Abstract
Persons with amblyopia, especially those with strabismus, are known to exhibit abnormal fixational eye movements. In this paper, we compared six characteristics of fixational eye movements among normal control eyes (n=16), the non-amblyopic fellow eyes and the amblyopic eyes of anisometropic (n=14) and strabismic amblyopes (n=14). These characteristics include the frequency, magnitude of landing errors, amplitude and speed of microsaccades, and the amplitude and speed of slow drifts. Fixational eye movements were recorded using retinal imaging while observers monocularly fixated a 1° cross. Eye position data were recovered using a cross-correlation procedure. We found that in general, the characteristics of fixational eye movements are not significantly different between the fellow eyes of amblyopes and controls, and that the strabismic amblyopic eyes are always different from the other groups. Next, we determined the primary factors that limit fixation stability and visual acuity in amblyopic eyes by examining the relative importance of the different oculomotor characteristics, adding acuity (for fixation stability) or fixation stability (for acuity), and the type of amblyopia, as predictive factors in a multiple linear regression model. We show for the first time that the error magnitude of microsaccades, acuity, amplitude and frequency of microsaccades are primary factors limiting fixation stability; while the error magnitude, fixation stability, amplitude of drifts and amplitude of microsaccades are the primary factors limiting acuity. A mediation analysis showed that the effects of error magnitude and amplitude of microsaccades on acuity could be explained, at least in part, by their effects on fixation stability.
Keywords: Amblyopia, fixational eye movements, fixation stability, visual acuity, microsaccades, slow drifts
INTRODUCTION
Amblyopia is a developmental abnormality resulting from physiological alternations in the visual cortex that impairs form vision (Ciuffreda, Levi & Selenow, 1991). It affects 2–4% of the population, and is usually associated with anisometropia, strabismus, or both conditions concurrently. Besides impaired form vision, amblyopia, especially when it is associated with strabismus, is often accompanied by oculomotor anomalies that include eccentric and unsteady fixation (Schor & Hallmark, 1978; Zhang et al, 2008), low pursuit gain (Bedell, Yap & Flom, 1990; Schor, 1975) and abnormal saccadic eye movements (reduced saccadic amplitudes, increased saccadic latencies and increased number of corrective saccades: Schor, 1975; Ciuffreda, Kenyon & Stark, 1978). In this study, our focus is on fixational eye movements.
For people with normal vision and normal oculomotor control, their eyes are constantly in motion even when they attempt to maintain stable fixation on a visual target. These involuntary eye movements during fixation comprise of tremors, slow drifts and microsaccades. Tremors are high-frequency oscillatory motion of the eye and are difficult to measure with most of the conventional eye movement measuring devices. However, tremors are generally considered to serve no functional purpose. Slow drifts are slow movements of the eyes, usually with an amplitude < 10 arc min (e.g. Ditchburn & Ginsborg, 1953; Krauskopf, Cornsweet & Riggs, 1960; Nachmias, 1961; Ratliff & Riggs, 1950; Steinman et al, 1975). Microsaccades are miniature saccades, or the fast eye movements that are interspersed among the slow drifts. They usually occur between 0.5 and 3 per second, with an amplitude < 30 arc min (e.g. Cherici et al, 2012; Ditchburn & Ginsborg, 1953; Ratliff & Riggs, 1950; Sansbury et al, 1973; Steinman et al, 1975).
For people with amblyopia, it is well known that the speed of slow drifts is higher than in normal eyes (Bedell et al, 1990; Ciuffreda et al, 1979; Schor & Hallmark, 1978) and that the amplitude of slow drifts is also larger (Ciuffreda, Kenyon & Stark, 1979; Schor & Hallmark, 1978). As for microsaccades, Ciuffreda et al (who referred to them as saccadic intrusions) reported a frequency of occurrence of ~1 per second (range: 0.3 – 2 per second), with an increased frequency and amplitude when the amblyopic eye monocularly fixates a visual target. Schor and Hallmark (1978) and Schor and Flom (1975) also reported similar ranges of frequency of microsaccades, which too, were higher than the values observed in normal controls. In contrast, a recent study reported no difference in the frequency or the amplitude of microsaccades among amblyopic and the non-amblyopic fellow eyes of amblyopes and normal control eyes (González et al, 2012). However, these studies examining the characteristics of fixational eye movements span several decades and used different techniques and devices (with different precision and resolution capabilities) for measuring fixational eye movements, thus it is unclear how comparable the findings are across different studies. Therefore, the first goal of this study was to use a novel method of measuring eye movements, viz., retinal imaging, combined with a cross-correlation technique (Stevenson & Roorda, 2005; Kumar & Chung, 2014) to evaluate the various characteristics of fixational eye movements in amblyopic observers.
A characteristic of fixational eye movements that is not captured by the properties of slow drifts and microsaccades is the variability of eye positions during fixation, or, the fixation stability. It has long been documented that amblyopic eyes have poor fixation stability (Schor & Hallmark, 1978; Zhang et al, 2008). However, to date, it is unclear what factors limit fixation stability in amblyopic eyes. Gonzalez et al (2012) claimed that the fixation instability in amblyopic eyes is due to slow drifts, as they found no difference in terms of the frequency and amplitude of microsaccades between their amblyopic and control groups. However, our recent work examining the relationship between fixation stability and fixational eye movements in people with macular disease suggests that the amplitude of microsaccades is the primary contributor to fixation instability (Kumar & Chung, 2014). Therefore, it is conceivable that fixation stability in persons with amblyopia is limited by the amplitude of microsaccades, just as in people with macular disease. As the second goal of this study, we sought to determine the primary factors that limit fixation stability in amblyopic eyes. This information is important if fixation stability is found to limit functional vision (see below). In this case, an effective treatment protocol to improve functional vision in amblyopic eyes should target the specific oculomotor components that are the major factors limiting fixation stability.
Form vision is impaired in the amblyopic eyes. Given that fixational eye movements are abnormal in amblyopic eyes, a logical question is whether the abnormal fixational eye movements limit form vision in persons with amblyopia. Many aspects of vision in the amblyopic eyes are reported to be affected by fixational eye movements, including but are not limited to: positional acuity (Levi & Klein, 1982; 1983; 1985; Hess & Holiday, 1992; Demanins & Hess, 1996), displacement thresholds (Levi, Klein & Aitsebaomo, 1984), contour integration (Hess & Demanins, 1998; Levi, Yu, Kuai & Rislove, 2007), and crowding (Flom, Weymouth & Kahneman, 1963; Hess & Jacobs, 1979; Levi & Klein, 1985; Bonneh, Sagi & Polat, 2007; Song, Levi & Pelli, 2014). However, since reduced visual acuity is the sine qua non of amblyopia, we were most interested in examining which characteristic(s) of fixational eye movements (if any) is the primary factor limiting acuity in persons with amblyopia. In addition, we were interested to determine whether there exists a positive correlation between visual acuity and fixation stability. Such a correlation is observed in people with macular disease (Reinhard et al, 2007; Tarita-Nistor, Brent, Steinbach & González, 2011), and there are some recent attempts to test whether such a relationship also exists in amblyopic eyes, with inconsistent results. On one hand, based on the results of 13 adult amblyopes (strabismic [n = 5], anisometropic [n = 4] and mixed [n = 4]), González et al (2012) concluded: “For the amblyopia group, visual acuity and fixation stability did not exhibit significant correlations.” (p. 5391). On the other hand, Subramanian, Jost and Birch (2013) obtained measurements from a large sample of children with amblyopia and found a significant positive correlation between visual acuity and fixation stability when data were considered for all groups together. The correlation was the strongest for the strabismic amblyopia group (p = 0.002, n = 7), followed by the mixed amblyopia group (p = 0.04, n = 24), but was not significant for the anisometropic amblyopia group (p = 0.26, n = 20). The question of whether a positive correlation exists between visual acuity and fixation stability is important because if such a correlation really exists, then treatment for amblyopia may benefit from procedures aimed at improving fixation stability. We note that correlation does not imply causation, and that several previous studies have addressed the role of fixational eye movements in limiting acuity (Schor & Flom, 1978; Ciuffreda et al, 1979; Hess, 1977) in a handful of amblyopic observers. The third goal of the study was to determine the oculomotor parameters (including fixation stability) that are the primary factors limiting visual acuity. To achieve this goal, we used robust statistical tools that go beyond simple correlational measurements.
METHODS
Forty-four adults participated in this study. Twenty-eight of them had amblyopia, defined as a difference in the best-corrected visual acuity between the two eyes of =0.2 logMAR (the logarithm of the minimum angle of resolution, where 0.0 logMAR = 20/20 Snellen acuity) and that the better-seeing eye (the fellow eye) had an acuity of at least 0.0 logMAR or better. The other 16 were control observers with normal vision (acuity in each eye at least 0.0 logMAR and normal stereoacuity). Among the 28 observers with amblyopia, 14 had amblyopia due to anisometropia (defined as a difference in refractive corrections of >0.75D spherical equivalent) with no strabismus; and 14 had amblyopia due to strabismus (four of them also had anisometropia), as revealed using the standard cover test procedure. Details of the observers’ characteristics are given in Table 1. All observers gave written informed consent before the commencement of data collection. This research followed the tenets of the Declaration of Helsinki and was approved by the Committee for Protection of Human Subjects at the University of California, Berkeley.
Table 1.
Visual and ocular characteristics of the 28 observers with amblyopia.
| Observer ID | Gender | Age (years) | Type | Eye | Visual Acuity (logMAR) | Refractive Errors | Eye Alignment |
|---|---|---|---|---|---|---|---|
| 2 | F | 48.9 | Aniso | OD | 0.24 | +1.75/−2.50×025 | |
| OS | −0.14 | pl/−0.75×160 | |||||
| 5 | M | 19.6 | Aniso | OD | −0.10 | pl/−0.25×005 | |
| OS | 0.20 | +4.25/−3.25×180 | |||||
| 9 | M | 34.2 | Aniso | OD | −0.12 | −1.25/−0.25×160 | |
| OS | 0.34 | +0.75/−0.75×160 | |||||
| 11 | F | 21.0 | Aniso | OD | 0.12 | +3.50/−0.25×165 | |
| OS | −0.16 | pl/−0.75×020 | |||||
| 12 | M | 28.5 | Aniso | OD | −0.18 | −2.50 | |
| OS | 0.50 | +0.75 | |||||
| 14 | F | 19.3 | Aniso | OD | 0.06 | +3.25/−0.25×135 | |
| OS | −0.22 | +1.25/−0.50×140 | |||||
| 15 | F | 26.1 | Aniso | OD | 0.22 | +1.25/−0.75×180 | |
| OS | −0.14 | −0.25/−0.25×030 | |||||
| 19 | M | 43.8 | Aniso | OD | −0.14 | −1.25/−0.25×040 | |
| OS | 0.70 | +5.50/−2.00×005 | |||||
| 20 | M | 48.4 | Aniso | OD | −0.08 | +0.25 | |
| OS | 0.18 | +1.75/−0.50×020 | |||||
| 22 | F | 22.7 | Aniso | OD | 0.24 | +4.00/−0.50×180 | |
| OS | −0.14 | +1.00/−0.50×180 | |||||
| 23 | M | 19.5 | Aniso | OD | −0.22 | −0.25 | |
| OS | 0.66 | +5.75/−1.25×045 | |||||
| 25 | F | 21.1 | Aniso | OD | 0.10 | +2.25/−1.00×120 | |
| OS | −0.14 | −0.25 | |||||
| 27 | F | 23.1 | Aniso | OD | −0.14 | +0.25 | |
| OS | 0.68 | +7.00 | |||||
| 28 | M | 33.7 | Aniso | OD | −0.16 | −1.00/−0.50×175 | |
| OS | 0.22 | +2.25/−1.00×175 | |||||
| 1 | F | 31.6 | Strab | OD | 0.38 | pl/−0.75×120 | 8Δ RET |
| OS | −0.08 | −4.00 | |||||
| 3 | F | 32.1 | Strab + | OD | −0.14 | −0.75/−0.50×140 | |
| Aniso | OS | 1.46 | +5.00/−1.75×170 | 4Δ LXT | |||
| 4 | M | 28.0 | Strab | OD | 0.00 | +1.25/−0.75×045 | |
| OS | 0.52 | +0.75/−0.50×160 | 12Δ LXT | ||||
| 6 | M | 31.4 | Strab | OD | 0.20 | pl/−2.75×120 | 16Δ RET, 10Δ RHyperT |
| OS | −0.10 | pl/−2.50×020 | |||||
| 7 | F | 36.2 | Strab | OD | 0.36 | −4.50 | 6Δ RET |
| OS | −0.12 | −4.00 | |||||
| 8 | F | 55.1 | Strab | OD | −0.12 | +1.50/−0.50×160 | |
| OS | 0.82 | +1.00/−0.50×160 | >20Δ LXT | ||||
| 10 | M | 40.4 | Strab | OD | −0.20 | +0.25 | |
| OS | 0.14 | +0.50/−0.25×180 | 10Δ LET | ||||
| 13 | M | 42.9 | Strab | OD | −0.26 | +0.25/−0.50×150 | |
| OS | 0.30 | −0.25/−0.25×130 | 4Δ LET | ||||
| 16 | M | 41.4 | Strab | OD | 0.60 | −1.25 | 25Δ RET |
| OS | −0.02 | −1.00 | |||||
| 17 | M | 79.1 | Strab | OD | −0.14 | +1.50/−3.00×180 | |
| OS | 0.62 | +1.25/−1.50×165 | 2Δ LET | ||||
| 18 | F | 24.7 | Strab | OD | 0.64 | +0.75/−1.50×085 | 10Δ RXT |
| OS | −0.18 | −0.25 | |||||
| 21 | F | 33.8 | Strab + | OD | −0.08 | −1.25 | |
| Aniso | OS | 1.16 | +3.00 | 6Δ LET | |||
| 24 | F | 28.9 | Strab + | OD | 0.96 | +2.75/−1.00×180 | 11Δ RXT, 3Δ RHypoT |
| Aniso | OS | −0.14 | +0.75/−0.50×175 | ||||
| 26 | M | 49.3 | Strab + | OD | 0.30 | +1.00/−0.75×025 | 14Δ RXT |
| Aniso | OS | −0.10 | −0.25 |
We elicited fixational eye movements by asking observers to maintain steady fixation on the center of a highly visible 1° cross, presented in the primary gaze of the observers, using a scanning laser ophthalmoscope (SLO, Rodenstock, Germany). The SLO, with a field of view of 32° by 24°, uses laser beams (a red Helium-Neon [632 nm] and a near-infrared wavelength that is invisible to human eyes) to present stimuli and to image the retina simultaneously, thus the fixation cross appears as a high-contrast red cross on a dimmer background. Observers were instructed to look at the center of the fixation cross while keeping the eye as still as possible, for a duration of 30 s. Although both eyes of observers remained open throughout testing, only the tested eye could see the fixation cross. Regardless of which eye was the amblyopic eye, we adhered to the same testing order. We first tested the right eye for 10 s as a practice trial (data of which were excluded for analysis). Then we began the actual data acquisition according to the following order: right eye, left eye, left eye, right eye, right eye and left eye, such that three 30-s trials were obtained from each eye. Retinal images were captured continuously at 30 Hz for the duration of each trial using a frame grabber (Meteor-II PCI Frame Grabber, Matrox Electronic Systems Ltd., Canada) interfaced with a TV-One CORIO scan converter (CS-450 Eclipse, Erlanger, KY). Software for generating and presenting the fixation cross, and controlling the flow of the experiment was custom-written in MATLAB 7.3 (The MathWorks, MA), under the control of a ViSaGe system (Cambridge Research Systems, Rochester, U.K.).
Recovery of Eye Position from Video Files
Eye positions as a function of time were recovered from the recorded videos using a cross-correlation procedure (Stevenson and Roorda, 2005) that was modified and described in detail in Kumar and Chung (2014). A characteristic of scanned raster images is that each full-frame image is scanned line by line, each at a different time than the others. Within a scan line, it is reasonable to assume that there is very little eye motion. Consequently, if we can determine the relative shift of each scan line (with respect to a reference image) within a video frame, we can sample eye position at a rate much higher than 30 Hz. For practical reasons, we determined the relative shifts of strips of scan lines (15 scan lines, corresponding to 0.75°, per strip) that provided more features for cross correlation than single scan lines. Each strip contained high-contrast features of part of the optic nerve head and/or retinal blood vessels, facilitating a robust cross-correlation procedure.
To perform the cross-correlation, for each trial, a high-fidelity reference image was first constructed by combining (using a cross-correlation procedure) between 20 and 40 video frames (out of a total of 900 frames) that had good image quality. For the purpose of comparing the characteristics of fixational eye movements, we only examined the eye movements during the first 10 s of each video. Each video frame of this 10-s epoch was then divided into 18 strips, effectively allowing us to sample eye movements at a rate of 540 Hz (Kumar & Chung, 2014). We cross-correlated each of strip number 1 to 14 with the reference image to determine the relative shifts in position (both horizontal and vertical) of that strip. Strips 15 to 18 were not used for analysis because they were “scanned” during the scanner fly back. Figure 1 presents the horizontal and vertical eye position traces for a 10-s epoch from two observers, one with anisometropic amblyopia (top) and the other with strabismic amblyopia (bottom).
Figure 1.
Sample eye position traces of an amblyopic observer with anisometropia (top) and another with strabismus (bottom). Traces are shown for the non-amblyopic fellow eye (FE) and the amblyopic eye (AE). For clarity, the vertical (blue) and horizontal (red) eye position traces are offset vertically in each panel.
Identifying Microsaccades and Slow Drifts
The resolution of the SLO is not high enough to accurately record tremors, therefore, we only examined microsaccades and slow drifts in this study. From the eye position traces recovered for each trial, we first removed the occurrences of blinks from analysis. We defined an onset[offset] of a blink as a rapid decrease[increase] in mean pixel luminance between two successive frames. All the frames within the range of two frames before the onset of a blink and two frames after the offset of the blink were removed from analysis. Next, we identified the occurrences of microsaccades. Eye movements occurring between each pair of microsaccades were then defined as slow drifts. To reduce the effect of noise on microsaccade detection, the eye position traces were smoothed with a 5-sample moving average filter prior to microsaccade identification. The smoothed traces were only used for identifying the occurrences of microsaccades, all subsequent analyses were performed using the raw (without smoothing) traces.
For a sequence of eye-position samples to qualify as a microsaccade, the following three criteria must be met: (1) the eye moved in the same direction for at least three consecutive samples; (2) the inter-sample speed between two consecutive samples was at least 8 deg/s; and (3) the inter-sample speed either was the same as, or higher than the value for the preceding pair of consecutive samples. The first sample of this sequence of eye-position samples was defined as the starting point of the microsaccade. The end point of this microsaccade referred to the first sample of this sequence of eye positions when the inter-sample speed was slower than 8 deg/s. The amplitude of a microsaccade refers to the shortest distance between the starting and the end points of the two-dimensional vector, while the speed of the microsaccade refers to the absolute value of the vector.
Although we defined the eye movements between a pair of microsaccades as a slow drift, the analysis of the characteristics of slow drifts excluded the first five eye-position samples immediately following the end point of one microsaccade and the five eye-position samples immediately before the starting point of the subsequent microsaccade. The amplitude of a slow drift refers to the shortest distance between the starting and the end points, while the speed refers to the average inter-sample speed of all the samples comprising the slow drift segment.
In this paper, each value reported for a characteristic of fixational eye movements for an observer represents the value averaged across all the occurrences of that characteristic within the 10-s epoch of each trial, and across multiple trials of the same observer (same eye for the amblyopic observers). Since we used an averaged value to represent a characteristic for a given observer, any potential relationship between two characteristics (e.g., amplitude and speed of microsaccades, which follow a linear main sequence relationship) is minimized by the individual variability across observers.
Quantifying Fixation Stability
Different methods have been used to quantify fixation stability. The most conventional method is to calculate the area of a bivariate contour ellipse (BCEA: Steinman, 1965; Timberlake et al, 2005) that includes a specified proportion of eye positions in the distribution. This method assumes a normal distribution of eye positions during fixation, an assumption that is usually violated (Castet & Crossland, 2012; Cherici et al, 2012; Kumar & Chung, 2014). Alternative methods to quantify fixation stability without assuming a normal distribution of eye positions include counting the number of “pixels” upon which the eye fixates and then summing all the pixels that yield a specified proportion (for example, 68%) of eye positions (Whittaker, Budd & Cummings, 1988). An improved method is to estimate the probability density function corresponding to eye positions during fixation, and then calculate the area yielding a specified proportion of fixations (Castet & Crossland, 2012; Cherici et al, 2012; Kumar & Chung, 2014). In a previous study, we compared fixation stability quantified using the BCEA method and the probability density function method for a group of people with normal vision and a group of observers with macular disorders, and found that the estimates based on the two methods are highly correlated (r = 0.99) and in excellent agreement with one another (based on a Bland-Altman plot: Bland & Altman, 1986), although the BCEA estimate is larger than the probability density function method by a constant amount (0.138 log deg2). Given the excellent correlation between the two methods, in this study, we only quantified fixation stability using BCEA, in order to facilitate comparison with previous work.
For each trial, we fit a bivariate contour ellipse to the eye position data over a 10-s epoch. The BCEA (in deg2) is defined as
where χ2 is the Chi-squared value corresponding to a probability of 0.68 (one standard deviation of the distribution), σx and σy refer to the standard deviations in the horizontal (x) and vertical (y) directions, and ρ is the correlation coefficient between x and y.
Analyses
To address our first goal of evaluating the various characteristics of fixational eye movements in amblyopic observers, we first compared six characteristics of microsaccades and slow drifts: frequency of microsaccades, error magnitude of microsaccades (distance between the landing position of a microsaccade and the mean retinal location upon which the fixation cross fell [the preferred retinal locus, PRL]), amplitudes of microsaccades and slow drifts, and speeds of microsaccades and slow drifts, across five groups: control, anisometropic fellow eyes (aniso FE), anisometropic amblyopic eyes (aniso AE), strabismic fellow eyes (strab FE) and strabismic amblyopic eyes (strab AE). In addition, we also compared fixation stability across these groups. For each characteristic of fixational eye movements, we used box plots to summarize the distributions of the values for each group. Then we used the Kruskal-Wallis test to test for differences among the different groups. Post-hoc pairwise comparisons using Wilcoxon Rank Sum test were performed when the Kruskal-Wallis test revealed a difference among the groups. All permutations of pairwise comparisons among the five groups were performed, except for the comparisons between the anisometropic fellow eyes (aniso FE) and the strabismic amblyopic eyes (strab AE), and between the anisometropic amblyopic eyes (aniso AE) and the strabismic fellow eyes (strab FE), because there is no a priori rationale to compare these pairs.
For our second and third goals, analyses were performed only on the amblyopic eyes, using multiple regression analysis combined with the relative importance package (“relaimpo” in R (Grömping, 2006)), to identify and rank the primary predictors of fixation stability and acuity. All statistical analyses reported in this paper were performed using the R software (R Development Core Team, 2014), which is free to the public.
RESULTS
Comparison of the Characteristics of Microsaccades and Slow Drifts
Figure 2 shows the box-plots comparing the six characteristics across the five groups: control, aniso FE, aniso AE, strab FE and strab AE. These six characteristics are the frequency and error magnitude of microsaccades, amplitude of microsaccades and slow drifts, and speed of microsaccades and slow drifts. Table 2 summarizes the group medians (ranges given within parentheses), Kruskal-Wallis Chi-square values, p-values and post-hoc pairwise comparisons using the Wilcoxon Rank Sum test. With the exception of the speed of slow drifts, Kruskal-Wallis test showed that the values of the other five characteristics of interest are different across the five groups. Post-hoc pairwise comparisons using the Wilcoxon Rank Sum test performed on these five characteristics of interest revealed that the differences among the five groups are generally due to the strab AE being different from the control group, the aniso AE and/or the strab FE. Clearly, the exact pairs that show differences are different for the five characteristics of interest, but there are a few consistent patterns in the results: (1) there is very little difference between the non-amblyopic eyes of the amblyopic observers (aniso FE and strab FE) and the control eyes; (2) the amblyopic eyes of strabismic amblyopes (strab AE) always show differences when compared with the control, or the aniso AE or the strab FE; and (3) the amblyopic eyes of anisometropic amblyopes (aniso AE) do not show as much differences when compared with the other groups as the strab AE.
Figure 2.
Box-and-whisker plots comparing the frequency of microsaccades, amplitude of microsaccades, speed of microsaccades, error magnitude of microsaccades, amplitude of slow drifts and speed of slow drifts for five groups of eyes: control eyes, non-amblyopic fellow eyes of anisometropic amblyopes (aniso FE), amblyopic eyes of anisometropic amblyopes (aniso AE), non-amblyopic fellow eyes of strabismic amblyopes (strab FE) and amblyopic eyes of strabismic amblyopes (strab AE). The upper and lower bound of each box represent the 75th and 25th percentiles of the distribution, and the median is represented by the thick line inside the box. The top and bottom ends of the whisker represent the 95th and 5th percentiles of the distribution, respectively. Outliers, if present, are represented by individual circles. The number of asterisks indicates the level of significance of the pairwise comparison according to the standard notation:* p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001, ***** p ≤ 0.0001.
Table 2.
Summary of the group medians and ranges, Kruskal-Wallis χ2 and the associated p-values, and the posthoc Wilcoxon Rank Sum Test for the various parameters.
| Group median (range) | Kruskal-Wallis χ2 (df = 4) | Post-hoc pairwise comparison using Wilcoxon Rank Sum Test (only pairs with a significant difference are listed) | |
|---|---|---|---|
| Fixation stability (BCEA, deg2) *statistical test performed on log BCEA | |||
| Control: 0.0625 (0.0263 – 0.4828) | 23.3468 (p = 0.00011) | control – strab AE | W = 24, p = 0.00010 |
| Aniso FE: 0.0525 (0.0206 – 0.4300) | aniso FE – aniso AE | W = 47, p = 0.01855 | |
| Aniso AE: 0.0770 (0.0405 – 1.8710) | strab FE – strab AE | W = 23, p = 0.00027 | |
| Strab FE: 0.0562 (0.0177 – 0.7307) | aniso AE – strab AE | W = 43, p = 0.01060 | |
| Strab AE: 0.2058 (0.0603 – 11.4099) | |||
| Frequency of microsaccades (number/s) | |||
| Control: 1.79 (0.56 – 3.11) | 12.5507 (p = 0.01369) | control – strab AE | W = 48, p = 0.00690 |
| Aniso FE: 1.37 (0.50 – 5.88) | aniso AE – strab AE | W = 52, p = 0.03504 | |
| Aniso AE: 1.74 (0.68 – 4.65) | |||
| Strab FE: 1.89 (0.62 – 3.86) | |||
| Strab AE: 2.82 (1.27 – 5.09) | |||
| Amplitude of microsaccades (arc min) | |||
| Control: 10.63 (6.72 – 19.21) | 11.1642 (p = 0.02478) | control – strab AE | W = 62, p = 0.03827 |
| Aniso FE: 7.89 (2.25 – 18.89) | strab FE – strab AE | W = 47, p = 0.01855 | |
| Aniso AE: 12.30 (5.44 – 27.79) | |||
| Strab FE: 8.78 (4.33–21.38) | |||
| Strab AE: 18.36 (6.10 – 53.85) | |||
| Speed of microsaccades (deg/s) | |||
| Control: 21.37 (15.39 – 42.15) | 20.8264 (p = 0.00034) | control – aniso AE | W = 56, p = 0.01940 |
| Aniso FE: 27.87 (17.68 – 82.31) | control – strab FE | W = 47, p = 0.00599 | |
| Aniso AE: 29.88 (19.05 – 45.49) | control – strab AE | W = 20, p = 0.00004 | |
| Strab FE: 34.15 (18.05 – 88.77) | aniso AE – strab AE | W = 44, p = 0.01225 | |
| Strab AE: 38.92 (24.41 – 65.78) | |||
| Error magnitude of microsaccades (arc min) | |||
| Control: 8.472 (5.716 – 23.780) | 18.3765 (p = 0.00104) | control – aniso AE | W = 58, p = 0.02454 |
| Aniso FE: 8.431 (4.794 – 15.480) | control – strab AE | W = 24, p = 0.00010 | |
| Aniso AE: 12.100 (6.183 – 35.800) | aniso FE – aniso AE | W = 53, p = 0.03948 | |
| Strab FE: 8.611 (5.136 – 31.740) | strab FE – strab AE | W = 35, p = 0.00296 | |
| Strab AE: 14.890 (8.738 – 94.080) | |||
| Amplitude of slow drifts (arc min) | |||
| Control: 5.716 (4.675 – 9.627) | 12.2495 (p = 0.01559) | control – aniso AE | W = 64, p = 0.04721 |
| Aniso FE: 7.216 (4.130 – 16.630) | control – strab AE | W = 39, p = 0.00176 | |
| Aniso AE: 7.296 (4.804 – 23.470) | strab FE – strab AE | W = 49, p = 0.02412 | |
| Strab FE: 7.164 (3.894 – 14.900) | |||
| Strab AE: 10.330 (4.174 – 38.080) | |||
| Speed of slow drifts (deg/s) | |||
| Control: 3.099 (2.331 – 3.329) | 7.8105 (p = 0.09877) | – | – |
| Aniso FE: 2.9211 (0.2699 – 3.2191) | |||
| Aniso AE: 2.962 (1.652 – 3.331) | |||
| Strab FE: 2.717 (1.203 – 3.569) | |||
| Strab AE: 2.582 (1.475 – 3.355) | |||
Fixation stability
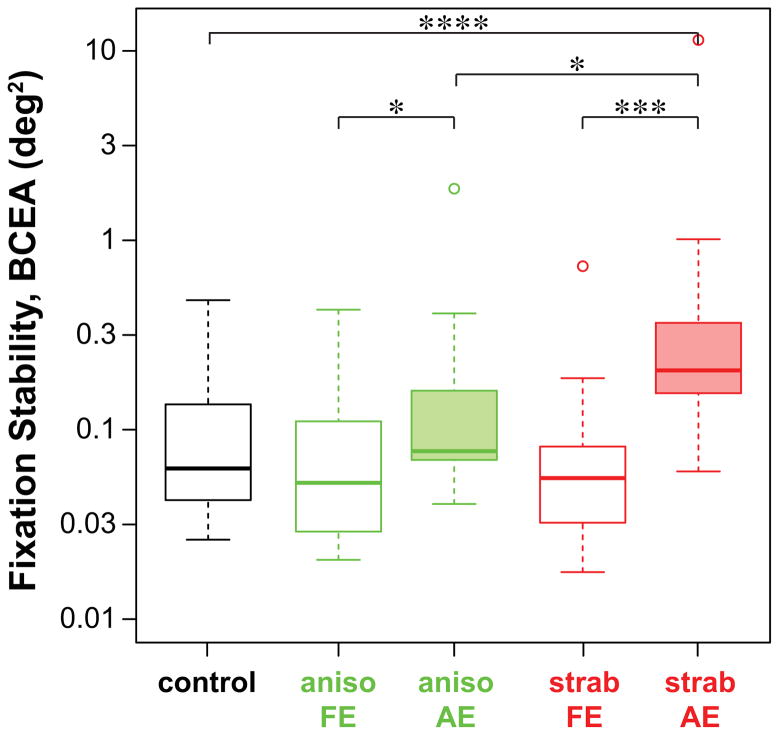
Fixation stability, quantified by the BCEA, is compared across the five groups in Figure 3. The statistical tests were performed on the log-transformed values of the BCEA. Kruskal-Wallis Chi-squared test showed that fixation stability varied across the five groups (see Table 2 for details). Post-hoc pairwise comparisons revealed that the differences are primarily due to the BCEA being larger in strab AE than in control eyes, aniso AE and strab FE. There is also a difference between the two eyes (AE vs. FE) of anisometropic amblyopes. Note that with the exception of a few outliers, the values of BCEA for the control group, the non-amblyopic eyes of amblyopic observers (aniso FE and strab FE) and the aniso AE are all highly comparable with the values reported for people with intact fovea and normal vision (0.022 – 0.36 deg2: Steinman et al., 1975; Rohrschneider et al., 1995; Crossland & Rubin, 2002). Even the BCEA for many of the strab AE fell within the reported range for normal eyes.
Figure 3.
Box-and-whisker plots comparing fixation stability, quantified as the bivariate contour ellipse area (BCEA, in deg2) for the five groups of eyes. Details of the boxes and whiskers are as in Figure 2.
Now that we have established that fixation stability is compromised in the amblyopic eyes, we can ask which characteristics of the fixational eye movements are the primary factors accounting for the fixation instability? To answer this question, we determined the “relative importance” of the contributions of different characteristics of fixational eye movements in explaining the variance of log BCEA in a multiple regression model. Because it is often assumed that people with better acuity also have better fixation stability, we included visual acuity (in logMAR units) as another predictor in the model. We were also interested in whether the type of amblyopia — anisometropic or strabismic — was a predictor for fixation stability, therefore, the type of amblyopia was included as a predictor in the model as well (anisometropic = 0; strabismic = 1). The analysis was accomplished using the relaimpo (relative importance) package in R (Grömping, 2006), and was performed only on the data of the amblyopic eyes (aniso AE and strab AE). The determination of the relative importance of the different characteristics of fixational eye movements (regressors in the model) using the relaimpo package is superior to other methods in identifying the important predictors in a model because it takes into account that predictors in a model are often correlated with one another, so that the total variance of the model accounted for by the predictors is not simply the sum of the proportion of variance accounted for by each individual regressor (from univariate regression). Instead, the relaimpo package considers the direct contribution (from univariate regression) and the indirect contribution (the additional contribution after other regressors are added to the model) of a regressor (Grömping, 2006). Before performing the relaimpo analysis, we used the Shapiro-Wilk test (Shapiro & Wilk, 1965) to test for normality of the six characteristics of fixational eye movements. The amplitude of microsaccades, amplitude of slow drifts and error magnitude of microsaccades failed the test. However, the log-transformed values of these data followed a normal distribution; as such, the log-transformed values of these three characteristics were used for subsequent data analyses.
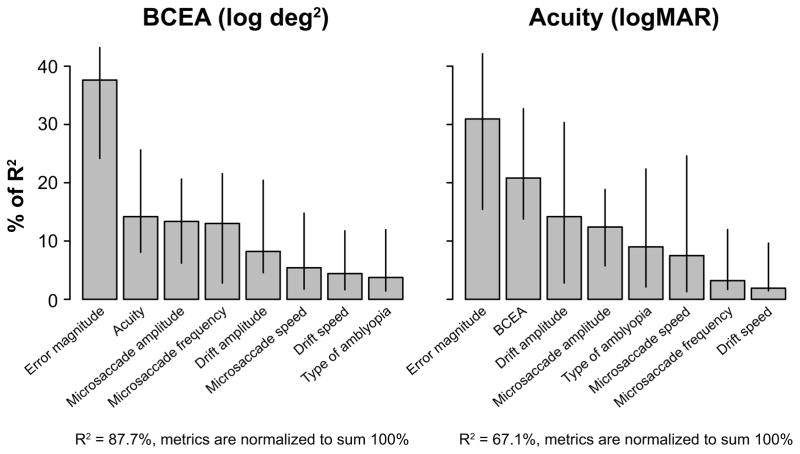
Results of the relaimpo analysis are shown in the left panel of Figure 4 where the relative importance of the predictors is ordered according to the lmg metric (Lindeman, Merenda & Gold, 1980). The lmg metric removes bias in the order effect of adding a regressor to the model by averaging sequential sums of squares over all orderings of regressors. With all the predictors, the model explains 87.7% of the variance of log BCEA. However, clearly some predictors are more important than others. If we only consider the predictors with a relative contribution of over 10%, then in the order of relative contribution, error magnitude of microsaccades (37.62% [95% confidence intervals: 24.18–43.19%]), acuity (14.19% [8.07–25.63%]), amplitude of microsaccades (13.36% [6.20–20.64%]) and frequency of microsaccades (13.02% [2.78–21.56%]) would be considered as “important” in predicting the value of log BCEA in amblyopic eyes. Together, these three predictors account for 68.57% (absolute, not relative) of the variance of log BCEA.
Figure 4.
Percentage of R2 of BCEA (log transformed values) and acuity (logMAR) accounted for by each of the parameter listed on the x-axes, as determined using the R package relaimpo. The relative importances of these parameters are ranked according to the lmg metric. Error bars represent ±95% confidence intervals estimated using bootstrappings, based on 10,000 resamplings.
Visual acuity
The third question of this study was to determine the predictive factors for visual acuity in the amblyopic eyes. Similar to our analysis for fixation stability, we used the relaimpo package in R to determine the relative contribution of the different predictive factors, including the different characteristics of fixational eye movements, log BCEA and the type of amblyopia. The right panel of Figure 4 shows the results of the analysis. With all the predictors, the model explains 67.1% of the variance of acuity. As before, if we only consider the predictors with a relative contribution of over 10%, then in the order of relative contribution, error magnitude of microsaccades (30.94% [15.44–42.13%]), fixation stability (20.81% [13.76–32.70%]), amplitude of slow drifts (14.19% [2.81–30.33%]) and amplitude of microsaccades (12.40% [5.75–18.85%]) would be considered as “important” in predicting visual acuity in amblyopic eyes. Together, these four predictors account for 52.56% (absolute, not relative) of the variance of acuity.
Figure 5 provides an overview of the pairwise comparisons among the important factors (based on our criterion that the relative contribution of the factor has to exceed 10% of explained variance of either fixation stability or visual acuity) that limit fixation stability and/or visual acuity. The correlation coefficients of each pair of variables are given in the lower-left half of the figure. Clearly, the correlation coefficient is significant when a factor (e.g. amplitude of microsaccade) is an important limitation on a measurement (in this case, either fixation stability or acuity).
Figure 5.
Scatter plots of the relationship between each “important factor” that can predict either fixation stability or visual acuity. These factors are: fixation stability (log-transformed BCEA values were used here), visual acuity (logMAR), error magnitude (log-transformed), amplitude of microsaccades (log-transformed), amplitude of slow drifts (log-transformed) and frequency of microsaccades. The correlation coefficients for each pair of variables are given in the lower half of the figure. All correlation coefficients are significant at the p ≤ 0.05 level except for the pairs between frequency of microsaccades and acuity, error magnitude, amplitude of microsaccades, and amplitude of slow drifts (correlation coefficients shown in light gray).
DISCUSSION
We examined the various characteristics of fixational eye movements in observers with anisometropic and/or strabismic amblyopia. We first compared these characteristics among normal control eyes (from observers with normal visual acuity and normal binocular vision), the non-amblyopic fellow eyes and the amblyopic eyes of observers with anisometropia or strabismus. With these characteristics of fixational eye movements, we then determined the primary factors limiting fixation stability and visual acuity in the amblyopic eyes.
We found that in general, there is very little difference between the non-amblyopic eyes of the amblyopic observers (aniso FE and strab FE) and the normal control eyes, implying that the oculomotor control in amblyopic observers could be as good as that in observers with normal vision. It is widely known that visual acuity is not significantly different between the non-amblyopic fellow eyes and normal control eyes (McKee, Levi & Movshon, 2003). Here, we provide evidence that fixational eye movement control is also not different between the non-amblyopic fellow eyes and normal control eyes. Note, however, that compared with normal controls, non-amblyopic fellow eyes have been shown to exhibit, for example, poorer contrast sensitivity at high spatial frequencies (Kelly, Chino, Cotter & Knuth, 1997), reduced Vernier acuity (Levi & Klein, 1985), more spatial uncertainty and distortion (Bedell, Flom & Barbeito, 1985), deficits in global motion processing (Simmers et al, 2003; Giaschi, Regan, Kraft & Hong, 1992; Ho, Giaschi, Boden, Dougherty, Cline & Lyons, 2005) and more spatial interference from nearby objects (Levi & Klein, 1985).
Considering that we used a novel technique (retinal imaging) to measure eye movements, how do our obtained values compare with those in the literature when more conventional eye movement measuring devices were used? In general, the values compare quite well. We found that the frequency of microsaccade is ~0.6 to 5 per second (for the fellow eyes and amblyopic eyes). The low end of the range matches well with published values while the high end is ~2x higher (Schor & Hallmark (1978): 0.6 – 2.3 per second; Ciuffreda et al (1979): 0.3 – 2 per second). We suspect the much higher end of the range in our study is due to the fact that we did not use a bite bar to stabilize observers’ head motion, as was used in previous studies. The amplitude of microsaccades is the characteristic that compares most favorably with published values. Our range of values is about 5 – 50 arc min in the amblyopic eyes, compared with 20 – 50 arc min in Schor and Hallmark (1978) and 40 – 200 arc min in Schor and Flom (1975). In the fellow eyes, our range, approximately 2 – 20 arc min, compares favorably with 5 – 25 arc min in Schor and Hallmark (1978). Considering individual variability among amblyopic observers, these values are remarkably similar.
As for slow drifts, Ciuffreda et al (1979; 1980) reported that drifts are not different in the fellow eyes and normal control eyes, but there is an increase in the drift amplitude and/or speed in the amblyopic eyes. On the contrary, Schor and Flom (1975) reported that drifts are similar in amplitude, duration, speed and direction between the amblyopic eyes and normal control eyes. In this study, despite drift amplitude not being different between fellow eyes and control eyes, it is higher in amblyopic eyes (range: ~4 – 38 arc min) than in the fellow eyes (range: ~4 – 16 arc min), consistent with the values reported by Ciuffreda et al (1979; 1980: maximum drift amplitude 42 arc min in amblyopic eyes). However, we found that the speed of drifts is not different between amblyopic and fellow eyes, or amblyopic and control eyes (and also not different between fellow eyes and control eyes). Note that our speed of drifts (maximum ~3.5 deg/s) is higher than previously reported values when measurements were obtained in only a few well-trained observers (e.g. Ciuffreda et al (1979; 1980): 0.33 deg/s; Srebro (1983): 0.14 – 1.49 deg/s). Previously, Cherici et al (2012) reported a three-fold increase in drift speed for untrained observers, compared with trained observers. They also found large individual variability among their sample of 14 observers (11 of whom were untrained). Therefore, the fact that our drift speed was higher than previously reported values could be due to individual observer variability, especially since our observers were all untrained. Other possible explanations include the use of different methods to measure and define drift speed among the different studies, and the use of bite bars to stabilize head motion in previous studies.
Factors limiting fixation stability
The second goal of this paper was to determine the primary factors limiting fixation stability in amblyopic eyes. Using a multiple linear regression model combined with the R package relaimpo to determine the relative importance of the different parameters in predicting fixation stability, we found that the error magnitude of microsaccades, visual acuity, amplitude and frequency of microsaccades are the four major limitations on fixation stability. Together, these four predictors account for 68.57% of the variance of fixation stability. The error magnitude of microsaccades represents the error between the landing position of a microsaccade and the PRL (where the microsaccade is supposed to land). Thus, a larger error magnitude means that the microsaccades are less accurate in their landing positions and takes the eye further away from the intended landing location. Consequently, a larger bivariate contour ellipse is required to describe the eye positions for the calculation of BCEA. Therefore, it is not surprising that the larger the error magnitude, the higher the fixation instability. In fact, we speculate that the reason the frequency of microsaccades is also a significant predictive factor of fixation stability is related to the error magnitude. Given that the direction of microsaccades is stochastic in nature, logically, if an observer is not precise in the landing positions following microsaccades, increasing the frequency of microsaccades would increase the imprecision, thus increasing fixation instability.
We have previously reported that for persons with macular disease, fixation stability is primarily limited by the amplitude of microsaccades (Kumar & Chung, 2014). Here, we showed that this limitation also applies to amblyopic eyes, suggesting that the limitation may be a general finding, instead of one that is specific to persons with macular disease. Our result is also consistent with previous reports that there are more “microsaccadic intrusions” in amblyopic eyes than in normal controls (Ciuffreda et al, 1980; Schor & Hallmark, 1978; Schor & Flom, 1979). Clearly, when the amplitude of microsaccades is large, each microsaccade takes the eye further away from the starting position than a microsaccade of smaller amplitude, thus increasing the fixation instability.
The other factor we identified as important in limiting fixation stability is visual acuity. As summarized in the Introduction, recent attempts to determine whether or not there exists a significant correlation between visual acuity and fixation stability in people with amblyopia yielded inconclusive results. On one hand, González et al (2012) reported a lack of significant correlation for a group of 13 adult amblyopes (5 strabismic, 4 anisometropic and 4 mixed). On the other hand, Subramanian et al (2013) found significant correlations for their amblyopia with strabismus group, and the mixed mechanism group, but not for the group with anisometropia alone. In this study, we found a significant correlation between visual acuity and fixation stability when all the amblyopic eyes were considered (r = 0.667 [95% confidence intervals: 0.391–0.833], p = 0.00011). Even if we consider only strabismic amblyopic eyes (with or without anisometropia), the correlation coefficient is still significant (r = 0.627 [0.145–0.869], p = 0.0164). Similarly, if we consider only anisometropic amblyopic eyes, the correlation coefficient is also significant (r = 0.555 [95% confidence intervals: 0.035–0.839], p = 0.0392). Thus, it is likely that the lack of a significant correlation in the study of González et al (2012) was a result of insufficient power to find an effect due to the small sample size.
A caveat in interpreting the significant correlation between visual acuity and fixation stability is that it is difficult to determine if acuity is the limiting factor on fixation stability, or vice versa. We shall further address this chicken and egg issue in the sections below.
Factors limiting visual acuity
The third question of this study was to determine the predictive factors for visual acuity in the amblyopic eyes. Using similar analyses as for fixation stability, we found that there are four important factors in predicting visual acuity in amblyopic eyes. These four factors are the error magnitude of microsaccades, fixation stability, amplitude of slow drifts and amplitude of microsaccades. However, together, these four factors only accounted for 52.56% of the variance of acuity. One factor that we did not consider in our study is the eccentricity of the fixation locus (PRL) of the amblyopic eyes. Given previous reports that acuity worsens with increased eccentricity of the PRL (Flom & Weymouth, 1961; Kandel, Grattan & Bedell, 1977),1 we expected that when combined with the other variables in our model, we would be able to account for a higher proportion of the variance of acuity. Unfortunately, because we did not specifically measure the location of the PRL, for instance, using the Maxwell’s spot, and that our SLO recordings do not allow us to identify the eccentricity of the PRL accurately,2 we were not able to add the eccentricity of the PRL location as another predictive variable.
As explained above, because of the significant correlation between visual acuity and fixation stability, it is difficult to tease apart whether acuity is the limiting factor on fixation stability, or vice versa. Nevertheless, there are several reasons to believe that fixation stability may place some limits on acuity, instead of the other way round. First, it has been shown that random jittering of an acuity target degrades acuity even in observers with normal vision (Chung & Bedell, 1995). This effect is minimal when the acuity stimulus is exposed for a short period of time, but is larger when the acuity target is exposed for a longer period of time (as is typically done in clinical measures). Presumably, when fixation is unsteady or when the acuity target is jittered, the image of the acuity target falls on different locations on the retina, which can result in positional uncertainty and may render it difficult for the observer to integrate sufficient information over time (and space) to form a global percept of the target. In this study, acuity was measured using the Bailey-Lovie Visual Acuity Chart (5 letters on each line) and observers were allowed unlimited amount of time to look at the letters. Therefore, an effect of random jittering of the retinal images of the letter targets due to fixation instability might be expected to affect the acuity measurement. This random jitter hypothesis is consistent with the largest contributors to acuity being error magnitude and fixation instability (BCEA). Increased error magnitude means that the microsaccades are less accurate in their landing positions and shift the eye further away from the intended fixation location, and increased BCEA reflects increased fixation instability. However, we note that Hess (1977) compared the grating acuity of two strabismic amblyopes under stabilized (using afterimages) and unstabilized conditions, and concluded that the abnormal fixational eye movements contributed little, if any, to the acuity deficit. Second, as mentioned earlier, there is published evidence that acuity correlates with the eccentricity of the PRL in amblyopic eyes (Flom & Weymouth, 1961; Kandel et al, 1977). Fixation instability would place the acuity stimulus on different extrafoveal locations, which may be further away from the actual PRL thus further degrading acuity.
Our analysis suggests that error magnitude and the amplitude of microsaccades are also important factors limiting acuity. The effects of error magnitude and amplitude of microsaccades on acuity could be indirect, due to each of their relationships with fixation stability. To examine this possibility, we use a mediation analysis to determine whether the effects of error magnitude and amplitude of microsaccades on acuity are mediated by a “mediator variable” — fixation stability in our case. Mediation analyses or models are statistical tools to help understand or explain the underlying relationship between an independent and a dependent variable, by including a third explanatory variable. The analyses are commonly used in social psychological research but have recently been applied to vision research (Calabrèse et al, 2014). Interested readers should refer to Calabrèse et al (2014) for an excellent description and explanation of the mediation analysis. Figure 6 is a schematic depiction of the model, listing the relationship between the independent variable (error magnitude or amplitude of microsaccades), the dependent variable (acuity) and the third explanatory variable, or, the mediator variable (fixation stability). At the core of the analysis is the use of multiple linear regression to estimate the regression coefficients between the independent and the dependent variables (c in Figure 6), the independent and the mediator variables (a), and the mediator and the dependent variables (b). The ratio of the indirect effect (a*b) to the total effect (c+a*b) provides an index of how much of the observed effect of the independent variable on the dependent variable is explained by the mediator (ratio of 1 means full mediation). We used the lavaan (“latent variable analysis”: Rosseel (2012)) package in R for this analysis. Before the analysis, we took the necessary steps to ensure that (1) each variable followed a normal distribution (log-transformed when necessary); (2) there was a significant correlation between the independent and dependent variables (see Figure 5); and (3) data were grand-mean centered.
Figure 6.

A schematic figure illustrating the mediation model. The mediation model decomposes the total effect of an independent variable (in our case, error magnitude or amplitude of microsaccades) on the dependent variable (in our case, visual acuity) into two components: the direct effect (c) and the indirect effect through a mediator (in our case, fixation stability), quantified by a*b. The total effect is then represented by c+a*b. The ratio of the indirect effect to the total effect represents the proportion of the effect explained by the mediator.
Applying this analysis to examine the effect of amplitude of microsaccades on acuity yielded a ratio of the indirect to the total effect of 1.062 (Table 3), implying that the effect is completely due to the effects of the amplitude of microsaccades on fixation stability and fixation stability on acuity. In other words, the amplitude of microsaccades has very little direct effect on acuity. In contrast, applying this analysis to the effect of error magnitude on acuity yielded a ratio of 0.403, implying that there are both direct effects of error magnitude on acuity, and indirect effects of error magnitude on acuity through the effect of error magnitudes on fixation stability.
Table 3a.
Results of the mediation analysis: Effect of amplitude of microsaccades on visual acuity through fixation stability. a represents the effect of amplitude of microsaccades on fixation stability; b represents the effect of fixation stability on visual acuity and c represents the direct effect of amplitude of microsaccades on visual acuity.
| Estimate | SE | z value | p-value | 95% lower percentile CI | 95% CI upper percentile | |
|---|---|---|---|---|---|---|
| Direct effect (c) | −0.032 | 0.199 | −0.163 | 0.870 | ||
| a | 1.271 | 0.446 | 2.854 | 0.004 | ||
| b | 0.434 | 0.168 | 2.590 | 0.010 | ||
| Indirect effect (a * b) | 0.552 | 0.220 | 2.506 | 0.012 | 0.155 | 1.041 |
| Total (c + a * b) | 0.520 | 0.207 | 2.505 | 0.012 | 0.107 | 0.942 |
| Ratio of indirect to total effects | 1.062 | 3.122 | 0.340 | 0.271 | 0.408 | 2.465 |
The 95% CIs are obtained by bootstrap with 10,000 resamples.
Our analysis also shows that the amplitude of slow drifts, which is not important in limiting fixation stability, is an important factor limiting acuity in amblyopic eyes. Presumably, larger drift amplitudes would smear the retinal stimulus across a greater retinal area, causing a degradation in acuity. Recent work suggests that in normal vision, slow drifts counter-balance the spectral distribution in natural scenes. This “whitening” of natural images results in enhanced high spatial-frequency information (Kuang, Poletti, Victor & Rucci, 2012). When eye movements are stabilized, the high spatial frequencies are no longer enhanced, thus impairing high-frequency discrimination (Rucci, Iovin, Poletti & Santini, 2007). This theory provides strong support for the important role of normal fixational eye movements in visual information processing.
In the case of abnormal fixation behavior, such as in the presence of increased amplitude of slow drifts as we found in this study, it is unclear whether the increased image motion due to slow drifts would further enhance the high spatial-frequency information in natural scenes, or reduce the sensitivity. Moreover, normal fixational eye movements can be approximated by Brownian motion (e.g., Cornsweet, 1956; Engbert & Kliegl, 2004), and in fact, the aforementioned theory requires that the slow drifts behave similar to Brownian motion (Kuang et al., 2012). However, it has been shown that fixational eye movements in persons with amblyopia (at least those with strabismic amblyopia) usually exhibit a predominant direction along the horizontal direction (Subramanian et al., 2013) and thus may not completely follow Brownian motion. As such, the theory proposed by Rucci and his colleagues linking slow drifts and functional vision may not be directly applicable to people with amblyopia. The functional consequence of slow drifts in amblyopia is no doubt an important and interesting question, a question that is currently under investigation in our laboratory.
Implications
The existence of a significant correlation between fixation stability and visual acuity is important on at least two accounts. There are indications from case studies that orthoptic treatment can result in both improved visual acuity and improved fixation stability (Selenow & Ciuffreda, 1983; 1986). It is currently unclear whether the improved fixation results from better acuity, or vice versa. Our results suggest that acuity might benefit from more stable fixation. Indeed, biofeedback training has been attempted in the past as a method to improve fixation stability in people with amblyopia (Flom, Kirschen & Bedell, 1980; Schor & Hallmark, 1978). However, the training was never widely adopted as a clinical procedure to improve fixation stability, presumably because the training was time consuming and because at the time, it was difficult to implement at home, since it involved sophisticated eye movement monitoring devices. With the current advances in technology, it might be possible to package the hardware and software required for biofeedback training in a small device (such as a smart phone) that amblyopic patients can use at home. If such a product were to be developed, the biofeedback training method should be targeted at minimizing the landing errors of microsaccades and/or reducing the size of the microsaccades. Furthermore, it is well known that strabismic amblyopes often exhibit much poorer acuity for a row of letters, than for isolated letters (Stuart & Burian, 1962). This “crowding” effect in strabismic amblyopes has been attributed in part, to poor eye movement control (Flom, 1991). Thus it would be interesting to know whether improved fixation stability would result in improvement in reading crowded text in strabismic amblyopes. These issues are currently under investigation in our laboratories.
Table 3b.
Results of the mediation analysis: Effect of error magnitude on visual acuity through fixation stability. a represents the effect of error magnitude on fixation stability; b represents the effect of fixation stability on visual acuity and c represents the direct effect of error magnitude on visual acuity.
| Estimate | SE | z value | p-value | 95% CI lower percentile | 95% CI upper percentile | |
|---|---|---|---|---|---|---|
| Direct effect (c) | 0.510 | 0.326 | 1.564 | 0.118 | ||
| a | 1.723 | 0.244 | 7.057 | 0.000 | ||
| b | 0.200 | 0.230 | 0.869 | 0.385 | ||
| Indirect effect (a * b) | 0.344 | 0.342 | 1.007 | 0.314 | −0.294 | 1.129 |
| Total (c + a * b) | 0.854 | 0.217 | 3.930 | 0.000 | 0.523 | 1.366 |
| Ratio of indirect to Total effects | 0.403 | 0.379 | 1.062 | 0.288 | −0.376 | 1.127 |
The 95% CIs are obtained by bootstrap with 10,000 resamples.
Highlights.
Fixational eye movements are similar in fellow eyes of amblyopes and normals
Characteristics of microsaccades and drifts are abnormal in strabismic amblyopic eyes
Fixation is more unstable in amblyopic eyes than in fellow eyes and control eyes
Microsaccade error, amplitude and frequency, and acuity limit fixation stability
Microsaccade error and amplitude, drift amplitude and fixation stability limit acuity
Acknowledgments
This study was supported in part by NIH research grants R01-EY012810 and R01-EY001728. We thank Ka-yee So and Kenneth Tran for their assistance in subject coordination, Daniel Coates for his helpful comments and Eric Castet for sharing his R code on mediation analysis.
Footnotes
Siepmann, Reinard and Herzau (2006) reported that some amblyopic observers used slightly different retinal locations to view acuity targets of different sizes, which could account for some variability of the relationship between visual acuity and eccentricity of fixation location.
In our previous study (Kumar & Chung, 2014), we were able to use the SLO images to estimate the location of the fovea based on some standard measurements between the optic nerve head and the fovea, and then calculate the eccentricity of the PRL with respect to the fovea, in a group of observers with macular disease. We are not able to adopt the same method in this study because several of the amblyopic observers showed a significant amount of torted eye positions in both eyes. Because we did not know by how much they eyes had torted, we could not estimate with certainty the location of the fovea, hence the eccentricity of the PRL.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Susana T.L. Chung, Email: s.chung@berkeley.edu.
Girish Kumar, Email: girish.kumar@berkeley.edu.
Roger W. Li, Email: oroger@berkeley.edu.
Dennis M. Levi, Email: dlevi@berkeley.edu.
References
- Aytekin M, Victor JD, Rucci M. The visual input to the retina during natural head-free fixation. Journal of Neuroscience. 2014;34:12701–12715. doi: 10.1523/JNEUROSCI.0229-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedell HE, Flom MC, Barbeito R. Spatial aberrations and acuity in strabismus and amblyopia. Investigative Ophthalmology & Visual Science. 1985;26:909–916. [PubMed] [Google Scholar]
- Bedell HE, Yap YL, Flom MC. Fixational drift and nasal-temporal pursuit asymmetries in strabismic amblyopes. Investigative Ophthalmology & Visual Science. 1990;31:968–976. [PubMed] [Google Scholar]
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- Bonneh YS, Sagi D, Polat U. Spatial and temporal crowding in amblyopia. Vision Research. 2007;47:1950–1962. doi: 10.1016/j.visres.2007.02.015. [DOI] [PubMed] [Google Scholar]
- Calabrèse A, Bernard JB, Faure G, Hoffart L, Castet E. Eye movements and reading speed in macular disease: the shrinking perceptual span hypothesis requires and is supported by a mediation analysis. Investigative Ophthalmology & Visual Science. 2014;55:3638–3645. doi: 10.1167/iovs.13-13408. [DOI] [PubMed] [Google Scholar]
- Castet E, Crossland M. Quantifying eye stability during a fixation task: a review of definitions and methods. Seeing and Perceiving. 2012;25:449–469. doi: 10.1163/187847611X620955. [DOI] [PubMed] [Google Scholar]
- Cherici C, Kuang X, Poletti M, Rucci M. Precision of sustained fixation in trained and untrained observers. Journal of Vision. 2012;12:31.1–31.16. doi: 10.1167/12.6.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung STL, Bedell HE. Effect of retinal image motion on visual acuity and contour interaction in congenital nystagmus. Vision Research. 1995;35:3071–3082. doi: 10.1016/0042-6989(95)00090-m. [DOI] [PubMed] [Google Scholar]
- Ciuffreda KJ, Kenyon RV, Stark L. Increased saccadic latencies in amblyopic eyes. Investigative Ophthalmology & Visual Science. 1978;17:697–702. [PubMed] [Google Scholar]
- Ciuffreda KJ, Kenyon RV, Stark L. Fixational eye movements in amblyopia and strabismus. Journal of the American Optometric Association. 1979;50:1251–1258. [PubMed] [Google Scholar]
- Ciuffreda KJ, Kenyon RV, Stark L. Increased drift in amblyopic eyes. British Journal of Ophthalmology. 1980;64:7–14. doi: 10.1136/bjo.64.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciuffreda KJ, Levi DM, Selenow A. Amblyopia: basic and clinical aspects. Stoneham, MA: Butterworth-Heinemann; 1991. [Google Scholar]
- Cornsweet TN. Determination of the stimuli for involuntary drifts and saccadic eye movements. Journal of the Optical Society of America. 1956;46:987–993. doi: 10.1364/josa.46.000987. [DOI] [PubMed] [Google Scholar]
- Crossland MD, Rubin GS. The use of an infrared eyetracker to measure fixation stability. Optometry and Vision Science. 2002;79:735–739. doi: 10.1097/00006324-200211000-00011. [DOI] [PubMed] [Google Scholar]
- Demanins R, Hess RF. Positional loss in strabismic amblyopia: interrelationship of alignment threshold, bias, spatial scale and eccentricity. Vision Research. 1996;36:2771–2794. doi: 10.1016/0042-6989(95)00318-5. [DOI] [PubMed] [Google Scholar]
- Ditchburn RW, Ginsborg BL. Involuntary eye movements during fixation. Journal of Physiology. 1953;119:1–17. doi: 10.1113/jphysiol.1953.sp004824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engbert R, Kliegl R. Microsaccades keep the eyes’ balance during fixation. Psychological Sciences. 2004;15:431–436. doi: 10.1111/j.0956-7976.2004.00697.x. [DOI] [PubMed] [Google Scholar]
- Flom MC. Contour interaction and the crowding effect. In: Rutstein RP, editor. Problems in Optometry. 2. Vol. 3. Philadelphia, Pa: Lippincott; 1991. pp. 237–257. [Google Scholar]
- Flom MC, Kirschen DG, Bedell HE. Control of unsteady, eccentric fixation in amblyopic eyes by auditory feedback of eye position. Investigative Ophthalmology & Visual Science. 1980;19:1371–1381. [PubMed] [Google Scholar]
- Flom MC, Weymouth FW. Centricity of Maxwell’s spot in strabismus and amblyopia. Archives of Ophthalmology. 1961;66:136–144. doi: 10.1001/archopht.1961.00960010262018. [DOI] [PubMed] [Google Scholar]
- Flom MC, Weymouth FW, Kahneman D. Visual resolution and contour interaction. Journal of the Optical Society of America. 1963;53:1026–1032. doi: 10.1364/josa.53.001026. [DOI] [PubMed] [Google Scholar]
- Giaschi DE, Regan D, Kraft SP, Hong XH. Defective processing of motion-defined form in the fellow eye of patients with unilateral amblyopia. Investigative Ophthalmology & Visual Science. 1992;33:2483–2489. [PubMed] [Google Scholar]
- González EG, Wong AMF, Niechwiej-Szwedo E, Tarita-Nistor L, Steinbach MJ. Eye position stability in amblyopia and in normal binocular vision. Investigative Ophthalmology & Visual Science. 2012;53:5386–5394. doi: 10.1167/iovs.12-9941. [DOI] [PubMed] [Google Scholar]
- Grömping U. Relative importance for linear regression in R: the package relaimpo. Journal of Statistical Software. 2006;17:1–27. [Google Scholar]
- Hess RF. Eye movements and grating acuity in strabismic amblyopia. Ophthalmic Research. 1977;9:225–237. [Google Scholar]
- Hess RF, Demanins R. Contour integration in anisometropic amblyopia. Vision Research. 1998;38:889–894. doi: 10.1016/s0042-6989(97)00233-2. [DOI] [PubMed] [Google Scholar]
- Hess RF, Holliday IE. The spatial localization deficit in amblyopia. Vision Research. 1992;32:1319–1339. doi: 10.1016/0042-6989(92)90225-8. [DOI] [PubMed] [Google Scholar]
- Hess RF, Jacobs RJ. A preliminary report of acuity and contour interactions across the amblyope’s visual field. Vision Research. 1979;19:1403–1408. doi: 10.1016/0042-6989(79)90214-1. [DOI] [PubMed] [Google Scholar]
- Ho CS, Giaschi DE, Boden C, Dougherty R, Cline R, Lyons C. Deficient motion perception in the fellow eye of amblyopic children. Vision Research. 2005;45:1615–1627. doi: 10.1016/j.visres.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Kandel GL, Grattan PE, Bedell HE. Monocular fixation and acuity in amblyopic and normal eyes. American Journal of Optometry and Physiological Optics. 1977;54:598–608. doi: 10.1097/00006324-197709000-00004. [DOI] [PubMed] [Google Scholar]
- Kelly SA, Chino YM, Cotter SA, Knuth J. Orientation anisotropy and strabismus. Vision Research. 1997;37:151–163. doi: 10.1016/s0042-6989(96)00081-8. [DOI] [PubMed] [Google Scholar]
- Krauskopf J, Cornsweet TN, Riggs LA. Analysis of eye movements during monocular and binocular fixation. Journal of the Optical Society of America. 1960;50:572–578. doi: 10.1364/josa.50.000572. [DOI] [PubMed] [Google Scholar]
- Kuang X, Poletti M, Victor JD, Rucci M. Temporal encoding of spatial information during active visual fixation. Current Biology. 2012;22:510–514. doi: 10.1016/j.cub.2012.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar G, Chung STL. Characteristics of fixational eye movements in people with macular disease. Investigative Ophthalmology & Visual Science. 2014;55:5125–5133. doi: 10.1167/iovs.14-14608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi DM, Klein SA. Hyperacuity and amblyopia. Nature. 1982;298:268–270. doi: 10.1038/298268a0. [DOI] [PubMed] [Google Scholar]
- Levi DM, Klein SA. Spatial localization in normal and amblyopic vision. Vision Research. 1983;23:1005–1017. doi: 10.1016/0042-6989(83)90011-1. [DOI] [PubMed] [Google Scholar]
- Levi DM, Klein SA. Vernier acuity, crowding and amblyopia. Vision Research. 1985;25:979–991. doi: 10.1016/0042-6989(85)90208-1. [DOI] [PubMed] [Google Scholar]
- Levi DM, Klein SA, Aitsebaomo AP. Detection and discrimination of the direction of motion in central and peripheral vision of normal and amblyopic observers. Vision Research. 1984;24:789–800. doi: 10.1016/0042-6989(84)90150-0. [DOI] [PubMed] [Google Scholar]
- Levi DM, Yu C, Kuai SG, Rislove E. Global contour processing in amblyopia. Vision Research. 2007;47:512–524. doi: 10.1016/j.visres.2006.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindeman RH, Merenda PF, Gold RZ. Introduction to bivariate and multivariate analysis. Scott, Foresman, Glenview, IL: 1980. p. 119. [Google Scholar]
- Nachmias J. Determiners of the drift of the eye during monocular fixation. Journal of the Optical Society of America. 1961;51:761–766. doi: 10.1364/josa.51.000761. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2014. Available at: http://www.R-project.org. [Google Scholar]
- Ratliff F, Riggs LA. Involuntary motions of the eye during monocular fixation. Journal of Experimental Psychology. 1950;40:687–701. doi: 10.1037/h0057754. [DOI] [PubMed] [Google Scholar]
- Reinhard J, Messias A, Dietz K, MacKeben M, Lakmann R, et al. Quantifying fixation in patients with Stargardt disease. Vision Research. 2007;47:2076–2085. doi: 10.1016/j.visres.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Rohrschneider K, Becker M, Kruse FE, Fendrich T, Vicker HE. Stability of fixation: results of fund us-controlled examination using the scanning laser ophthalmoscope. German Journal of Ophthalmology. 1995;4:197–202. [PubMed] [Google Scholar]
- Rosseel Y. lavaan: An R package for structural equation modeling. Journal of Statistical Software. 2012;48:1–36. [Google Scholar]
- Rucci M, Iovin R, Poletti M, Santini F. Miniature eye movements enhance fine spatial detail. Nature. 2007;447:851–854. doi: 10.1038/nature05866. [DOI] [PubMed] [Google Scholar]
- Sansbury RV, Skavenski AA, Haddad GM, Steinman RM. Normal fixation of eccentric targets. Journal of the Optical Society of America. 1973;63:612–614. doi: 10.1364/josa.63.000612. [DOI] [PubMed] [Google Scholar]
- Schor C. A directional impairment of eye movement control in strabismus amblyopia. Investigative Ophthalmology. 1975;14:692–697. [PubMed] [Google Scholar]
- Schor C, Hallmark W. Slow control of eye position in strabismic amblyopia. Investigative Ophthalmology & Visual Science. 1978;17:577–581. [PubMed] [Google Scholar]
- Schor CM, Flom MC. Eye position control and visual acuity in strabismus and amblyopia. In: Lennerstrand G, Bach-y-Rita P, editors. Basic mechanisms of ocular motility and their clinical implications. New York: Pergamon Press; 1975. pp. 555–559. [Google Scholar]
- Selenow A, Ciuffreda KJ. Vision function recovery during orthoptic therapy in an exotropic amblyope with high unilateral myopia. American Journal of Optometry and Physiological Optics. 1983;60:659–666. doi: 10.1097/00006324-198308000-00003. [DOI] [PubMed] [Google Scholar]
- Selenow A, Ciuffreda KJ. Vision function recovery during orthoptic therapy in an adult esotropic amblyope. Journal of the American Optometric Association. 1986;57:132–140. [PubMed] [Google Scholar]
- Shapiro SS, Wilk MB. An analysis of variance test for normality (complete samples) Biometrika. 1965;3–4:591–611. [Google Scholar]
- Siepmann K, Reinhard J, Herzau V. The locus of fixation in strabismic amblyopia changes with increasing effort of recognition as assessed by scanning laser ophthalmoscope. Acta Ophthalmologica Scandinavica. 2006;84:124–129. doi: 10.1111/j.1600-0420.2005.00550.x. [DOI] [PubMed] [Google Scholar]
- Simmers AJ, Ledgeway T, Hess RF, McGraw PV. Deficits to global motion processing in human amblyopia. Vision Research. 2003;43:729–738. doi: 10.1016/s0042-6989(02)00684-3. [DOI] [PubMed] [Google Scholar]
- Song S, Levi DM, Pelli DG. A double dissociation of the acuity and crowding limits to letter identification, and the promise of improved visual screening. Journal of Vision. 2014;14(5) doi: 10.1167/14.5.3. article number 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srebro R. Fixation of normal and amblyopic eyes. Archives of Ophthalmology. 1983;101:214–217. doi: 10.1001/archopht.1983.01040010216006. [DOI] [PubMed] [Google Scholar]
- Steinman RM. Effect of target size, luminance, and color on monocular fixation. Journal of the Optical Society of America. 1965;55:1158–1165. [Google Scholar]
- Steinman RM, Haddad GM, Skavenski AA, Wyman D. Miniature eye movement. Science. 1975;181:810–819. doi: 10.1126/science.181.4102.810. [DOI] [PubMed] [Google Scholar]
- Stevenson SB, Roorda A. Correcting for miniature eye movements in high resolution scanning laser ophthalmoscopy. Proceedings of SPIE. 2005;5688A:145–151. [Google Scholar]
- Stuart JA, Burian HM. A study of separation difficulty. American Journal of Ophthalmology. 1962;53:471–477. [PubMed] [Google Scholar]
- Subramanian V, Jost RM, Birch EE. A quantitative study of fixation stability in amblyopia. Investigative Ophthalmology & Visual Science. 2013;54:1998–2003. doi: 10.1167/iovs.12-11054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarita-Nistor L, Brent MH, Steinbach MJ, González EG. Fixation stability during binocular viewing in patients with age-related macular degeneration. Investigative Ophthalmology & Visual Science. 2011;52:1887–1893. doi: 10.1167/iovs.10-6059. [DOI] [PubMed] [Google Scholar]
- Timberlake GT, Sharma MK, Grose SA, Gobert DV, Gauch JM, Maino JH. Retinal location of the preferred retinal locus relative to the fovea in scanning laser ophthalmoscope images. Optometry and Vision Science. 2005;82:177–185. doi: 10.1097/01.opx.0000156311.49058.c8. [DOI] [PubMed] [Google Scholar]
- Whittaker SG, Budd J, Cummings RW. Eccentric fixation with macular scotoma. Investigative Ophthalmology & Visual Science. 1988;29:268–278. [PubMed] [Google Scholar]
- Zhang B, Stevenson SB, Cheng H, Laron M, Kumar G, Tong J, Chino YM. Effects of fixation instability on multifocal VEP (mfVEP) responses in amblyopes. Journal of Vision. 2008;8(3):16.1–16.14. doi: 10.1167/8.3.16. [DOI] [PubMed] [Google Scholar]