Summary
Aim
Hospital-acquired disability causes decreased quality of life for patients with dementia and family caregivers, and increased societal costs.
Materials & methods
A comparative, repeated measures study tested the feasibility and preliminary efficacy of the family-centered, function-focused care intervention (Fam-FFC) in dyads of hospitalized, medical patients with dementia and family caregivers (FCGs).
Results
The intervention group demonstrated better activities of daily living and walking performance, and less severity/duration of delirium and hospital readmissions, but no significant differences in gait/balance. FCGs showed increased preparedness for caregiving and less anxiety but no significant differences in depression, strain and mutuality.
Conclusion
Fam-FFC presents a possible pathway to meeting the Triple Aim of improved patient care, improved patient health and reduced costs for persons with dementia.
Keywords: acute care, dementia, family caregivers, familyengagement, physicaland cognitive function, postacute recovery
An estimated 5 million Americans have Alzheimer's disease or another type of dementia [1]; around 3.2 million enter the hospital each year [2]. Persons with dementia are about two times more likely to be hospitalized than their peers who are cognitively healthy [1,2]. Moreover, once hospitalized they are more likely to experience delirium and behavioral manifestations of distress, as well as pressure ulcers, falls and nutritional problems [3,4]. These complications put them at increased risk for hospital-acquired disability (manifest in loss of activities of daily living [ADL] function and mobility), and increased morbidity/mortality [5,6]. During the postacute period, persons with dementia are more likely to experience protracted delirium and increased care dependency [7,8] with lower quality of life for both themselves and their family caregivers (FCGs) [9,10]. Consequently, they utilize more postacute care [1] and are at increased risk for rehospitalizations, transitions to long-term nursing home stays and mortality than persons without dementia [6,8].
Risk factors for hospital acquired disability & complications
A combination of intrinsic and extrinsic factors increases the risk for hospital-acquired disability and other complications in hospitalized persons with dementia. Intrinsic vulnerabilities include co-morbidities, low baseline physical and cognitive function and delirium [11,12]. Extrinsic risk factors include limited opportunity for physical activity related to environmental (e.g., lack of safe walking areas and seating, unsafe bed/toilet height and restricted allocation of therapy resources) and policy issues (limited access to areas for ambulation, enforced bedrest, use of tethering devices such as indwelling urinary catheters and monitoring devices) contribute to hospital-acquired deconditioning [13]. The unfamiliar, confusing and sometimes frightening environment of the hospital and lack of meaningful cognitive stimulation during the acute stay increases anxiety and boredom, which may contribute to delirium and associated functional decline [14]. Bedrest and restricted mobility [15], the use of physical and chemical restraints despite risk of associated complications and inadequate attention to nutrition, mood and pain management, are also commonly cited culprits contributing to functional decline and other complications [16–18].
The role of the family caregiver of the hospitalized person with dementia
Approximately 75% of persons with dementia are cared for in the community by family members and friends [1,10]. Although no guidelines are currently available, FCGs can play a key role during hospitalization in evaluation, treatment decisions and care delivery, acting either as care managers (informants, advocates and coordinators) and/or care providers (offering direct care, encouragement and emotional support) [19,20]. When engaged to do so, FCGs of persons with dementia provide essential information on baseline cognitive and functional status necessary to guide care delivery and track treatment response [21,22]. Although they may be limited in the acute care setting, discussions with close family members may be useful in predicting and preventing excessive stress in persons with dementia, with the potential to prevent unnecessary antipsychotic use and mitigate progressive physical or cognitive decline [23].
Further, FCGs can play a positive role by motivating and encouraging patients during the hospitalization [24] and subsequently in the home setting [25]. However, lack of understanding of the ramifications of inactivity and how to prevent deconditioning and functional loss may cause families to attempt to restrict physical activity. FCGs may believe that bedrest will promote recovery from an acute illness, or fear that the older adult will fall if physical activity is encouraged. FCGs are consulted for medical treatment decisions and placement issues but less frequently for care-related issues with teaching of appropriate care interactions (physical activity, cognitive stimulation and self-care) to optimize function and physical activity [24].
Although hospitalization may be expected to provide respite for FCGs, the experience is often associated with increased stress [22,26]. The patient's functional loss, presence of delirium and neuropsychiatric symptoms increase FCG burden during the hospitalization of the person with dementia [26]. Additionally, the pre-existing, chronic strain borne by FCGs of persons with dementia is compounded by anxiety about the comfort and safety of the patient during their hospital stay and the potential for increased care needs at discharge [22]. Interventions that allow the FCG to practice appropriate care interventions during the hospital stay that optimize function and physical activity are greatly needed. These interventions can improve the cognition, physical function and emotional response of the patient and thereby also improve the FCG experience.
Family-centered function-focused care
Extensive research in multiple settings demonstrated the efficacy of a theory-based philosophy of care, function-focused care (FFC), in maximizing function and physical activity [27]. Guided by a social–ecological model [28] and social–cognitive theory [29], FFC is implemented into settings of care using four components including: environmental and policy assessment; staff education; individualized FFC goals and mentoring and motivating nursing staff and patients [27]. Combining FFC with a family-centered approach, we developed family-centered FFC (Fam-FFC). Fam-FFC recognizes that the hospitalization may represent an opportunity to strengthen the FCG's focus on function in their role as both care manager and care provider, by incorporating an educational–empowerment intervention for FCGs provided within the four evidence-based FFC steps [24]. Initial testing of Fam-FFC resulted in improved postacute function, delirium abatement and reduced hospital readmissions for older patients without increasing FCG strain or negatively impacting the relationship with the patient [24]. We have adapted Fam-FFC to make it ‘dementia-capable’, by customizing the Fam-FFC steps to the specialized needs of persons with dementia and their family caregivers.
The purpose of this study was to test the feasibility of Fam-FFC and examine its impact among hospitalized persons with dementia and their FCGs at discharge, 14 days and 60 days postdischarge. Our primary hypothesis was that Fam-FFC would improve ADL performance in patients with dementia at 60 days postdischarge. We also hypothesized that exposure to Fam-FFC would result in positive patient outcomes including less severity and duration of delirium; better walking performance, gait and balance; a greater return to prehospitalization ADL performance; shorter lengths of stay in acute care; less utilization of postacute rehabilitation services; fewer discharges to nursing homes; fewer 30-day hospital readmissions and positive caregiver outcomes including better preparedness for caregiving, less anxiety and depression, reduced role strain and better mutuality with the person with dementia
Methods
Design
This study utilized a comparative repeated measures design and was implemented on five medical units of two hospitals in the Northeast USA over 18 months (2013–2014). The units were matched based on size, staffing and physical configuration; none of the medical units included in the study had previously implemented dementia initiatives or FFC. One unit served as the intervention unit in each hospital and three units served as control units. We utilized two control units in one hospital in order to facilitate equal recruitment between the control and intervention arm.
Upon referral from the hospital staff, a research evaluator provided study information to patients and FCGs within 24 h of admission to the unit. The hospital staff referred only older adults age 65 and above, consistent with the age criteria for the study. If the patient and FCG agreed to the study, the evaluation to sign consent (ESC) form [30] was used to determine ability to sign consent. Patients who were unable to pass the ESC but were able to provide assent were enrolled when the legally authorized representative provided consent. In addition to age, patient eligibility included: English-speaking/reading, a positive mini-cog [31] and an AD8 greater than or equal to 2. The AD8 contains eight items that test for memory, orientation, judgment and function validated as an informant-based interview used to screen for dementia [32]. Patients who were known to be terminally ill and/or receiving hospice care or surgery were excluded. Family members age 21 and above whose relatives meet inclusion criteria were eligible if they could speak and read English; were related to the patient by blood, marriage, adoption or affinity as a significant other and were primary family caregivers who either lived with the patient or continued to provide caregiving from an alternate residence. The study was approved by the Institutional Review Board of the New York University School of Medicine and the study sites. Written informed consent was obtained from all participants prior to determining eligibility.
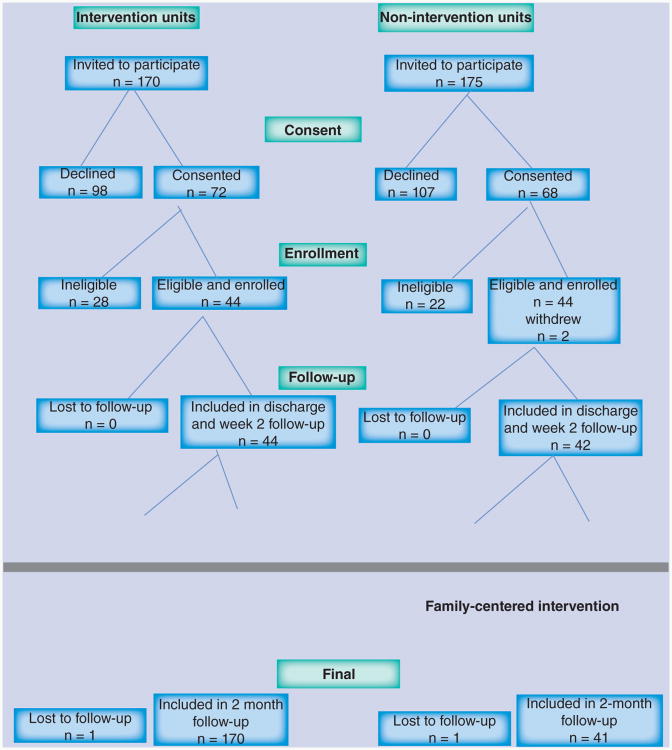
Of the 345 potentially eligible dyads, 140 (40%) consented to be screened for participation in the study. Fifty-two dyads were excluded for the following reasons: the patient was did not meet the inclusion criteria for a positive mini-cog or AD8 (n = 37; 71%); the patient had a terminal medical diagnosis (n = 7; 14%) and scheduling problems interfered with timely enrollment in the study (n = 8; 15%). Thus, 88 dyads were enrolled, 44 on the intervention units and 44 on the control units. Two dyads withdrew during initial data collection yielding a final sample of 86 with 44 in the intervention arm and 42 in the control arm. Between 2 weeks and 2 months after discharge two dyads were lost to follow-up, yielding 43 in the final intervention arm and 41 in the final control arm. Figure 1 shows participant recruitment and study participation.
Figure 1. Participant recruitment and study participation.
Implementation of the intervention
The Fam-FFC intervention aimed to create an enabling care environment for persons with dementia that promoted patient engagement in functional recovery while actively engaging the patient and FCG in care-related decisionmaking. The family-centered resource nurse (FCRN), a registered nurse, devoted 10–15 h a week to implement the Fam-FFC intervention with the support of the research team. In addition, the study units were asked to appoint a nurse as the unit champion to work with the FCRN, in order to support implementation and sustainability of the intervention. The FCRN, with the support of the unit champion, implemented the components of Fam-FFC described in Table 1. To assure setting readiness, Component I (Environmental and Policy Evaluation) and II (Staff Education) were initiated with the nursing staff prior to Component III (Ongoing Training and Motivation of Nursing Staff) and Component IV (FamCare). The first three components continued throughout the course of the intervention and overlapped with the FamCare component (FCG/patient education, jointly developed bedside goals and treatment plans and postacute follow-up).
Table 1. Components of family-centered function-focused care for dementia.
| Component | When | By whom | Description |
|---|---|---|---|
| Environmental and policy assessment | Beginning of the study; at 3 and 9 months during implementation | Fam-FFC Research Nurse with unit champions; recommendations for change discussed with administration and initiated as approved | Modifications included: a policy to safely label glasses/hearing aids; bedside white boards to promote FCG/patient communication with the interdisciplinary team and access to inexpensive hearing amplifiers, activity cart/supplies and mobility devices |
|
| |||
| Staff education and training (delivery options included: instructor-led PowerPoint presentations and one on one review) | Beginning of the study | Fam-FFC Research Nurse on intervention units; co-investigator on control units | Content includes: hospital experience for person with dementia and family; cognitive and functional assessment, and evidence-based approaches to prevent delirium and functional decline; FFC (incorporating into routine care, specific techniques/equipment, safety considerations, goal setting/discharge planning) and partnership with families† (assessment of preferences, information-sharing, care planning, promoting advocacy and patient/family engagement in decision-making and discharge planning) |
| At month 2, 3 and 5: educational reminders of educational key points provided in Staff mailboxes and posted on the educational board of the intervention unit | |||
|
| |||
| Ongoing training and motivation of nursing staff | Following initial education of the staff; during 12 months of implementation | Fam-FFC Research Nurse mentors the unit champions and nursing staff | Assistance to champions and nurses was provided on consented patients to: assess physical capability; establish and update goals with input from FCGs/patients and develop a care plan with FCG/patient addressing factors that impede FFC (e.g., acute illness, sedation, pain, fear/anxiety, pain, apathy, neuropsychiatric symptoms and depression) and support the unit champions to mentor and motivate nursing Staff (RN, LPN and nursing assistants) |
|
| |||
| FamPath care pathway | During the 12 months of implementation | Fam-FFC Research Nurse | Family/patient education: provided in lay terms (cueing and motivating techniques, support of physical activity, meals, cognitive stimulation and safety) linked to joint FCG/nurse assessment (baseline cognition, physical function and social profile); jointly developed bedside, individualized goals and treatment plans (updated daily; discharge checklist); coaching of primary nurse to communicate and provide a copy of FamPath plan to postacute providers and postacute follow-up to provide ongoing education and modification of the FFC plan (home visit within 48 h of discharge, weekly telephone calls for a total of 7 additional weeks, then monthly for 4 months), coaching of FCGs to communicate FFC goals and expectations to the postacute providers as indicated |
Not included in the control unit education.
FCG: Family caregiver; FFC: function-focused care; LPN: Licensed practical nurse; RN: Registered nurse.
The control units received FFC education using a modified version of the staff education component, as described in Table 1. The possibility of data collection contamination between units was averted by avoiding data collection during the times of FamCare implementation.
Measures
Descriptive measures
Descriptive information for patients included age, race, sex, education, marital status, type of residence prior to hospitalization and use of a mobility device. Upon admission, the presence of delirium was assessed with the Confusion Assessment Method [33] and co-morbidities were measured using Charlson co-morbidity scale [34]. Additionally, baseline physical function (family caregiver report of status 2 weeks prior to admission) was collected using the Barthel Index, a 14-item measure of the person's self-care performance [35]. For family members information was obtained on age, race, sex, education, marital status, work status and role in the family (spouse, child and other). All measures have established evidence of reliability and validity for use with older adults and FCGs, as noted below.
Patient outcome measures
Trained data collectors collected patient data within 48 h of admission, at discharge, 14 days postdischarge and 60 days postdischarge. Patient outcome measures included ADL performance, walking performance, gait and balance and delirium severity. The primary outcome, ADL performance, was measured using the Barthel Index [35] from the verbal report of the assigned nurse in acute care and the FCG after discharge. Additionally, the single item from the Barthel Index that measures the ability to walk 50 feet was used to measure walking performance. Gait and balance was measured using the Tinetti Scale [36], comprised of 12 items evaluating gait and 16 items evaluating balance. The severity of delirium was evaluated using the Delirium Severity Scale [37], a 10-item scale that ranges from 0 (no delirium) to 32 (highest degree of severity).
Family caregiver measures
Outcome measures for FCGs were collected using pen and paper questionnaires completed within 48 h of patient admission to the unit, within 48 h of discharge and 14 and 60 days postdischarge. Preparedness for caregiving was evaluated using the Preparedness for Caregiving Scale [38], an eight-item self-rated instrument that asks caregivers how well prepared they believe they are for multiple domains of caregiving such as providing physical care and emotional support, setting up in-home support services and dealing with the stress of caregiving. Items are rated 0 (not at all prepared) to 4 (very well prepared); scores can range from 0 to 32. Caregiver anxiety and depression were evaluated using the two subscales within the Hospital Anxiety and Depression Scale (HADS). Specifically this included a seven-item anxiety subscale (HADS-A) and a seven-item Depression subscale scale (HADS-D) [39]. Scores for each subscale can range from 0 to 21 with scores categorized as follows: normal (0–7), mild (8–10), moderate (11–14) and severe (15–21) for each of the HADS subscales.
Mutuality, defined as the positive quality of the relationship between caregiver and care receiver, and the ability of the caregiver to find meaning in the caregiving situation, was measured using the Mutuality Scale [38]. The Mutuality Scale includes dimensions of reciprocity, love, shared pleasurable activities and shared values. Fifteen items are rated on a five-point scale that ranges from 0 (not at all) to 4 (a great deal) with scores ranging from 0 to 60 (high mutuality) [38]. Caregiver strain was evaluated using the Modified Caregiver Strain Index (CSI), a 13-question tool that measures strain related to the following domains affected by caregiving: employment, financial, physical, social and time [40]. Caregivers were also encouraged to complete a bedside log to detail FCG involvement in care by type of activity and time spent.
Treatment fidelity was evaluated in terms of the delivery (environmental/policy assessments, staff training and goal setting; care planning as planned); receipt (change in environment and policy, knowledge scores and goal attainment scaling) and enactment of the intervention by staff and FCGs (see Table 2).
Table 2. Treatment fidelity.
| Focus | Data | Evidence of treatment fidelity |
|---|---|---|
| Delivery | Environment/policy assessments (component I) | Completion of assessments by Fam-FFC Research Nurse |
| Education: percentage of nurses exposed (component II) | 80% of all nursing Staff working on participating nursing units (no. exposed/total no. nursing staf)† | |
| Goal attainment (component III) | Forms completed on all recruited patients in treatment units | |
| Fam-Path Audit (component IV) | Completion of all tools: bedside goals and treatment plans, discharge checklist, communication with postacute provider, postacute follow-up and plan update | |
|
| ||
| Receipt | Knowledge of Fam-FFC test | Mean score of ≥90% and ≥80% for nurses and nursing assistants, respectively, after exposure to education† |
| Environment/policy assessments | Evidence of change(s) made over the course of the study | |
| Goal attainment scaling | Positive goal attainment scores incorporated into care plans and expected outcomes evaluated at discharge The Goal Attainment Scaling [41] evaluated the FCG/patient views on the degree to which goals were met using the following scale:-2 (much less than expected), -1 (less than expected), 0 (expected), +1 (greater than expected) and +2 (much greater than expected) | |
|
| ||
| Enactment | FFC behavior checklist | Performance of Fam-FFC by nurses based on observations of care interactions in the hospital |
| FCG logs | FCG involvement in care and follow-through with the care plan in the hospital and postacute setting | |
Both treatment and control sites; everything else is treatment site only.
FCG: Family caregiver; FFC: Function-focused care
Data analysis
Based on the primary endpoint of ADL performance, a total sample size of n = 84 is required to detect a difference of at least 20 points at 60 days between the nonintervention and intervention groups, with an overall standard deviation of 32, α = 5% and power = 80%. Descriptive analysis of the data according to intervention arm was performed to assess for differences in demographics and baseline variables. Correlations between sample characteristics and outcome variables were examined to identify potential covariates. A two (group) by four (time) repeated measures analysis of variance technique was used with patient and family study outcomes as the dependent variable. For each outcome, scatterplots, frequencies and boxplots were evaluated to assess model assumptions. Mauchly's test was used to evaluate sphericity. When the sphericity assumption was violated, the Greenhouse-Geisser F-test was used when drawing conclusions regarding the time–treatment interaction effect. Post hoc analyses were conducted using the Bonferroni correction when preceded by significant analysis of variance effects. Analysis of variance was used to compare length of hospital stay by treatment groups. χ2 analysis was performed to compare the impact of treatment on the following postacute outcomes: admission to the nursing home posthospital discharge, unplanned hospital readmissions within 30 days of discharge, return to baseline function and delirium at 2 months postdischarge.
Missing value analysis was conducted to examine missing pattern and mechanism of data. All the variables included in this analysis had none or very little missing data (2% for education, 2% marital status and 0.2% for mutuality), which did not exceed the 5–10% recommended allowable limit for missing data [42]. Analyses followed intention to treat such that all subjects providing data were included in analyses regardless of study participation level.
Results
Patient characteristics
Patient and family characteristics are shown in Table 3. The mean age of the patients was 82.4 (± 7.6). The majority were female (59%), widowed (52%), with at least a high school education (94%) and admitted from home (98%). The sample was equally represented by black (45%) and white (45%) patients. In total, 42% demonstrated delirium upon admission and the mean Charlson co-morbidity was 3.7 (± 2.5). The average AD8 and Barthel Index (2 weeks prior to admission) was 4.2 (± 2.1) and 84.9 (± 20.7), respectively. No significant differences were found between the treatment groups with regard to demographics and baseline characteristics.
Table 3. Dyad characteristics.
| Characteristics | Total (n = 86) | Nonintervention group (n = 42) | Intervention group (n = 44) | p-value |
|---|---|---|---|---|
| Patient characteristics, n (%) or mean (SD) | ||||
|
| ||||
| Female | 51 (59) | 28 (67) | 23 (52) | 0.08 |
|
| ||||
| Race: | 1.0 0 | |||
| – Black | 39 (45) | 19 (45) | 20 (45) | |
| – White | 39 (45) | 19 (45) | 20 (45) | |
|
| ||||
| Widowed | 45 (52) | 24 (57) | 21 (48) | 0.30 |
|
| ||||
| High school education or above | 81 (94) | 41 (98) | 40 (91) | 0.40 |
|
| ||||
| Delirium at admission | 36 (42) | 17 (4 0) | 19 (4 4) | 0.75 |
|
| ||||
| Admitted from private residence | 84 (98) | 41 (98) | 43 (98) | 1.00 |
|
| ||||
| Age, years | 82.4 (7.6) | 83.8 (6.5) | 81.0 (8.5) | 0.18 |
|
| ||||
| AD8 score | 4.2 (2.1) | 4.4 (2.0) | 4.0 (2.1) | 0.49 |
|
| ||||
| Preadmission Barthel score | 84.9 (20.7) | 80.6 (25.1) | 88.9 (14.9) | 0.15 |
|
| ||||
| Charlson co-morbidity index | 3.7 (2.5) | 3.2 (2.0) | 4.0 (2.6) | 0. 31 |
|
| ||||
| Family caregiver characteristics, n (%) | ||||
|
| ||||
| Relationship to patient: | 0.02 | |||
| – Female spouse | 20 (23) | 12 (2 9) | 8 (18) | |
| – Male spouse | 13 (15) | 9 (21) | 4 (9) | |
| – Daughter | 33 (39) | 17 (4 0) | 16 (36) | |
| – Son | 12 (14) | 2 (5) | 10 (23) | |
| – Other | 8 (9) | 2 (5) | 6 (14) | |
|
| ||||
| Race: | 0.33 | |||
| – Black | 40 (46) | 19 (45) | 21 (48) | |
| – White | 39 (45) | 19 (45) | 20 (46) | |
|
| ||||
| Married | 59 (69) | 31 (74) | 28 (64) | 0.60 |
|
| ||||
| Some college education or higher | 58 (67) | 28 (67) | 30 (68) | 0.58 |
|
| ||||
| Age, years: | 0.70 | |||
| – 31–45 | 5 (6) | 3 (7) | 2 (5) | |
| – 46–65 | 45 (52) | 20 (48) | 25 (57) | |
| – 66–80 | 19 (22) | 10 (24) | 9 (20) | |
| – Over 80 | 17 (20) | 9 (21) | 8 (18) | |
|
| ||||
| Employment outside the home | 46 (54) | 22 (52) | 24 (55) | 0.47 |
SD: Standard deviation.
Family caregiver characteristics
Daughters comprised the largest group of FCGs in both study arms. Male spouse FCGs were more represented in the control group, while son FCGs were more represented in the intervention group. Most FCGs were married (69%) with a college education or higher (67%). Most FCGs were in the age range of 46–65 (52%) and a little more than half (54%) were employed outside the home.
Patient outcomes
Patient functional outcomes at discharge, 14 days and 60 days postdischarge are demonstrated in Table 4. There were no significant differences between the groups with regard to outcome variables evaluated on admission. Patients who participated in Fam-FFC demonstrated better ADL performance (F [2.0] = 4.2; p = 0.02, partial η2 = 0.08), with improvement evident at 2 months after discharge. Mean walking performance differed significantly between intervention arms (F [2.5] = 6.1; p = 0.001, partial η2 = 0.11); Fam-FFC elicited less decrease in walking performance at 2 months postdischarge.
Table 4. Patient outcomes at discharge, 2 weeks and 2 months postdischarge.
| Outcome, mean (SD) | Nonintervention | Intervention | F (df) | p-value |
|---|---|---|---|---|
| ADL performance (Barthel Index): | 4.2 (2.0) | 0.02 | ||
| – Admission | 70.8 (29.9) | 78.0 (24.8) | ||
| – Discharge | 67.1 (28.9) | 72.9 (24.8) | ||
| – 2 weeks | 64.5 (30.5) | 81.0 (20.9) | ||
| – 2 months | 67.6 (30.9) | 88.3 (15.3) | ||
|
| ||||
| Walking performance (50 yards): | 6.1 (2.5) | 0.001 | ||
| – Admission | 9.0 (6.6) | 12.0 (5.1) | ||
| – Discharge | 8.4 (6.3) | 11.0 (5.7) | ||
| – 2 weeks | 7.9 (5.9) | 10.3 (5. 8) | ||
| – 2 months | 6.8 (5.5) | 12.4 (4.9) | ||
|
| ||||
| Gait and balance (Tinetti Scale): | 0.3 (2.4) | 0.79 | ||
| – Admission | 16.3 (8.8) | 17.8 (8.8) | ||
| – Discharge | 15.8 (8.0) | 16.2 (8.9) | ||
| – 2 weeks | 15.3 (9.1) | 16.7 (9.4) | ||
| – 2 months | 15.8 (8.8) | 17.3 (9.6) | ||
|
| ||||
| Delirium severity (Delirium Severity Scale): | 4.1 (1.4) | 0.03 | ||
| – Admission | 7.2 (8.5) | 7.9 (8.3) | ||
| – Discharge | 7.0 (6.9) | 8.3 (2.6) | ||
| – 2 weeks | 3.3 (4.0) | 1.2 (1.7) | ||
| – 2 months | 3.0 (3.5) | 1.0 (0.9) | ||
ADL: Activities of daily living; SD: Standard deviation.
There was no significant effect of the intervention on Tinetti Gait and Balance (F [2.4] = 0.9; p = 0.79). Additionally, the intervention was also associated with a significant decrease in overall delirium severity (F [1.4] = 4.1; p = 0.03, partial η2 = 0.08). The Fam-FFC arm demonstrated less delirium severity from admission to both 2 weeks and 2 months postdischarge.
Discharge outcomes are shown in Table 5. The average hospital length of stay did not differ significantly between the intervention group (4.0 ± 2.1) and the control group (4.4 ± 2.0). The number of patients utilizing postacute rehabilitation services or transferred to a nursing home was not significantly different. There was a modest treatment effect upon the number of 30-day hospital readmissions, which was lower in the treatment group as compared with the control group (χ2 = 5.8; p = 0.02). Patients exposed to Fam-FFC showed less delirium 2 months after discharge (χ2 = 3.8; p = 0.05) and the number of patients who returned to baseline ADL performance was significantly higher in the group exposed to Fam-FFC (χ2 = 8.6; p = 0.003).
Table 5. Patient discharge outcomes.
| Outcome, n (%) or mean (SD) | Nonintervention | Intervention | χ2 | p-value |
|---|---|---|---|---|
| Discharge to nursing home | 11 (26) | 12 (2 7) | 2.0 | 0.56 |
| Utilization of postacute rehabilitation | 27 (64) | 29 (66) | 1. 5 | 0.69 |
| Readmission to hospital within 30 days | 10 (24) | 3 (7) | 5.8 | 0.02 |
| Delirium present 2 months postdischarge | 12 (29) | 3 (7) | 3.8 | 0.05 |
| Failed to return to baseline function by 2 months postdischarge | 21 (15) | 5 (12) | 8.6 | 0.003 |
| Length of stay | 4.4 (2.0) | 4.0 (2.1) |
SD: Standard deviation.
Family caregiver outcomes
The outcome variables for FCGs are presented in Table 6. There were no significant differences between the groups with regards to FCG baseline assessments. Role relationship was significantly correlated to FCG strain at all time points and was controlled for in the analysis. The intervention was associated with a significant increase in preparedness for caregiving (F [2.6] = 3.0; p = 0.04, partial η2 = 0.06); post hoc tests revealed that Fam-FFC was associated with increased preparation for caregiving from admission to 2 months postdischarge. The mean FCG anxiety differed significantly between groups (F [1.7] = 5.5; p < 0.008, partial η2 = 0.10); Fam-FFC was associated with less anxiety from admission to 2 months postdischarge. There was no significant effect of the intervention on depression (F [2.1] = 2.7; p = 0.07), mutuality (F [1.9] = 1.7; p = 0.19) or role strain (F [1.6] = 1.7; p = 0.20), controlling for FCG role relationship.
Table 6. Family caregiver outcomes.
| Outcome, mean (SD) | Nonintervention | Intervention | F (df) | p-value |
|---|---|---|---|---|
| Preparedness for caregiving: | 3.0 (2.6) | 0.04 | ||
| – Admission | 22.8 (5.5) | 21.9 (8.0) | ||
| – Discharge | 21.8 (6.5) | 23.3 (6.7) | ||
| – 2 weeks | 23.0 (4.9) | 22.7 (4.9) | ||
| – 2 months | 23.2 (7.0) | 26.3 (5.3) | ||
|
| ||||
| Anxiety (HADS-A): | 5.5 (1.7) | 0.008 | ||
| – Admission | 6.2 (4.2) | 7.0 (4.6) | ||
| – Discharge | 5.8 (4.0) | 6.5 (5.0) | ||
| – 2 weeks | 6.1 (4.6) | 5.9 (4.6) | ||
| – 2 months | 6.3 (5.2) | 4.7 (4.0) | ||
|
| ||||
| Depression (HADS-D): | 2.7 (2.1) | 0.07 | ||
| – Admission | 3.8 (2.9) | 4.7 (3.9) | ||
| – Discharge | 4.0 (3.4) | 5.0 (4.1) | ||
| – 2 weeks | 4.5 (4.0) | 4.6 (4.9) | ||
| – 2 months | 4.5 (4.1) | 3.8 (4.6) | ||
|
| ||||
| Role strain (Modified Caregiver Strain Index): | 1.7 (1.6) | 0.20 | ||
| – Admission | 6.2 (5.5) | 6.2 (5.8) | ||
| – Discharge | 6.8 (6.0) | 7.0 (6.7) | ||
| – 2 weeks | 6.8 (5.9) | 6.0 (5.8) | ||
| – 2 months | 6.7 (5.0) | 5.9 (5.7) | ||
|
| ||||
| Mutuality: | 1.7 (1.9) | 0.19 | ||
| – Admission | 4 7.3 (11.9) | 4 8.4 (10.2) | ||
| – Discharge | 46.9 (11.8) | 47.4 (12.7) | ||
| – 2 weeks | 43.3 (15.3) | 48.2 (10.1) | ||
| – 2 months | 45.1 (13.6) | 48.6 (9.7) | ||
HADS-A: Hospital Anxiety and Depression Scale – Anxiety Subscale; HADS-D: Hospital Anxiety and Depression Scale – Depression Subscale; SD: Standard deviation.
Treatment fidelity
The evaluation of the physical environment demonstrated consistency between three time points: the beginning of the study, 3 months and 9 months. Receipt of the training was supported based on average post scores on the Knowledge of Fam-FFC for Dementia Care Test of 90 (± 7.1) for nurses and 87.5 (± 6.2) for nursing assistants. The FCRN implemented all components of FamCare, based upon a review of documentation, patient goals and interactions with patients and FCGs.
Observation of care delivery by nursing staff showed 88% agreement between the care plan and enactment of the plan. The most common area of nonadherence was supporting patients to walk to the bathroom rather than using a urinal or bedpan. Comparison of the logs describing FCG role in care showed 92% agreement with the care plan. FCGs indicated that goals were met in 95% of the cases, with 64% (n = 28) stating outcomes were greater than expected.
The postacute follow-up by the FCRN was consistently provided. Postacute adherence to the care plan occurred in 82% (n = 71) of the patients; problems with adherence were in patients who were transferred to a nursing home and one person discharged to home. Examples of nonadherence with the care plan included use of hoyer lift without indication, eating meals in bed, use of the wheelchair for prolonged sitting and lack of involvement in activities adapted to person's cognitive level.
Discussion
The findings from this study support the feasibility of Fam-FFC and show preliminary efficacy that implementation of Fam-FFC can improve outcomes for both hospitalized patients with dementia and their FCGs. Specifically, patients on Fam-FFC units had better ADL and walking performance, less severity and duration of delirium and less hospital readmissions. FCGs on Fam-FFC units showed a significant increase in preparedness for caregiving and less anxiety from admission to 2 months postdischarge. In addition, the study offers methodological and measurement considerations for future research.
The improvements in ADL and walking performance suggest the benefits of engaging families and patients in individualized plans that focus on self-care and physical activity within an enabling care environment. The trend to delirium abatement with Fam-FFC suggests that a systemic-approach to FFC may promote cognitive recovery posthospitalization. Given that the resolution of delirium among postacute patients appears to be a prerequisite for functional recovery [7], these results are clinically relevant and warrant further investigation.
The rate of hospital readmissions was lower in the intervention arm; this could have potential ramifications on healthcare costs. This outcome is consistent with research conducted in long-term care, demonstrating the benefits of FFC on reducing hospital admissions while simultaneously improving functional outcomes [27]. Further, findings are consistent with other research demonstrating the benefits of nurse-implemented transitional interventions with persons with dementia [43]. Future research examining the treatment effect in a larger sample, in a randomized controlled trial over longer periods of time is warranted to further evaluate clinical efficacy.
There were no improvements in gait and balance associated with exposure to Fam-FFC. The Tinetti Gait and Balance scores indicated moderate fall risk [36] in both the intervention and control groups. These results indicate the need to incorporate fall prevention strategies, and targeted, gait, balance, flexibility, endurance and strength training in discharge planning and follow-up. Additionally, future research could provide more aggressive physical activity and evaluation with actigraphy as an objective measure of physical activity.
The improvements in FCG preparation for caregiving suggest that the hospitalization may present the opportunity to support FCG knowledge and skills. Further, those FCGs exposed to Fam-FFC demonstrated less anxiety. These findings underscore the value of shared decision-making and involvement in care, described by family members as central characteristics of quality care [20,30]. Importantly, the intervention was not associated with an increase in FCG strain or negative effects upon the FCG–patient relationship. We will continue to evaluate these measures in future research, within an expanded social ecological model which considers factors such as the FCG's health, role relationship, competing demands, health literacy and cultural preferences.
The intervention's feasibility was evaluated within the four major components. The environmental and policy assessment was not deemed to be too time-consuming by the nurse champions who felt that it could be integrated into routine safety rounds. The educational intervention was provided at multiple times in multiple formats so as to minimize the cost and inconvenience of replacing staff during times of training. The FCRN played a central role in FamPath implementation and ongoing mentoring of staff. The costs associated with FCRN functions and the potential of integrating these activities into other existing roles is planned for a future cost-benefit analysis of Fam-FFC.
Administrative support of the unit champion role was integral to implementation of the intervention, facilitating not only logistics (training and communication) but also follow-through by nursing staff on the care plan. This support and oversight was not extended to the patients discharged to a nursing home, evident in the inconsistent follow-through in that setting in contrast to the home setting. Thus, the Fam-FFC intervention will be expanded to include a partnership with the postacute care setting facilitated by the use of a facility-based champion and collaboration with nursing home staff, including the rehabilitation team.
Study limitations
This study was limited by a small sample size. Additionally, data collectors were not blinded though they were not informed of the intervention's goals and content. Finally, the type and intensity of rehabilitation services provided was not considered and could be a confounding variable.
Conclusion & future perspective
Dementia places significant burden on patients, caregivers, providers and healthcare delivery systems. As hospitalization is increasingly common in this vulnerable population, economic impact, societal financial burden and care dependency will likewise increase without a considerable redesign of the healthcare delivery for dementia. Despite limitations, this study supports the use of Fam-FFC to engage hospitalized patients with dementia, their families and nursing staff in promoting functional and cognitive recovery. Fam-FFC offers benefits to FCGs, who have much at stake when their relatives with dementia are hospitalized with reduced anxiety and more preparedness for caregiving. Further, we were able to demonstrate possible medicoeconomic benefits of Fam-FFC by significantly reducing the 30-day readmission rate, and increasing the likelihood of patients with dementia returning to baseline function 2 months after discharge. Future research in a larger sample of diverse, representative hospitals will support its efficacy and afford the possibility of understanding methods of uptake, dissemination and sustainability. Approaches such as Fam-FFC could address the Center for Medicare and Medicaid triple aim [44] by improving patient (and caregiver) health and care while reducing healthcare costs.
Practice points.
Persons with dementia have a higher likelihood of hospitalization than persons without cognitive impairment.
Dementia increases risk for functional decline in the hospitalized person which can persist along with delirium in the postacute time period.
Family can play a vital role in assessment, care delivery and decision-making when the person with dementia is hospitalized.
A source of stress for the family caregiver is the worry that the person with dementia will have increased care dependency at discharge.
The family-centered, function-focused care intervention (Fam-FFC) incorporates an educational empowerment model for family caregivers that focuses on promoting functional recovery during and after the acute stay.
Patients who participated in Fam-FFC demonstrated less delirium severity, better activities of daily living performance and less decrease in walking ability at 2 months after discharge.
Patients in the Fam-FFC group experience fewer 30-day hospital readmissions than the comparison group which can have potential savings to healthcare delivery systems.
Family caregivers who participated in Fam-FFC showed a significant increase in preparedness for caregiving and less anxiety but no significant differences in depression, strain and mutuality.
This study highlights the advisability of including family members in care delivery and decision-making of hospitalized persons with dementia.
Acknowledgments
This project was supported by grants NIRG-12-242090 from the Alzheimer's Association (M Boltz) and R01 AG040211-A1 from the National Institute on Aging (JE Galvin).
Footnotes
Financial & competing interests disclosure: The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest; •• of considerable interest
- 1.Alzheimer's Association. 2014 facts and figures. Alzheimers Dement. 2014;10(2):e47–e92. doi: 10.1016/j.jalz.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 2•.Phelan EA, Borson S, Grothaus L, Balch S, Larson EB. Association of incident dementia with hospitalizations. JAMA. 2012;307:165–117. doi: 10.1001/jama.2011.1964. In a longitudinal cohort study of 3019 adults aged 65 years or older, incident dementia was significantly associated with increased risk of hospitalization. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zekry D, Herrmann FR, Grandjean R, et al. Demented versus non-demented very old inpatients: the same co-morbidities but poorer functional and nutritional status. Age Ageing. 2008;37:83–89. doi: 10.1093/ageing/afm132. [DOI] [PubMed] [Google Scholar]
- 4.Mecocci P, von Strauss E, Cherubini A, et al. Cognitive impairment is the major risk factor for development of geriatric syndromes during hospitalization: results from the GIFA study. Dement Geriatr Cogn Disord. 2005;20(4):262–269. doi: 10.1159/000087440. [DOI] [PubMed] [Google Scholar]
- 5••.Watkin L, Blanchard MR, Tookman A, Sampson EL. Prospective cohort study of adverse events in older people admitted to the acute general hospital: risk factors and the impact of dementia. Int J Geriatr Psychiatry. 2012;(1):76–82. doi: 10.1002/gps.2693. Patient-related reported adverse events were associated with male gender, delirium, mild/moderate cognitive impairment and functional impairment. Hospital staff showed lack of understanding of the impact of cognitive impairment. [DOI] [PubMed] [Google Scholar]
- 6.Sampson EL, Blanchard MR, Jones L, Tookman A, King M. Dementia in the acute hospital: prospective cohort study of prevalence and mortality. Br J Psychiatry. 2009;195(1):61–66. doi: 10.1192/bjp.bp.108.055335. [DOI] [PubMed] [Google Scholar]
- 7.Kiely DK, Jones RN, Bergmann MA, Murphy KM, Oray EJ, Marcantonio ER. Association between delirium resolution and functional recovery among newly admitted postacute facility patients. J Gerontol A Biol Sci Med Sci. 2006;61(2):204–208. doi: 10.1093/gerona/61.2.204. [DOI] [PubMed] [Google Scholar]
- 8.Daiello LA, Gardner R, Epstein-Lubow G, Butterfield K, Gravenstein S. Association of dementia with early rehospitalization among Medicare beneficiaries. Arch Gerontol Geriatr. 2014;59(1):162–168. doi: 10.1016/j.archger.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Campbell P, Wright J, Oyebode J, et al. Determinants of burden in those who care for someone with dementia Int J Geriatr Psychiatry. 2008;23:1078–1085. doi: 10.1002/gps.2071. [DOI] [PubMed] [Google Scholar]
- 10.Brodaty H, Donkin M. Family caregivers of people with dementia. Dialogues Clin Neurosci. 2009;11(2):217–228. doi: 10.31887/DCNS.2009.11.2/hbrodaty. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inouye SK, Wagner DR, Acampora D, et al. A predictive index for functional decline in hospitalized elderly medical patients. J Gen Intern Med. 1993;8(12):645–652. doi: 10.1007/BF02598279. [DOI] [PubMed] [Google Scholar]
- 12••.Pedone C, Ercolani S, Catani M. Elderly patients with cognitive impairment have a high risk for functional decline during hospitalization: the GIFA Study. J Gerontol A Biol Sci Med Sci. 2005;60(12):1576–1580. doi: 10.1093/gerona/60.12.1576. In a multisite study (n = 9061), multivariate analysis demonstrated that cognitive impairment upon admission was an important risk factor for functional decline, independent of age, gender, comorbidity, polypharmacy and disability on admission. [DOI] [PubMed] [Google Scholar]
- 13.Brown CJ, Woodby LL, Davis LL, Allman RM. Barriers to mobility during hospitalization from the perspectives of older patients and their nurses and physicians. J Hosp Med. 2007;2(5):305–313. doi: 10.1002/jhm.209. [DOI] [PubMed] [Google Scholar]
- 14.Moyle W, Borbasi S, Wallis M, Olorenshaw R, Gracia N. Acute care management of older people with dementia: a qualitative perspective. J Clin Nurs. 2010;20:420–428. doi: 10.1111/j.1365-2702.2010.03521.x. [DOI] [PubMed] [Google Scholar]
- 15.Brown CJ, Redden DT, Flood KL, Allman RM. The under-recognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc. 2009;57(9):1660–1665. doi: 10.1111/j.1532-5415.2009.02393.x. [DOI] [PubMed] [Google Scholar]
- 16.Gill SS, Bronskill SE, Normand SL, et al. Antipsychotic drug use and mortality in older patients with dementia. Ann Intern Med. 2007;146:775–786. doi: 10.7326/0003-4819-146-11-200706050-00006. [DOI] [PubMed] [Google Scholar]
- 17.Minnick AF, Mion LC, Johnson ME, Catrambone C, Leipzig R. Prevalence and variation of physical restraint use in US acute care settings. J Nurs Scholarsh. 2007;39(1):30–37. doi: 10.1111/j.1547-5069.2007.00140.x. [DOI] [PubMed] [Google Scholar]
- 18•.Jurgens FJ, Clissett p, Gladman JRF, Harwood RH. Why are family carers of people with dementia dissatisfied with general hospital care? A qualitative study. BMC Geriatrics. 2012;12:57. doi: 10.1186/1471-2318-12-57. Family caregivers describe negative hospital experiences including patients' adverse occurrences and poor communication with staff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li H, Powers BA, Melnyk BM, et al. Randomized controlled trial of CARE: an intervention to improve outcomes of hospitalized elders and family caregivers. Res Nurs Health. 2012;35:533–549. doi: 10.1002/nur.21491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leung D, Todd J. Dementia care in the acute district general hospital. Clin Med. 2010;10(3):220–222. doi: 10.7861/clinmedicine.10-3-220. [DOI] [PubMed] [Google Scholar]
- 21.Clissett P, Porock D, Harwood RH, Gladman JRF. Experiences of family carers of older people with mental health problems in the acute general hospital: a qualitative study. J Adv Nurs. 2013;69(12):2707–2716. doi: 10.1111/jan.12159. [DOI] [PubMed] [Google Scholar]
- 22••.Boltz M, Chippendale T, Resnick B, Galvin J. Anxiety in family caregivers of hospitalized persons with dementia: contributing factors and responses. Alzheimer Dis Assoc Disord. 2015 doi: 10.1097/WAD.0000000000000072. (Epub ahead of print). Lower patient physical function of hospitalized persons with dementia and higher caregiver strain were associated with higher caregiver anxiety. Family caregivers described care-related worries especially related to patients' functional loss and desire to be informed and involved in care decisions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Howard R, Ballard C, O'Brien JO, Burns A. Guidelines for the management of agitation in dementia. Int J Geriatr Psychiatry. 2011;16(7):714–717. doi: 10.1002/gps.418. [DOI] [PubMed] [Google Scholar]
- 24••.Boltz M, Resnick B, Chippendale T, Galvin J. Testing a family-centered intervention to promote functional and cognitive recovery in hospitalized older adults. J Am Geriatr Soc. 2014:2398–2407. doi: 10.1111/jgs.13139. Pilot study demonstrated the feasibility and preliminary positive outcomes associated with family-centered, function-focused care. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pretzer-Aboff I, Galik E, Resnick B. Testing the feasibility and long term impact of a function focused care intervention for people with Parkinson's in the community setting. Nurs Res. 2011;60(4):276–228. doi: 10.1097/NNR.0b013e318221bb0f. [DOI] [PubMed] [Google Scholar]
- 26••.Shankar KN, Hirschman KB, Hanlon AL, Naylor MD. Burden in caregivers of cognitively impaired elderly adults at time of hospitalization: a cross-sectional analysis. J Am Geriatr Soc. 2014;62(2):276–284. doi: 10.1111/jgs.12657. Caregivers of older adults who were cognitively impaired at hospital admission experience burden, more so when the older adult has delirium and functional deficits. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Resnick B, Galik E, Boltz M. Function focused care approaches to care for older adults: literature review of progress and future possibilities. J Am Med Dir Assoc. 2013;14(5):313–318. doi: 10.1016/j.jamda.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 28.Sallis JF, Cervero RB, Ascher W, Henderson KA, Kraft MK, Kerr J. An ecological approach to creating active living communities. Annu Rev Public Health. 2006;27:297–322. doi: 10.1146/annurev.publhealth.27.021405.102100. [DOI] [PubMed] [Google Scholar]
- 29.Bandura A. Self-Efficacy: The Exercise of Control. WH Freeman Company; NY, USA: 1997. [Google Scholar]
- 30.Resnick R, Gruber-Baldinie AL, Pretzer-Aboff I, et al. Reliability and validity of the evaluation to sign consent measure. Gerontologist. 2007;47(1):69–77. doi: 10.1093/geront/47.1.69. [DOI] [PubMed] [Google Scholar]
- 31.Borson S. The mini-cog: a cognitive vital signs measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry. 2000;15(11):1021. doi: 10.1002/1099-1166(200011)15:11<1021::aid-gps234>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 32.Galvin JE, Roe CM, Xiong C, Morris JC. Validity and reliability of the AD8 informant interview in dementia. Neurology. 2006;67(11):1942–1948. doi: 10.1212/01.wnl.0000247042.15547.eb. [DOI] [PubMed] [Google Scholar]
- 33.Inouye S, Van Dyck C, Alessi C, Balkin S, Siegal A, Horwitz R. Clarifying confusion: the confusion assessment method: a new method for detection of delirium. Ann Intern Med. 1990;113(12):941–948. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 34.Van Doorn C, Bogardus S, Williams CS, Concato J, Towle VR, Inouye SK. Risk adjustment for older hospitalized persons: a comparison of two methods of data collection for the Charlson Index. J Clin Epid. 2001;54(7):694–701. doi: 10.1016/s0895-4356(00)00367-x. [DOI] [PubMed] [Google Scholar]
- 35.Sainsbury A, Seebass G, Bansal A, Young J. Reliability of the Barthel Index when used with older people. Age Ageing. 2005;34(33):228–232. doi: 10.1093/ageing/afi063. [DOI] [PubMed] [Google Scholar]
- 36.Tinetti ME. Performance oriented assessment of mobility problems in elderly patients. J Am Geriatr Soc. 1986;34(2):119–126. doi: 10.1111/j.1532-5415.1986.tb05480.x. [DOI] [PubMed] [Google Scholar]
- 37.Trzepacz PT. The Delirium Rating Scale: its use in consultation-liaison research. Psychosomatics. 1999;40(3):193–204. doi: 10.1016/S0033-3182(99)71235-1. [DOI] [PubMed] [Google Scholar]
- 38.Archbold PG, Stewart BJ, Greenlick MR, Harvath TA. Key Aspects of Eldercare. Springer Publishing Company; NY, USA: 1992. The clinical assessment of mutuality and preparedness in family caregivers to frail older people; pp. 328–339. [Google Scholar]
- 39.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale: an updated literature review. J Psychos Res. 2002;52(2):69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 40.Thornton M, Travis SS. Analysis of the reliability of the Modified Caregiver Strain Index. J Gerontol Ser A. 2003;58(2):S127–S132. doi: 10.1093/geronb/58.2.s127. [DOI] [PubMed] [Google Scholar]
- 41.Kiresuk TJ, Smith A, Cardillo JE. Goal Attainment Scaling: Applications, Theory and Measurement. Lawrence-Erlbaum; NJ, USA: 1994. [Google Scholar]
- 42.Fidell LS, Tabachnick BG. Using Multivariate Statistics. Harper and Row; NY, USA: 2006. [Google Scholar]
- 43•.Naylor MD, Hirschman KB, Hanlon AL, et al. Comparison of evidence-based interventions on outcomes of hospitalized cognitively impaired older adults. J Comp Eff Res. 2014;3(3):245–257. doi: 10.2217/cer.14.14. As compared with Augmented Standard Care (ASC; lower dose) and Resource Nurse Care (RNC; medium dose), the Transitional Care Model (higher dose), demonstrated lower mean rehospitalization rates per patient compared with the RNC and ASC groups at 30 days posthospitalization and the ASC group at 90-day posthospitalization. No significant group differences in functional status were observed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berwick DM, Nolan TW, Whittington J. The triple aim: care, health, and cost. Health Aff (Millwood) 2008;27(3):759–769. doi: 10.1377/hlthaff.27.3.759. [DOI] [PubMed] [Google Scholar]