Abstract
Abstract
Cardiorenal syndrome type 1 (CRS-1) is the acute kidney disfunction caused by an acute worsening of cardiac function. CRS-1 is the consequence of renal vasoconstriction secondary to renin–angiotensin system (RAS) activation. No animal models of CRS-1 are described in literature.
Purpose
To characterize a murine model of CRS-1 by using a high-resolution ultrasound echo-color Doppler system (VEVO2100).
Materials
Post-ischemic heart failure was induced by coronary artery ligation (LAD) in seven CD1 mice. Fifteen and thirty days after surgery, mice underwent cardiac and renal echo-color Doppler. Serum creatinine and plasma renin activity were measured after killing. Animals were compared to seven CD1 control mice.
Results
Heart failure with left ventricle dilatation (end diastolic area, p < 0.05 vs. controls) and significantly reduced ejection fraction (EF; p < 0.01 vs. controls) was evident 15 days after LAD. We measured a significant renal vasoconstriction in infarcted mice characterized by increased renal pulsatility index (PI; p < 0.05 vs. controls) associated to increased creatinine and renin levels (p < 0.05 vs. controls)
Conclusions
The mice model of LAD is a good model of CRS-1 evaluable by Doppler sonography and characterized by renal vasoconstriction due to the activation of the renin–angiotensin system secondary to heart failure.
Keywords: Cardiorenal syndrome, EcoDoppler, Pulsatility index
Abstract
RIASSUNTO
Per sindrome cardiorenale tipo-1 (SCR-1) si intende il peggioramento acuto della funzione renale dovuto alla riduzione della funzione cardiaca. La SCR-1 è conseguenza della vasocostrizione renale dovuta all’attivazione del sistema renina-angiotensina. In letteratura non sono descritti modelli animali che riproducono tale patologia.
Obiettivo di questo studio è caratterizzare mediante l’utilizzo di un sistema ecografico ad alta risoluzione (VEVO2100) un modello murino di CRS-1.
Materiali e Metodi
lo scompenso cardiaco post ischemico è stato indotto in 7 topi CD1 mediante legatura dell’arteria coronaria sinistra discendente anteriore. 15 e 30 giorni dopo la procedura chirurgica gli animali sono stati valutati mediante ecografia ed ecoDoppler cardiaco e renale. Gli animali sono stati sacrificati il trentesimo giorno e sono stati effettuati il dosaggio della creatinina sierica e dell’attività reninica plasmatica. I risultati sono stati confrontati con quelli ottenuti da 7 topi CD1 di controllo.
Risultati
la presenza di scompenso cardiaco, valutato mediante misurazione ecocardiografica dell’area telediastolica del ventricolo sinistro (EDA) e della frazione di eiezione (FE), è risultata evidente già 15 giorni dopo la LAD (rispettivamente p < 0.05 e p < 0.01 vs. controlli). E’ risultata inoltre una significativa vasocostrizione renale nei topi infartuati con aumento degli indici di pulsatilità renali (PI) nei topi infartuati (PI, p < 0.01 vs. controlli), associata ad un significativo aumento della creatinina e della attività reninica plasmatica (p < 0.05).
In conclusione
il modello murino di legatura di coronaria è un buon modello di SCR valutabile mediante ecoDoppler e caratterizzato da vasocostrizione renale e attivazione del sistema renina angiotensina.
Introduction
Cardiorenal syndrome (CRS) is the acute (type 1) or chronic (type 2) worsening of renal function due to heart failure (HF) and to the activation of renin–angiotensin system. Cardiorenal syndrome (CRS) type 1 is characterized by an acute worsening of cardiac function leading to acute kidney disfunction.
Several pathophysiological mechanisms have been proposed to explain the complicated and mutual interaction between heart and kidney during heart failure [1, 2]. One of the most known models is based on the balance of four interrelated cardiorenal connectors: the renin–angiotensin–aldosterone (RAS) system, the sympathetic nervous system (SNS), inflammation, and NO/reactive oxygen species [3].
During acute heart failure, the reduced cardiac output leads to RAS and SNS activation in an attempt to balance the hypoperfusion of the kidney. The result of RAS and SNS activation is renal vasoconstriction [3] which, in humans, can be noninvasively evaluated by renal Doppler sonography [4].
However, the reduced cardiac output is not the only physiopathological mechanism involved in renal vasoconstriction, as the inflammatory state induced by the systemic response to myocardial necrosis is directly involved in renal vascular response to myocardial infarction (MI) [5–8].
The interaction between oxidative stress, inflammation, and activation of RAS is well documented in the failing heart. RAS and its effector molecule, angiotensin (ANG) II, have been shown to play a major role in cardiac remodeling. Many of the downstream effects of ANGII signaling are mediated by elevated levels of reactive oxygen species (ROS) and oxidative stress, which are implicated in the pathology of heart failure and thus CRS-1 [9]. Various experimental models of excess ANG II demonstrate myocardial and renal fibrosis secondary to increased inflammation [10].
There are no specific animal models of CRS. Animal studies usually focus either on heart or kidney. Few studies have described the combined dysfunction of both, heart and kidney [11]. Moreover, all these studies use animals with a combined damage: myocardial infarction plus uninephrectomy or subnephrectomy [11]. The most used model of CRS is myocardial infarction plus uninephrectomy. The major limit of this model is that it does not reproduce the real physiopathology of CRS-1. Few studies have evaluated the effect of post-ischemic heart failure on renal function, and they are based on serum creatinine levels, and results are discordant. Lu et al. [12] describe an early increase in serum creatinine 3 days after coronary artery ligation and a subsequent tendency to the normalization of the value. The evaluation of serum creatinine requires blood sampling, an invasive procedure not always practicable in infarcted mice. There is a need for new and better models of the cardiorenal interaction to improve our understanding of CRS and thus to find new effective therapies.
High-resolution ultrasound allows the study of anatomical structures and hemodynamic functions in small animal, in vivo, longitudinally, in a noninvasive manner. The Vevo high resolution in vivo micro-imaging systems used in this study provides high-resolution imaging down to 30 microns [13]. Dedicated softwares for highly detailed analysis of cardiac function have been validated in mice models of myocardial infarction [14].
Purpose of this study was to evaluate whether ecoDoppler measurement of renal resistance can be used as noninvasive marker of CRS-1 in the mice model of acute post-ischemic heart failure obtained by LAD and thus whether this model can be used as a physiopathological model of CRS-1
Materials and methods
The study was performed in Padova (Department of Medicine, University of Padova). All described procedures were conducted in accordance with all institutional and Italian guidelines for the care and use of laboratory animals and were approved by the local animal care and use committee (CEASA) and by the Italian Ministry of Health.
Design of the study
Post-ischemic heart failure was induced by left anterior descending coronary artery ligation (LAD) in seven CD1 mice.
Mice with surgically induced myocardial infarction (MI) were compared to seven CD1 control mice.
Mice underwent cardiac and renal ecoDoppler evaluation 15 and 30 days after surgery.
Cardiac morphology and function and renal pulsatility index (PI) were measured.
After killing serum creatinine and plasma renin activity were measured.
Detailed procedures
Left anterior descending coronary artery ligation (LAD): animals were anesthetized using ketamine and xylazine (80 and 10 mg/Kg, respectively), chest shaved, endotracheal intubated, and ventilated with 100 % oxygen and isoflurane (0.75–1.5 %) at 180 breaths per minute using a MiniVent Mouse Ventilator (type 845, Harvard Apparatus). Heating pad was used to maintain body temperature at 37 °C. Under sterile conditions, a left thoracotomy was performed in the 3th intercostal space, the hearth exposed, and the pericardium opened. An 8.0 prolene ligature (Ethicon) was passed and tied around the proximal left coronary artery, just distal to the left atrial appendage border. Blanching of the anterolateral region of the left ventricle (LV) confirmed infarction. The chest was then closed with a single 5.0 silk suture between the third and fourth ribs, the muscle layers were recomposed, and the skin closed whit continuous suture. Postoperatively, all mice were hydratated with saline.
Echocardiographic evaluation: transthoracic echocardiography was performed using a high-resolution echo machine with a 30 MHz probe (VEVO 2100 Visualsonics). Animals were chest shaved and anesthetized with 3 % isoflurane, and temperature-controlled anesthesia was maintained with 1.5 % isoflurane. Two-dimensional cine loops and M-mode cine loops of a long-axis view and a short-axis view of the LV were recorded. M-mode cine loop of aortic valve was recorded and Doppler analysis of LV flow was performed from the long-axis B-mode image, placing the sample volume in the left ventricle, below the mitral annulus.
End diastolic area (EDA) and end systolic area (ESA) were measured from the long-axis B-mode images. Anterior wall (AWT) and left ventricular posterior wall (PWT) thicknesses, left ventricle end systolic and diastolic diameter, and maximal left ventricular length (LV length) were measured in systole and in diastole (ESD, EDD, LV length s, LV length d) from the long-axis and short-axis M-mode images, according to standard procedures. Cardiac contractility, measured as fraction area change (FAC) and as fractional shortening (FS), was calculated using the following formulas FAC = (EDA−ESA)/EDA; FS = (EDD/ESD)/EDD. End diastolic (EDV) and end systolic volumes (ESV) and ejection fraction (EF) were determined using the following simplified formulas: EDV = (3.14/6)*EDD*EDD*EDL ESV = (3.14/6)*ESD*ESD*ESL EF = (EDV−ESV)/EDV.
Isovolumetric contraction and relaxation times (IVCT, IVRT), and ejection time (ET) were measured from transmitral Doppler analysis, and myocardial performance index was calculated using the following formula MPI = IVCT + IVRT/ET.
E wave, A wave and E wave deceleration time (DT) were measured from transmitral Doppler flow profile.
Renal echoDoppler evaluation: immediately after echocardiographic evaluation, abdomens of the mice were shaved, and renal B-mode cine loops of a transversal section of both kidneys were recorded. Color Doppler was used to identify renal interlobar arteries, then Doppler analysis of identified arteries’ flow was performed, placing the sample volume in the renal cortex. Peak velocity (Vmax), end diastolic velocity (Vmin) and mean velocity (VM) were measured and pulstility index (PI) was calculated using the following formula PI = (Vmax−Vmin)/Vmean. The mean value of tree different measurements was calculated.
All mice were imaged by a single operator.
Serum creatinine and plasma renin measurements were performed by ELISA test (Assay Gate, Inc. Ijamsville, MD).
Statistical analysis
The data are presented as mean ± standard error (SEM). For comparison between treatment groups, the null hypothesis was tested by unpaired t test. Statistical significance (p < 0.05) between the experimental groups was determined by the Fisher’s method of analysis for multiple comparisons.
Results
The main echocardiographic parameters are summarized in Table 1. Body weight and heart rate during echocardiographic analysis were similar in the two groups.
Table 1.
CD1 mice: echocardiographic and renal echoDoppler measurements
| Controls (n = 7) | MI 15 days (n = 7) | MI 30 days (n = 7) | |
|---|---|---|---|
| Body weight (g) | 29 ± 2 | 30 ± 3 | 31 ± 3 |
| Heart rate (bpm) | 420 ± 28 | 456 ± 41 | 432 ± 36 |
| EDA (mm2) | 25 ± 3 | 29 ± 4** | 33 ± 3** |
| ESA (mm2) | 13 ± 2 | 19 ± 4** | 22 ± 4** |
| EDD (mm) | 3.89 ± 0.3 | 4.21 ± 0.2 | 4.59 ± 0.09** |
| ESD (mm) | 2.42 ± 0.4 | 3.16 ± 0.2* | 3.28 ± 0.3* |
| PWTd (mm) | 0.77 ± 0.07 | 0.91 ± 0.4 | 0.86 ± 0.17 |
| PWTs (mm) | 1.23 ± 0.1 | 1.17 ± 0.4 | 1.13 ± 0.21 |
| AWTd (mm) | 0.82 ± 0.07 | 0.64 ± 0.1* | 0.69 ± 0.15* |
| AWTs (mm) | 1.32 ± 0.12 | 0.87 ± 0.1** | 1.00 ± 0.29* |
| LV vol d (ul) | 66.3 ± 14.6 | 78 ± 17 | 96.7 ± 4.6**+ |
| LV vol s (ul) | 21.5 ± 8.8 | 41 ± 12** | 44.2 ± 11** |
| FAC (%) | 47 ± 6 | 34 ± 4* | 32 ± 2** |
| EF (%) | 70 ± 6 | 49 ± 2** | 54 ± 1* |
| FS (%) | 38 ± 6 | 25 ± 5** | 28 ± 5* |
| MPI | 0.6 ± 0.07 | 1.03 ± 0.16** | 0.87 ± 0.3** |
| MV ET (msec) | 65 ± 9 | 57 ± 6 | 49 ± 5* |
| PI | 0.87 ± 0.1 | 1.33 ± 0.2** | 1.09 ± 0.1*+ |
Values are expressed as mean ± standard error; n = number of animals; *P < 0.05 and **P < 0.01 versus controls + P<0.05 and ++ P < 0.01 versus MI 15 gg
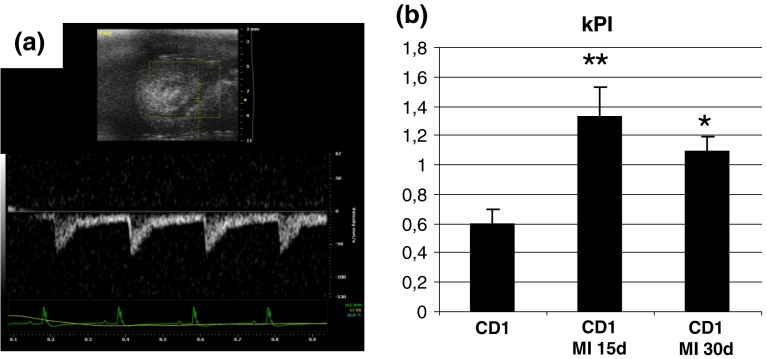
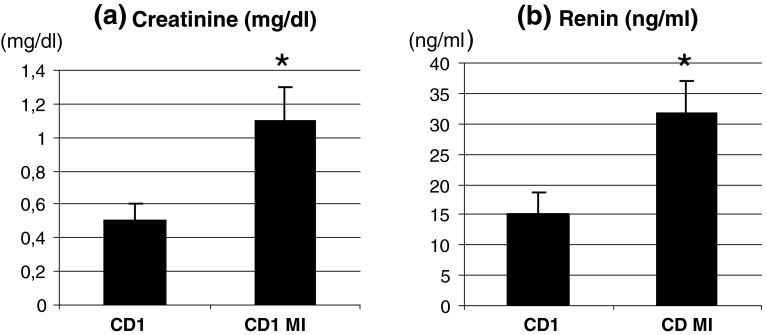
In infarcted mice, renal PI was significantly increased 15 days after surgery. Thirty days after surgery, PI was still significantly increased but less than after 15 days (p < 0.05) (Table 1, Fig. 1a, b). Renal vasoconstriction was associated with an increased creatinine and renin (creatinine ctr 0.5 ± 0.1 mg/dl, MI 1.1 ± 0.2 mg/dl, p < 0.05; Renin ctr 15.2 ± 3.5 ng/ml, MI 31.7 ± 5.2 ng/ml, p < 0.05) (Fig. 2a, b).
Fig. 1.
a High-resolution EcoDoppler image of renal perfusion obtained with the VEVO2100 echo machine using a 30 MHz probe; b renal PIs: basal value, 15 and 30 days after LAD *P < 0.05 and **P < 0.01 versus controls
Fig. 2.
a serum creatinine levels assessed by ELISA test, *P < 0.05 versus controls; b serum renin levels assessed by ELISA test, *P < 0.05 versus controls
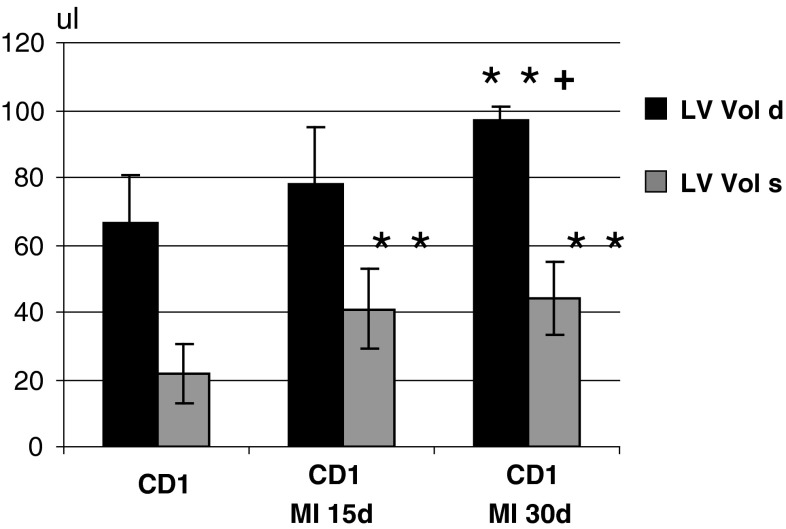
Echocardiographic alterations after LAD were evident 15 days after surgery (Table 1, Fig. 3). A significant dilatation of the left ventricle (EDA, ESA, LV vol d, s: p < 0.01 vs. controls) and a thinning of the anterior wall (p < 0.05) were evident. Moreover, FE, FS, and FAC were significantly reduced (p < 0.01 vs. controls) as result of the impaired systolic function of the left ventricle. Anterior wall thinning of MI group was significant (AWT, s: p < 0.05 vs. controls), whereas there were no significant changes in the thickness of the posterior wall. The use of the high-resolution ultrasound system allowed the assessment of transmitral flow (Fig. 4) and the study of the diastolic function of the left ventricle through the calculation of MPI (Table 1). MPI was significantly increased in infarcted mice 15 and 30 days after surgery (<0.01 vs. controls). Increased MPI was due to a reduction in ET. The DT of E wave was reduced, but the reduction was not statistically significant. Both parameters indicate an impairment of diastolic function. There were no significant differences in the echocardiographic measurements 15 and 30 days after MI.
Fig. 3.
Left ventricle end diastolic and end systolic volumes (LV vol d; LV vol s) in control mice and in mice with LAD evaluated 15 and 30 days after surgery, *P < 0.05 and **P < 0.01 versus controls + P<0.05 and versus MI 15 gg
Fig. 4.
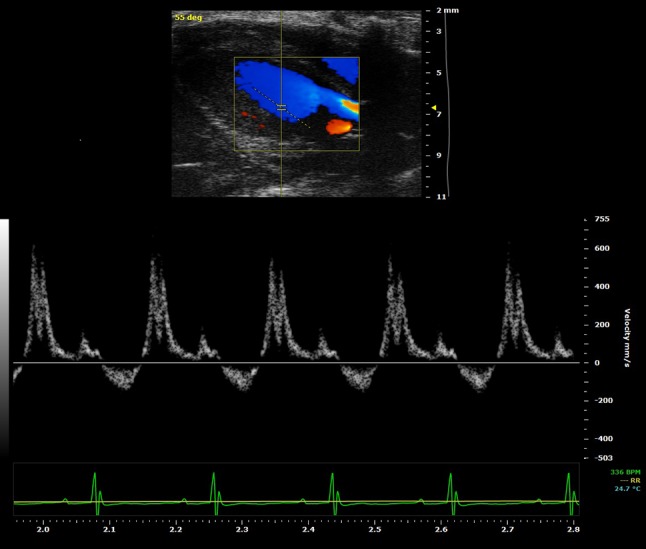
High-resolution EcoDoppler image of transmitral flow in a control mice obtained with the VEVO2100 echo machine using a 30 MHz probe
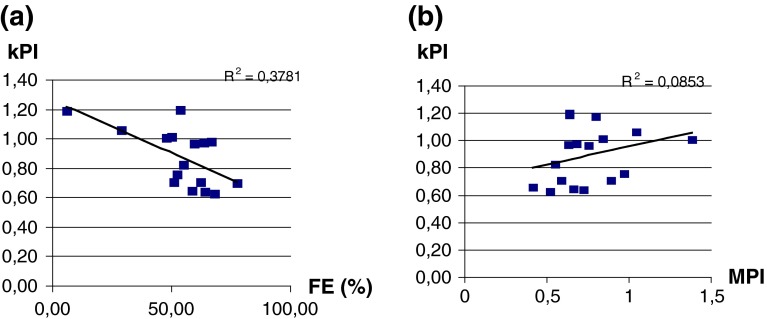
No significant correlations between PI and systolic (FE) or diastolic (MPI) cardiac indexes were evident (Fig. 5a, b)
Fig. 5.
Correlation between PIs and EF and between PIs and MPI
Discussion
In this study, we characterize renal perfusion in infarcted mice using an high-resolution ultrasound system. This study demonstrates that in mice with MI, there is a significant increase in renal resistances measured noninvasively by the Doppler method (Table 1, Fig. 1a, b). Increased PIs were associated with increased creatinine and renin levels (Fig. 2a, b). This finding allows to propose this model as an useful model of CRS-1. Compared to the most commonly used models of CRS-1 (based on myocardial ischemia and subnephrectomy or uninephrectomy) [11], it reproduces the real physiopathology of CRS-1, as renal damage is the consequence of a primary myocardial disfunction and not of a direct renal injury. The reduced cardiac output causes an activation of the RAS and SNS, with a consequent vasoconstriction of renal arterioles in order to contain fluid excretion and increase the effective circulating volume [1, 15]. The finding of increased values of renin and creatinine in infarcted mice (Fig. 2a, b) supports this hypothesis and allows the diagnosis of CRS-1 that is based on the criteria for the definition of renal failure and hence on the values of creatinine [16]. In literature, there are few data, often discordant, on the values of creatinine in mice with myocardial infarction. However, an early increase in serum creatinine 3 days after surgical ligation of the coronary artery has already been described by Lu et al. [12], with a subsequent tendency to normalization. This finding is interpreted as related to the acute phase of post-ischemic heart failure, but it could also be due to the surgical procedure and anesthesia-induced hypoperfusion. In agreement with Lu’s findings, it is interesting to notice that in our study, CD1 mice showed higher values of PI 15 days after myocardial infarction than 30 days after. This finding supports our proposal that the mice model of LAD-related renal dysfunction is a model of type 1 CRS as the acute cardiac damage leads to an acute renal dysfunction.
The evaluation of renal PIs was possible in all mice, and the whole procedure (echocardio plus renal ecoDoppler) lasted less than 10 min for each animal. It was not painful because mice were anesthetized and the evaluation could be easily repeated throughout the experiment while collecting multiple blood samples for creatinine measurement in mice with myocardial infarction is not recommended. Moreover, renal echoDoppler allows the direct evaluation of the kidney while creatinine evaluation is an indirect tool and may be affected by age, diet, and metabolic disease and should be used with caution especially in patients with cardiovascular disease [17].
In our study, there were no significant correlations between renal PIs and cardiac indexes of systolic and diastolic function of the left ventricle even if there was an upward trend in PIs related to the decrease of EF (index of systolic dysfunction) and increase of MPI (index of diastolic dysfunction) (Fig. 5a, b). This finding leads to the assumption that the increased renal resistance is only partly related to the pumping dysfunction of the left ventricle as already described in literature [5–8]. Recent studies have shown that renal vasoconstriction is related also to the systemic pro-inflammatory state consequent to myocardial necrosis [5–8]. We can hypothesize that, together with the reduced cardiac output, the release of ROS from the necrotic myocardium amplifies ANGII effect on renal arteries. Moreover, it is known that ANGII itself stimulates the production of reactive oxygen species [10] that are directly involved in renal vasoconstriction.
EcoDoppler cardiac evaluation confirmed what has already been reported in literature in relation to the LAD model. Myocardial ischemia obtained by ligation of the left coronary artery determines a morphological and functional alteration of the left ventricle characterized by a thinning of the anterior (ischemic) wall, a reactive hypertrophy of the posterior wall and a dilation of the left ventricle associated with a reduction in ejection fraction and fractional shortening [18–21]. The thinning of the anterior wall (AWT) is due to scar formation at the level of the ischemic myocardium where the necrotic tissue is replaced by fibrous tissue. The hypertrophy of the posterior wall (PWTd) is described in the literature as a compensatory response of viable myocardium to mechanical stress due to the higher diastolic filling secondary to fluid retention after myocardial infarction (Frank-Starling mechanism) [22–24]. The use of a high-resolution equipment allowed the evaluation of Doppler transmitral flow and, thus, left ventricle’s diastolic function. In murine models, the evaluation of transmitral flow has actually some limitations due to the elevated heart rate at which the examination is performed (usually around 400 bpm). At these frequencies, there is often a fusion of E wave and A wave; therefore, the evaluation of the E/A ratio is not always applicable [24–26]. To overcome this problem in this study, we analyzed the myocardial performance index (MPI or Tei index). The MPI is a parameter that evaluates the relationship between the time of isovolumetric contraction and relaxation and the ejection time: MPI = IVCT + IVRT/ET. This index provides an index of both diastolic and systolic ventricular function [27]. MPI is independent from heart rate and from the morphology of the ventricle [27, 28]. In our study, mice with heart failure had a significant increase of MPI (Table 1) in agreement with data reported in literature about dilated cardiomyopathy and ischemic heart diseases [29–31]. The MPI reduction is mainly due to a shortening of ET, indicating a reduced stroke volume [28].
Conclusions
Mice with LAD develop acute renal dysfunction with renal vasoconstriction. In mice, renal vasoconstriction can be easily and noninvasively evaluated and monitored by high-resolution ecoDoppler sonography; thus, LAD is a good model of CRS-1. This model reproduces the physiopathology of CRS with activation of the renin–angiotensin system. The use of this model could open new perspectives for CRS-1 study and treatment.
Conflict of interest
The authors declare that they have no conflict of interest.
Animal studies
The study was conducted in accordance with all institutional and national guidelines for the care and use of laboratory animals.
References
- 1.Bongartz LG, Cramer MJ, Braam B. The cardiorenal connection. Hypertension. 2004;43:e14. doi: 10.1161/01.HYP.0000118521.06245.b8. [DOI] [PubMed] [Google Scholar]
- 2.Bongartz LG, Cramer MJ, Doevendans PA, Joles JA, Braam B. The severe cardiorenal syndrome: ‘Guyton revisited’. Eur Heart J. 2005;26:11–17. doi: 10.1093/eurheartj/ehi020. [DOI] [PubMed] [Google Scholar]
- 3.Badzyńska B, Sadowski J. Moderate intrarenal vasoconstriction after high pressor doses of norepinephrine in the rat: comparison with effects of angiotensin II. Kidney Blood Press Res. 2011;34:307–310. doi: 10.1159/000328328. [DOI] [PubMed] [Google Scholar]
- 4.Sacerdoti D, Bolognesi M, Merkel C, Angeli P, Gatta A. Renal vasoconstriction in cirrhosis evaluated by duplex Doppler ultrasonography. Hepatology. 1993;17:219–224. [PubMed] [Google Scholar]
- 5.Monu SR, Pesce P, Sodhi K, Boldrin M, Puri N, Fedorova L, Sacerdoti D, Peterson SJ, Abraham NG. Kappas A.HO-1 induction improves the type-1 cardiorenal syndrome in mice with impaired angiotensin II-induced lymphocyte activation. Hypertension. 2013;62(2):310–316. doi: 10.1161/HYPERTENSIONAHA.111.00495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mullens W, Abrahams Z, Francis GS, et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol. 2009;53:589–596. doi: 10.1016/j.jacc.2008.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Damman K, van Deursen VM, Navis G, Voors AA, van Veldhuisen DJ, Hillege HL. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol. 2009;53:582–588. doi: 10.1016/j.jacc.2008.08.080. [DOI] [PubMed] [Google Scholar]
- 8.Sinkeler SJ, Damman K, van Veldhuisen DJ, Hillege H, Navis G. A re-appraisal of volume status and renal function impairment in chronic heart failure: combined effects of pre-renal failure and venous congestion on renal function. Heart Fail Rev. 2012;17(2):263–270. doi: 10.1007/s10741-011-9233-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zablocki D, Sadoshima J. Angiotensin II and oxidative stress in the failing heart. Antioxid Redox Signal. 2013;19(10):1095–1109. doi: 10.1089/ars.2012.4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chu PY, Zatta A, Kiriazis H, Chin-Dusting J, Du XJ, Marshall T, Kaye DM. CXCR4 antagonism attenuates the cardiorenal consequences of mineralocorticoid excess. Circ Heart Fail. 2011;4:651–658. doi: 10.1161/CIRCHEARTFAILURE.110.960831. [DOI] [PubMed] [Google Scholar]
- 11.Szymanski MK, de Boer RA, Navis GJ, van Gilst WH, Hillege HL. Animal models of cardiorenal syndrome: a review. Heart Fail Rev. 2012;17:411–420. doi: 10.1007/s10741-011-9279-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu J, Wang X, Wang W, Muniyappa H, Deshmukh A, Hu C, Das K, Mehta JL. Abrogation of lectin-like oxidized LDL receptor-1 attenuates acute myocardial ischemia-induced renal dysfunction by modulating systemic and local inflammation. Kidney Int. 2012;82(4):436–444. doi: 10.1038/ki.2012.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.VisualSonics VEVO2100 Operator Manual, Copyright © 2001–2008 by VisualSonics Inc
- 14.Benavides-Vallve C, Corbacho D, Iglesias-Garcia O, Pelacho B, Albiasu E, Castaño S, Muñoz Barrutia A, Prosper F, Ortiz-de-Solorzano C. New strategies for echocardiographic evaluation of left ventricular function in a mouse model of long-term myocardial infarction. PLoS One. 2012;7(7):e41691. doi: 10.1371/journal.pone.0041691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Francio SG, Gassler PJ Sonnenblick HE Fisiopatologia dello scompenso cardiaco. Il Cuore 10 ed. Hurst esd, McGraw-Hill Pubs 200; 743–79
- 16.Adiyanti SS, Loho T. Acute kidney injury (AKI) biomarker. Acta Med Indones-Indones J Intern Med. 2012;44(3):246–255. [PubMed] [Google Scholar]
- 17.Verhave JC, Gansevoort RT, Hillege HL, De Zeeuw D, Curhan GC, De Jong PE. Drawbacks of the use of indirect estimates of renal function to evaluate the effect of risk factors on renal function. J Am Soc Nephrol. 2004;15(5):1316–1322. [PubMed] [Google Scholar]
- 18.Pfeffer MA, Pfeffer JM, Fishbein MC. Myocardial infarct size and ventricular function in rats. Circ Res. 1979;44(4):503–512. doi: 10.1161/01.RES.44.4.503. [DOI] [PubMed] [Google Scholar]
- 19.Sjaastad I, Sejersted OM, Ilebekk A, Bjonerheim R. Echocardiographic criteria for detection of postinfarction congestive heart failure in rats. J App Phisiol. 2000;89:1445–1454. doi: 10.1152/jappl.2000.89.4.1445. [DOI] [PubMed] [Google Scholar]
- 20.Morgan E, Faulx M, McElfresh T, Kung T, Zawaneh M, Stanley W, Chandler M, Hoit B. Validation of echocardiographic methods for assessing ventricular dysfunction in rats with myocardial infarction. Am J Phisiol Heart Circ Physiol. 2004;287(5):H2049–H2053. doi: 10.1152/ajpheart.00393.2004. [DOI] [PubMed] [Google Scholar]
- 21.Fayssoil A Tournoux F (2012) Analyzing left ventricular function in mice with Doppler echocardiography. Heart Fail Rev [DOI] [PubMed]
- 22.Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101(2981–2988):41. doi: 10.1161/01.cir.101.25.2981. [DOI] [PubMed] [Google Scholar]
- 23.Braunwald E. Congestive heart failure: a half century perspective. Eur Heart J. 2001;22:825–836. doi: 10.1053/euhj.2001.2614. [DOI] [PubMed] [Google Scholar]
- 24.Scherrer-Crosbie M, Thibault HB. Echocardiography in translation research: of mice and men. J Am Soc Echocardiogr. 2008;21:1083–1092. doi: 10.1016/j.echo.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ganau A, Devereux RB, Roman MJ, de Simone G, Pickering TG, Saba PS, Vargiu P, Simongini I, Laragh JH. Patterns of left ventricular hypertrophy and geometric remodeling in essential hypertension. J Am Coll Cardiol. 1992;19:1550–1558. doi: 10.1016/0735-1097(92)90617-V. [DOI] [PubMed] [Google Scholar]
- 26.Yuan Lijun, Wang Tao, Liu Fang, Cohen Ethan D, Patel Vickas V. An evaluation of transmitral and pulmonary venous Doppler indices for assessing murine left ventricular diastolic function. Cardiol Rev. 2002;10(4):218–229. doi: 10.1097/00045415-200207000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 Appropriate use criteria for echocardiography a report of the American college of cardiology foundation appropriate use criteria task force, American society of echocardiography, American heart association, American Society of nuclear cardiology, heart failure society of America, heart rhythm society, society for cardiovascular angiography and interventions, society of critical care medicine, society of cardiovascular computed tomography, and society for cardiovascular magnetic resonance. Journal of the American College of Cardiology Vol. 57, No. 9, 2011 44 [DOI] [PubMed]
- 28.Lax JA, Bermann AM, Cianciulli TF, Morita LA, Masoli O, Prezioso HA. Estimation of the ejection fraction in patients with myocardial infarction obtained from the combined index of systolic and diastolic left ventricular function: a new method. J Am Soc Echocardiogr. 2000;13(2):116. doi: 10.1016/S0894-7317(00)90022-1. [DOI] [PubMed] [Google Scholar]
- 29.Lakoumentas JA, Panou FK, Kotseroglou VK, Aggeli KI, Harbis PK. The Tei index of myocardial performance: applications in cardiology. Hellenic J Cardiol. 2005;46(1):52–58. [PubMed] [Google Scholar]
- 30.Dujardin KS, Tei C, Yeo TC, Hodge DO, Rossi A, Seward JB. Prognostic value of a Doppler index combining systolic and diastolic performance in idiopathic-dilated cardiomyopathy. Am J Cardiol. 1998;82(9):1071–1076. doi: 10.1016/S0002-9149(98)00559-1. [DOI] [PubMed] [Google Scholar]
- 31.Ascione L, De Michele M, Accadia M, Rumolo S, Damiano L, D’Andrea A, Guarini P, Tuccillo B. Myocardial global performance index as a predictor of in- hospital cardiac events in patients with first myocardial infarction. J Am Soc Echocardiogr. 2003;16(10):1019–1023. doi: 10.1016/S0894-7317(03)00589-3. [DOI] [PubMed] [Google Scholar]