Abstract
Purpose
Total hip arthroplasty (THA) is a widespread option for treating hip osteoarthritis. Peri-prosthetic complications after THA represent a common event influencing patient outcome and costs. The purpose of this paper is to report the use of ultrasonography (US) to detect peri-prosthetic complications in symptomatic patients who underwent THA.
Methods
We retrospectively reviewed the records of patients with THA who underwent imaging evaluation between January 2009 and December 2012 at two different institutions. We evaluated the presence/absence of superficial and/or deep peri-prosthetic collections as well as the presence/absence of a cutaneous sinus tract. For patients who underwent both MRI and US, a concordance correlation analysis between US and MR findings was performed.
Results
In the reference period, 532 symptomatic patients (mean age ± standard deviation 74 ± 12 years) underwent X-ray and MRI examinations for suspected peri-prosthetic complications. Among them, 111 (20.9 %) underwent also US. Overall, 108 patients underwent both US and MRI. US findings included 67 superficial collections, 48 subcutaneous fistulas, 74 deep peri-prosthetic collections. Twenty-four patients had solid, mass-like peri-prosthetic collections. In 11 patients, no peri-prosthetic complications were seen. MRI findings included 68 superficial collections, 49 subcutaneous fistulas, 79 deep peri-prosthetic collections. Twenty-four patients had solid, mass-like peri-prosthetic collections. In four patients, no peri-prosthetic complications were seen. Concordance analysis between US and MRI findings showed almost perfect agreement (k ≥ 0.89).
Conclusion
US is an efficient and practical imaging modality to evaluate peri-prosthetic complications in patients with THA, being almost comparable to MRI in detecting and characterizing these complications.
Keywords: Ultrasound, Total hip arthroplasty, Hip infection, Magnetic resonance imaging
Riassunto
Obiettivo
L’artroprotesi totale di anca (PTA) rappresenta una valida opzione per il trattamento della patologia osteoartrosica. L’incidenza di complicanze post-intervento è una problematica che influenza costi e risultato terapeutico. Scopo del lavoro è stato riportare la nostra esperienza nella valutazione ecografica di complicanze peri-protesiche in pazienti sottoposti a PTA.
Metodi
Abbiamo riesaminato retrospettivamente i referti di pazienti con sospetto di complicanze peri-protesiche che hanno eseguito follow up diagnostico presso due ospedali da Gennaio 2009 e Dicembre 2012. Abbiamo valutato la presenza/assenza di raccolte superficiali e/o profonde e la presenza di eventuali fistole cutanee. Per ogni paziente che ha eseguito risonanza magnetica (RM) ed ecografia abbiamo stimato la concordanza dei dati.
Risultati
In tale periodo, 532 pazienti (età media ± deviazione standard 74 ± 12 anni) hanno eseguito follow-up radiologico e RM presso gli istituti di riferimento. Centoundici pazienti (20.9 %) hanno anche effettuato ecografia. I pazienti che hanno eseguito ecografia e RM sono stati 108. I reperti ecografici includevano: 67 raccolte superficiali e 74 profonde, 48 fistole sottocutanee. Ventiquattro pazienti mostravano raccolte solide. Undici pazienti non mostravano complicanze. I reperti di risonanza magnetica includevano: 68 raccolte superficiali e 79 profonde, 49 fistole cutanee. Ventiquattro pazienti mostravano raccolte solide. Quattro pazienti non mostravano complicanze. L’analisi statistica dei dati ha dimostrato un’alta concordanza tra reperti ecografici e RM (k ≥ 0.89).
Conclusioni
l’ecografia è una modalità di imaging pratica ed efficace nel valutare pazienti sottoposti ad artoprotesi d’anca con sospetto di complicanze peri-protesiche, con alta correlazione con l’esame RM.
Introduction
Total hip arthroplasty (THA) is a common and widespread option for treating hip osteoarthritis [1]. Peri-prosthetic complications after THA represent a relatively common event [2] and, despite numerous prophylactic measures, cases of early and late infections still occur, with an incidence ranging from 0.5 to 2 % [3].
Several imaging modalities are currently available to perform post-operative follow-up in patients who underwent THA. Plain radiographs are limited to assess the correct positioning of hip implants, implant loosening or dislocation. However, this modality can be helpful only at later stages, when at least 30 % of bone mineralization is lost. Computed tomography (CT) has several limitations in evaluating soft tissue surrounding hip prosthesis, due to striking artifacts induced by metallic implants. Magnetic resonance imaging (MRI) may also suffer from susceptibility artifact induced by metallic implants. Although the recent introduction of specific sequences and parameters (i.e., metal artifacts reduction sequences, MARS) improved the visualization of peri-prosthetic soft tissues, image degradation may be relevant, especially with certain types of metallic alloys [4]. Furthermore, MRI is relatively expensive and time consuming. Ultrasonography (US) is considered as a valuable tool in the assessment of orthopedic surgical complications and infections [5]. The ability to study soft tissues as well availability, low cost, and lack of ionizing radiation are advantages of this technique over others [6]. Being capable of real-time evaluation, US is also a valid option to guide interventional procedures, such as needle biopsy or aspiration, not only to confirm diagnosis, but also to perform therapeutic loco-regional treatments [7, 8].
The purpose of our study is to report our experience in the use of US to detect peri-prosthetic complications during follow-up of patients suspected to have prosthesis complications.
Materials and methods
Two radiologists with 10 and 5 years experience in musculoskeletal ultrasound retrospectively reviewed in consensus the records of our picture archiving and communication system of patients with THA undergone US evaluation for clinical suspicion of peri-prosthetic infection between January 2009 and December 2012 at two different institutions. US examination was performed in addition to conventional post-operative imaging including conventional radiography and MRI. All patients had abnormal white blood cell count and/or erythrocyte sedimentation rate.
Different US systems (MyLab 70 XvG, Esaote, Italy; iU22, Philips, The Netherlands) equipped with convex (3–6 MHz) and high-resolution broadband linear array transducers (13-6 and 12-5 MHz, respectively) were used. Examinations were performed by different radiologists with different experience (5–25 years) in musculoskeletal US.
Patients were asked to lie on a bed in supine position to evaluate the anterior aspect of the hip joint. Longitudinal images were first obtained by placing the convex or the linear transducer parallel to the femoral neck of the prosthesis. Then, transverse images, perpendicular to the major axis of the implant were performed covering the entire area of the hip, from the anterior superior iliac spine up to the middle portion of the thigh. Then, patients were asked to turn to lie on the side opposite to the affected prosthesis to assess the peri-trochanteric tissues. There, longitudinal and transverse images were obtained by moving the linear transducer in cranio-caudal and antero-posterior directions.
For each US examination, the presence/absence of superficial and/or deep peri-prosthetic collections, classifying them according to their consistency (fluid, solid, particulate) as well as the presence/absence of a cutaneous sinus tract was recorded. At US, a fluid collection was defined as an anechoic area with increased through transmission. A solid mass was defined as a hypo/isoechoic mass with space-occupying appearance. A particulate collection was defined as a hypo/anechoic area containing spotty bright internal echoes.
For patients who underwent both MRI and US, a concordance correlation analysis between US findings and their corresponding MR findings as indicated on MR reports was performed using Cohen’s kappa statistics and subgroup analysis was performed using the Fisher’s exact test. A P value lower than 0.05 was considered as statistically significant. The SPSS (SPSS Inc., Harmonk, USA) was used.
Results
In the reference period, 532 patients with symptomatic hip prosthesis (mean age ± standard deviation 74 ± 12 years) underwent X-ray and MRI examinations for suspected peri-prosthetic complications. Among them, 111 underwent also US (20.9 %). Overall, 108 patients underwent both US and MRI.
US findings included 67 superficial collections, 48 subcutaneous fistulas, 74 deep peri-prosthetic collections. Twenty-four patients had solid, mass-like peri-prosthetic collections. In 11 patients, no peri-prosthetic complications were seen.
MRI findings included 68 superficial collections (fluid, n = 61; particulate, n = 5; solid, n = 2), 49 subcutaneous fistulas, 79 deep peri-prosthetic collections (fluid, n = 34; particulate, n = 45). Twenty-four patients had solid, mass-like peri-prosthetic collections. In four patients, no peri-prosthetic complications were seen. Concordance analysis between US and MRI findings showed almost perfect agreement (k ≥ 0.89). Subgroup analysis regarding the nature of the collections revealed no significant differences between US and MRI evaluation (P ≥ 0.87). Full data are reported in Table 1.
Table 1.
Agreement between ultrasound and magnetic resonance imaging in detecting peri-prosthetic hip complications
| Ultrasound (%) | Magnetic resonance imaging (%) | Statistical analysis | |
|---|---|---|---|
| Superficial collections | 67/108 (62) | 68/108 (63) | k = 0.96 |
| Fluid | 60 | 61 | P = 0.99 |
| Particulate | 5 | 5 | |
| Solid | 2 | 2 | |
| Subcutaneous fistulas | 48/108 (44) | 49/108 (45) | k = 0.94 |
| Deep collections | 74/108 (69) | 79/108 (73) | k = 0.89 |
| Fluid | 30 | 34 | P = 0.87 |
| Particulate | 44 | 45 |
Figures 1, 2, 3 and 4 show the spectrum of findings in our series.
Fig. 1.
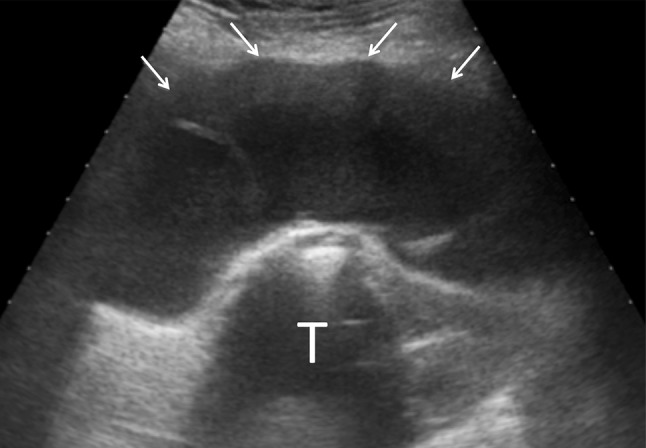
Coronal scan of a large fluid collection (arrows) around the greater trochanter (T)
Fig. 2.
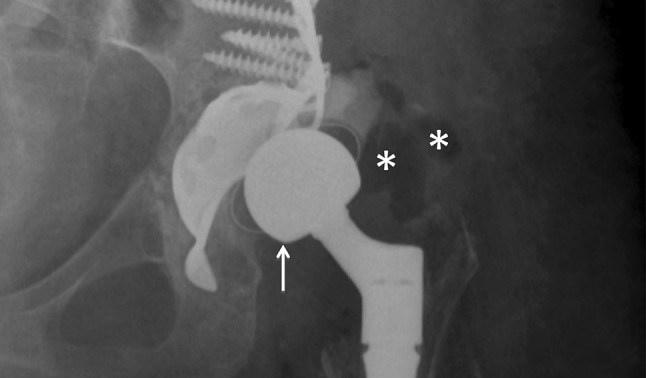
X-ray of a patient with hip dislocation and suspected infection. Antero-posterior projection shows cranial dislocation of implant femoral head (white arrow) and peri-prosthetic soft tissues swelling. Asterisks show air within peri-prosthetic soft tissues
Fig. 3.
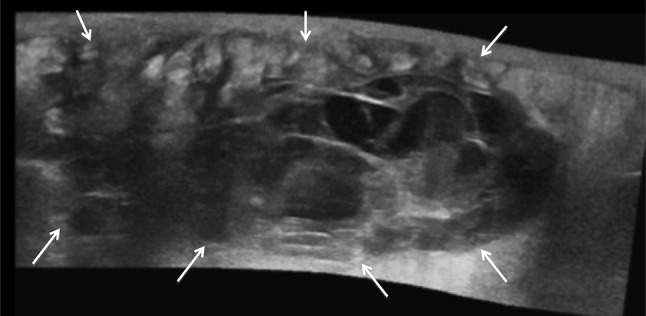
Extended field of view ultrasound image of a large solid mass with fluid area within soft tissues around an infected hip implant
Fig. 4.
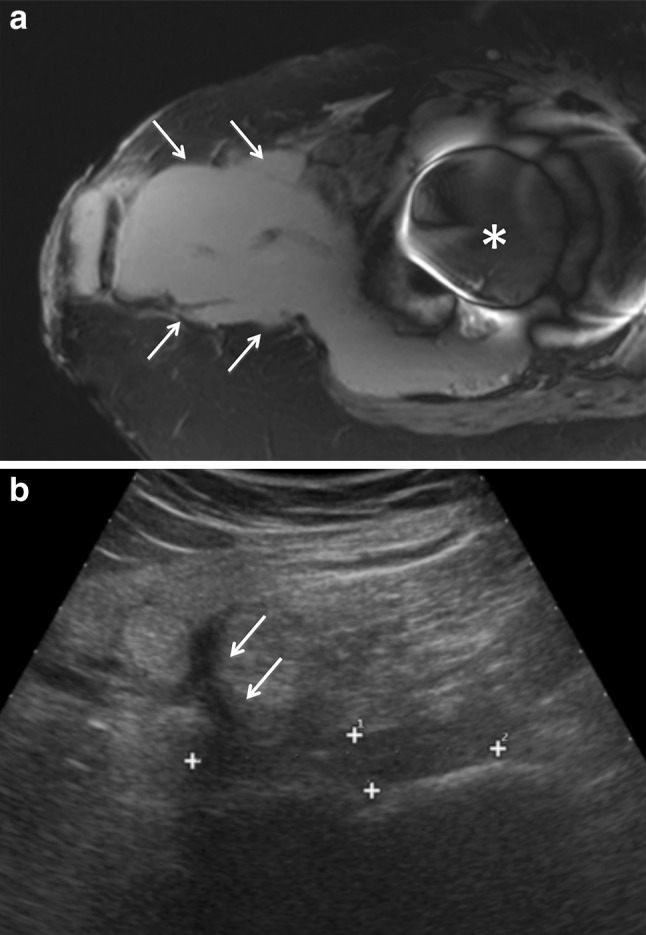
a T2-weighted axial magnetic resonance image of a large fluid peri-prosthetic collection (arrows) that reach the skin surface. Note the remarkable susceptivity artifact induced by the metal implant (asterisk). b Ultrasound image obtained after aspiration and antibiotic treatment. The size of the collection is remarkably reduced (calipers). However, a sinus tract (arrows) can be clearly seen
Discussion
In recent years, THA has become a widely accepted option for treating hip osteoarthritis. The demand for primary THA is estimated to grow by almost 175 % in the next 15 years [9]. Although relatively uncommon, infection is one undesirable complication that can occur after THA, during early and late follow-up, often leading to surgical revision of the implant [10, 11]. For these reasons, early diagnosis of peri-prosthetic complications is essential for improving patient outcome and reducing costs due to revision surgery. In this setting, the prompt and correct management of symptomatic patients is of great importance [12, 13].
Radiographic examination still represents the first approach when a prosthesis complication/infection is suspected, despite its low sensitivity and specificity. Radiograms may be negative in the first weeks from complication onset and cannot be used for early detection. Early sign of infection may be soft tissue swelling. Only at later stages it may reveal periostal reaction or the presence of osteolysis as a radiolucent area around the prosthesis [14].
As previously reported, the use of CT in the evaluation of hip implants is limited by the presence of implant-related striking artifacts on images, particularly around the implant. However, CT demonstrated to have increased diagnostic performance compared to radiography in detecting bone abnormalities and prosthesis loosening. Overall, CT has limited accuracy in evaluating peri-prosthetic soft tissues and cannot detect bone edema. In addition, ionizing radiation makes the use of CT questionable, especially when repeated scans are needed [15].
MRI performed with metal artifact reduction sequences has been demonstrated to be excellent in evaluating the involvement of the superficial and deep soft tissues surrounding hip prosthesis, the presence of fistulae and bone edema [15]. MRI is also helpful in characterizing the nature of collection as serous, purulent, or hematic. Great limitations of MRI are high costs and low availability.
US is not recommended as first-line examination to assess peri-prosthetic bone complications [16], being limited by the deep location of the hip implant. However, US can be efficiently used to detect the presence of peri-prosthetic fluid collections, being also occasionally able to differentiate septic from aseptic collections. Also, US is able to detect the presence of sinus tracts within soft tissues [5, 17, 18].
Despite all the above-mentioned limitations, in the present series we demonstrated that US has a high correlation with MRI in detecting peri-prosthetic hip complications. In particular, we note that for superficial collections, US seems to have comparable outcome compared to MRI. When dealing with deeper collections, US was able to detect the presence of peri-prosthetic collections in a lower percentage of patients (−4 %). However, this was probably related to the small size of these collections and their clinical implication is yet to be demonstrated. At any rate, the almost perfect concordance between US and MRI for both superficial and deep collections, as well as the absence of any significant differences regarding the subgroup analysis, indicate that US is mostly comparable to MRI in this setting, with the advantages of reduced costs and higher availability.
In the last few years several authors analyzed the role of US in the assessment of peri-prosthetic complications. In 2006, Miller et al. [19] provided a broad review of the sonographic appearances of complications after arthroplasty of the hip, as well as the shoulder and knee.
More recently, in 2011 and 2012, Long [20] and Douis [21], respectively, reviewed the most common US findings in a series of patients after THA. They concluded their works emphasizing the great advantages of US compared to other imaging modalities. They established that US is a valuable tool in patients with THA because the soft tissue surrounding the prosthetic joint are not obscured by metallic artifacts and because US enables hands-on examination of the painful site, dynamic evaluation of moving structures, and comparison with the opposite side. Certainly, US is not ideally suited to evaluating the prosthesis and periprosthetic bone because of the inability of sound beams to penetrate metal or bone. However, US is an excellent modality in evaluating for joint effusion and extra-articular fluid collections, as well as to visualize the soft tissues surrounding the hip such as the iliopsoas tendon/bursa, gluteal tendons, greater trochanteric bursa, and iliotibial band.
Other advantages of US are the ability to perform US-guided diagnostic and therapeutic procedures, the lack of ionizing radiation, lower cost, and the possibility to perform in patients with MR contraindications such as claustrophobia or presence of pacemakers.
This work has certainly limitations. First, it is a retrospective review of a case series that partially limits the value of our results. Second, we compared the US images with what were included in MRI reports. This was done for uniformity, as images were not available for all patients for review. As we did not reanalyze MR images, we had to rely on what the radiologist included in the report. Last, despite the high experience of the operators in performing musculoskeletal examinations, the implication of different radiologists scanning patients at different institutions may represent a non-negligible bias in the acquisition of the presented data. However, images were reviewed in consensus by two experienced operators, thus limiting this issue.
In conclusion, we think that US is an efficient and practical imaging modality to detect peri-prosthetic complications in patients with THA. US has been demonstrated to be comparable to MRI in detecting and characterizing these complications.
Conflict of interest
Silvana Sdao, Davide Orlandi, Alberto Aliprandi, Francesca Lacelli, Luca Maria Sconfienza, Filippo Randelli, Francesco Sardanelli, Giovanni Serafini declare that they have no conflict of interest.
Informed consent
All procedures were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Patients’ informed consent was waived for the present study.
References
- 1.National Joint Registry for England and Wales (2014) 8th annual report 2011—National Joint Registry. http://www.njrcentre.org.uk/NjrCentre/Portals/0/Documents/NJR%208th%20Annual%20Report%202011.pdf. Accessed 2 April 2014
- 2.Tormenta S, Sconfienza LM, Iannessi F, Bizzi E, Massafra U, Orlandi D, Migliore A. Prevalence study of iliopsoas bursitis in a cohort of 860 patients affected by symptomatic hip osteoarthritis. Ultrasound Med Biol. 2012;38:1352–1356. doi: 10.1016/j.ultrasmedbio.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Anagnostakos K, Schmid NV, Kelm J, Grün U, Jung J. Classification of hip joint infections. Int J Med Sci. 2009;6:227–233. doi: 10.7150/ijms.6.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gibbon WW, Long G, Barron DA, O’Connor PJ. Complications of orthopedic implants: sonographic evaluation. JCU. 2002;30:288–299. doi: 10.1002/jcu.10065. [DOI] [PubMed] [Google Scholar]
- 5.Van Holsbeeck MT, Eyler WR, Sherman LS, Lombardi TJ, Mezger E, Verner JJ, Schurman JR, Jonsson K. Detection of infection in loosened hip prostheses: efficacy of sonography. AJR. 1994;163:381–384. doi: 10.2214/ajr.163.2.8037036. [DOI] [PubMed] [Google Scholar]
- 6.Carbó S, Rosón N, Vizcaya S, Escribano F, Zarcero M, Medrano S. Can ultrasound help to define orthopedic surgical complications? Curr Probl Diagn Radiol. 2006;35:75–89. doi: 10.1067/j.cpradiol.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Sconfienza LM, Perrone N, Lacelli F, Lentino C, Serafini G. Ultrasound-guided injection of botulinum toxin A in the treatment of iliopsoas spasticity. J Ultrasound. 2008;11:113–117. doi: 10.1016/j.jus.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robotti G, Canepa MG, Bortolotto C, Draghi F. Interventional musculoskeletal US: an update on materials and methods. J Ultrasound. 2013;16:45–55. doi: 10.1007/s40477-013-0018-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005–2030. J Bone Joint Surg. 2007;89:780–785. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 10.Antti-Poika I, Joseffsson G, Konttinen Y, Lidgren L, Santavirta S, Sanzén L. Hip arthroplasty infection: current concepts. Acta Orthop Scand. 1990;61:163–169. doi: 10.3109/17453679009006513. [DOI] [PubMed] [Google Scholar]
- 11.Pandey R, Drakoulakis E, Athanasou NA. An assessment of the histologic criteria used to diagnose infection in hip revision arthroplasty tissues. J Clin Pathol. 1999;52:118–123. doi: 10.1136/jcp.52.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jutte PI, Lazzeri E, Sconfienza LM, Cassar-Pullicino V, Trampuz A, Petrosillo N, Signore A. Diagnostic flowcharts in osteomyelitis, spondylodiscitis and prosthetic joint infection. Q J Nucl Med Mol Imaging. 2014;58:2–19. [PubMed] [Google Scholar]
- 13.Aliprandi A, Di Pietto F, Minafra P, Zappia M, Pozza S, Sconfienza LM. Femoro-acetabular impingement: what the general radiologist should know. Radiol Med. 2014;119:103–112. doi: 10.1007/s11547-013-0314-7. [DOI] [PubMed] [Google Scholar]
- 14.Aliprandi A, Sconfienza LM, Randelli P, Bandirali M, Tritella S, Di Leo G, Sardanelli F. Magnetic resonance imaging of the knee after medial unicompartmental arthroplasty. Eur J Radiol. 2011;80:416–421. doi: 10.1016/j.ejrad.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Aliprandi A, Sconfienza LM, Randelli F, Bandirali M, Di Leo G, Sardanelli F. Magnetic resonance imaging of painful total hip replacement: detection and characterisation of periprosthetic fluid collection and interobserver reproducibility. Radiol Med. 2012;117:85–95. doi: 10.1007/s11547-011-0706-5. [DOI] [PubMed] [Google Scholar]
- 16.Klauser AS, Tagliafico A, Allen GM, Boutry N, Campbell R, Court-Payen M, Grainger A, Guerini H, McNally E, O’Connor PJ, Ostlere S, Petroons P, Reijnierse M, Sconfienza LM, Silvestri E, Wilson DJ, Martinoli C. Clinical indications for musculoskeletal ultrasound: a Delphi-based consensus paper of the European Society of Musculoskeletal Radiology. Eur Radiol. 2012;22:1140–1148. doi: 10.1007/s00330-011-2356-3. [DOI] [PubMed] [Google Scholar]
- 17.Petchprapa CN, Rosenberg ZS, Sconfienza LM, Cavalcanti CF, Vieira RL, Zember JS. MR imaging of entrapment neuropathies of the lower extremity. Part 1. The pelvis and hip. Radiographics. 2010;30:983–1000. doi: 10.1148/rg.304095135. [DOI] [PubMed] [Google Scholar]
- 18.Sofka CM, Adler RS. Sonographic evaluation of shoulder arthroplasty. AJR Am J Roentgenol. 2003;180:1117–1120. doi: 10.2214/ajr.180.4.1801117. [DOI] [PubMed] [Google Scholar]
- 19.Miller TT. Sonography of joint replacements. Semin Musculoskelet Radiol. 2006;10:79–85. doi: 10.1055/s-2006-934218. [DOI] [PubMed] [Google Scholar]
- 20.Long SS, Surrey D, Nazarian LN. Common sonographic findings in the painful hip after hip arthroplasty. J Ultrasound Med. 2012;31:301–312. doi: 10.7863/jum.2012.31.2.301. [DOI] [PubMed] [Google Scholar]
- 21.Douis H, Dunlop DJ, Pearson AM, O’Hara JN, James SL. The role of ultrasound in the assessment of post-operative complications following hip arthroplasty. Skelet Radiol. 2012;41:1035–1046. doi: 10.1007/s00256-012-1390-9. [DOI] [PubMed] [Google Scholar]


