task with arbitrary stimulus-response associations and equal probabilities of stimuli. We recorded EEG with 64 electrodes and used Laplace transformation of the event-related potentials (ERPs) to improve spatial resolution (Babiloni et al., 1995) and more accurately define frontal and parietal contributions. Laplacian transformation, in addition to its advantage of being reference-free, has been proven useful to improve localization of both sensory and response- or feedback-related processes (Tenke and Kayser, 2012). Previously studied effects include auditory and visual N1, N2, error-related negativity and P3 (Babiloni et al., 2004; Cavanagh et al., 2009; Kayser et al., 2009; Kayser et al., 2010). Laplacian transformation was also used in a recent paper by our group on stop-signal data in frontal lesion patients. As explained above, stop-signals typically elicit a broad, centrally maximal N2 around 200 to 250 ms after the stop-signal. Laplacian transformed stop-signal data revealed two distinct effects in the same time-range, a negativity with a parieto-occipital maximum around 180 ms and a negativity with a frontocentral maximum peaking around 220 ms (Krämer et al., 2013). In this study, visual stop-signals were presented lateralized and the parieto-occipital negativity was found to be maximal contralateral to the stimulus presentation consistent with activity in extrastriate visual areas. Importantly, the parieto-occipital effect was found to be reduced in frontal lesion patients whereas patients showed an increased frontal negativity over the intact hemisphere (Krämer et al., 2013). Although Laplacian transformation is hence clearly useful to study condition- and group-differences, there are other challenges of stop-signal EEG data that it might not be able to resolve, namely the strong overlap between go- and stop-ERPs. Go- and stop-signals are typically presented in rapid and jittered succession of only a few hundred milliseconds which results in stop-ERPs being influenced by the preceding stimulus (Krämer et al., 2011). Methods to correct for this overlap such as ADJAR or similar (Krämer et al., 2011; Woldorff, 1993) will compensate for this to some extent but not fully and are moreover based on the assumption of purely additive effects.
Here, we present an alternative approach to standard ERP analyses of stop-signal data by performing independent component analysis (ICA) of the surface potentials. ICA is a method of mathematical blind source separation which decomposes the EEG signal from a number of electrodes into the same number of statistically maximally independent time-courses (Makeig et al., 2004; Onton et al., 2006). The output of an ICA is a matrix with the independent components’ time-courses and a weights matrix which reflects the independent components’ spatial distribution across electrodes (Onton et al., 2006). ICA has been proven useful to study the contributions of the error-related negativity (ERN) to the feedback-related negativity (Gentsch et al., 2009) and the correct-related negativity (Roger et al., 2010), to relate the single-trial ERN to fMRI data (Debener, 2005) and to study connectivity in the SSP (Huster et al., 2014). It has also previously been suggested as alternative method to Laplacian transformation for “deblurring” EEG data (Foffani et al., 2004). Although other decomposition approaches as PCA or a combined CSD-PCA approach would have been possible (Kayser and Tenke, 2006a; Kayser et al., 2009), we decided to apply ICA to allow for more direct comparisons with other recent ICA studies on the stop-signal task (Huster et al., 2013; Wessel and Aron, 2014). Here, we applied ICA to examine the contribution of stop-signal related components to change-signal activity and to study the relevant components’ single-trial activity relative to motor responses.
In summary, we aimed to resolve previous inconsistent findings regarding stopping or changing a motor response (Coxon et al., 2007; Kenner et al., 2010; Krämer et al., 2011). Whereas fMRI-data pointed to a similar network recruited in both types of response control (Boecker et al., 2011; Kenner et al., 2010), EEG-results suggested different mechanisms invoked by stop- and change-signals (Krämer et al., 2011). The studies differed, though, in aspects of response selection difficulty and probability of trials with incongruent stimulus-response mapping. In the present study, we used stimuli with weaker response association and did not include flanker stimuli. We focused on the stop-N2 as electrophysiological marker of response control and improved SSP analyses by applying Laplace transformation and by performing independent component analysis of the surface ERPs.
2. Material and methods
2.1 Participants
The study included 22 right-handed participants. As we implemented a tracking algorithm in the stop-change paradigm aiming for 50% inhibition rates (see below for explanation), data of four participants were rejected because they had less than 40 % (three participants) or more than 60 % (one participant) failed stop- or change-trials. Two participants were excluded because of extensive EEG artifacts. The final sample consisted of 16 participants (age range 18 – 38 y, mean: 23.1; 9 women). All volunteers were recruited from the student population of the University of California in Berkeley.
The participants gave informed consent and received money for participation (US$10 per hour). None of the participants had psychiatric or neurological disorders (self-report). The study was performed in agreement with the Declaration of Helsinki and approved by the ethics committee of the University of California, Berkeley.
2.2 Design and Stimuli
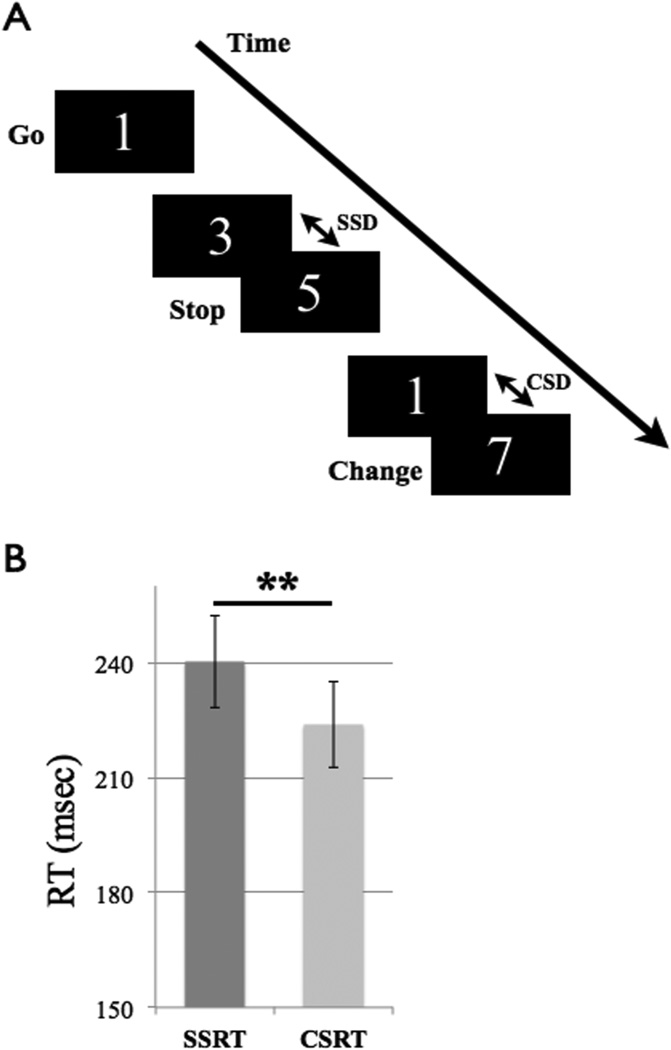
The stimuli were numbers from 1 to 9, with numbers from 1 to 4 indicating to press the left button of a mouse, and numbers from 6 to 9 indicating to press the right button (GO condition). Participants used their left and right index fingers for button presses. Each number appeared with the same probability. In stop-trials, the number 5 was presented after a short delay, indicating to withhold the response. In change-trials, a high (low) number was presented shortly after a low (high) number, indicating to respond with the left (right) instead of the right (left) mouse button. A schematic representation of the task is shown in Figure 1A.
Figure 1. Task and behavioral results.
A Schematic representation of the task, showing the three conditions, Go, Stop and Change (SSD stop-signal delay; ChSD change-signal delay). B Results for the Stop Signal Reaction Time (SSRT) and Change Signal Reaction Time (CSRT), which differed significantly (** p < 0.001). Error bars denote standard errors.
The participants performed two practice blocks (168 trials each) with the first including only go-trials and the second including go-, stop- and change-trials. The experiment proper consisted of 12 blocks, each one including 168 trials, of which 71.4 % of the trials were go-trials, 14.3 % stop-trials and 14.3 % change-trials. This yielded 1440 go-trials, and 288 trials each of both stop- and change-trials. Regarding stimulus probabilities, the stop-stimulus (letter “5”) appeared 288 times, whereas every other letter appeared 252 times in total during the experiment.
The duration of each stimulus was 100 ms and the inter-trial-interval (ITI) varied randomly between 1.3 s to 1.7 s. The stop and change signal delay was adjusted based on a staircase-tracking algorithm. The signal delay started with 140 ms and increased by 10 ms after a successful and decreased by 10 ms after failed trials. Such algorithm was implemented to yield 50 % successful stop- and change-trials (adapted from De Jong et al., 1995).
2.3 Procedures
The experiment was performed using the Presentation® software (Version 11.3). Stimuli were presented in the center of a 17” screen, about 1m away from the participant. After each of the experimental blocks the participant had a short break.
Participants were instructed to be as fast and accurate as possible. Participants were told that the task was designed in such a way that made it impossible to be successful in every stop and change trial. This was done to prevent participants from slowing down to the primary go-task.
2.4 EEG recordings and data preprocessing
The electrophysiological recordings were performed using 64 active scalp electrodes, with the BioSemi Active2 system (BioSemi, Amsterdam, The Netherlands). Electrodes were placed according to an extension of the International 10–20 system (Nuwer et al., 1998). Vertical and horizontal eye movements (VEOG and HEOG) were recorded, the latter using electrodes located on the outer canthus of each eye, and the former using electrodes placed below and above the right eye. Reference electrodes were placed on the right and left earlobes. The sampling rate was 512 Hz.
2.5 Behavioral data analyses
Reaction times and failed trial rates were calculated for the go, stop-and change-conditions. SSRT and CSRT were computed based on the distribution of reaction times in go-trials and the average stop and change signal delays (SSD and ChSD; Logan et al., 1984). First, the reaction times in go-trials are rank ordered; second, the reaction time that corresponds to the nth centile is chosen with n being the probability to respond in stop- (change-) trials; and third, the average of the SSD (or ChSD) is subtracted from the nth reaction time. The SSRT and CSRT were subjected to a paired samples t-test.
2.6 EEG data analyses
EEG data analysis was performed with EEGlab (Delorme and Makeig, 2004) and custom written Matlab scripts. EEG data was re-referenced to the average of the signal from the two earlobe electrodes, resampled to 256 Hz, and high-pass filtered with 0.5 Hz. The data were epoched for the different conditions (go, change- and stop-trials). Epochs included one second before and two seconds after the stimulus. The baseline was defined as the 100 ms preceding the stimulus. An Independent Components Analysis (ICA) was performed on the epoched data including all conditions. Independent components accounting for blink artifacts were visually identified and removed from the data (Delorme et al., 2007; Jung et al., 2000a; Jung et al., 2000b). Trials affected by other artifacts caused e.g. by muscle tension were rejected from further analysis.
2.6.1 Event Related Potentials (ERPs)
The ERP analysis focused on identifying the differences in processing the stop and change stimuli; since this processing may be affected by the GO signal presented previously, a technique similar to the ADJAR proposed by Woldorff (1993) was used. In contrast to the procedure suggested by Woldorff, the correct GO trials were divided in fast and slow trials (division made based on the SSRT and CSRT), these trials were shifted from their original distribution according to the stop/change signal delays. The resultant ’delay-corrected’ go-ERPs were subtracted from the successful and failed stop/change trials (fast GO trials with failed and slow GO trials with successful stop/change trials). The difference ERPs reflect the processing of the stop/change signal regardless of the GO signal presented before (Krämer et al., 2011). In order to increase the spatial resolution and distinguish frontal and parietal contributions to stop-signal processing, Current Source Density (CSD) was estimated using Laplacian transformation based on spherical splines interpolation with a spline order of 4 (Kayser and Tenke, 2006b). We used the CSD toolbox developed by Jürgen Kayser (2009). We present the data before and after Laplacian transformation to demonstrate how this approach helps distinguishing frontal and parieto-occipital activity in the N2 time-range. This was done for go- and inhibited stop-trials in which the effect of Laplacian transformation can best be evaluated.
To assess the stop-related activity over parieto-occipital and frontocentral sites, we ran repeated measures ANOVAs of the mean amplitude in go- and inhibited stop-trials for the early parieto-occipital negativity (150 – 200 ms) and later differences (200 – 250 ms). To assess statistical differences for our main comparison of interest, mean amplitudes were computed for every participant and the stop- and change-conditions (separately for successful and failed trials) during time-windows of interest. To accurately analyze the spatial distribution of the effects and to increase signal-to-noise ratio, we formed clusters of 4 electrodes each. We performed separate repeated measures ANOVAs for the early and late task effects. The early negativity ANOVA was applied for two lateral posterior clusters (left/right), and the late negativity ANOVA for the three frontal clusters (left/center/right), both with Stop (stop-all/stop-change), Inhibition (failed/inhibited) and Laterality as within-subject factors.
For all statistical effects involving more than one degree of freedom in the numerator, the Greenhouse-Geisser correction was applied to correct for possible violations of the sphericity assumption (Greenhouse and Geisser, 1959). We report the uncorrected degrees of freedom, corrected probabilities and the ε-value.
2.6.2 Independent component analysis
Analysis of stop- or change-signal related ERPs is complicated by the fact that go-signal and stop-/change-signal ERPs are overlapping as the stimuli are presented in rapid succession. Moreover, ERP components as the stop-N2 likely result from several brain processes, such as visual attention to the stop-signal or response inhibition. Computing difference waves (see above) and performing Laplace transformation of the ERPs are first steps to deal with these problems. However, these methods are limited, as they are prone to effects of noise and have not generally been used for analyses of single-trial data with respect to single-trial behavioral performance. Here, we followed an alternative analysis approach by using independent component analyses of the stop- and change-signal data. Note that ICA was performed on surface-potentials and not on Laplacian transformed data.
We performed independent component analysis (ICA) in two steps. First, we applied ICA to identify blink-related components (see EEG data analysis paragraph above), removed respective components and performed artifact rejection on the data that was uncontaminated by blinks. Second, we performed a spatial principal component analysis on the artifact-free data-sets to reduce the data dimensionality to 30 components and ran a second ICA. ICA was computed on the stimulus-locked data from all conditions. ICA has frequently been applied to EEG analysis, particularly in order to remove artifacts. However, there is no consensus on how to best use ICA in group studies, i.e. how to compare independent components across subjects. As ICA has mostly been done on single-subject level, this yields a different component structure in each subject. Previous EEG-ICA studies all used different methods to select comparable ICs in single subjects to then compare them across subjects with respect to condition differences. Critically, the suitable method to identify relevant components across subjects depends also on the kind of research question or components of interest. For instance, most studies using ICA in group studies focused on the error-related negativity (ERN). These studies used a set of well-established criteria characterizing the ERN (topography, latency, differences between failed and succeed trials) to identify the component(s) of interest (Debener, 2005; Gentsch, et al., 2009; Roger, et al., 2010) and study their single-trial activity or differences between conditions.
Here, we used ICA to examine components that are related to response inhibition. Such components should show stronger activity in successful stop-trials compared to go-trials in the relevant time-window (~ 0–300 ms after stimulus-presentation). In the first step, we compared for each participant and component the activity in successful stop-trials and go-trials with a non-parametric test (Wilcoxon rank-sum test). Note that change-trials were not included in the selection of ICs. This comparison was done for three consecutive time-windows (each 100 ms length) after stimulus presentation by comparing the average amplitude in these time-windows. We evaluated the first 20 components in each participant (sorted by explained variance of the signal) and accordingly corrected for multiple comparison (20 components and 3 time-windows) using a Bonferroni correction. To compare results across participants, we clustered the identified components using clustering functions of EEGlab (Delorme & Makeig, 2004). First, an N-dimensional distance matrix is created based on selected component measures, which are reduced to N dimensions using PCA. For the present study, we considered the components’ ERPs and the equivalent dipole model solution as relevant measures. Second, the components are clustered using the k-means algorithm of MATLAB (MathWorks, Natick, MA). The k-means algorithm partitions the components into k clusters with the goal to minimize across all clusters the sum of each component’s distance within a cluster to the cluster’s centroid. We included only those components with a dipole solution with less than 10% residual variance. The dipoles were fitted using the dipfit2 toolbox implemented in EEGlab (provided by Robert Oostenveld, Donders Institute, Nijmegen), assuming a boundary element head model (Oostendorp and van Oosterom, 1989).
3. Results
3.1 Behavioral data
Participants had an average reaction time of 617 ms (s.d.± 54) in go-trials and had 6.7 % (s.d.± 5.7) of failed trials. Participants were slower in the correct go-trials than in the failed stop- and change-trials (stop-all: t15 = 9.44, p < 0.001; change: t15 = 20.37, p < 0.001), but significantly faster than in successful change trials (t15 = 42.13, p < 0.001). In stop-trials, the average reaction time of failed trials was 543 ms (± 63) and the percentage of failed trials was 48 % (± 3). In change-trials, the average reaction time of failed trials was 515 ms (± 46), and 981 ms (± 75) in successfully changed trials. Even when subtracting the ChSD from the reaction times for successful changed trials (yielding 638 ms ± 44) to estimate the reaction time relative to the change signal, the participants were faster in the go-trials than in successful change trials (t15 = 2.21, p < 0.05). The percentage of failed change-trials was 49 %.
The results indicate that the inhibitory process in the change condition (CSRT = 224 ms± 26) was faster than in the stop condition (SSRT = 240 ms ± 29; t15= 6.72, p < 0.001; see Figure 1B).
3.2 Event Related Potentials (ERP)
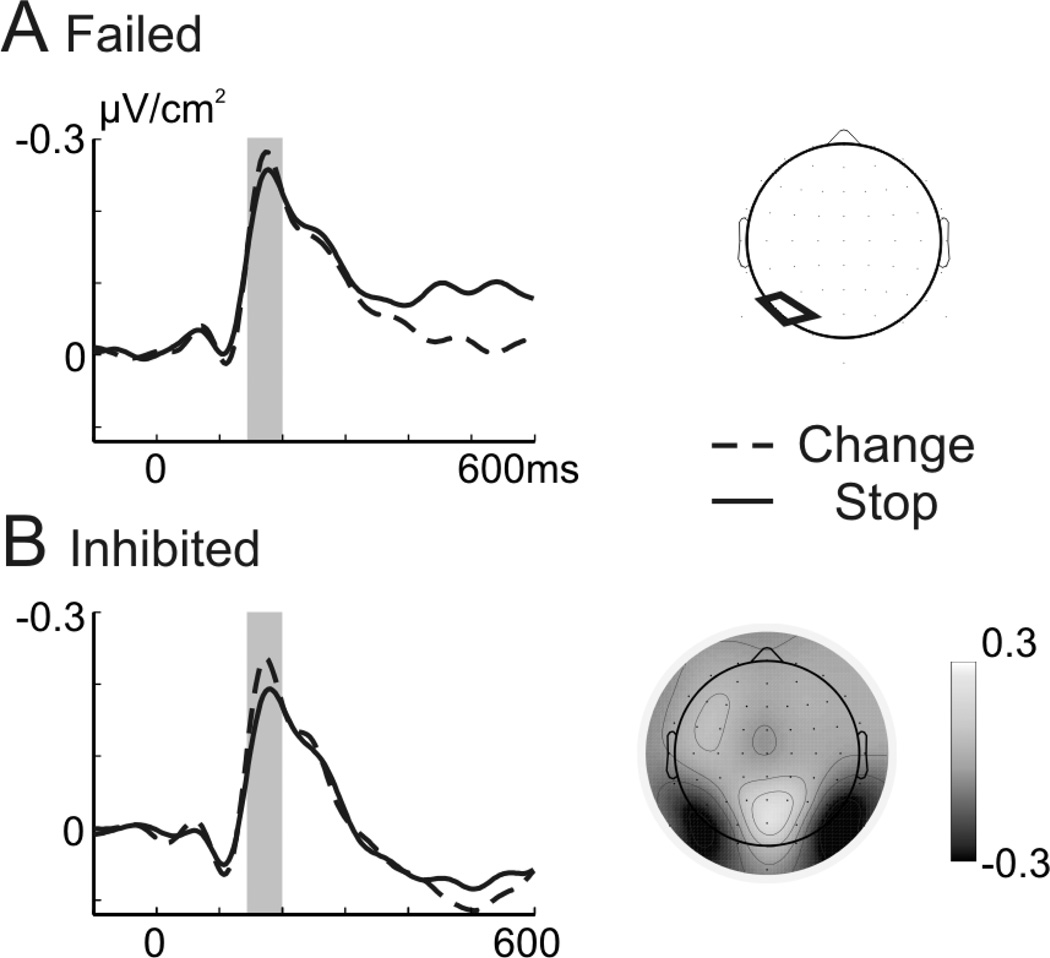
We first compared inhibited stop- with go-trials to evaluate the effect of Laplacian transformation on the N2 in terms of its topography and specificity to stop-trials. As can be observed in Figure 2B, surface potentials show the typical, broadly distributed N2 to stop-signals. The N2 amplitude is increased relative to go-trials in most electrodes as can be seen in the topography on the right side (Figure 2). Notably, go- and stop-trials differ already before the N2, beginning with stimulus onset, likely caused by remaining overlap with go-stimulus ERPs. In the CSD data, the frontocentral and parieto-occipital negativity can be distinguished (Figure 2A) and go- and stop-signals show less differences before the N2 time-window. Comparably to go-trials, stop-signals showed a strong negativity (visual N1 sink) over parieto-occipitals electrodes (maximal around 180 ms). Stop-signals elicited an increased negativity over lateral parieto-occipital and frontocentral electrodes (200 – 250 ms).
Figure 2. Surface and Laplace ERPs of go- and inhibited stop-trials.
Shown are the ERPs without current source density transformation (B) and after CSD transformation (A), both for go- (black) and inhibited stop-trials (red). The time-point 0 ms refers to onset of the go-stimulus for the go-trials and to the onset of the stop-stimulus for the stop-trials. The upper row shows the data of the midfrontal electrode cluster and the middle row shows the signal of the left parietal electrode cluster (indicated on the map on the right side). The grey bar indicates the analyzed time-window for the fronto-central electrodes (200 – 250 ms) and the parietal electrodes (150 – 250 ms). The lower row depicts the topography of the difference between inhibited stop- and go-trials between 180 and 220 ms for CSD ERPs (left side) and surface ERPs (right side).
To analyze these effects, we performed repeated measures ANOVAs with the factors Condition (Go vs. Stop inhibited) and Laterality (left vs. right parietal cluster) for the earlier (150 – 200 ms) and later time-window (200 – 250 ms) on the CSD data. Activity in stop-signals did not differ from go-signals in the early time-window (main effect Condition: F1,15 = 1.2, p = 0.285; Condition × Laterality: F1,15 = 2.7, p = 0.12), but was increased in the late time-window (Condition: F1,15 = 17.26; p = 0.001). We also performed an ANOVA for the frontocentral N2 sink (200 – 250 ms) with the factors Condition (Go vs. Stop-inhibited) and Laterality (left, midfrontal and right). This resulted in a significant main effect of Condition (F1,15 = 18.28; p = 0.001) and interaction of Condition with Laterality (F2,30 = 5.55; p = 0.013, ε = 0.851) reflecting an increased effect of central and right electrode sites. Summarizing, these results show the advantage of Laplacian transformation for sharpening the waveforms and distinguishing frontocentral and parietal stop-related effects as well as replicating the stop-related frontocentral N2 sink. In the following, we compared stop- and change-related Laplacian potentials in the N2 time-range to address our main research question.
Both stop- and change-signal related ERPs showed the pronounced negativity peaking around 180 ms over parieto-occipital electrodes bilaterally and, slightly later around 220 ms, over frontocentral electrodes (Figure 3). To assess condition effects during these time-windows, we subjected average amplitudes to repeated measures ANOVA separately for the early (150 – 200 ms) and late time-window (200 – 250 ms). The first ANOVA comprised the factors Stop (stop vs. change), Inhibition (succeed vs. failed trials) and Laterality (left vs. right parietal cluster). For the second time-window, we ran one ANOVA with the same factors to assess parieto-occipital effects and one ANOVA comprising the same factors, but the Laterality factor had three levels (left, central and right frontal cluster) to assess differences in the frontal N2 sink. Greenhouse-Geisser correction was applied when required (Greenhouse and Geisser, 1959).
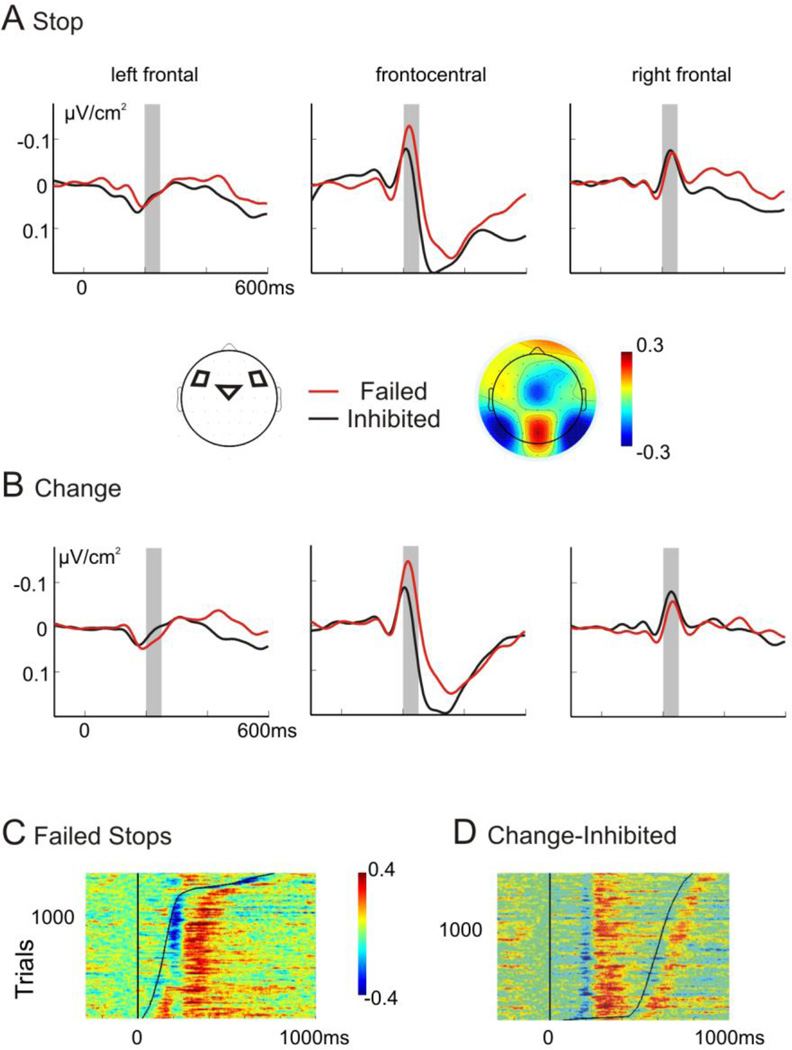
Figure 3. Stop- and change-signal-locked Laplace-ERPs.
Shown are the ERPs for Change (dashed lines) and Stop trials (solid lines) for the left posterior electrode cluster, separately for failed (A) and successful (B) trials. The time-point 0 ms refers to the stop-/change-stimulus onset. The time-window used for the analysis, 150 to 200 ms, is highlighted in grey. On the right side, the location of the posterior electrode cluster is indicated and topographical map of the average amplitude between 150–200 ms is presented for successful stop. A 15 Hz low-pass filter was applied for plotting purposes, but not for the analysis.
The ANOVA of the early posterior time window revealed a slightly higher negativity in failed compared to successful trials (Figure 3), yielding a significant main effect of the factor Inhibition (F1,15= 4.58, p = 0.049). Additionally, an interaction of Stop × Laterality (F1,15= 4.78, p = 0.045) demonstrated an increased negativity in change-compared to stop-trials over left parietal areas (Stop: F1,15 = 5.13, p = 0.039; right cluster: F < 1). The ANOVA on mean amplitudes at parietal electrodes in the later time-window (200 – 250 ms) did not yield any significant condition effects (main effect Condition: F1,15 = 0.33, p = 0.57; interaction Condition × Laterality: F1,15 = 1.049, p = 0.33).
For the frontal N2 sink, we analyzed data from the three frontocentral electrode clusters (Figure 4). The negativity could be observed over the right and central cluster, but was not detectable over the left hemisphere (right cluster: −4.9 µV vs. left cluster: 2.7 µV; main effect Laterality: F2,30 = 8.01, p = 0.005, ε = .717). The effect differed between successful and failed trials depending on the electrode location (Inhibition × Laterality: F2,30 = 4.56, p = 0.045, ε = .553). Over right frontal areas, the negativity was enhanced in successful compared to failed trials (F1,15 = 6.21, p = 0.025), but no differences were observed over left electrodes (F1,15 = 1.26, p = 0.28). Over central electrodes, we detected a tendency for an increased negativity in failed compared to successful trials, but this did not yield significance (F1,15 = 3.20, p = 0.09) due to a larger amplitude variability at these electrode sites. Main effect of Condition (F < 1) and interaction with Inhibition (F < 1) or Laterality (F < 1) were not significant. To assess the consistency of the N2 sink across trials, its temporal relationship to the stop-stimulus and responses and to allow comparisons with the ICA results, we also plotted single-trial data across all subjects of the failed stop- and inhibited change-trials sorted by response time (relative to the stop-/change-stimulus; Figure 4C and D). The N2 sink shows a clear temporal relationship to the stimulus and not the response although this is less clear in failed stop-trials.
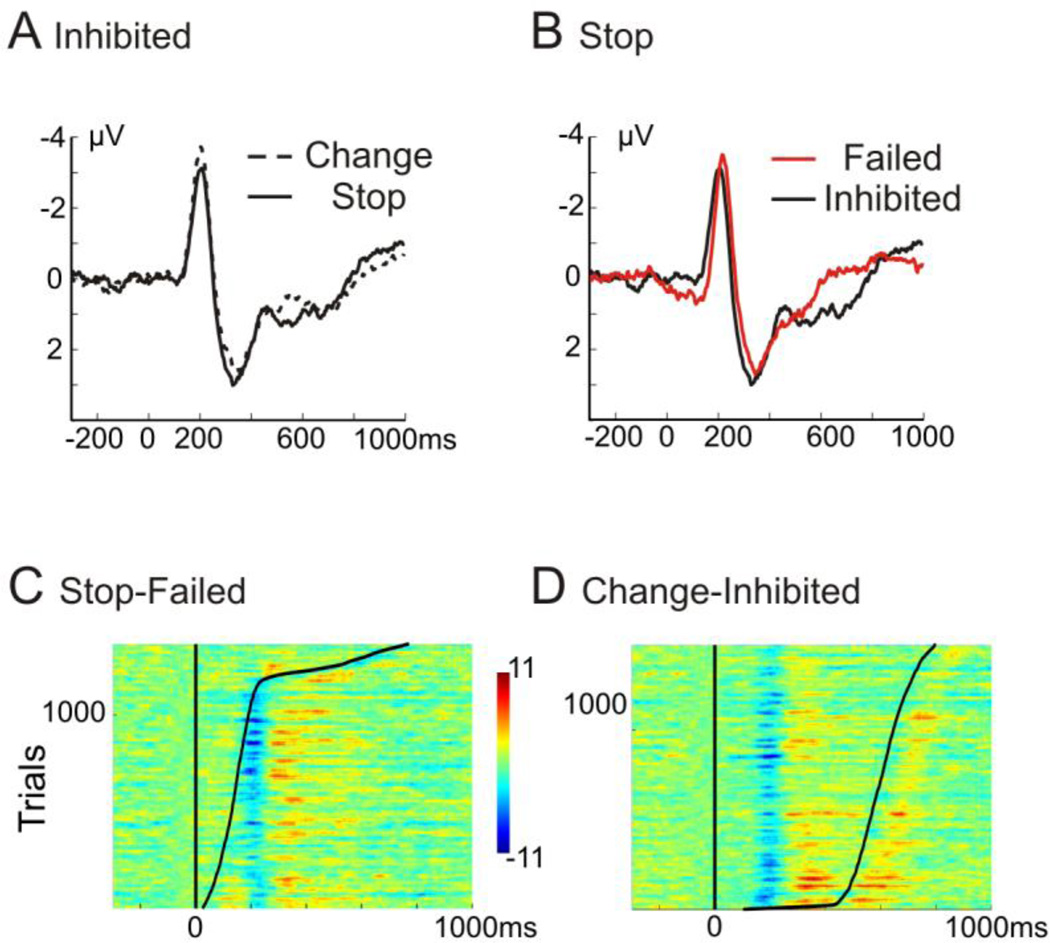
Figure 4. A Stop-signal-locked Laplace-ERPs.
Shown are ERPs for the left, frontocentral and right frontolateral electrode cluster, separately for failed (red) and successful trials (black). The analyzed time-window (200 – 250 ms) is highlighted with a grey frame. The topographical map of the mean amplitude in the same time-window for successful stop-trials is shown. B Change-signal locked ERPs for left, frontocentral and right frontolateral electrode clusters, separately for failed (red) and successful trials (black). C+D Plotted are single-trial Laplacian ERPs for all stop-failed (C) and change-succeed (D) trials across all participants (~ 1400 trials) at electrode Cz, sorted by reaction time (relative to stop-/change-stimlus; using a smoothing window of 20 trials), which is depicted with a solid black line.
3.3 Independent component analysis
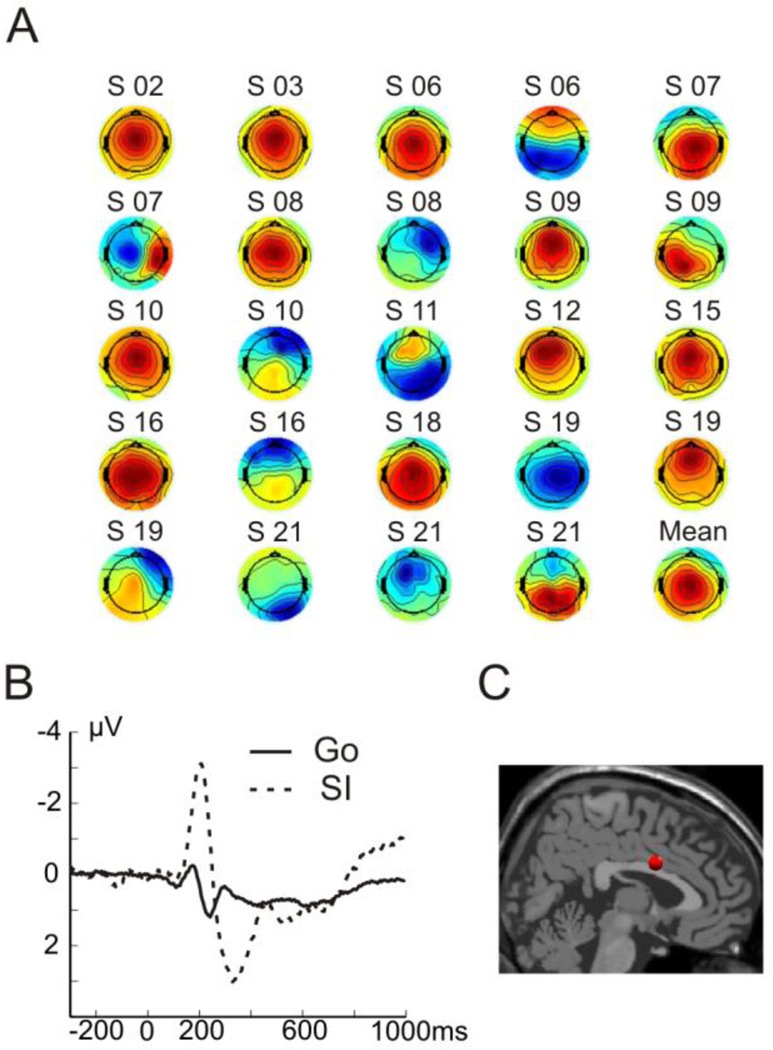
The ICA was performed on surface potentials (see methods). The comparison of stop-signal and go-signal ERPs for each participant’s components resulted in 2 to 7 significant components per participant. Visual inspection of the components highlighted one component, characterized by a mediofrontal maximum and a prominent negativity around 200 ms, which was present in most participants. This observation was supported by the k-means clustering algorithm. When clustering the components into four clusters (corresponding to the average number of components per subject), we identified one mediofrontal cluster which comprised 24 components from 14 (out of 16) participants (Figure 5A). The average topography showed a maximum over central electrodes, the centroid of estimated dipoles was in the anterior cingulate gyrus and the cluster’s time-course was characterized by a negativity peaking around 200 ms and an ensuing positivity maximal around 330 ms (Figure 5). For visualization purposes, Figure 5B shows the average backprojected time-course of the mediofrontal cluster’s components at electrode Cz for successful stop-trials and go-trials. As the components were selected based on statistical comparisons between Go and Stop on the single-subject level and as the components’ time-course is consistent across participants, the ERPs in the two conditions differ also on the group level. The other three clusters showed maximum activity over posterior electrodes with centroids of estimated dipoles in the superior temporal gyrus or precuneus (supplementary material, Figure 1). As these clusters each comprised less than two-thirds of participants, they were not further considered for statistical comparisons between conditions.
Figure 5. Results of the independent component analysis.
A Shown are the topographic maps for the components included in the mediofrontal cluster with the participants’ codes noted above each component. Note that the direction of the maps (positive vs. negative) is arbitrary. The directionality of the component’s activity is split into the weight matrix (spatial map) and the time-course. B Average time-course of the depicted components back-projected to electrode Cz, separately for go-trials (solid line) and successful stop-trials (SI, dashed line). C Centroid of the estimated sources of the included components shown in anterior cingulate cortex.
In the next step, we examined whether the identified mediofrontal cluster’s components differentiate between conditions of stop- and change-conditions and between failed and successful inhibitions (Figure 6A & B). We averaged the amplitude in a 100 ms time-window around the maximum of the negativity (150 – 250 ms), backprojected to electrode Cz, and subjected it to a repeated-measures ANOVA with the factors Stop (stop-all vs. change) and Inhibition (failed vs. inhibited). Note that these analyses included only the 14 participants who were represented in the mediofrontal cluster. If more than one component of a participant was included in the cluster, we back-projected both components’ time-courses. The mediofrontal negativity did not differ between stop- and change-trials or between failed and successful inhibitions (Condition: F1,13 = 2.02, p = 0.18; Inhibition: F1,13 = 1.66, p = 0.22; Condition × Inhibition: F1,13 = 0.608, p = 0.45; Figure 6A & B). As can be observed in Figure 5, while most components show a clear frontocentral topography, some have a different, more lateralized topography. We also performed the statistical analyses with only including those components with a clear frontocentral topography. This analysis did not show significant differences between stop- and change-conditions (F1,13 = 3.15, p = 0.1) or between failed and successful inhibitions (F1,13 = 2.32, p = 0.15).
Figure 6. ICA results for stop- and change-trials.
A Average time-course of the mediofrontal cluster’s components back-projected to electrode Cz, separately for stop-succeed (solid line) and change-succeed trials (dashed line). B Shown is the average time-course of the mediofrontal cluster’s components back-projected to electrode Cz, separately for stop-succeed (black line) and stop-failed (red line). C+D Plotted are single-trial ERPs for all stop-failed (C) and change-succeed (D) trials across all participants (~ 1400 trials) from the mediofrontal components, sorted by reaction time (relative to stop-/change-stimulus; using a smoothing window of 20 trials), which is depicted with a solid black line. The negativity at 200 ms is clearly stimulus-related and maximal shortly after or around the erroneous motor response.
The latency of the mediofrontal cluster negativity, about 200 ms, is in line with a critical role in inhibitory motor control, considering an SSRT and CSRT of about 220 – 240 ms. In this case, one might expect enhanced activity or a shorter latency in successful compared to failed inhibitions, which we did not observe. Alternatively and in line with the Horse-Race Model of inhibition (Logan et al., 1984) and previous electrophysiological data (Krämer et al., 2011), one might argue that the success of the inhibition depends more on the speed of response preparation. In failed stop- or change-trials, the response would have been executed before the maximum of the mediofrontal activity. To examine this, we plotted single-trial activity of the mediofrontal components sorted by reaction times (relative to the stop-/change-stimulus) in failed stop-trials (Figure 6C). This was done across all 14 participants, yielding about 1400 total trials. As can be assessed from the figure, the negativity was maximal at the time of or after the motor response and reduced or absent in trials with late responses (reaction times > 300 ms). Notably, the reaction time distribution shows a considerable number of responses later than 300 ms after the stop-signal, i.e. after the SSRT. The horse-race model predicts fast errors when the response preparation was faster than the inhibition process. Slow errors or failed inhibitions on the other hand might be rather caused by slips of attention. We also plotted the negativity in successful change trials (Figure 6D) which confirmed the stimulus-locked activity around 200 ms and no evident relationship to the reaction times of the succeed responses. The direct comparison of single-trial CSD-data (Figure 4C–D) and single-trial ICA data (Figure 6C–D) revealed a similar pattern. In both cases, the negativity around 200 ms showed a clear relationship with the stimulus and not the response time. However, the negativity in failed stops was less consistent in single-trial CSD data compared to the ICA based data which underscores the strength of the ICA approach for single-trial analyses.
4. Discussion
In this study, we sought to identify the underlying brain mechanisms engaged when stopping a response or switching to an alternate response. We compared neural activity elicited by stop-vs. change-signals and focused on the stop-N2 as a neural correlate of response inhibition. We observed a faster CSRT than SSRT, but no differences were found between the neural correlates of stopping and changing a response. There are several noteworthy results of the EEG analysis. Laplacian transformation of the data allowed direct comparisons of stop- and go-ERPs and revealed comparable amplitudes of the visual N1 sink but increased activity for stop-trials over lateral parietal electrodes between 200 – 250 ms and an increased stop-N2 sink over frontocentral sites. Neither of these components showed differences between trials that required stopping vs. changing the response. Finally, independent component analyses supported the notion of an important role of mediofrontal structures in response control.
4.1 Behavioral results
The latency to inhibit in change- and stop-trials differed significantly as previously reported by Krämer and colleagues (2011) with the CSRT being faster than the SSRT. It should be noted that the SSRT and CSRT are assumed to only estimate the latency of the inhibition (Logan et al., 1984; Verbruggen and Logan, 2008), but not the additional response selection required in change-trials. As all stimuli were almost equally probable, any behavioral or electrophysiological differences between stop- and change-trials cannot be explained by stimulus probabilities. Previous studies reported differences in the opposite direction, with CSRTs higher than SSRTs (e.g., Coxon et al., 2009; van de Laar et al., 2010). This indicates that behavioral differences depend on the specific task demands. If the task implies withholding one response but not another (Coxon et al., 2009) or if the change implies bimodal responding (i.e. inhibiting the manual response but executing pedal response), the CSRT appears to be higher than the SSRT (De Jong et al., 1995). This shows that the CSRT depends on whether the alternative response is part of the current primary task-set or not. Generally, most previous studies with the change-signal task used a change-response that was not used in go-trials (Boecker et al., 2007; De Jong et al., 1995; Logan and Burkell, 1986). The probability of the change-response in these paradigms hence corresponded to the probability of stopping the response. In the current paradigm, the response required in change-trials corresponded to responses in go-trials. The overall probability of the responses given in change-trials was thus considerably higher (here: 42% of either right- or left-hand response) compared to stopping the response (14%). Studies directly comparing these different change-conditions are needed to answer whether response probabilities are sufficient to explain CSRT and SSRT differences. However, an influence of the nature of the change-response on the CSRT would argue against the assumption of serial processes in change-trials and point to parallel processing of stopping and preparation of the second response (Verbruggen et al., 2008). Finally, whether stop- and change-trials are presented in the same blocks as in the present study or in alternating blocks (e.g. Kenner et al. (2011)) may additionally contribute to SSRT vs. CSRT differences.
4.2 Electrophysiology of motor inhibition: Laplacian ERPs and ICA
We presented two ways to examine stop-signal ERPs by applying Laplace transformation of the data and by performing independent component analyses. Laplace transformation improves the spatial resolution of EEG, especially in combination with higher density recordings (> 64 electrodes) as in the present data set (Babiloni et al., 1995). This analysis helped to distinguish bilateral parietal and frontocentral contributions to stop-activity. The parieto-occipital negativity has been linked to perceptual processing of the attended and task-relevant stop-signal and likely emanates from extrastriate visual areas and/or inferior parietal cortex (Krämer et al., 2013; Schmajuk et al., 2006). The parieto-occipital negativity has previously been reported in Laplace transformed stop-signal data (Krämer et al., 2013). In this study, the negativity was strongest contralateral to the laterally presented stop-signals and was reduced in patients with focal frontal lesions. This is consistent with activity in extrastriate areas which is modulated by prefrontal cortex. These results converge with fMRI-findings of enhanced inferior parietal activity in response to stop- and nogo-signals, especially if they are task-relevant (Boehler et al., 2010). The posterior negativity was slightly enhanced in failed compared to successful trials under both stop- and change-conditions, which was unexpected if assuming that it reflects orienting of attention towards the stop-signal. It might be that the computation of difference waves controls only partly for overlap with go-signal ERPs, which causes a shift of ERPs in failed and successful trials because of baseline differences. Additionally, we observed a difference in lateralization of the parietal negativity between stop- and change-trials such that stop-trials showed a slightly right-lateralized negativity, whereas no lateralization was evident for change-trials. This resulted in an increased negativity for change-compared to stop-trials over left parietal areas. A right-lateralization is consistent with fMRI results of a right-lateralized network in stop-trials (Aron et al., 2004; Rubia et al., 2001; Wager et al., 2005).
Besides the posterior negativity, Laplacian potentials revealed a frontocentral negativity around 230 ms. The frontal N2 sink was maximal at central electrodes (Cz) and higher over right than left frontal areas. Previous Laplacian data of the stop-signal paradigm also reported a fronto-central N2 (Krämer et al., 2013). In this study, healthy controls presented a centrally maximal N2 to stop-signals whereas patients with focal frontal lesions had an increased N2 in their intact hemisphere, ipsilateral to the side of the stop-stimulus. This increased frontal N2 was interpreted as compensatory activity (Krämer et al., 2013). Interestingly, successful trials elicited in the present study an enhanced N2 over right frontal electrodes, whereas failed trials tended to elicit a larger N2 over central electrodes. Again, inspection of the ERPs suggested differences between failed and successful trials before the N2, likely due to differences in go-ERPs (see Krämer et al., 2011 for further discussion of this point), which renders interpretation of the effects difficult.
One approach to overcome these limitations is an independent component analysis, which decomposes the EEG signal into components with maximally independent time-courses, supposedly reflecting distinct brain processes (Makeig et al., 2004; Onton et al., 2006). ICA results of the present study highlighted a component with a central maximum and a time-course characterized by a negativity at 200 ms and ensuing positivity at 330 ms. The centroid of the components’ estimated dipoles was located in the anterior cingulate cortex. This component was identified based on the comparison of go- and successful stop-trials and showed clear stop-signal specific activity. However, the component did neither distinguish failed and successful inhibitions nor stop- and change-trials. Additionally, components with a parietal or occipital maximum significantly differed between stop- and go-trials. However, these components appeared less consistent across participants, resulting in few components and participants in respective clusters. This makes statistical comparisons across participants difficult and we refrained from further analyses of these components. This shows a limitation of the ICA approach as analyses of condition effects require the comparison and grouping of components across participants (see Huster et al., 2014 for a different approach). As previously mentioned, different approaches have been suggested on how to best perform ICA group analyses of EEG data (Onton et al., 2006). Here, we chose a step-wise procedure by first identifying within participants components which differ between go- and successful stop-trials and then clustering the specified components across participants.
As alluded to in the introduction, ICA and Laplacian transformation are very different analyses methods which make different assumptions. As shown here and in previous work (Krämer et al., 2013), Laplacian transformation resulted in a sharpening of stop-signal related neural effects and helped to specify differences between go- and stop-signal processing as well as differentiating parieto-occipital and frontocentral stop-signal effects within the same time-window. Together with previous reports of differential effects of prefrontal lesions on parietal and frontal inhibition-related ERP effects which were observed after Laplacian transformation (Krämer et al., 2013), these results strongly advocate Laplacian transformation for (high-resolution) EEG recordings of the stop-signal paradigm. However, Laplacian signals of the stop-signal data still depend on the correction for overlap with go-ERPs through ADJAR-like procedures. As could be observed in the current data, the correction might not work perfectly resulting in remaining unspecific condition differences (cf. Figure 4). ICA on the other hand aims to identify distinct, statistically independent neural processes and does not depend on such correction. With this approach it was possible to separate frontocentral and parietal effects in distinct component cluster. The observed frontocentral component cluster showed temporally more specific activity which could best be assessed in the single trial time-courses (cf. Figures 4 and 6). As can be observed when comparing Figure 4C and Figure 6C, the ICA based single-trial data of particularly failed stop-trials showed more consistent effects across trials than the single-trial CSD-data. The ICA might thus be more promising to relate condition differences and inter- and intraindividual variability across trials to specific neural processes. However, as we did not combine CSD with PCA as suggested by others (Kayser and Tenke, 2006a; Kayser et al., 2010), we cannot exclude that other methodological approaches might have provided similar advantages. Importantly, ICA has also many drawbacks, especially the challenge how to identify equivalent components across subjects (Onton et al., 2005). Despite these differences, the results of Laplacian ERPs and ICA were quite similar which is consistent with a previous comparison of these methods (Foffani et al., 2004) and strengthens the conclusions.
With this approach, we present further evidence for the relevance of frontocentral areas, likely ACC or pre-SMA, for inhibitory response control (Floden and Stuss, 2006; Li et al., 2006; Mostofsky and Simmonds, 2008; Picton et al., 2006). Although the Laplacian ERPs indicated a right-lateralized N2 effect, we did not observe inhibition-related components with a right-lateralized frontal maximum, which were consistent across participants. The present results thus not speak to the hypothesis of a critical influence of right IFG on response inhibition. However, recent data from patients with lateral prefrontal lesions who were not impaired in inhibitory speed provided evidence against this hypothesis (Krämer et al., 2013).
As mentioned in the introduction, it is debated whether the stop-N2 reflects an inhibitory process or a more general conflict-monitoring process (Huster et al., 2013). In the present study we did not observe clear differences between inhibited and failed stop- or change-trials or even an enhanced N2 activity for failed trials which seems difficult to reconcile with an inhibitory process. A recently suggested unifying theoretical framework for inhibitory control might account for many of the stop-N2 findings (Munakata et al., 2011). The authors argue that conflict signals activate the pre-SMA and/or ACC, possibly reflected in the stop N2, which then inhibit the motor output via the STN. Whether or not the inhibition is successful or not will still depend on whether motor preparation in the cortico-striatal-thalamo-cortical loop crossed a critical threshold (Schmidt et al., 2013).
4.3 Stopping vs. changing a motor response
Results from Laplace transformed ERPs and ICA showed no evidence for differences between stop- and change-trials for the frontocentral N2. This suggests a common neural mechanism, possibly involving the hyperdirect or indirect fronto-basal ganglia pathway and posterior medial frontal cortex, accounting for stopping and changing a response in the current task. This is in accord with recent fMRI data (Kenner et al., 2010), but different from previous ERP results (Krämer et al., 2011). How can these discrepancies be explained? There are key differences between paradigms that need to be considered. In the present study, the task required an association between a number and a response hand, whereas in the previous EEG-study the association was made between the direction of an arrow and a response hand. This implies for the present task a much weaker stimulus-response association, which was reflected in generally higher reaction times. However, the task of Kenner et al. also involved a higher stimulus-response association for stop-trials, which required stopping after a color change to red. More importantly, the stop- and change-trials were embedded in Flanker trials in the previous EEG-study (Krämer et al., 2011), with a high probability (50 %) of incongruent stimulus-response mapping. If considering incongruent Flanker trials as similar to change-trials with a ChSD of 0 ms, this would strongly increase the probability of change-relative to stop-trials. A high probability of conflictive stimulus-response mappings might drive the system towards alternative response control mechanisms such as biased competition in the cortex (Miller and Cohen, 2001). Whereas response-switching in change-trials in the previous study might have been easier to accomplish, this was not the case in the present paradigm. Interestingly, we still observed a faster CSRT than SSRT in the current data-set, which is difficult to reconcile with the neural effects we observed. Unfortunately, we were unable to manipulate stimulus-response mappings to compare neural and behavioral effects due to limitations in the length of the recording sessions. The results of the two EEG-studies and fMRI-study, however, suggest that neural mechanisms of response inhibition depend not only on the selectivity of the inhibitory control, but also on the task context (e.g. probability of response conflict) and stimulus-response mapping.
It should be noted that the finding of no condition differences essentially confirms the null hypothesis which limits the conclusions one can derive from the results. As the number of subjects and number of trials per condition were comparable to our previous study with the stop-change-paradigm (Krämer et al., 2011), we believe that the power was sufficient to detect these conditions differences. Moreover, the converging results from different analysis approaches gives further credibility to the stability of the effects.
4.4 Conclusions
The present study provides further support for the importance of posterior mediofrontal areas in inhibitory response control and is consistent with a common neural pathway underlying stopping and changing a motor response. The data also show the advantages of using high-density EEG recordings together with Laplace transformation or ICA to disentangle activity of parietal and frontal sources involved in response inhibition. Based on the ICA, we identified a mediofrontal component with a negativity peaking at 200 ms after the stop- or change-stimulus with a likely source in ACC.
Supplementary Material
Highlights.
-
-
We studied neurophysiologial response to stop-signals and change-signals
-
-
Laplacian transformation revealed bilateral parietal and midfrontal activity
-
-
Using ICA, a mediofrontal component with likely source in ACC was identified
-
-
Stop- and change-trials did not differ in neurophysiological activity
Acknowledgments
The study was supported by the German Research Foundation (KR 3691/1-1 to UMK), the National Institute of Neurological Disorders and Stroke (R37NS21135 to RTK) and the Nielsen Corporation. The sponsors had no role in the design of the study or collection, analysis or interpretation of the data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:3743–3752. doi: 10.1523/JNEUROSCI.0519-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. Cortical and subcortical contributions to Stop signal response inhibition: role of the subthalamic nucleus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends in cognitive sciences. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex: one decade on. Trends Cogn Sci. 2014 doi: 10.1016/j.tics.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Azizian A, Freitas AL, Parvaz MA, Squires NK. Beware misleading cues: perceptual similarity modulates the N2/P3 complex. Psychophysiology. 2006;43:253–260. doi: 10.1111/j.1469-8986.2006.00409.x. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Brancucci A, Arendt-Nielsen L, Babiloni F, Capotosto P, Carducci F, Cincotti F, Romano L, Chen AC, Rossini PM. Alpha event-related desynchronization preceding a go/no-go task: a high-resolution EEG study. Neuropsychology. 2004;18:719–728. doi: 10.1037/0894-4105.18.4.719. [DOI] [PubMed] [Google Scholar]
- Babiloni F, Babiloni C, Fattorini L, Carducci F, Onorati P, Urbano A. Performances of surface Laplacian estimators: a study of simulated and real scalp potential distributions. Brain Topogr. 1995;8:35–45. doi: 10.1007/BF01187668. [DOI] [PubMed] [Google Scholar]
- Band GP, van Boxtel GJ. Inhibitory motor control in stop paradigms: review and reinterpretation of neural mechanisms. Acta psychologica. 1999;101:179–211. doi: 10.1016/s0001-6918(99)00005-0. [DOI] [PubMed] [Google Scholar]
- Boecker M, Buecheler MM, Schroeter ML, Gauggel S. Prefrontal brain activation during stop-signal response inhibition: an event-related functional near-infrared spectroscopy study. Behavioural brain research. 2007;176:259–266. doi: 10.1016/j.bbr.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Boecker M, Drueke B, Vorhold V, Knops A, Philippen B, Gauggel S. When response inhibition is followed by response reengagement: an event-related fMRI study. Hum. Brain Mapp. 2011;32:94–106. doi: 10.1002/hbm.21001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boecker M, Gauggel S, Drueke B. Stop or stop-change - Does it make any difference for the inhibition process? International journal of psychophysiology : official journal of the International Organization of Psychophysiology. 2012 doi: 10.1016/j.ijpsycho.2012.09.009. [DOI] [PubMed] [Google Scholar]
- Boehler CN, Appelbaum LG, Krebs RM, Hopf JM, Woldorff MG. Pinning down response inhibition in the brain — Conjunction analyses of the Stop-signal task. Neuroimage. 2010;52:1621–1632. doi: 10.1016/j.neuroimage.2010.04.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broere F, du Pre MF, van Berkel LA, Garssen J, Schmidt-Weber CB, Lambrecht BN, Hendriks RW, Nieuwenhuis EE, Kraal G, Samsom JN. Cyclooxygenase-2 in mucosal DC mediates induction of regulatory T cells in the intestine through suppression of IL-4. Mucosal Immunol. 2009;2:254–264. doi: 10.1038/mi.2009.2. [DOI] [PubMed] [Google Scholar]
- Cavanagh JF, Cohen MX, Allen JJ. Prelude to and resolution of an error: EEG phase synchrony reveals cognitive control dynamics during action monitoring. Journal of Neuroscience. 2009;29:98–105. doi: 10.1523/JNEUROSCI.4137-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers CD, Garavan H, Bellgrove MA. Insights into the neural basis of response inhibition from cognitive and clinical neuroscience. Neuroscience and biobehavioral reviews. 2009;33:631–646. doi: 10.1016/j.neubiorev.2008.08.016. [DOI] [PubMed] [Google Scholar]
- Coxon JP, Stinear CM, Byblow WD. Selective inhibition of movement. Journal of neurophysiology. 2007;97:2480–2489. doi: 10.1152/jn.01284.2006. [DOI] [PubMed] [Google Scholar]
- Coxon JP, Stinear CM, Byblow WD. Stop and go: the neural basis of selective movement prevention. Journal of cognitive neuroscience. 2009;21:1193–1203. doi: 10.1162/jocn.2009.21081. [DOI] [PubMed] [Google Scholar]
- De Jong R, Coles MG, Logan GD. Strategies and mechanisms in nonselective and selective inhibitory motor control. Journal of experimental psychology: Human perception and performance. 1995;21:498–511. doi: 10.1037//0096-1523.21.3.498. [DOI] [PubMed] [Google Scholar]
- Debener S. Trial-by-Trial Coupling of Concurrent Electroencephalogram and Functional Magnetic Resonance Imaging Identifies the Dynamics of Performance Monitoring. J. Neurosci. 2005;25:11730–11737. doi: 10.1523/JNEUROSCI.3286-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Delorme A, Sejnowski T, Makeig S. Enhanced detection of artifacts in EEG data using higher-order statistics and independent component analysis. NeuroImage. 2007;34:1443–1449. doi: 10.1016/j.neuroimage.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donkers FC, van Boxtel GJ. The N2 in go/no-go tasks reflects conflict monitoring not response inhibition. Brain and cognition. 2004;56:165–176. doi: 10.1016/j.bandc.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Hohnsbein J. ERP components in Go/Nogo tasks and their relation to inhibition. Acta psychologica. 1999;101:267–291. doi: 10.1016/s0001-6918(99)00008-6. [DOI] [PubMed] [Google Scholar]
- Floden D, Stuss DT. Inhibitory control is slowed in patients with right superior medial frontal damage. Journal of cognitive neuroscience. 2006;18:1843–1849. doi: 10.1162/jocn.2006.18.11.1843. [DOI] [PubMed] [Google Scholar]
- Foffani G, Bianchi AM, Cincotti F, Babiloni C, Carducci F, Babiloni F, Rossini PM, Cerutti S. Independent component analysis compared to Laplacian filtering as "deblurring" techniques for event related desynchronization/synchronization. Methods of information in medicine. 2004;43:74–78. [PubMed] [Google Scholar]
- Gentsch A, Ullsperger P, Ullsperger M. Dissociable medial frontal negativities from a common monitoring system for self- and externally caused failure of goal achievement. Neuroimage. 2009;47:2023–2030. doi: 10.1016/j.neuroimage.2009.05.064. [DOI] [PubMed] [Google Scholar]
- Greenhouse SW, Geisser S. On Methods in the Analysis of Profile Data. Psychometrika. 1959;24:95–112. [Google Scholar]
- Huster RJ, Enriquez-Geppert S, Lavallee CF, Falkenstein M, Herrmann CS. Electroencephalography of response inhibition tasks: Functional networks and cognitive contributions. International journal of psychophysiology : official journal of the International Organization of Psychophysiology. 2012 doi: 10.1016/j.ijpsycho.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Huster RJ, Enriquez-Geppert S, Lavallee CF, Falkenstein M, Herrmann CS. Electroencephalography of response inhibition tasks: functional networks and cognitive contributions. International journal of psychophysiology : official journal of the International Organization of Psychophysiology. 2013;87:217–233. doi: 10.1016/j.ijpsycho.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Huster RJ, Plis SM, Lavallee CF, Calhoun VD, Herrmann CS. Functional and effective connectivity of stopping. Neuroimage. 2014;94:120–128. doi: 10.1016/j.neuroimage.2014.02.034. [DOI] [PubMed] [Google Scholar]
- Jung TP, Makeig S, Humphries C, Lee TW, McKeown MJ, Iragui V, Sejnowski TJ. Removing electroencephalographic artifacts by blind source separation. Psychophysiology. 2000a;37:163–178. [PubMed] [Google Scholar]
- Jung TP, Makeig S, Westerfield M, Townsend J, Courchesne E, Sejnowski TJ. Removal of eye activity artifacts from visual event-related potentials in normal and clinical subjects. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2000b;111:1745–1758. doi: 10.1016/s1388-2457(00)00386-2. [DOI] [PubMed] [Google Scholar]
- Kayser J. Current source density (CSD) interpolation using spherical splines - CSD. Toolbox (Version 1.1) 2009 [Google Scholar]
- Kayser J, Tenke CE. Principal components analysis of Laplacian waveforms as a generic method for identifying ERP generator patterns: I. Evaluation with auditory oddball tasks. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2006a;117:348–368. doi: 10.1016/j.clinph.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Kayser J, Tenke CE. Principal components analysis of Laplacian waveforms as a generic method for identifying ERP generator patterns: II. Adequacy of low-density estimates. Clin. Neurophysiol. 2006b;117:369–380. doi: 10.1016/j.clinph.2005.08.033. [DOI] [PubMed] [Google Scholar]
- Kayser J, Tenke CE, Gil RB, Bruder GE. Stimulus- and response-locked neuronal generator patterns of auditory and visual word recognition memory in schizophrenia. International journal of psychophysiology : official journal of the International Organization of Psychophysiology. 2009;73:186–206. doi: 10.1016/j.ijpsycho.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser J, Tenke CE, Kroppmann CJ, Fekri S, Alschuler DM, Gates NA, Gil R, Harkavy-Friedman JM, Jarskog LF, Bruder GE. Current source density (CSD) old/new effects during recognition memory for words and faces in schizophrenia and in healthy adults. International journal of psychophysiology : official journal of the International Organization of Psychophysiology. 2010;75:194–210. doi: 10.1016/j.ijpsycho.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenner NM, Mumford JA, Hommer RE, Skup M, Leibenluft E, Poldrack RA. Inhibitory Motor Control in Response Stopping and Response Switching. J. Neurosci. 2010;30:8512–8518. doi: 10.1523/JNEUROSCI.1096-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krämer UM, Knight RT, Münte TF. Electrophysiological Evidence for Different Inhibitory Mechanisms When Stopping or Changing a Planned Response. Journal of cognitive neuroscience. 2011;23:2481–2493. doi: 10.1162/jocn.2010.21573. [DOI] [PubMed] [Google Scholar]
- Krämer UM, Solbakk AK, Funderud I, Lovstad M, Endestad T, Knight RT. The role of the lateral prefrontal cortex in inhibitory motor control. Cortex; a journal devoted to the study of the nervous system and behavior. 2013;49:837–849. doi: 10.1016/j.cortex.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Huang C, Constable RT, Sinha R. Imaging response inhibition in a stop-signal task: neural correlates independent of signal monitoring and post-response processing. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:186–192. doi: 10.1523/JNEUROSCI.3741-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan GD, Burkell J. Dependence and Independence in Responding to Double Stimulation: A Comparison of Stop, Change, and Dual-Task Paradigms. Journal of Experimental Psychology: Human Perception and Performance. 1986;12:549–563. [Google Scholar]
- Logan GD, Cowan WB. On the ability to inhibit thought and action: A theory of an act of control. Psychological review. 1984;91:295–327. doi: 10.1037/a0035230. [DOI] [PubMed] [Google Scholar]
- Logan GD, Cowan WB, Davis KA. On the ability to inhibit simple and choice reaction time responses: a model and a method. Journal of experimental psychology. Human perception and performance. 1984;10:276–291. doi: 10.1037//0096-1523.10.2.276. [DOI] [PubMed] [Google Scholar]
- Makeig S, Debener S, Onton J, Delorme A. Mining event-related brain dynamics. Trends Cogn Sci. 2004;8:204–210. doi: 10.1016/j.tics.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An Integrative Theory of Prefrontal Cortex Function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Simmonds DJ. Response inhibition and response selection: two sides of the same coin. J. Cogn. Neurosci. 2008;20:751–761. doi: 10.1162/jocn.2008.20500. [DOI] [PubMed] [Google Scholar]
- Munakata Y, Herd SA, Chatham CH, Depue BE, Banich MT, O'Reilly RC. A unified framework for inhibitory control. Trends in cognitive sciences. 2011;15:453–459. doi: 10.1016/j.tics.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachev P, Wydell H, O’Neill K, Husain M, Kennard C. The role of the pre-supplementary motor area in the control of action. Neuroimage. 2007;36:T155–T163. doi: 10.1016/j.neuroimage.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambu A, Tokuno H, Takada M. Functional significance of the cortico-subthalamo-pallidal 'hyperdirect' pathway. Neurosci. Res. 2002;43:111–117. doi: 10.1016/s0168-0102(02)00027-5. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Yeung N, van den Wildenberg W, Ridderinkhof KR. Electrophysiological correlates of anterior cingulate function in a go/no-go task: effects of response conflict and trial type frequency. Cogn Affect Behav Neurosci. 2003;3:17–26. doi: 10.3758/cabn.3.1.17. [DOI] [PubMed] [Google Scholar]
- Nuwer MR, Comi G, Emerson R, Fuglsang-Frederiksen A, Guerit JM, Hinrichs H, Ikeda A, Luccas FJ, Rappelsburger P. IFCN standards for digital recording of clinical EEG. International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol. 1998;106:259–261. doi: 10.1016/s0013-4694(97)00106-5. [DOI] [PubMed] [Google Scholar]
- Onton J, Delorme A, Makeig S. Frontal midline EEG dynamics during working memory. NeuroImage. 2005;27:341–356. doi: 10.1016/j.neuroimage.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Onton J, Westerfield M, Townsend J, Makeig S. Imaging human EEG dynamics using independent component analysis. Neurosci. Biobehav. Rev. 2006;30:808–822. doi: 10.1016/j.neubiorev.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Oostendorp TF, van Oosterom A. Source parameter estimation in inhomogeneous volume conductors of arbitrary shape. IEEE transactions on bio-medical engineering. 1989;36:382–391. doi: 10.1109/10.19859. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Ford JM, Weller BJ, Kopell BS. ERPs to response production and inhibition. Electroencephalogr Clin Neurophysiol. 1985;60:423–434. doi: 10.1016/0013-4694(85)91017-x. [DOI] [PubMed] [Google Scholar]
- Picton TW, Stuss DT, Alexander MP, Shallice T, Binns MA, Gillingham S. Effects of Focal Frontal Lesions on Response Inhibition. Cereb. Cortex. 2006;17:826–838. doi: 10.1093/cercor/bhk031. [DOI] [PubMed] [Google Scholar]
- Ramautar JR, Kok A, Ridderinkhof KR. Effects of stop-signal modality on the N2/P3 complex elicited in the stop-signal paradigm. Biological psychology. 2006;72:96–109. doi: 10.1016/j.biopsycho.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Roger C, Bénar CG, Vidal F, Hasbroucq T, Burle B. Rostral Cingulate Zone and correct response monitoring: ICA and source localization evidences for the unicity of correct- and error-negativities. Neuroimage. 2010;51:391–403. doi: 10.1016/j.neuroimage.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Russell T, Overmeyer S, Brammer MJ, Bullmore ET, Sharma T, Simmons A, Williams SC, Giampietro V, Andrew CM, Taylor E. Mapping motor inhibition: conjunctive brain activations across different versions of go/no-go and stop tasks. NeuroImage. 2001;13:250–261. doi: 10.1006/nimg.2000.0685. [DOI] [PubMed] [Google Scholar]
- Schmajuk M, Liotti M, Busse L, Woldorff MG. Electrophysiological activity underlying inhibitory control processes in normal adults. Neuropsychologia. 2006;44:384–395. doi: 10.1016/j.neuropsychologia.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Leventhal DK, Mallet N, Chen F, Berke JD. Canceling actions involves a race between basal ganglia pathways. Nature neuroscience. 2013;16:1118–1124. doi: 10.1038/nn.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds DJ, Pekar JJ, Mostofsky SH. Meta-analysis of Go/No-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia. 2008;46:224–232. doi: 10.1016/j.neuropsychologia.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swick D, Ashley V, Turken U. Are the neural correlates of stopping and not going identical? Quantitative meta-analysis of two response inhibition tasks. NeuroImage. 2011;56:1655–1665. doi: 10.1016/j.neuroimage.2011.02.070. [DOI] [PubMed] [Google Scholar]
- Tenke CE, Kayser J. Generator localization by current source density (CSD): implications of volume conduction and field closure at intracranial and scalp resolutions. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2012;123:2328–2345. doi: 10.1016/j.clinph.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Boxtel GJM, van der Molen MW, Jennings JR, Brunia CHM. A psychophysiological analysis of inhibitory motor control in the stop-signal paradigm. Biological psychology. 2001;58:229–262. doi: 10.1016/s0301-0511(01)00117-x. [DOI] [PubMed] [Google Scholar]
- van de Laar MC, van den Wildenberg WP, van Boxtel GJ, van der Molen MW. Processing of global and selective stop signals: application of Donders' subtraction method to stop-signal task performance. Exp Psychol. 2010;57:149–159. doi: 10.1027/1618-3169/a000019. [DOI] [PubMed] [Google Scholar]
- Verbruggen F, Logan GD. Response inhibition in the stop-signal paradigm. Trends in cognitive sciences. 2008;12:418–424. doi: 10.1016/j.tics.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F, Schneider DW, Logan GD. How to stop and change a response: the role of goal activation in multitasking. Journal of experimental psychology. Human perception and performance. 2008;34:1212–1228. doi: 10.1037/0096-1523.34.5.1212. [DOI] [PubMed] [Google Scholar]
- Wager TD, Sylvester CY, Lacey SC, Nee DE, Franklin M, Jonides J. Common and unique components of response inhibition revealed by fMRI. NeuroImage. 2005;27:323–340. doi: 10.1016/j.neuroimage.2005.01.054. [DOI] [PubMed] [Google Scholar]
- Wessel JR, Aron AR. It's not too late: The onset of the frontocentral P3 indexes successful response inhibition in the stop-signal paradigm. Psychophysiology. 2014 doi: 10.1111/psyp.12374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiecki TV, Frank MJ. A computational model of inhibitory control in frontal cortex and basal ganglia. Psychol. Rev. 2013;120:329–355. doi: 10.1037/a0031542. [DOI] [PubMed] [Google Scholar]
- Woldorff MG. Distortion of ERP averages due to overlap from temporally adjacent ERPs: analysis and correction. Psychophysiology. 1993;30:98–119. doi: 10.1111/j.1469-8986.1993.tb03209.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.