Abstract
Purpose
Unstirred water layers (UWLs) present an unavoidable complication in the measurement of transport kinetics in cultured cells and the high rates of transport achieved by overexpressing heterologous transporters exacerbate the UWL effect. This study examined the correlation between measured Jmax and Kt values and the effect of manipulating UWL thickness or transport Jmax on the accuracy of experimentally determined kinetics of the multidrug transporters, OCT2 and MATE1.
Methods
Transport of TEA and MPP was measured in CHO cells that stably expressed human OCT2 or MATE1. UWL thickness was manipulated by vigorous reciprocal shaking. Several methods were used to manipulate maximal transport rates.
Results
Vigorous stirring stimulated uptake of OCT2-mediated transport by decreasing apparent Kt (Ktapp) values. Systematic reduction in transport rates was correlated with reduction in Ktapp values. The slope of these relationships indicated a 1500 µm UWL in multiwell plates. Reducing the influence of UWLs (by decreasing either their thickness or the Jmax of substrate transport) reduced Ktapp by 2-fold to >10-fold.
Conclusions
Failure to take into account the presence of UWLs in experiments using cultured cells to measure transport kinetics can result in significant underestimates of the affinity of multidrug transporters for substrates.
Keywords: transport, organic cation, unstirred water layers, kinetics
INTRODUCTION
The kinetics of substrate uptake into cells can provide context for understanding the role of a transporter in cellular or systemic physiology/pharmacology, as well as insight into the transport mechanism itself. Certainly the measurement of transport kinetics is a central element in the physiological characterization of the process. Furthermore, in recent years the kinetics of ligand interaction with multidrug transporters (i.e., the relationship between ligand concentration and activity of the transporter) has been used to identify potential candidates for unwanted drug-drug interactions (DDIs) and make recommendations concerning the need for further study, including recommendations for conducting clinical DDI studies (1). However, the interpretation of the kinetic parameters of transport, including the maximal rate of substrate transport (Jmax), the substrate concentration that results in half-maximal transport (Kt), and the concentration of inhibitor that reduces transport by 50% (IC50), relies on confidence in their accuracy.
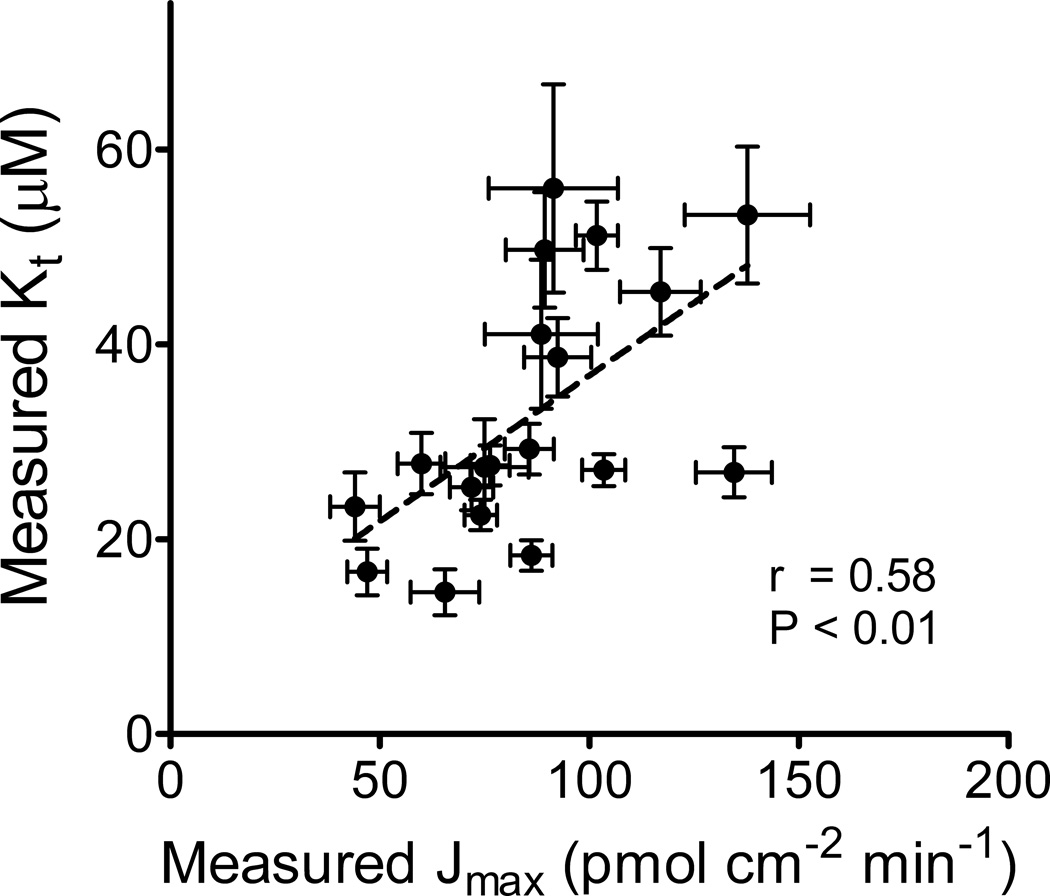
Cultured mammalian cells, including HEK 293, CHO, MDCK, and CaCo-2 cells, are widely used as platforms to study the activity of cloned, heterologous transport proteins, especially the study of the kinetics of inhibitory ligand interaction with pharmacologically important transporters (e.g., (2, 3)). These systems offer a comparatively straightforward means to focus analytical attention on one (or more) experimentally defined processes without the complicating influence of undefined transporters that may contribute to substrate flux in native tissues (see (1)). However, common approaches to the use of such ‘over expression’ models also carry the risk of introducing experimental artifacts into the measurement of transport kinetics. Our own interest has focused on the application of computational tools to probe the kinetics and selectivity of the Organic Cation Transporters (OCTs) and Multidrug And Toxin Extruders (MATEs) that play significant roles in the renal clearance of cationic drugs (e.g., (4–8)). While analyzing a data set on the kinetics of OCT2-mediated transport of the prototypical substrate tetraethylammonium (TEA) collected over the course of several studies, we noted a correlation between measured values of Jmax and Kt, namely, higher Jmax values were routinely associated with higher Kt values (Fig. 1). The Kt, i.e., the apparent ‘affinity’ of a transporter for substrate, is generally assumed to be an intrinsic property of the transport protein (in that it reflects protein structure). Consequently, we were concerned that the observed systematic variation in the measured Kt value for TEA’s interaction with OCT2 reflected an experimental artifact in its measurement. The data presented here show that the primary explanation for this Jmax-Kt correlation is the influence of an unstirred water layer (UWL; also referred to as the aqueous boundary layer) on the measurement of transport kinetics.
Figure 1.
Relationship between experimentally determined values for Jmax and Kt for transport of TEA in CHO cells that stably expressed human OCT2. Each point is the Jmax and Kt value as calculated by GraphPad from data obtained in single experiments (of a total of 19) that measured (generally in triplicate in 24 or 48 well plates) [3H]TEA transport as a function of increasing concentration of unlabeled TEA. Error bars indicate SE as determined (by GraphPad) for fitting the Michaelis-Menten equation to the data using nonlinear regression analysis. The dashed line was fit to these data using linear regression.
That UWLs can introduce error into the measurement of the kinetics of carrier-mediated transport has been recognized since at least the early 1970’s (e.g., (9–11)). For carrier-mediated uptake into cells, transport activity reduces the concentration of substrate in the layer of effectively static fluid immediately adjacent to the extracellular face of the membrane, thereby establishing a gradient of substrate concentration between the surface of the membrane and the (well-stirred) bulk medium. Consequently, the concentration of substrate in the experimental solution is greater than that exposed to the transporter, and so ‘apparent’ Kt values (Ktapp) determined experimentally overestimate the actual concentration of substrate required to half-saturate the transporter. To describe the influence of UWLs on the apparent kinetics of carrier-mediated transport, Winne (9) introduced an equation for the substrate concentration in the well-stirred bulk medium that results in half-maximal transport:
| eq. 1 |
where Jmax is the maximal rate of mediated transport, Kt is the true Michaelis constant of the transporter, δ is the thickness of the UWL above the membrane surface, and D is the diffusion coefficient of the substrate. This concentration corresponds to the apparent Michaelis constant (Ktapp):
| eq. 2 |
Thus, the bias introduced to experimentally determined Ktapp values is proportional to (i) the thickness of the UWL and (ii) the maximal rate of transport. In qualitative terms, at least, a positive correlation between measured values of Kt and Jmax, such as that evident in Figure 1, is an expected consequence of the influence of UWLs on experimentally determined transport kinetics. Indeed, such a correlation between empirically determined Jmax and Kt values was recently noted for transepithelial bile salt transport across MDCK monolayers that express the apical bile salt transporter, ASBT (13).
The Winne relationship is based on the assumption that the concentration profile near the membrane is constant with time. In fact, this assumption does not hold for typical uptake studies because achieving that steady state takes longer than the course of typical incubation times (a few tens of seconds to minutes). Despite this limitation, the Winne relationship provides a useful basis for assessing the potential influence of UWLs on the experimentally determined kinetics of carrier-mediated transport.
In addition to its dependence on Jmax, the impact of UWLs on transport kinetics is a function of the thickness (depth) of the UWL. The depth of UWLs associated with commonly used cell culture plates has been thoroughly examined in the context of their impact on estimates of passive permeability of drugs (14). Without vigorous mixing (of a type difficult to achieve with transport protocols that use cultured cell models), the UWL thickness above the planar surface of a multiwell plate is typically between 1000 and 2000 µm (e.g., (12, 15–17)), making diffusional resistance through this layer a potentially significant element in the measurement of the kinetics of substrate uptake using cultured cells.
In the current study we assessed the quantitative impact of UWLs on the kinetics of substrate transport mediated by human OCT2 and MATE1 expressed in a cultured mammalian cell. The observations suggest that rates of transport commonly achieved in studies using heterologous expression systems can result in overestimates of Kt values by a factor of 2 to 10 or more. Reducing the Jmax of transport by, for example, reducing levels of transporter expression, can provide more accurate estimates of Kt.
MATERIALS AND METHODS
Chemicals
[3H]tetraethylammonium ([3H]TEA; 54 Ci/mmol) was prepared by American Radiolabeled Chemicals (St. Louis, MO). [3H]1-methyl-4-phenylpyridinium ([3H]MPP; 80 Ci/mmol) was prepared by the Synthesis Core of the Southwest Environmental Health Sciences Center/Department of Chemistry of the University of Arizona (Tucson, AZ). The fluorescent organic cation and transported OCT2 substrate (8), N,N,N-trimethyl-2-[methyl(7-nitrobenzo[c][l,2,5]oxadiazol-4-yl)amino]ethanaminium (NBD-MTMA; (18)), was also prepared by the Department of Chemistry of the University of Arizona, as was unlabeled MPP. TEA chloride and other chemicals were obtained from Sigma-Aldrich (St. Louis, MO).
Cell culture and stable expression of OCT2
Chinese Hamster Ovary (CHO) cells containing the Flp recombination target site were obtained from Invitrogen (Carlsbad, CA). CHO cells were grown in Ham’s F-12 Nutrient Mixture (Sigma-Aldrich) with 10% fetal bovine serum (Fisher Scientific, Pittsburg, PA) and supplemented with 100 µg/ml Zeocin (Invitrogen). Cells that stably expressed OCT2 or MATE1 were prepared using methods described previously (19, 20). In all the studies presented here the OCT2 protein expressed in CHO cells included the V5 epitope (GKPIPNPLLGLDST) added to the C-terminus (immediately following the wild type hOCT2 sequence) or the N-terminus (immediately preceding the hOCT2 sequence). Preliminary experiments showed that the kinetics of MPP transport in both of the constructs did not differ from those measured in cells that expressed the wild type OCT2 sequence without the epitope tag (data not shown). The MATE1 construct used in these studies did not include an added epitope tag. The cells expressing OCT2 or MATE1 were grown and maintained under hygromycin (Invitrogen) pressure (100 µg/ml) in plastic cell culture flasks at 37°C in a humidified atmosphere with 5% CO2. Cells were passed every 3–4 days and seeded into 12-well plates (500,000 cells/well), 24-well plates (275,000 cells/well), 48-well plates (137,500 cells/well), or 96-well plates (25,000 cells/well) as needed, and typically used 24–48 hours later.
Measurement of transport
For studies that used CHO cells grown to confluence in 12, 24 or 48-well plates, the wells were rinsed twice with Waymouth buffer (WB) (135 mM NaCl, 13 mM HEPES, 2.5 mM CaCl2•2H2O, 1.2 mM MgCl2, 0.8 mM MgSO4•7H2O, 5 mM KCl, and 28 mM D-glucose). For experiments measuring OCT2-mediated transport, buffer (room temperature) containing radiolabeled substrate (typically 10–20 nM for [3H]-labeled compounds), plus the desired concentration of a test compound (substrate or inhibitor), was added individually to each well, removed after a set amount of time, after which the wells were washed three times with cold WB to stop transport. Following the cold WB rinses the cells were solubilized in 200 µl of 0.5 N NaOH with 1% SDS per well and shaken for 15 min. The solubilized cells were neutralized with 100 µL of 1 N HCl and 250 µL were placed in a scintillation vial. The radioactivity in each sample was determined using scintillation spectroscopy (Beckman model LS6000IC). Some studies measured uptake of NBD-MTMA using its native fluorescence (18). Following exposure of cells to WB containing NBD-MTMA (typically 25 µM), accumulated substrate was determined using a fluorescence plate-reader (VarioScan, Thermo) (8). Individual transport experiments were conducted in triplicate and typically repeated at least 3 times. Experiments were conducted using cells between passages 4 and 35 with no consistent difference in transport rates between earlier and later passages.
Studies that measured transport in cells grown in 96-well plates (Greiner; VWR Intl., Arlington Heights, IL) used an automatic fluid aspirator/dispenser (Model 406, BioTek, Winooski, VT). Plates containing culture media were placed in the unit and automatically rinsed/aspirated three times with room temperature WB, after which transport buffer (60 µl) was automatically introduced into each well (the composition and timing of the addition varied based on the experimental protocol; refer to figure legends). In some experiments, following addition of the transport buffer the plate was shaken (reciprocating; 13 or 19 Hz) for the duration of the exposure (up to 30 sec). In studies measuring MATE1-mediated transport, the cells were first preincubated for 5, 10 or 20 min in 10 or 20 mM NH4Cl. Transport was initiated by aspirating this medium and replacing it with an NH4Cl-free medium (thereby rapidly establishing an outwardly-directed H+ gradient; (21)) containing radiolabeled substrate (w/ or w/o additional ligand), as described for the OCT2 experiments. The transport reaction was stopped by the rapid addition (and simultaneous aspiration) of cold (4°C) WB. Following aspiration of the cold stop, 250 µl of scintillation cocktail (Microscint 20, Perkin-Elmer, Waltham, MA) was added to each well and the plates were sealed (Topseal-A; Perkin-Elmer) and allowed to sit for at least 2 hrs before radioactivity was assessed in a 12 channel, multiwell scintillation counter (Wallac Trilux 1450 Microbeta, Perkin-Elmer). Transport was typically normalized to surface area of the confluent monolayer. For the purpose of comparing transport rates expressed per cm2 to those in the literature expressed per milligram of cell protein, we have found the conversion factor of 0.035 mg/cm2 (22) to be reasonably accurate.
shRNA knockdown of OCT2 expression
hOCT2-expressing CHO cells and non-expressing wild type CHO cells were seeded in a 96-well plate at a density of 12,000 cells/well. The cells were exposed to lentiviral (LV) transduction particles (multiplicity of infection – MOI – of 2) containing either (i) one of five distinct DNA sequences each coding for a short hairpin RNA sequence designed for the knockdown of hOCT2 (Sigma product SHCLNV-NM_003058); or (ii) an LV transduction particle containing a non-target shRNA sequence (Sigma product SHC002). After 24 hours puromycin (60 µg/ml) was added to the wells to select for cells that stably expressed the LV-introduced shRNA sequence (per manufacturer instructions).
Measurement of RNA
Isolation of total RNA from the cell monolayers and synthesis of cDNA were performed using the Cells-to-cDNA™ II Kit (Applied Biosystems, Grand Island, NY) according to the manufacturer's protocol. Real-time quantitative PCR (qPCR) was performed using the Applied Biosystems 7300 Real-Time PCR System. Each PCR reaction solution was prepared using the TaqMan Gene Expression Assays (Hs00537914_m1) and TaqMan Gene Expression Master Mix according to the manufacturer’s protocol (Applied Biosystems). The PCR was run at 95°C for 10 min, followed by 40 amplification cycles of 95°C for 15 s and 60°C for 1 minute. Quantification of relative gene expression was performed using the ΔΔCT method. The housekeeping gene 18s rRNA was used for normalization.
SDS-PAGE and Western blotting
Crude membranes were prepared from OCT2-expressing cells according to Pelis et al. (23). Proteins (0.1 µg/lane) were separated on 8% SDS-PAGE gels and electrophoretically transferred to a polyvinylidene fluoride membrane. The membrane was blocked for 1 h in blocking buffer [5% non-fat dry milk in PBS-T (PBS containing 0.05% Tween-20)] at room temperature, followed by overnight incubation (4°C) with mouse anti-V5 antibody (0.1 µg/ml; Invitrogen) diluted in blocking buffer. After extensive washing with PBS-T, the membrane was incubated with HRP-conjugated goat anti-mouse IgG (0.01 µg/ml) diluted in blocking buffer. Following extensive washing with PBS-T, the membrane was incubated in SuperSignal West Femto Maximum Sensitivity Substrate (Pierce Protein Biol, Rockford, Il), and the secondary antibody was detected on high performance chemiluminescence film (Amersham ECL; GE Healthcare Bio-Sciences, Pittsburgh, PA).
Statistical analysis
Data are expressed as means ± SE, with calculations of standard errors based on the number of replicates within an experiment (typically 3–5), or based on mean values determined in separate experiments using cells at a different passage number. Statistical comparison of differences between sets of experimental observations used two-tailed t-tests (Excel 2007; Microsoft, Redmond, WA); comparison of slopes and intercepts used an extra sum-of-squares F test ((Prism 5; GraphPad Software, La Jolla, CA). Observed differences were deemed significant when P < 0.05.
RESULTS
Manipulation of δ: the effect of shaking on OCT2-mediated transport in cultured CHO cells
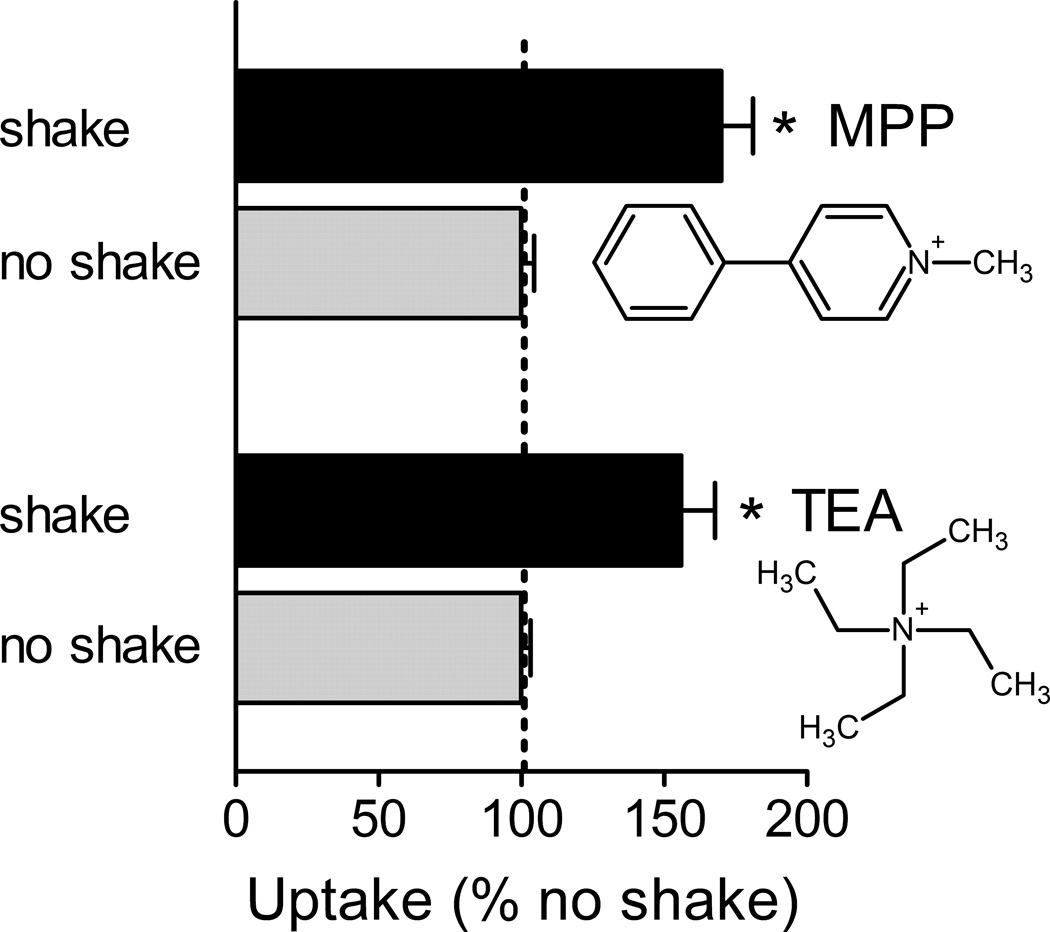
If an UWL is of sufficient depth to influence the rate of carrier-mediated substrate transport, then it follows that reducing its depth should increase substrate uptake. Although the type of stirring/shaking that can be applied to transport experiments with cells cultured in multiwell plates cannot eliminate UWLs (14), it should be possible to reduce their influence. We tested this by assessing the effect of shaking multiwell plates on rates of OCT2-mediated transport of MPP and TEA. The mixing arising from placing a 24 well culture plate on a flat-bed orbital shaker (GeneMate MP4, BioExpress, Kaysville, UT) rotating at 300 rpm (5 Hz) increased the 30 sec uptake of [3H]TEA (by 16%; P < 0.05) and [3H]MPP (by 50% P < 0.05). The more vigorous shaking produced by reciprocal agitation at 13 Hz of a 96 well plate (via the BioTek 406 fluid dispenser), resulted in larger increases in the 30 sec accumulation of both TEA (15 nM) and MPP (8 nM) (56% and 72%, respectively; Fig. 2). As a control for the possibility that the mechanical agitation of the cells stimulated the transport process itself (e.g., secondary to activation of mechanically sensitive channels/receptors), we assessed the effect of measuring transport immediately after shaking the cells for 30 sec. This treatment had no effect on the subsequent rate of uptake (data not shown), suggesting that the stimulation of transport resulting from agitation reflected a decrease in UWL thickness rather than an intrinsic change in activity of the transporter.
Figure 2.
Effect of shaking on the rate of transport of [3H]TEA (15 nM) or [3H]MPP (8 nM) into OCT2-expressing CHO cells. Uptake (30 sec) was measured in cells grown in wells of a 96 well plate. Following addition of the medium containing labeled substrate (60 µl) the plates were either shaken (13 Hz) or left unshaken. The length of each bar represents the average (+SE) uptake, normalized to that measured without shaking, determined in three (TEA) or five (MPP) separate experiments (each measured in 6 wells). Each uptake was corrected for that measured into cells in which mediated transport was blocked by the addition of 1 mM unlabeled MPP. Asterisks (*) indicate differences at the level of P < 0.05.
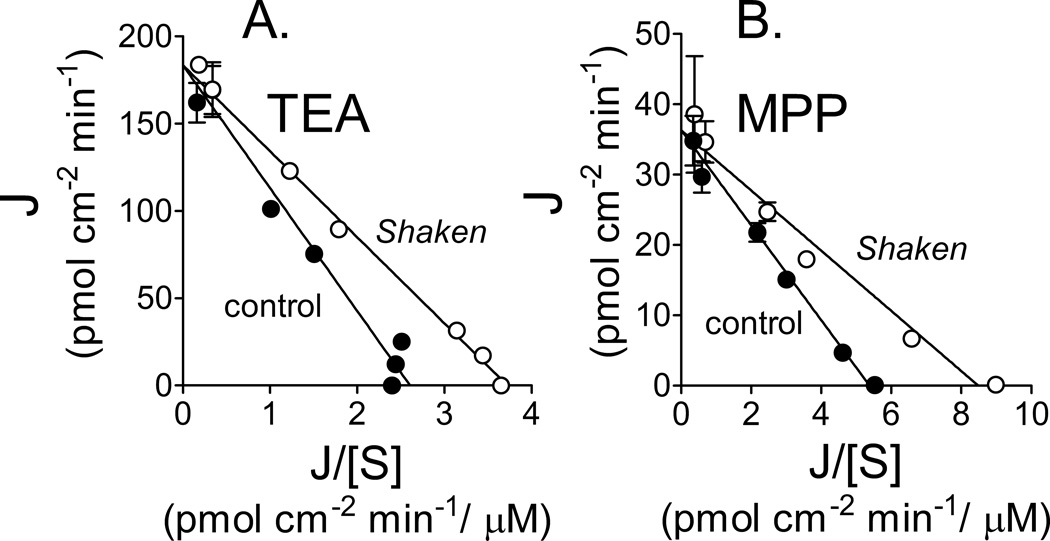
If reduction of the depth of the UWL caused the stimulation of uptake produced by shaking, then the kinetic basis of that stimulation should be a decrease in the apparent Michaelis constant for transport; Jmax should remain unchanged (see (24)). This proved to be the case. Figure 3A shows the effect of reciprocal shaking (13 Hz) on the relationship between TEA concentration and the rate of TEA transport into OCT2-expressing CHO cells (corrected for accumulation in wild-type CHO cells). Consistent with the influence of an UWL on the kinetic profile of TEA transport, in three separate experiments shaking reduced the Ktapp of uptake (from 69.5 ± 4.1 to 50.8 ± 2.4 µM; P < 0.05) with no change in Jmax (181 ± 7.2 and 188 ± 5.7 pmol cm−2 min−1 for control and shaken, respectively). Shaking produced a similar effect on the kinetics of MPP transport (Fig. 3B), reducing the Ktapp of uptake (from 6.5 ± 0.4 to 4.5 ± 0.5 µM; n=3, P < 0.05) with no change in Jmax (35.4 ± 1.4 and 37.4 ± 2.5 pmol cm−2 min−1 for control and shaken, respectively).
Figure 3.
Effect of shaking (13 Hz) on the kinetics of OCT2-mediated transport of (A.) TEA or (B.) MPP. In the Eadie-Hofstee plots of the transport data, each point represents the average (±SE) of three separate experiments that determined the rate of transport as a function of increasing substrate concentration. In each separate experiment rates of transport at each substrate concentration were determined from 30 sec uptakes measured in six wells of a 96 well plate. Open circles show rates of uptake resulting from continuous shaking of the plate following addition of the transport buffer; filled circles show rates of uptake in wells of cells that were shaken for three sec after addition of the transport buffer and then left unshaken for the remainder of the incubation. OCT2-mediated transport was calculated from total uptake less that occurring by non-saturable pathways (incl. diffusion and non-specific binding).
Effect of reduced Jmax on the impact of UWL on transport kinetics
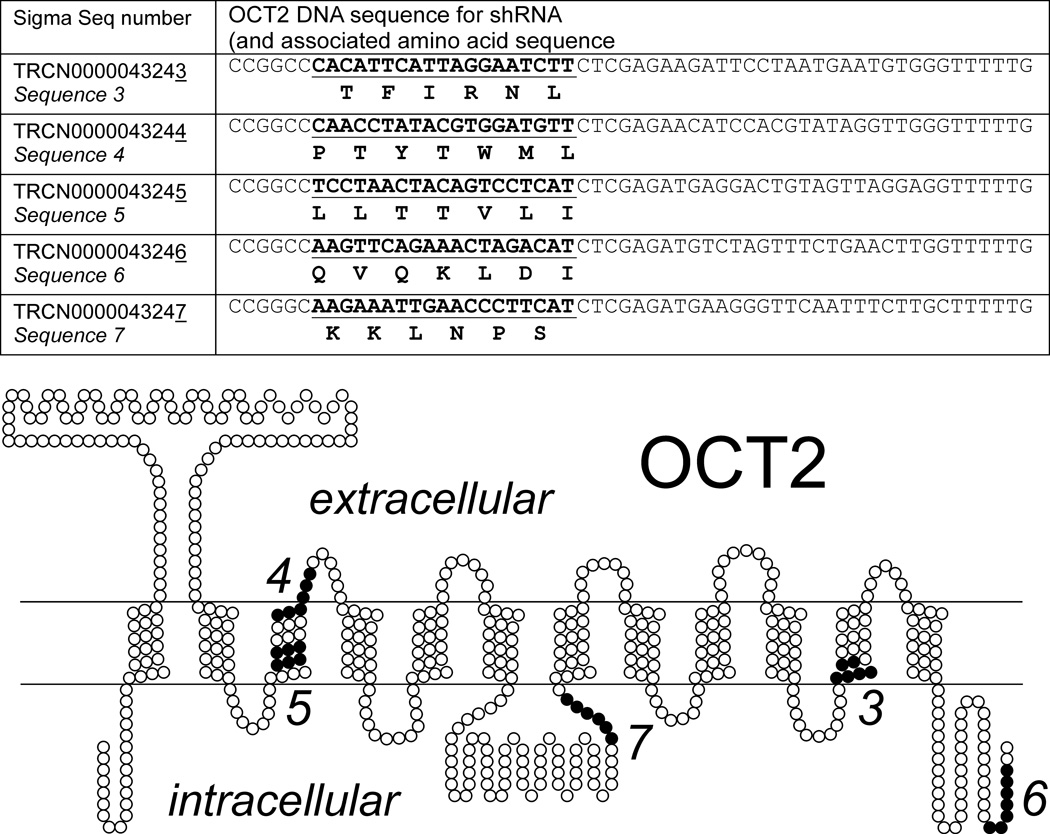
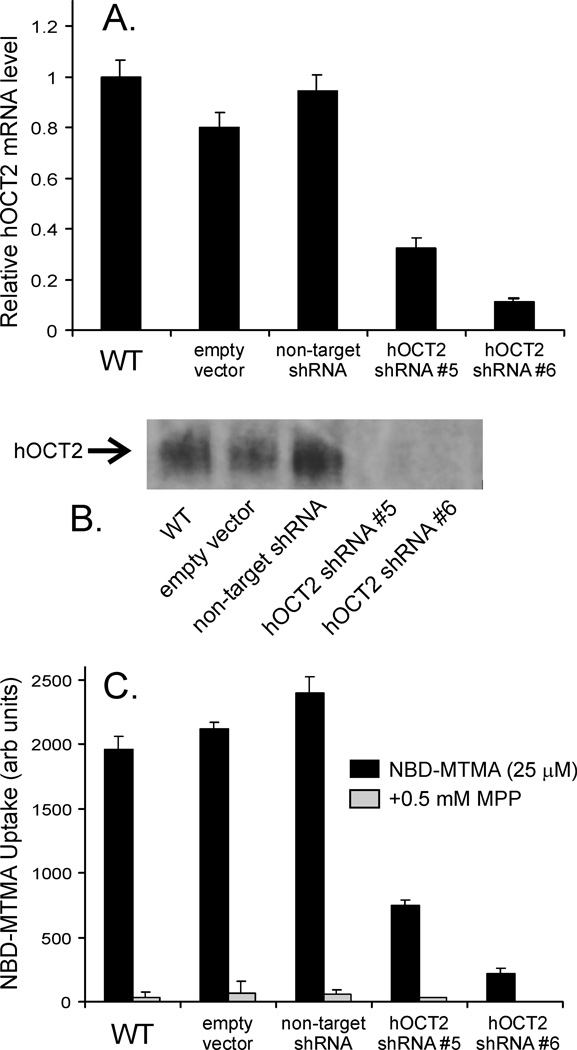
According to the Winne relationship, the bias introduced to measured values of Ktapp is proportional to UWL depth. While shaking can decrease UWL depth, the type of shaking that is practical for use in transport experiments with cultured cells cannot eliminate the UWL. But the bias introduced to Ktapp is also proportional to Jmax. It follows, therefore, that rather than using an experimental system that maximizes rates of transport (e.g., by maximizing transport protein expression), the kinetics of transport could be more accurately assessed using an experimental system with lower transport rates. To test this hypothesis we used lentivirus-introduced shRNA to knockdown the level of expression of OCT2 in CHO cells that stably expressed the transporter. Of the five shDNA sequences we tested, one (shRNA #6; Fig. 4) was effective in producing a (stable) reduction in OCT2 mRNA (>90%; Fig. 5A), total OCT2 protein (Fig. 5B), and transport function (Fig. 5C). Figure 6 compares the time course of TEA and MPP transport into control cells (OCT2-expressing CHO cells that also stably expressed a ‘non-target’ shRNA sequence; Fig. 5A) and into the ‘knockdown’ cells (cells stably transfected with shDNA sequence #6; Fig. 5B). The reduced level of OCT2 expression in the knockdown cells was associated with 80% and 55% reductions in the 15 sec uptake of TEA and MPP, respectively.
Figure 4.
DNA sequence introduced into OCT2-expressing CHO cells, using lentiviral transfection. For each of the five sequences identified in the left hand column with their Sigma-Aldrich lentiviral particle designation the coding sequence found in OCT2 (accession no. NM_003058) is underlined in bold. The corresponding portion of the amino acid sequence of OCT2 is also shown, as is the position of that sequence in the proposed secondary structure of OCT2 (58).
Figure 5.
Effect of lentiviral-introduced shRNAi on the expression of OCT2 mRNA (A.), protein (B.), and transport function (C.). (A.) OCT2 mRNA levels (relative to 18s) of CHO cells that stably expressed OCT2 (WT) or OCT2 plus a lentivirus-introduced vector that was either empty; or that generated a non-target shRNA; or one of two shRNA1 sequences that targeted OCT2. (B.) Western blot of V5-epitope-tagged OCT2 from WT OCT2-expressing cells or cells that stably expressed either the empty lentivirus vector or one of three shRNA sequences (as indicated). (C.) Transport of the fluorescent organic cation, NBD-MTMA (expressed in arbitrary fluorescence units) in wild-type OCT2 cells or cells that stably transfected with lentivirus containing one of the indicated sequences. Cells were exposed to 25 µM NBD-MTMA for 10 minutes in the absence or presence of 0.5 µM MPP. The height of each bar represents the average (+SE) of uptake measured in three wells (of a 12 well plate) from a single, representative experiment.
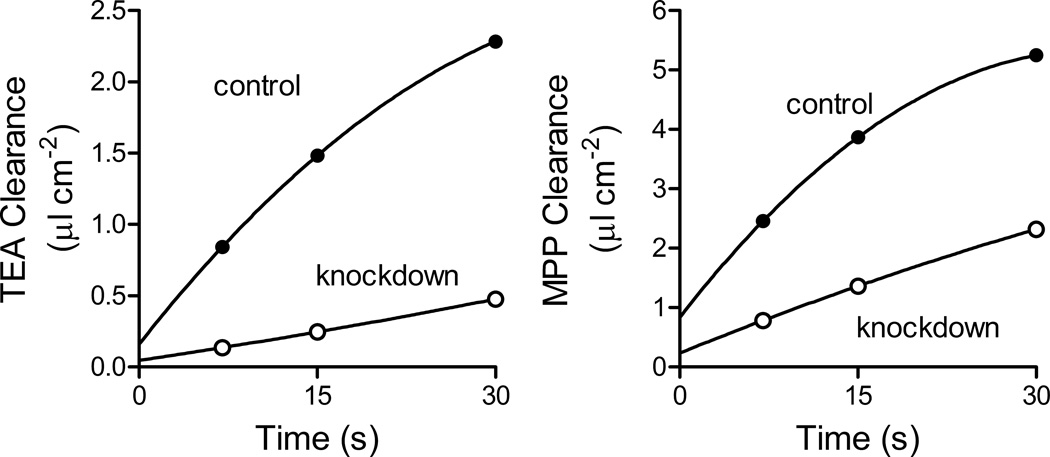
Figure 6.
Time course of uptake of OCT2-mediated MPP and TEA in CHO cells stably transfected with either the empty lentiviral vector (control) or shRNAi sequence #6 (knockdown; see Figs. 4 and 5). Uptakes of the two substrates were measured during simultaneous exposures to the two substrate (1.3 µM [14C]TEA plus 1.0 nM [3H]MPP). Each point is the average (SE) of uptake measured in triplicate wells of a 12 well plate and expressed as clearance of substrate from the transport buffer. Results shown are from a single representative experiment.
We reasoned that the reduced rate of transport supported by the knockdown OCT2 cells should be evident in a reduction in Jmax for substrate transport and, consequently, the apparent Kt values for this transport, and this proved to be the case. Figure 7A shows the kinetics of TEA transport into control and OCT2 knockdown cells. In three paired studies, the Jmax was reduced by about 90% (from 123 ± 4.4 to 10.8 ± 3.8 pmol cm−2 min−1), and the apparent Kt was reduced from 36.0 ± 4.0 to 17.6 ± 0.2 µM. The OCT2 knockdown also displayed reduced Jmax and apparent Kt values for MPP transport (Fig. 7B). In four paired studies the Jmax was also reduced by 90% (64.7 ± 16.6 to 6.1 ± 1.2 pmol cm−2 min−1), as expected from the reduction of transporter expression, and the apparent Kt was reduced from 16.6 ± 4.0 to 3.8 ± 0.5 µM.
Figure 7.
Kinetics of OCT2-mediated TEA transport (A.) or MPP transport in cells control (fully expressing) cells or in cells from which OCT2 expression was reduced by stable expression of shRNAi sequence #6 (knockdown). Each point is the average (±SE) of uptake (30 sec) from single experiments in which uptake at each substrate concentration was measured either in triplicate (TEA) or quadruplicate (MPP). For each experiment OCT2-specific uptake was determined by correcting total uptake for that measured in wild type CHO cells. Each curve reflects a non-linear regression fit of the data to the Michaelis-Menten equation. Insets show the Eadie-Hofstee plots for each data set.
We also determined the effect on Ktapp of increasing Jmax by increasing expression of OCT2 in stably transfected cells. Dimethyl sulfoxide (DMSO) has been used as a chemical chaperone to increase membrane expression of mutant proteins (25) and we have used a 24 hr exposure to 2% DMSO to produce 2- to 4-fold increases in plasma membrane expression of OCT2 (23)(R Pelis, personal communication). In three experiments the Jmax and Ktapp for TEA transport in the control cells exposed to DMSO were 351 ± 84.5 pmol cm−2 min−1 and 87.8 ± 31.9 µM, which were both larger than the values determined in parallel studies with DMSO-exposed OCT2 knockdown cells: 22.0 ± 5.4 pmol cm−2 min−1 and 22.9 ± 2.6 µM.
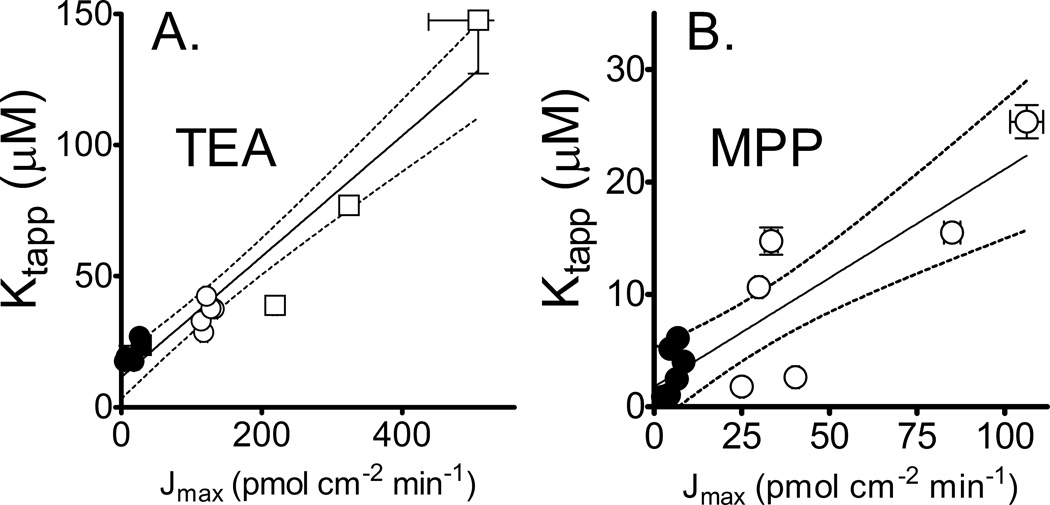
Figure 8A shows the relationship between Jmax and Ktapp for OCT2-mediated TEA transport determined in the several experiments (n = 14) that ‘titrated’ plasma membrane expression of OCT2 as a means to vary Jmax. The intercept of this relationship represents an estimate of the true Michaelis constant for OCT2-mediated TEA transport, i.e., the substrate concentration at the membrane at ½Jmax when the transport capacity is sufficiently low that diffusion through the UWL is not rate-limiting. For OCT2-mediated TEA transport, the estimated true Kt was 11.5 ± 3.8 µM, a value not significantly different from the Ktapp for TEA transport measured in the OCT2 knockdown cells (17.6 ± 0.2 µM; n=3). In addition, the same degree of reciprocal shaking that produced a 56% stimulation of transport into the control cells (Fig. 2) had no effect on the rate of OCT2-mediated transport of 17 nM [3H]TEA into the knockdown cells (data not shown), suggesting that the rate of transport into the knockdown cells was sufficiently reduced to effectively eliminate the influence of UWLs on measurement of Ktapp.
Figure 8.
Relationship between experimentally determined Jmax and apparent Kt for OCT2-mediated transport of TEA (8A) and MPP (8B). Kinetic parameters were determined in a series of experiments using either control OCT2-expressing cells (open symbols) or in cells in which OCT2 expression was reduced through stable transfection of a lentivirus-introduced shRNAi sequence from OCT2 (filled symbols). Square symbols show kinetic parameters determined from experiments in which OCT2 expression was increased by 24 hr exposure of cells to 2% DMSO prior to measurement of TEA transport. Each point shows the kinetic parameter values (±SE) as determined by non-linear regression analysis (GraphPad). The solid line is a linear regression of Jmax vs. Ktapp; dashed lines show the 95% confidence interval (for TEA, n = 14; for MPP, n = 14).
Figure 8B shows the relationship between Jmax and Ktapp for OCT2-mediated MPP transport determined in six paired experiments that measured MPP transport in control and OCT2-knockdown cells (n=12) . The y-intercept of the resulting relationship between these parameters was consistent with a true Kt of 1.8 ± 1.6 µM, a value not significantly different from the Ktapp for MPP transport measured in the OCT2 knockdown cells (3.3 ± 0.9 µM, n = 6).
According to equation 1 the slope of the Ktapp vs. Jmax relationship is proportional to the thickness of the UWL for the experimental system. Assuming a diffusion coefficient for TEA (and all OCs of MW of 150 to 300) of 6 × 10−6 cm2/sec (the measured diffusion coefficient for glucose, MW 180; (26)), the relationship between Ktapp and Jmax observed in all the experiments associated with this study can be accounted for by the influence of an UWL 1656 ± 147 µm, which did not differ from the value of 1193 ± 117 µm determined in the experiments with control and knockdown cells. The effective UWL determined from the slope of the Ktapp vs. Jmax relationship for MPP (Fig. 8B) was 1394 ± 257 µm, which was not different from the value determined from the compiled TEA kinetic data.
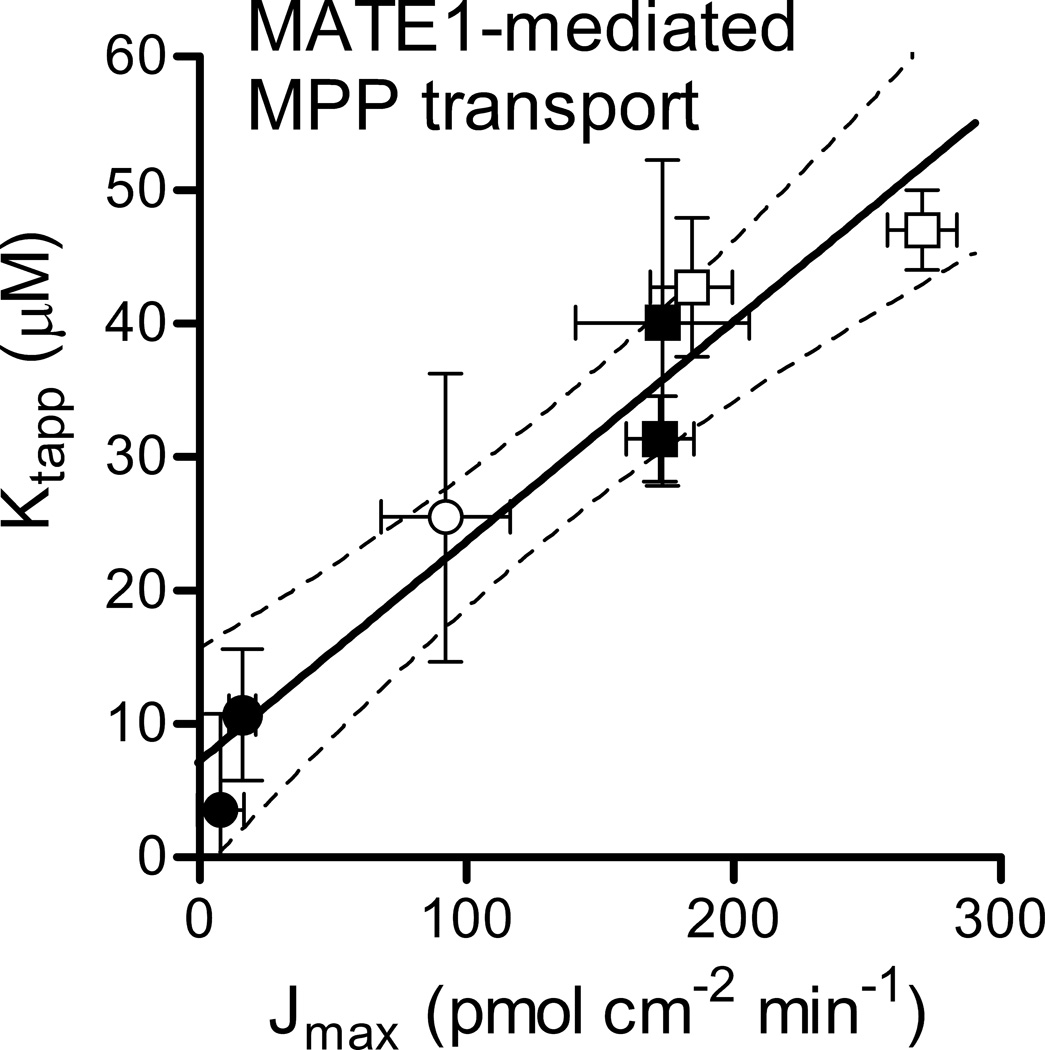
We also examined the effect of reduced rates of transport on the kinetics of transport mediated by a mechanistically distinct process, i.e., the organic cation-H+ exchanger, MATE1. Figure 9 shows the relationship between Jmax and apparent Kt determined in seven experiments. Jmax was varied by altering the outwardly-directed H+ gradient, which was achieved by preincubating the cells in different concentrations of NH4Cl for different lengths of time (see figure legend). The slope of this relationship was consistent with an unstirred layer of 1186 ± 149 µm, and the intercept suggested a true Kt for MATE-mediated MPP uptake of 7.1 ± 3.3 µM.
Figure 9.
Relationship between experimentally determined Jmax and apparent Kt for MATE1-mediated MPP transport. Kinetic parameters were determined in a series of experiments in which the maximal rate of MPP transport was manipulated by varying intracellular [H+] by manipulating the preexposure to NH4Cl: open squares, 20 min preincubation in 20 mM NH4Cl; filled squares, 10 min in 20 mM; open circle, 5 min in 20 mM; filled circles, 10 min in 10 mM. Each point shows the kinetic parameter values (±SE) as determined by non-linear regression analysis (GraphPad). The solid line is a linear regression of Jmax vs. Ktapp; dashed lines show the 95% confidence interval.
Discussion
The substrate concentrations that determine rates of transport are those immediately adjacent to the membrane, at the interface between the membrane and the overlying solution. Above the membrane is an unstirred water layer (UWL); operationally this is the static water layer immediately adjacent to the membrane and the overlying layers of slow laminar flow that extend to the bulk solution and through which movement of solutes is limited to diffusion (27). As noted by Barry and Diamond (24), the UWL results in concentrations at the membrane that can differ from that in the bulk solution by 10-fold or more. For transport processes that support net substrate entry across the plasma membrane, the inevitable consequence of an UWL is a substrate concentration at the membrane that is less than that in the overlying, well-stirred bulk-solution. It follows that empirically determined kinetic ‘constants,’ including values for Kt, overestimate the ‘true’ values reflective of substrate (or inhibitor) concentrations at the membrane. In some instances, the extent of the overestimation may be negligible for all practical purposes. The data presented here, however, show that commonly used approaches for measuring transport activity of cloned transport proteins heterologously expressed in cultured cells can overestimate Kt values by 2 to 10-fold or more.
Winne’s theoretical assessment of the impact of UWLs on measurement of Michaelis constants (9) (see equation 1) identified two parameters that functionally define the extent to which transport constants are exaggerated: (i) the operational thickness of the UWL, or δ; and (ii) the rapidity with which substrate can be removed from the fluid at the membrane, or Jmax. The factors that influence δ in experimental systems have been examined extensively (14, 24, 28), but two points are worth emphasizing here. First, in the multiwell cultured cell systems most commonly used to study rates of transport supported by cloned proteins (29), without (very) vigorous stirring, UWLs are 1000 to 2000 µm in thickness (14). Second, even vigorous stirring (using orbital or reciprocal shaking) cannot eliminate UWLs (14); the most vigorous stirring we were able to use still left UWLs that were likely to have been 500–1000 µm in thickness. Nevertheless, the modest stimulation of OCT2-mediated transport of TEA and MPP that was produced by shaking (Fig. 2) was accounted for by decreases in the apparent Michaelis constants for this transport, rather than by changes in Jmax for either substrate (Fig. 3), and UWLs influence the apparent Kt, not the Jmax, of a transport process (24). It is also worth noting that the level of stirring/shaking tolerated by our experiments reflected the use of CHO cells (because they adhere so well to the plastic substrate of culture plates). Unfortunately, HEK 293 cells, which are the most commonly used expression system for studies of OCT- and MATE-mediated transport, frequently lift from the plate upon application of comparatively mild agitation (Morales and Wright, personal observation). Consequently, we suggest that experimental results obtained using cells cultured in unshaken multiwall plates should be interpreted in the light of the likely presence of UWLs that, on average, are on the order of 1500 µm in depth.
The ubiquity of UWLs and the practical inability to substantially reduce them in many experimental situations means that their impact on measurement of transport constants is effectively defined by the Jmax for substrate transport. The Jmax of a transport process is the product of transporter turnover number and the number of functional transporters expressed at the plasma membrane. For practical experimental reasons, common practice has been to increase the level of heterologous transporter expression as a means to increase rates of substrate uptake, thereby increasing the transport ‘signal’ compared to the ‘noise’ represented by the level of substrate accumulation observed in cells that do not express the heterologous transport protein. Several methods have been used to maximize expression of heterologous transporter protein in cultured cells, including selection of clonal lines of cells that display the highest rates of transport following transfection and antibiotic selection (e.g., (30)), and induction of protein expression, either selectively (e.g., using zinc-inducible promoters upstream of the transfected transporter sequence; (31)) or, more commonly, through use of sodium butyrate-driven induction of gene expression (32). Indeed, for cells that stably express a heterologous transporter adding 5 mM sodium butyrate to the culture media for 24 hrs prior to experimental use can increase transport activity by 2- to 5-fold or more (33) and is commonly used to increase rates of OCT- and MATE-mediated substrate uptake (e.g., (34, 35)). It is, however, ironic that efforts to increase the precision of transport measurements (by increasing Jmax values) can compromise the accuracy of the resulting estimates of transport kinetics (by increasing the influence of UWLs on measurement of those same values).
Our examination of the influence of Jmax on apparent Michaelis constants for transport mediated by OCT2 and MATE1 focused on two prototypic substrates for these processes, TEA and MPP. TEA was among the first substrates used to characterize OC transport in isolated renal proximal tubules (36) and in membrane vesicles isolated from renal cortex (37, 38). TEA was subsequently used to characterize transport by the first cloned OCT (39, 40) and MATE (41) transporters. Use of MPP as a test substrate for renal OC transporters followed the observation (42) that it is transported with substantially higher transport efficiency (i.e., ratio of Jmax/Kt) than is TEA. MPP is now frequently used as a ‘well-transported’ substrate for both OCTs and MATEs (22, 43). In fact, the increasing use of MPP transport as a measure of activity made it the recommended substrate for use in assays of OCT-based drug-drug interactions in the Food and Drug Administration’s 2012 draft Guidance for Industry for Drug Interaction Studies (44). Finally, the structural dissimilarity of TEA and MPP (MCS Tanimoto coefficient of 0.16; see Fig. 2) is correlated with quantitatively distinctive kinetic profiles (Fig. 7), and that provided us with a means to apply a broader test of the influence of UWLs on transport than would a focus on any single substrate.
A decrease in Jmax for transport of TEA and MPP should reduce the impact of UWLs on measurement of their respective apparent Kt values. Indeed, according to the Winne relationship, the degree of bias introduced to measured estimates of an apparent Kt should be directly proportional to the Jmax for transport of a given substrate. We decreased the Jmax for OCT2-mediated TEA and MPP transport by introducing an RNAi sequence (Fig. 4) that reduced mRNA and translated protein for OCT2 (Fig. 5), and the resulting line of cells with reduced OCT2 expression displayed maximum transport rates for TEA and MPP that were 11-fold lower than those of the ‘parental’ line of OCT2-expressing cells (Fig. 7). Consistent with the Winne relationship, the decreases in Jmax also produced decreases in the apparent Kt values for TEA and MPP (Fig. 8).
The conclusion that the observed changes in Ktapp simply reflected the influence of UWLs rather than changes in the transport process itself was ultimately predicated on the assumption that there is, in fact, a unique Kt value for the interaction of a particular substrate molecule with its transporter. In other words, we assume the Kt for interaction of a transport protein with a particular substrate is an intrinsic property of the protein that reflects the protein’s structure under a particular set of physiological conditions (as defined by the prevailing relevant forces, including membrane potential, local ionic conditions and the status of any regulatory process that may influence protein structure). Alternatively, increases in expression of OCT2 protein could lead to increased formation of OCT2 oligomers that display reduced affinity for substrate. However, whereas OCTs have been shown to form homomultimers in the plasma membrane (45, 46), the Ktapp values for OCT-mediated transport of both TEA and MPP are not influenced by oligomerization (45).
Further support for UWLs as the probable explanation for the correlation between Jmax and Ktapp was the observation that changes in the Jmax for MATE1-mediated transport that were independent of the amount of protein in the membrane were also associated with changes in Ktapp (Fig. 9). In the experiments using MATE1-expressing cells we varied Jmax for MPP transport by taking advantage of the characteristic stimulation of MATE-mediated OC transport produced by increasing intracellular [H+] (41, 47). That stimulation reflects an increase in the Jmax of OC/H+ exchange brought about by elevation of the trans concentration of hydrogen ion (presumably due to an increase in turnover of the transporter; (48)). Systematic variation of the ‘ammonia pulse’ used to acidify the cytoplasm of MATE1-expressing cells varied the Jmax for transport by more than 20-fold, and decreases in Jmax were strongly correlated with decreases in Ktapp for MPP transport (Fig. 9). Importantly, if the causal link between changes in Jmax and Ktapp is an UWL that is a common property of the experimental system, then the slope of that relationship should be proportional to the depth of the UWL and, therefore, the same for all transport. Thus it was significant that the slopes of the relationship between Jmax and Ktapp for transport of the same substrate (MPP) by two processes that have distinct energetic mechanisms (i.e., OCT2 and MATE1; Figs. 8B and 9), did not differ significantly from the slopes that described the correlation observed for transport of two distinct substrates (TEA and MPP) by the same process (i.e., OCT2; Fig. 8A and 8B). It is also worth emphasizing that the slopes of the three Jmax vs. Ktapp relationships (Figs. 8 and 9) did not differ from the average value of 1500 µm previously reported for UWLs in unstirred multiwell culture plates (14, 16).
Interpreting the kinetic parameters for OC transport measured here in the context of those reported in the literature is challenging given the substantial degree of variability in the published values (see (43)). It is, however, instructive to examine them in the light of the expectation that UWLs should influence all estimates of the Ktapp for transported substrates. Table I lists published values for Jmax and apparent Kt for transport of TEA and MPP mediated by human OCT2 expressed in cells (CHO or HEK-293) that stably expressed the transporter and were grown on multiwell culture plates. Table I also lists the calculated Winne bias, in µM, predicted to be included in the Ktapp values owing to the influence of the associated Jmax and the presence of a 1500 µm UWL. The Jmax and Ktapp values reported in the present study were within the range of these previously published values. It is noteworthy that there is a marked correlation between reported Jmax and Ktapp values; the highest and lowest values for Ktapp for OCT2-mediated TEA and MPP transport (500 and 20 µM for TEA; 29 and 8 µM for MPP) were associated with the highest and lowest Jmax values for transport of these substrates (805 vs. 63 pmol cm−2 min−1 for TEA; 101 vs. 13 pmol cm−2 min−1 for MPP).
Table I.
Literature values for experimentally determined kinetic parameters for OCT2-mediated transport of TEA, MPP and metformin.
| Substrate | Jmax (pmol cm−2 min−1)* |
Ktapp (µM) |
Winne ‘bias’ (µM) |
reference |
|---|---|---|---|---|
| TEA | 805 | 500 | 168 | (50) |
| 132 | 431 | 28 | (51) | |
| 99 | 54 | 21 | (52) | |
| 97 | 35 | 20 | present study | |
| 63 | 20 | 13 | (19) | |
| MPP | 101 | 29 | 21 | (53) |
| 88 | 25 | 18 | (50) | |
| 35 | 9 | 7 | present study | |
| 23 | 9 | 5 | (8) | |
| 20 | 20 | 4 | (3) | |
| 16 | 11 | 3 | (5) | |
| 13 | 8 | 3 | (54) | |
| Metformin | 417 | 1380 | 87 | (55) |
| 412 | 3356 | 86 | (3) | |
| 350 | 1066 | 73 | (56) | |
| 265 | 378 | 55 | Morales & Wright, unpublished |
|
| 67 | 215 | 14 | (57) |
Jmax values expressed per mg of membrane protein were converted to values expressed per cm2 of cell surface area by using the factor 0.035 mg/cm2 (22).
As noted earlier, the influence of UWLs on kinetic constants can be small relative to the probable value of the true Kt for transport of a substrate. For example, Table I also lists published values for the kinetics of OCT2-mediated metformin transport. The median Jmax for metformin transport (~350 pmol cm−2 min−1) is substantially greater than that for either TEA or MPP transport (97 and 35 pmol cm−2 min−1, respectively). Consequently, the bias introduced to measured Ktapp values for metformin (around 75 µM) should be substantially larger than that introduced to Ktapps for TEA (~20 µM) and MPP (~5 µM). However, the ‘metformin bias’ represents only about 7% of the ~1066 µM Ktapp reported for OCT2-mediated metformin transport, which is negligible in the face of a true Kt of, perhaps, ~1000 µM. The complicating influence of UWLs is, however, more substantial when dealing with substrates that have transport properties like those of TEA and MPP. The data presented here suggest that the true Kt for OCT2-mediated TEA transport is ~10 to 15 µM, so a 20 µM bias is ≥150% larger than the actual Kt. The values of ~50 µM routinely reported in the literature (Table I), reflect an underestimate of the affinity of OCT2 for TEA of >300%. With respect to MPP, our data suggest a true Kt of ~1 to 2 µM, indicating that literature values underestimate the affinity of OCT2 for MPP by 250% to >700%. There are, of course, many potential sources of inter-study variation in the kinetic parameters generated by different groups (see (49)), and it is unlikely that UWLs are the basis of all (or in some cases, perhaps, even most) of the exaggeration of apparent Michaelis constants for OC transport that appears common in the literature. While it is beyond our means to discern all the reasons for the variability in kinetic constants for OC transport evident in the literature (e.g., (43)), the potential influence of UWLs on such measurements can and should be a part of the interpretation of all transport kinetics.
Although UWLs cannot be eliminated from experimental systems commonly used to characterize membrane transport, their influence on the measurement of the kinetic parameters of drug transport can be significantly reduced, thereby increasing the accuracy of experimentally determined Michaelis constants. However, before applying methods to reduce the influence of UWLs on experimental estimates of transport kinetics, we suggest that the Winne relationship be used to obtain an order-of-magnitude estimate of the bias that UWLs may introduce to measured kinetic parameters. It is simple to estimate the potential bias that the presence of an UWL may introduce into an empirically determined Kt: divide the product of the measured Jmax (expressed in moles per cm2-sec) and an estimate of the UWL (e.g., 0.15 cm; (14)) by 2D (where D is ~6 × 10−6 cm2/sec). The result, when converted to moles per liter from its value in moles/cm3, can be compared to the measured kinetic constant. In the event that UWLs may be introducing unacceptably large error to the estimate, use of vigorous stirring (>>10 Hz), or cells with limited expression of the transport protein (or both) can significantly reduce the error that UWLs introduce to the measurement of transport kinetics.
CONCLUSION
Heterologous expression systems that use cultured cells grown in multiwell plates offer the means to measure the kinetics of multidrug transport with substantial precision. The accuracy of the resulting values is, however, in question; the physical characteristics of such systems, particularly the ubiquitous presence of unstirred water layers, make them vulnerable to overestimating substrate and inhibitor binding constants. The present study showed that commonly used methods to measure the kinetics of organic cation interaction with OCT2 and MATE1 can result in 2 to 10-fold overestimates of Kt values. The accuracy of these measurements can be improved through vigorous stirring of the experimental system or by substantial reduction in the level of expression of the transport protein. Regardless, the increasing use of in vitro assessment of ligand binding interactions in clinical, pharmaceutical and regulatory decision-making underscores the importance of interpreting experimental results fully informed of the issues that may influence their accuracy.
Acknowledgments
ACKNOWLEDGEMENTS AND DISCLOSURES
This work was supported in part by NIH grants 5R01DK058251, 1R01DK080801, 5P30ES006694, and 5T32HL07249. The authors extend their thanks to Dr. William H. Dantzler, University of Arizona, and Dr. Ryan M. Pelis, Dalhousie University, for helpful discussions.
ABBREVIATIONS
- CHO
Chinese hamster ovary
- MDCK
Madin-Darby canine kidney
- DDI
Drug-drug interaction
- HEK-293
Human embryonic kidney 293
- Jmax
Maximal rate of transport
- Kt
Michaelis constant
- Ktapp
Apparent Michaelis constant
- hMATE1
human Multidrug And Toxin Extruder 1
- MPP
1-Methyl-4-phenylpyridinium
- NBD-MTMA
N,N,N-trimethyl-2-[methyl(7-nitrobenzo[c][l,2,5]oxadiazol-4-yl)amino]ethanaminium
- hOCT2
human Organic Cation Transporter 2
- TEA
Tetraethylammonium
- UWL
Unstirred Water Layer
References
- 1.Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, Dahlin A, et al. Membrane transporters in drug development. Nat Rev Drug Discov. 2010;9:215–236. doi: 10.1038/nrd3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kido Y, Matsson P, Giacomini KM. Profiling of a prescription drug library for potential renal drug-drug interactions mediated by the organic cation transporter 2. J Med Chem. 2011;54:4548–4558. doi: 10.1021/jm2001629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zolk O, Solbach TF, Konig J, Fromm MF. Structural determinants of inhibitor interaction with the human organic cation transporter OCT2 (SLC22A2) Naunyn Schmiedebergs Arch Pharmacol. 2009;379:337–348. doi: 10.1007/s00210-008-0369-5. [DOI] [PubMed] [Google Scholar]
- 4.Astorga B, Ekins S, Morales M, Wright SH. Molecular determinants of ligand selectivity for the human Multidrug And Toxin Extrusion proteins, MATE1 and MATE-2K. J Pharmacol Exp Ther. 2012;341:743–755. doi: 10.1124/jpet.112.191577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harper JN, Wright SH. Multiple mechanisms of ligand interaction with the human organic cation transporter, OCT2. Am J Physiol Renal Physiol. 2012;304:F56–F67. doi: 10.1152/ajprenal.00486.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suhre WM, Ekins S, Chang C, Swaan PW, Wright SH. Molecular determinants of substrate/inhibitor binding to the human and rabbit renal organic cation transporters hOCT2 and rbOCT2. Mol Pharmacol. 2005;67:1067–1077. doi: 10.1124/mol.104.004713. [DOI] [PubMed] [Google Scholar]
- 7.Bednarczyk D, Ekins S, Wikel JH, Wright SH. Influence of molecular structure on substrate binding to the human organic cation transporter, hOCT1. Mol Pharmacol. 2003;63:489–498. doi: 10.1124/mol.63.3.489. [DOI] [PubMed] [Google Scholar]
- 8.Belzer M, Morales M, Jagadish B, Mash EA, Wright SH. Substrate-dependent ligand inhibition of the human Organic Cation Transporter, OCT2. J Pharmacol Exp Ther. 2013;346:300–310. doi: 10.1124/jpet.113.203257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winne D. Unstirred layer, source of biased Michaelis constant in membrane transport. Biochim Biophys Acta. 1973;298:27–31. doi: 10.1016/0005-2736(73)90005-9. [DOI] [PubMed] [Google Scholar]
- 10.Thomson AB, Dietschy JM. Derivation of the equations that describe the effects of unstirred water layers on the kinetic parameters of active transport processes in the intestine. J Theor Biol. 1977;64:277–294. doi: 10.1016/0022-5193(77)90357-5. [DOI] [PubMed] [Google Scholar]
- 11.Dietschy JM, Sallee VL, Wilson FA. Unstirred water layers and absorption across the intestinal mucosa. Gastroenterology. 1971;61:932–934. [PubMed] [Google Scholar]
- 12.Avdeef A, Nielsen PE, Tsinman O. PAMPA--a drug absorption in vitro model 11. Matching the in vivo unstirred water layer thickness by individual-well stirring in microtitre plates. Eur J Pharm Sci. 2004;22:365–374. doi: 10.1016/j.ejps.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Balakrishnan A, Hussainzada N, Gonzalez P, Bermejo M, Swaan PW, Polli JE. Bias in estimation of transporter kinetic parameters from overexpression systems: Interplay of transporter expression level and substrate affinity. J Pharmacol Exp Ther. 2007;320:133–144. doi: 10.1124/jpet.106.107433. [DOI] [PubMed] [Google Scholar]
- 14.Korjamo T, Heikkinen AT, Monkkonen J. Analysis of unstirred water layer in in vitro permeability experiments. J Pharm Sci. 2009;98:4469–4479. doi: 10.1002/jps.21762. [DOI] [PubMed] [Google Scholar]
- 15.Adson A, Burton PS, Raub TJ, Barsuhn CL, Audus KL, Ho NF. Passive diffusion of weak organic electrolytes across Caco-2 cell monolayers: uncoupling the contributions of hydrodynamic, transcellular, and paracellular barriers. J Pharm Sci. 1995;84:1197–1204. doi: 10.1002/jps.2600841011. [DOI] [PubMed] [Google Scholar]
- 16.Karlsson J, Artursson P. A method for the determination of cellular permeability coeffcients and aqueous boundary layer thickness in monolayers of intestinal epithelial (Caco-2) cells grown in permeable filter chambers. Int J Pharmaceut. 1991;71:55–64. [Google Scholar]
- 17.Ruell JA, Tsinman KL, Avdeef A. PAMPA--a drug absorption in vitro model. 5. Unstirred water layer in iso-pH mapping assays and pKa flux--optimized design (pOD-PAMPA) Eur J Pharm Sci. 2003;20:393–402. doi: 10.1016/j.ejps.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Aavula BR, Ali MA, Bednarczyk D, Wright SH, Mash EA. Synthesis and fluorescence of n , n , n -trimethyl-2-[methyl(7-nitrobenzo[c][1,2,5]oxadiazol-4-yl)amino]ethanaminium iodide, a pH-insensitive reporter of organic cation transport. Synthetic Comm. 2006;36:701–705. [Google Scholar]
- 19.Pelis RM, Dangprapai Y, Wunz TM, Wright SH. Inorganic mercury interacts with cysteine residues (C451 and C474) of hOCT2 to reduce its transport activity. Am J Physiol Renal Physiol. 2007;292:F1583–F1591. doi: 10.1152/ajprenal.00496.2006. [DOI] [PubMed] [Google Scholar]
- 20.Zhang X, He X, Baker J, Tama F, Chang G, Wright SH. Twelve transmembrane helices form the functional core of mammalian MATE1 (Multidrug and Toxin Extruder 1) protein. J Biol Chem. 2012;287:27971–27982. doi: 10.1074/jbc.M112.386979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kapus A, Grinstein S, Wasan S, Kandasamy R, Orlowski J. Functional characterization of three isoforms of the Na+/H+ exchanger stably expressed in Chinese hamster ovary cells. ATP dependence, osmotic sensitivity, and role in cell proliferation. J Biol Chem. 1994;269:23544–23552. [PubMed] [Google Scholar]
- 22.Schomig E, Lazar A, Grundemann D. Extraneuronal monoamine transporter and organic cation transporters 1 and 2: a review of transport efficiency. Handb Exp Pharmacol. 2006:151–180. doi: 10.1007/3-540-29784-7_8. [DOI] [PubMed] [Google Scholar]
- 23.Pelis RM, Suhre WM, Wright SH. Functional influence of N-glycosylation in OCT2-mediated tetraethylammonium transport. Am J Physiol Renal Physiol. 2006;290:F1118–F1126. doi: 10.1152/ajprenal.00462.2005. [DOI] [PubMed] [Google Scholar]
- 24.Barry PH, Diamond JM. Effects of unstirred layers on membrane phenomena. Physiol Rev. 1984;64:763–872. doi: 10.1152/physrev.1984.64.3.763. [DOI] [PubMed] [Google Scholar]
- 25.Papp E, Csermely P. Chemical chaperones: mechanisms of action and potential use. Handb Exp Pharmacol. 2006:405–416. doi: 10.1007/3-540-29717-0_16. [DOI] [PubMed] [Google Scholar]
- 26.Stein WD. Channels, Carriers, and Pumps: An Introduction to Membrane Transport. San Diego: Academic Press, Inc; 1990. [Google Scholar]
- 27.Dainty J. Water relations of plant cells. Adv Bot Res. 1963;1:279–326. [Google Scholar]
- 28.Pedley TJ. Calculation of unstirred layer thickness in membrane transport experiments: a survey. Q Rev Biophys. 1983;16:115–150. doi: 10.1017/s0033583500005060. [DOI] [PubMed] [Google Scholar]
- 29.Giacomini KM, Huang SM. Transporters in drug development and clinical pharmacology. Clin Pharmacol Ther. 2013;94:3–9. doi: 10.1038/clpt.2013.86. [DOI] [PubMed] [Google Scholar]
- 30.Urakami Y, Okuda M, Masuda S, Saito H, Inui KI. Functional characteristics and membrane localization of rat multispecific organic cation transporters, OCT1 and OCT2, mediating tubular secretion of cationic drugs. J Pharmacol Exp Ther. 1998;287:800–805. [PubMed] [Google Scholar]
- 31.Shi X, Bai S, Ford AC, Burk RD, Jacquemin E, Hagenbuch B, Meier PJ, et al. Stable inducible expression of a functional rat liver organic anion transport protein in HeLa cells. J Biol Chem. 1995;270:25591–25595. doi: 10.1074/jbc.270.43.25591. [DOI] [PubMed] [Google Scholar]
- 32.Palermo DP, DeGraaf ME, Marotti KR, Rehberg E, Post LE. Production of analytical quantities of recombinant proteins in Chinese hamster ovary cells using sodium butyrate to elevate gene expression. J Biotechnol. 1991;19:35–47. doi: 10.1016/0168-1656(91)90073-5. [DOI] [PubMed] [Google Scholar]
- 33.Briz O, Serrano MA, Rebollo N, Hagenbuch B, Meier PJ, Koepsell H, Marin JJ. Carriers involved in targeting the cytostatic bile acid-cisplatin derivatives cis-diammine-chloro-cholylglycinate-platinum(II) and cis-diammine-bisursodeoxycholate-platinum(II) toward liver cells. MolPharmacol. 2002;61:853–860. doi: 10.1124/mol.61.4.853. [DOI] [PubMed] [Google Scholar]
- 34.Ito S, Kusuhara H, Yokochi M, Toyoshima J, Inoue K, Yuasa H, Sugiyama Y. Competitive inhibition of the luminal efflux by multidrug and toxin extrusions, but not basolateral uptake by organic cation transporter 2, is the likely mechanism underlying the pharmacokinetic drug-drug interactions caused by cimetidine in the kidney. J Pharmacol Exp Ther. 2012;340:393–403. doi: 10.1124/jpet.111.184986. [DOI] [PubMed] [Google Scholar]
- 35.Muller F, Konig J, Hoier E, Mandery K, Fromm MF. Role of organic cation transporter OCT2 and multidrug and toxin extrusion proteins MATE1 and MATE2-K for transport and drug interactions of the antiviral lamivudine. Biochem Pharmacol. 2013;86:808–815. doi: 10.1016/j.bcp.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 36.Schäli C, Schild L, Overney J, Roch-Ramel F. Secretion of tetraethylammonium by proximal tubules of rabbit kidneys. Am J Physiol. 1983;245:F238–F246. doi: 10.1152/ajprenal.1983.245.2.F238. [DOI] [PubMed] [Google Scholar]
- 37.Takano M, Inui KI, Okano T, Saito H, Hori R. Carrier-mediated transport systems of tetraethylammonium in rat renal brush-border and basolateral membrane vesicles. Biochim Biophys Acta. 1984;773:113–124. doi: 10.1016/0005-2736(84)90556-x. [DOI] [PubMed] [Google Scholar]
- 38.Wright SH, Wunz TM. Transport of tetraethylammonium by rabbit renal brush-border and basolateral membrane vesicles. Am J Physiol. 1987;253:F1040–F1050. doi: 10.1152/ajprenal.1987.253.5.F1040. [DOI] [PubMed] [Google Scholar]
- 39.Gründemann D, Gorboulev V, Gambaryan S, Veyhl M, Koepsell H. Drug excretion mediated by a new prototype of polyspecific transporter. Nature. 1994;372:549–552. doi: 10.1038/372549a0. [DOI] [PubMed] [Google Scholar]
- 40.Okuda M, Saito H, Urakami Y, Takano M, Inui KI. cDNA cloning and functional expression of a novel rat kidney organic cation transporter, OCT2. Biochem Biophys Res Commun. 1996;224:500–507. doi: 10.1006/bbrc.1996.1056. [DOI] [PubMed] [Google Scholar]
- 41.Otsuka M, Matsumoto T, Morimoto R, Arioka S, Omote H, Moriyama Y. A human transporter protein that mediates the final excretion step for toxic organic cations. Proc Natl Acad Sci USA. 2005;102:17923–17928. doi: 10.1073/pnas.0506483102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lazaruk KDA, Wright SH. MPP+ is transported by the TEA+-H+ exchanger of renal brush-border membrane vesicles. Am J Physiol. 1990;258:F597–F605. doi: 10.1152/ajprenal.1990.258.3.F597. [DOI] [PubMed] [Google Scholar]
- 43.Nies AT, Koepsell H, Damme K, Schwab M. Organic cation transporters (OCTs, MATEs), in vitro and in vivo evidence for the importance in drug therapy. Handb Exp Pharmacol. 2011;201:105–167. doi: 10.1007/978-3-642-14541-4_3. [DOI] [PubMed] [Google Scholar]
- 44.Huangand SM, Zhang L, editors. U.S.Food, Drug A. Guidance for Industry: Drug Interaction Studies - Study Design, Data Analysis, Implications for Dosing, and Labeling Recommendations. Draft Guidance. 2012:1–79. [Google Scholar]
- 45.Keller T, Egenberger B, Gorboulev V, Bernhard F, Uzelac Z, Gorbunov D, Wirth C, et al. The large extracellular loop of organic cation transporter 1 influences substrate affinity and is pivotal for oligomerization. J Biol Chem. 2011;286:37874–37886. doi: 10.1074/jbc.M111.289330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brast S, Grabner A, Sucic S, Sitte HH, Hermann E, Pavenstadt H, Schlatter E, et al. The cysteines of the extracellular loop are crucial for trafficking of human organic cation transporter 2 to the plasma membrane and are involved in oligomerization. FASEB J. 2011;26:976–986. doi: 10.1096/fj.11-180679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Masuda S, Terada T, Yonezawa A, Tanihara Y, Kishimoto K, Katsura T, Ogawa O, et al. Identification and functional characterization of a new human kidney-specific H+/organic cation antiporter, Kidney-Specific Multidrug and Toxin Extrusion 2. J Am Soc Nephrol. 2006;17:2127–2135. doi: 10.1681/ASN.2006030205. [DOI] [PubMed] [Google Scholar]
- 48.Wright SH, Wunz TM. Mechanism of cis- and trans- substrate interactions at the tetraethylammonium/H+ exchanger of rabbit renal brush-border membrane vesicles. J Biol Chem. 1988;263:19494–19497. [PubMed] [Google Scholar]
- 49.Bentz J, O'Connor MP, Bednarczyk D, Coleman J, Lee C, Palm J, Pak YA, et al. Variability in P-glycoprotein inhibitory potency (IC50) using various in vitro experimental systems: implications for universal digoxin drug-drug interaction risk assessment decision criteria. Drug Metab Dispos. 2013;41:1347–1366. doi: 10.1124/dmd.112.050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Umehara KI, Iwatsubo T, Noguchi K, Kamimura H. Comparison of the kinetic characteristics of inhibitory effects exerted by biguanides and H2-blockers on human and rat organic cation transporter-mediated transport: Insight into the development of drug candidates. Xenobiotica. 2007;37:618–634. doi: 10.1080/00498250701397705. [DOI] [PubMed] [Google Scholar]
- 51.Urakami Y, Akazawa M, Saito H, Okuda M, Inui K. cDNA cloning, functional characterization, and tissue distribution of an alternatively spliced variant of organic cation transporter hOCT2 Predominantly Expressed in the Human Kidney. J Am Soc Nephrol. 2002;13:1703–1710. doi: 10.1097/01.asn.0000019413.78751.46. [DOI] [PubMed] [Google Scholar]
- 52.Pelis RM, Dangprapai Y, Cheng Y, Zhang X, Terpstra J, Wright SH. Functional significance of conserved cysteines in the human organic cation transporter 2. Am J Physiol Renal Physiol. 2012;303:F313–F320. doi: 10.1152/ajprenal.00038.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kikuchi R, Lao Y, Bow DA, Chiou WJ, Andracki ME, Carr RA, Voorman RL, et al. Prediction of Clinical Drug-Drug Interactions of Veliparib (ABT-888) with Human Renal Transporters (OAT1, OAT3, OCT2, MATE1, and MATE2K) J Pharm Sci. 2013;102:4426–4432. doi: 10.1002/jps.23737. [DOI] [PubMed] [Google Scholar]
- 54.Gründemann D, Hahne C, Berkels R, Schomig E. Agmatine is efficiently transported by non-neuronal monoamine transporters extraneuronal monoamine transporter (EMT) and organic cation transporter 2 (OCT2) J Pharmacol Exp Ther. 2003;304:810–817. doi: 10.1124/jpet.102.044404. [DOI] [PubMed] [Google Scholar]
- 55.Kimura N, Okuda M, Inui K. Metformin transport by renal basolateral organic cation transporter hOCT2. Pharm Res. 2005;22:255–259. doi: 10.1007/s11095-004-1193-3. [DOI] [PubMed] [Google Scholar]
- 56.Kusuhara H, Ito S, Kumagai Y, Jiang M, Shiroshita T, Moriyama Y, Inoue K, et al. Effects of a MATE protein inhibitor, pyrimethamine, on the renal elimination of metformin at oral microdose and at therapeutic dose in healthy subjects. Clin Pharmacol Ther. 2011;89:837–844. doi: 10.1038/clpt.2011.36. [DOI] [PubMed] [Google Scholar]
- 57.Wang K, Sun S, Li L, Tu M, Jiang H. Involvement of organic cation transporter 2 inhibition in potential mechanisms of antidepressant action. Prog Neuropsychopharmacol Biol Psychiatry. 2014;53:90–98. doi: 10.1016/j.pnpbp.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 58.Zhang X, Shirahatti NV, Mahadevan D, Wright SH. A conserved glutamate residue in transmembrane helix 10 influences substrate specificity of rabbit OCT2 (SLC22A2) J Biol Chem. 2005;280:34813–34822. doi: 10.1074/jbc.M506342200. [DOI] [PubMed] [Google Scholar]