Abstract
Introduction
Many pathological causes are responsible for the sonographic presentation of myometrium cysts and cyst-like lesions, where the distinction between these etiologies is required.
The aim of the work
The current work is aimed at discerning between different etiologies of myometrium cysts and cyst-like lesions for an optimum management.
Methodology
In the course of daily practice of gynecological transvaginal ultrasound, 66 cases of myometrium cysts and cyst-like lesions have been discerned, where all were examined with endovaginal ultrasound using a multifrequency endocavitary probe having color Doppler capability.
Results
Adenomyosis uteri detected in 15 cases, invasive mole in 4 cases, congested arcuate veins in 20 cases, incidental cysts in 4 cases, cystic degeneration of myoma in 3 cases, C-section scar cysts and cyst-like lesions in 13 cases, interstitial ectopic pregnancy in 2 cases, incomplete abortion with congested myometrium vessels in 4 cases, and arteriovenous malformation in 1 case. The number of cases with cervical nabothian cysts was not considered since they were too frequent.
Conclusion
Endosonography is an important tool in differentiating between the various diseases that are responsible for benign myometrium cysts and cyst-like lesions, which are all important since some of them are visualized as serious clinical situations and others turn out to be of little clinical significance.
Keywords: Endosonography, Myometrium, Cysts, Adenomyosis
Riassunto
Introduzione
Molte patologie sono responsabili della presentazione ecografica di cisti del miometrio e di lesioni simil-cistiche ed è necessaria la distinzione tra le varie eziologie.
Scopo del lavoro
Scopo del lavoro è distinguere tra le diverse eziologie delle cisti del miometrio e delle lesioni simil-cistiche per una loro gestione ottimale.
Materiali e metodi
Durante la pratica quotidiana di ecografia transvaginale ginecologica sono stati riscontrati 66 casi di cisti del miometrio e di lesioni simil-cistiche, tutti sono stati esaminati con ecografia endovaginale con sonda endocavitaria multifrequenza e modulo Doppler.
Risultati
In 15 casi è stata rilevata adenomiosi dell’utero, in 4 casi mola invasiva, in 20 casi congestione venosa, in 4 casi riscontro incidentale di cisti, in 3 casi degenerazione cistica dei miomi, in 13 casi cicatrice cistica (CS) e lesioni simil-cistiche, in 2 casi gravidanza ectopica, in 4 casi aborto incompleto con vasi del miometrio congestionati e in 1 caso malformazioni artero-venose. I casi di cisti Naboth non sono stati considerati in quanto di troppo frequente riscontro.
Conclusioni
L’ecoendoscopia è uno strumento importante nella differenziazione delle varie malattie che sono responsabili della formazione di cisti benigne del miometrio come di lesioni simil-cistiche, alcune gravi, altre di scarsa rilevanza clinica.
Introduction
The presence of a myometrium cyst or a cyst-like lesion may be a sign of a serious clinical situation or an underlying disease process responsible for the patient’s symptoms.
Many pathological causes are responsible for the sonographic presentation of myometrium cysts and cyst-like lesions, where the differentiation between these etiologies is required for optimum management.
Cysts appear by endosonography as well as defined lesions with thin wall, anechoic contents, distal acoustic enhancement, and showing no internal flow signal during the Doppler study. Complicated cysts showed internal turbidity, thick walls, soft tissue-like internal components, and capsular hyperemia during the Doppler study. Cyst-like lesions are those lesions that mimic cysts by ultrasound but bear additional criteria or standards that apply to other diagnoses rather than true cysts. Congested arcuate uterine veins mimic cystic lesions, but showed internal flow with color Doppler. The gestational sac resembles a cyst but showed the fetal node inside and the yolk sac.
The aim of the work
The current study aimed to discern between different etiologies of myometrium cysts and cyst-like lesions for the sake of optimum management.
Methodology
During the daily practice of gynecological transvaginal ultrasound, 66 cases of myometrium cysts from January 2013 to February 2014 have been distinguished, where their ages ranged 20–50 years and the average age was 34 years. Their complaints were primary infertility, abnormal uterine bleeding, amenorrhea, dysmenorrhagia, dyspareunia, lower abdominal pain, or asymptomatic.
All were examined with endovaginal ultrasound using a multifrequency endocavitary probe of 4–9 MHz and a SonoAce x8 ultrasound machine (Medison, Korea) assisted with color Doppler and three-dimensional capabilities. Myometrium cysts were assessed for the site, size, shape, number, the distribution within the myometrium, wall thickness, color flow, and the pulsed Doppler criteria.
The final diagnosis was established based on clinical history, image findings, lab results, surgical outcome, and the histopathological reports.
Results
Adenomyosis uteri were detected in 15 cases, invasive mole in 4 cases, congested arcuate veins in 20 cases, incidental cysts in 4 cases, cystic degeneration of myoma in 3 cases, C-section scar cysts and cyst-like lesions in 13 cases, interstitial ectopic pregnancy in 2 cases, incomplete abortion with congested myometrium vessels in 4 cases, and arteriovenous malformation in 1 case (Table 1). The number of cases with cervical nabothian cysts was not considered since they were too frequent.
Table 1.
Number of cases with myometrium cysts and cyst-like lesions and their sonographic diagnosis
| Sonographic diagnosis | Number of cases | % |
|---|---|---|
| Adenomyosis uteri | 15 | 22.8 |
| Invasive mole | 4 | 6 |
| Congested arcuate veins | 20 | 30.3 |
| Incidental cysts | 4 | 6.1 |
| Cystic degeneration of myoma | 3 | 4.5 |
| CS scar cysts and cyst-like lesions | 13 | 19.7 |
| Interstitial ectopic pregnancy | 2 | 3 |
| Incomplete abortion | 4 | 6.1 |
| Arteriovenous malformation (AVM) | 1 | 1.5 |
| Total | 66 | 100 |
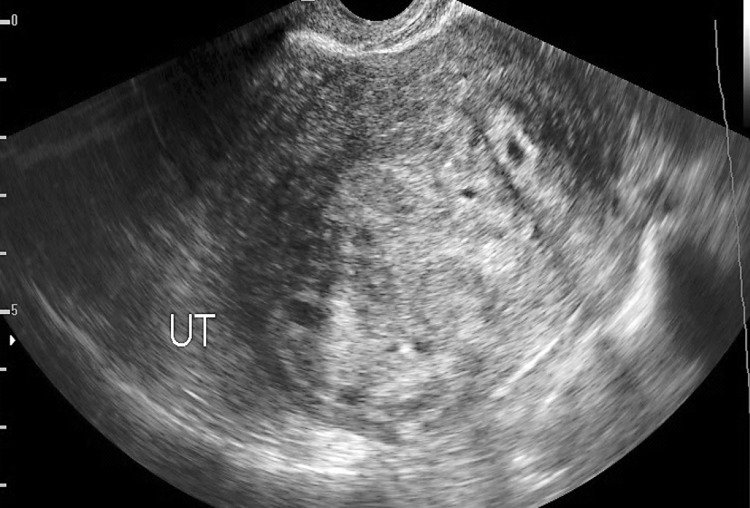
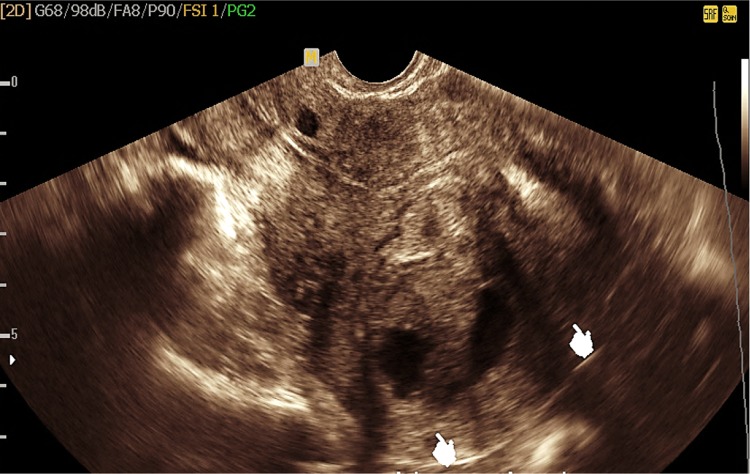
Adenomyosis uteri (15 cases) (Fig. 1)
Fig. 1.
Transvaginal ultrasound showing bulky uterus (UT) with inhomogeneous myometrium texture, an ill-defined foci of hyperechogenicity, and small myometrium cysts suggesting adenomyosis
Sonographic features of adenomyosis showed a bulky uterus with asymmetrical posterior myometrium wall thickening, small subendometrial and diffuse myometrium cysts less than 5 mm in diameter, abnormal ill-defined myometrium foci of hyperechogenicity, and thickening of subendometrial zone greater than 12 mm [1–3].
Cystic adenomyoma
Extremely rare and represents the focal form of adenomyosis with cystic changes inside that may attain a large size and can be mistaken for leiomyoma with cystic degeneration [4–7].
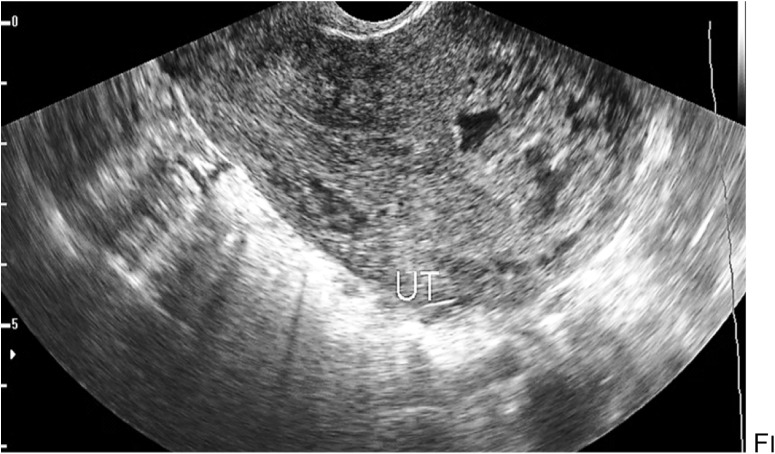
Invasive mole (4 cases) (Fig. 2)
Fig. 2.
Endosonography showing bulky uterus (UT) with irregular large anterior myometrium area of hyperechoic pattern with small cysts inside in a patient with a history of vesicular mole and presented with abnormal uterine bleeding suggesting an invasive mole
Invasive mole can be detected as multiple variable size myometrium cysts with increased background parenchymal echogenicity in association with abnormally thickened endometrium that showed cystic changes inside, where the patients typically showed signs of amenorrhea, followed by bleeding and high serum HCG levels [8].
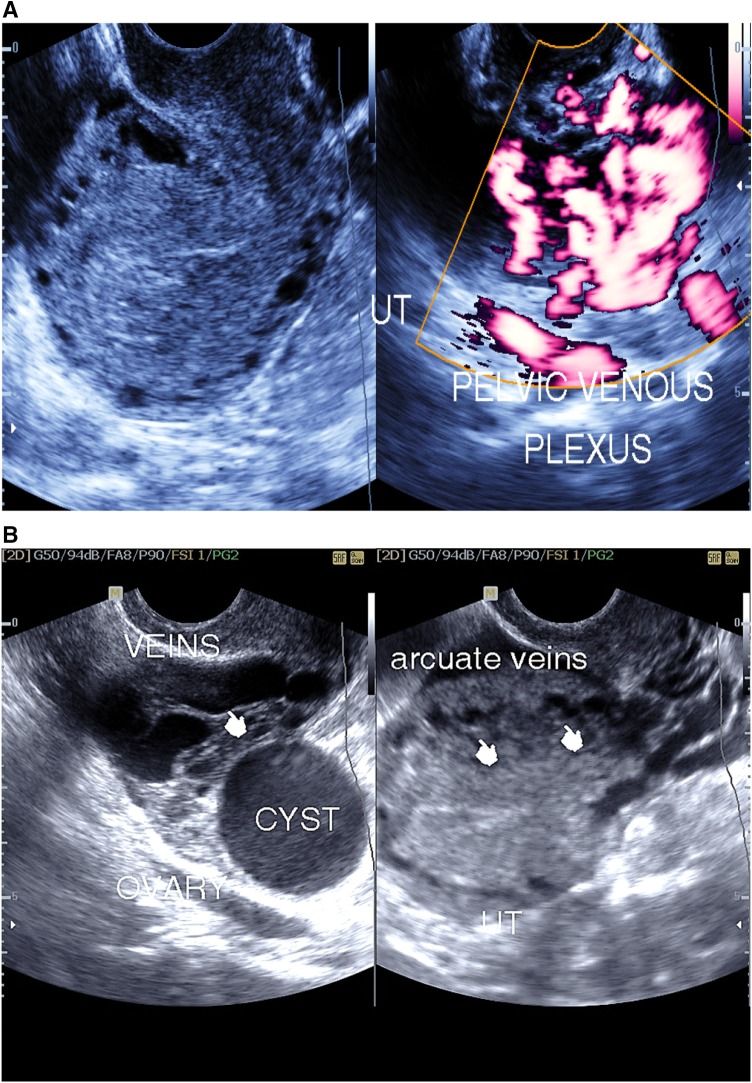
Congested myometrium arcuate veins (20 cases) (Fig. 3a, b)
Fig. 3.
a Endosonography assisted with color Doppler study, showing congested pelvic venous plexus and retroverted uterus (UT) with congested arcuate veins. b Endosonography showing congested pelvic and arcuate uterine veins (cysts) in association with an ovarian cyst
Multiple dilated serpentine tubular structures seen in the myometrium with venous flow signal inside during the color Doppler study associated with dilated parauterine and pelvic venous plexus.
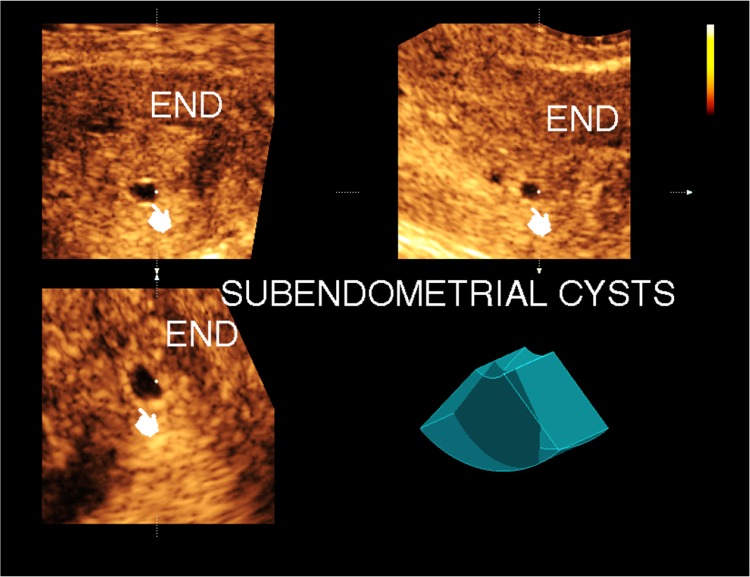
Incidental cysts (4 cases) (Fig. 4)
Fig. 4.
Transvaginal 3-D ultrasonography with multiplanar image analysis showing subendometrial incidental cysts
Small cysts that are detected in the subendometrial region in an asymptomatic young patient with a uniform myometrium texture that showed no abnormality with endosonography.
Cystic degeneration of uterine myoma (3 cases) (Fig. 5)
Fig. 5.
Transvaginal ultrasound showing the uterus with a well-defined posterior wall mural myoma with gross cystic changes inside (indicators)
Uterine myomas can be seen in subserous, submucosal, and mural locations. Uncommonly, the uterine mural myoma may show gross cystic changes inside [9].
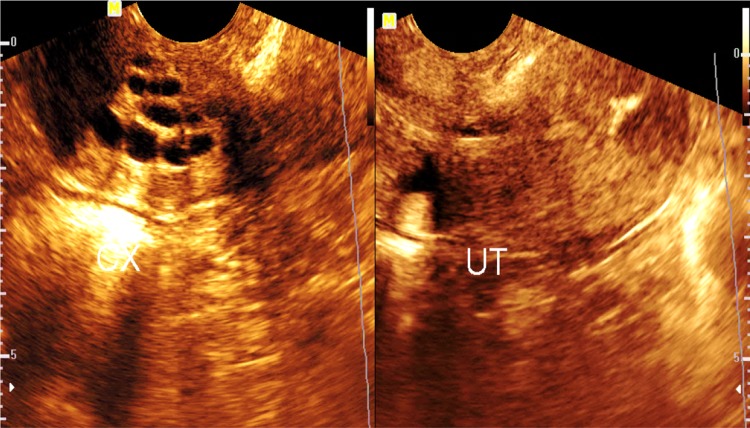
Cervical nabothian cysts (Fig. 6)
Fig. 6.
Endosonography showing the cervix (CX) with multiple cervical nabothian cysts
These are usually small cysts at the myometrium of the cervix, usually close to the cervical canal and showing clear anechoic contents. They may arise close to the external cervical os and project into the vagina.
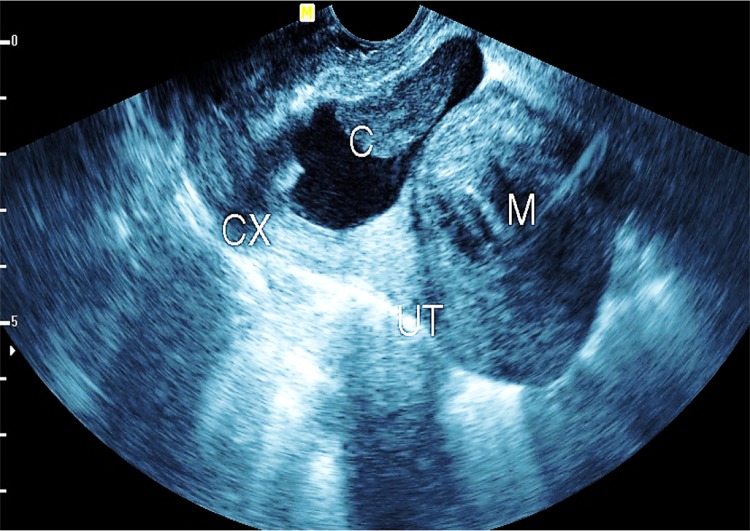
Caesarean section (CS) scar cystic changes (13 cases) (Figs. 7, 8)
Fig. 7.
Endosonography showing a case of CS scar ectopic pregnancy
Fig. 8.
Transvaginal ultrasound showing cyst with medium- and low-level echoes at the site of the CS scar, suggesting endometriosis cyst and anterior wall mural myoma
Caesarean section scar is the myometrium fibrous scar seen in the anterior aspect of the cervico-corporeal junction; it may show cystic changes inside as gapping endometriotic cyst, ectopic gestational sac, missed or incomplete abortion of the CS scar ectopic gestation.
Gapping of CS scar (7 cases) may result in postmenstrual spotting and could be associated with chronic pelvic pain and may alert for complicated future pregnancies.
CS scar endometriosis (2 cases) is very rare; however, it could be associated with uterine adenomyosis and can be delivered as a cystic lesion with low-level echoes at the scar site.
Ectopic pregnancy or complicated ectopic pregnancy of the CS scar (4 cases) can be seen as a cystic lesion representing the gestational sac with or without a fetal node inside and could represent a medical emergency [10–15].
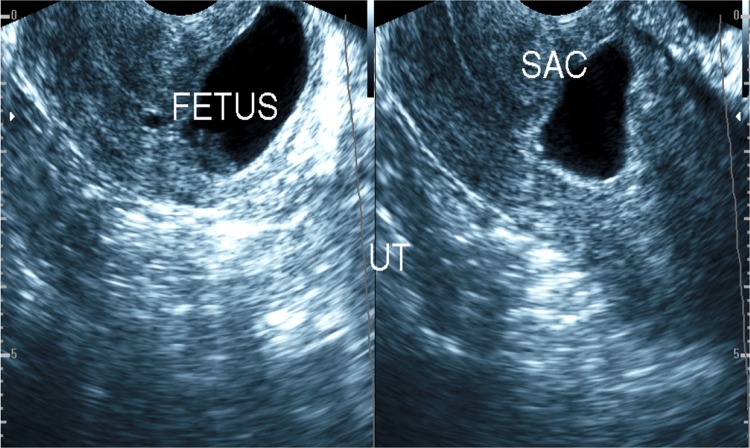
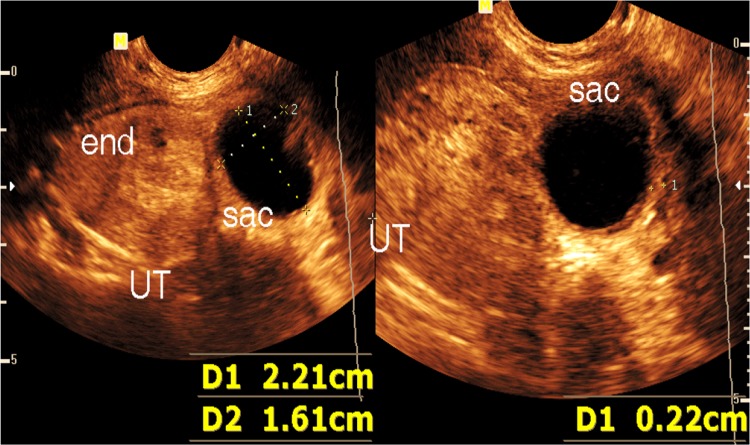
Interstitial ectopic pregnancy (2 cases) (Fig. 9)
Fig. 9.
Transvaginal ultrasound showing interstitial ectopic gestational sac with no fetal node (missed abortion of ectopic gestation)
A rare type of ectopic gestation with high incidence of complications presented as eccentric gestational sac within the myometrium or as a cystic lesion of the interstitial part of the Fallopian tube with or without a fetal node inside (blighted ovum or neglected missed abortion), where the residual myometrium tissue around the sac at its outer aspect does not exceed 5 mm [16].
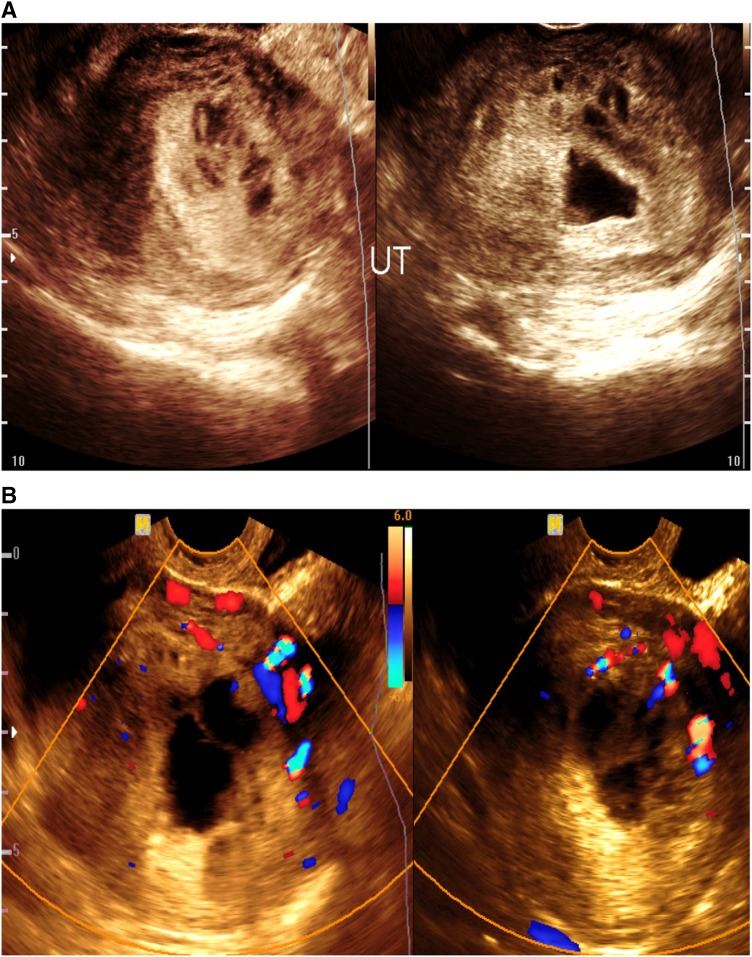
Incomplete abortion with neglected intrauterine retained parts (4 cases) (Fig. 10a, b)
Fig. 10.
a, b Transvaginal ultrasound assisted with color Doppler showing intrauterine irregular gestational sac and retained parts with small myometrium cystic changes (a). With color flow signal inside (b)
The endometrial cavity appears dilated and filled with inhomogeneous retained parts of inhomogeneous echo pattern and associated with dilated serpentine vascular structures within the myometrium, showing high-velocity, low-resistant flow pattern inside with Doppler study.
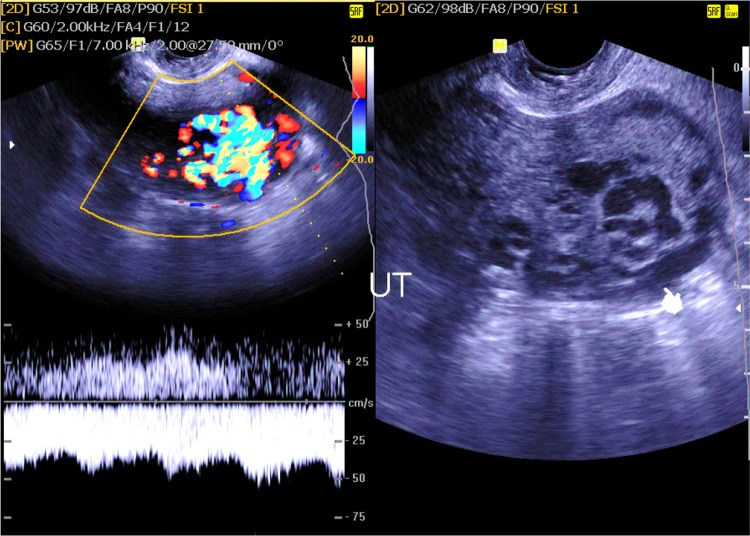
Arteriovenous malformation (AVM) (1 case) (Fig. 11)
Fig. 11.
B mode and color duplex study of an arteriovenous malformation, showing high-velocity, low-resistant arterial flow pattern seen involving the posterior myometrial wall (indicator)
It could be congenital or acquired. Congenital AVM usually shows multiple communicating channels between the arteries and veins and may extend to extrauterine regions, whereas acquired post-traumatic AVM shows single fistulous communication between an artery and a vein, where it exhibits marked turbulence with high velocity.
It shows low-resistant flow pattern on the arterial side and pulsatile venous flow signal at the venous side with multiple serpentine cyst-like lesions within the myometrium on a grayscale ultrasound [17, 18].
Other causes of benign myometrial cysts not seen among our patients but should be mentioned
Pseudoaneurysm of uterine artery Rare cause of abnormal uterine bleeding presented as a cystic lesion with arterial color flow signal inside during color duplex study. It can be managed with endovascular embolization or percutaneous ultrasound-guided thrombin injection [19].
Cystic adenomatoid tumor A rare tumor of mesothelial origin may occur in the myometrium and appear similar to leiomyoma with cystic changes or as a multilocular cystic mass lesion similar to lymphangioma [20, 21].
Lymphangioma of uterus An extremely rare tumor that may originate from the uterine fundus and presented as a large multilocular cystic mass lesion [22].
Discussion
Many etiologies are responsible for the myometrium cysts and cyst-like lesions. The distinction between these etiologies is essential for the sake of optimum management, wherein some cases of myometrium cysts are of little clinical significance and others are regarded as serious clinical situations that require early accurate diagnosis and rapid intervention.
Magnetic resonance imaging (MRI) is a useful tool in the evaluation of uterine myometrial cysts in adjunct with ultrasound; however, it is expensive and not readily available in many hospitals, especially in low-income nations.
The subendometrial cysts could be an incidental finding or could be caused by adenomyosis. The conflict between both will depend on the myometrial texture, which appears uniform in cases of incidental cysts or inhomogeneous with scattered echogenic ill-defined foci in cases with adenomyosis. MRI showed a similar accuracy to the transvaginal ultrasound in the diagnosis of adenomyosis with reported sensitivity and specificity of 77.5 and 92.5 %, respectively. The diagnostic criteria include maximum junctional zone thickness greater than 12 mm, maximum junctional zone thickness and myometrial ratio greater than 40 %, and high-signal intensity myometrial spots [23].
Cystic lesion of CS scar could be endometriosis cyst or gestational sac. The differentiation between both is easy when the sac shows a fetus inside or a yolk sac, but when the sac does not show a fetus, as in cases of neglected missed abortion or in cases with anembryonic pregnancy, where no fetal node or yolk sac is present, the distinction between both will be difficult depending on the imaging criteria. The serum human chorionic gonadotropin (hCG) levels could be low, indeterminate, or abnormally elevated due to factors other than pregnancy, and the clinical feature of amenorrhea will be misleading due to the presence of irregular uterine bleeding, but still the differentiation can be accomplished depending on the sonographic image as an endometriotic cyst will be filled with medium- to low-level echoes inside, which is not experienced in instances of missed abortion or anembryonic pregnancy, and the endometriotic cyst usually has a regular wall thickness, whereas the gestational sac has asymmetrical wall thickening. MRI may be helpful in differentiation as an endometriotic cyst elicits high-signal intensity in the T1W image and an ISO-low intense signal in the T2W image. These signal intensities resulted from the high-iron concentration within the lesion [24].
The differentiation between interstitial ectopic pregnancy and mural myoma with cystic changes situated on the upper-lateral uterine wall will be difficult unless supported by clinical features and a positive serum pregnancy test; but when they are misleading, the differentiation can be achieved through endosonography as the thickness of the soft tissue planes outside the cyst will often exceed 5 mm in cases of myoma with cystic changes, and less than 5 mm in cases with interstitial ectopic pregnancy. As well, the marginal vascularity at the wall of the cyst will be higher in cases with pregnancy. MRI features of interstitial pregnancy include the heterogeneous mass of predominant hyperintense signal in the T2W image situated lateral to the uterine cornu with clear junctional zone with the uterine cavity [25, 26].
The differentiation between myoma with cystic changes and cystic adenomyoma is very difficult by endosonography; however, cystic adenomyoma is very rare and shows bloody contents, which appears as medium- and low-level echoes inside the cyst. MRI can be utilized to differentiate between cystic adenomyoma and myoma with cystic changes, as cystic adenomyoma shows hemorrhage inside, which elicits a high-intensity signal in the T1W image and a decreased ISO-intense signal in the T2W image. Myoma with cystic changes showed a low-signal intensity in the T1W image and a high-signal intensity in the T2W image [27].
The presentation of the myometrium cyst with the bidirectional arterial flow in color duplex sonography will suggest pseudoaneurysm of the uterine artery, while the presence of multiple small cystic lesions with venous flow signal will suggest congested arcuate veins.
In summation, the value of the color duplex ultrasound in evaluating uterine vascular abnormalities (such as AVM), congested arcuate veins, and uterine artery pseudoaneurysm, it proves useful in assessing cases of incomplete abortion with neglected intrauterine retained parts, where increased myometrial hypervascularity was noted, which may help in differentiating the condition from other endocavitary lesions, such as polyps and submucosal myoma. It was helpful in evaluating cases with invasive moles where myometrial hypervascularity was noted at the background parenchyma of myometrial cysts, so it can be employed to assess the presence of myometrial invasion in the absence of definitive cystic lesions.
3-D ultrasonography with multiplanar image analysis gives valuable anatomical information about the cystic lesions, as it can accurately evaluate the relation of the cysts to the endometrium, which allows differentiation of endometrial cysts seen in cystic endometrial hyperplasia from subendometrial cysts noted in cases with adenomyosis. It can also accurately localize the site of a gestational sac in cases with ectopic interstitial pregnancy, it assists in the assessment of the relation of the cystic lesion to the site of the CS scar and aids in sorting out uterine endocavitary lesions from myometrial lesions.
Conclusion
Endosonography is an important tool in distinguishing between the various diseases that are responsible for benign myometrium cysts and cyst-like lesions, which is essential since some of them are regarded as serious clinical situations and others appear of little clinical significance.
Conflict of interest
The author declares that he has no conflict of interest.
Informed consent
All patients provided written informed consent to enrolment in the study and to the inclusion in this article of information that could potentially lead to their identification.
References
- 1.Atri M, Reinhold C. Mehio AR et al. Adenomyosis: US features with histologic correlation in an in vitro study. Radiology. 2000;215(3):783–790. doi: 10.1148/radiology.215.3.r00jn06783. [DOI] [PubMed] [Google Scholar]
- 2.Reinhold C, Tafazoli F, Mehio A et al. (1999) Uterine adenomyosis: endovaginal US and MR imaging features with histopathologic correlation. Radiographics;19 Spec No : S147–60 [DOI] [PubMed]
- 3.Sakhel K, Abuhamad A. Sonography of adenomyosis. J Ultrasound Med. 2012;31(5):805–808. doi: 10.7863/jum.2012.31.5.805. [DOI] [PubMed] [Google Scholar]
- 4.Jain N, Goel S. Cystic Adenomyoma simulates uterine malformation: A diagnostic dilemma: Case report of two unusual cases. J Hum Reprod Sci. 2012;5:285–288. doi: 10.4103/0974-1208.106342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tamai K, Koyama T, Umeoka S, Saga T, Fujii S, Togashi K. Spectrum of MR features in adenomyosis. Best Pract Res Clin Obstet Gynaecol. 2006;20:583–602. doi: 10.1016/j.bpobgyn.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 6.Kataoka M, Togashi K, et al. MRI of adenomyotic cyst of uterus. J Comput Assist Tomogr. 1998;22:555–559. doi: 10.1097/00004728-199807000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Sakai Y, Matsukuma S. Large cystic uterine adenomyoma showing marked epithelial metaplasia and exophytic polypoidgrowth. Arch Gynecol Obstet. 2003;269(1):74–76. doi: 10.1007/s00404-003-0538-1. [DOI] [PubMed] [Google Scholar]
- 8.Jauniaux Mr E. Ultrasound diagnosis and follow-up of gestational trophoblastic disease. Ultrasound Obstet Gynecol. 1998;11(5):367–377. doi: 10.1046/j.1469-0705.1998.11050367.x. [DOI] [PubMed] [Google Scholar]
- 9.Kaushik C, Prasad A, Singh Y, Baruah BP. Case series: cystic degeneration in uterine leiomyomas. Indian J Radiol Imaging. 2008;18(1):69–72. doi: 10.4103/0971-3026.35820. [DOI] [Google Scholar]
- 10.Jurkovic D, Hillaby K, Woelfer B, Lawrence A, Salim R, Elson CJ. First-trimester diagnosis and management of pregnancies implanted into the lower uterine segment Caesarian section scar. Ultrasound Obstet Gynecol. 2003;21:220–227. doi: 10.1002/uog.56. [DOI] [PubMed] [Google Scholar]
- 11.Cheng P-J, Chueh H-Y, Soong Y-K. Sonographic diagnosis of a uterine defect in a pregnancy at 6 weeks gestation with a history of curettage. Ultrasound Obstet Gynecol. 2003;21:501–503. doi: 10.1002/uog.109. [DOI] [PubMed] [Google Scholar]
- 12.Fylstra DL. Ectopic pregnancy within a Caesarian scar: a review. Obstet Gynecol Surv. 2002;57:537–543. doi: 10.1097/00006254-200208000-00024. [DOI] [PubMed] [Google Scholar]
- 13.Armstrong V, Hansen WF, Van Voorhis BJ, Syrop CH. Detection of Caesarian scars by transvaginal ultrasound. Obstet Gynecol. 2003;101:61–65. doi: 10.1016/S0029-7844(02)02450-X. [DOI] [PubMed] [Google Scholar]
- 14.Goel P, Sood SS, Dalal A, Romilla (2005) Cesarean scar endometriosis: Report of two cases. Indian J Med Sci;59:495–8 [PubMed]
- 15.Lahiri AK, Sharma K, Busiri N. Endometriosis of the uterine cesarean section scar: A case report. Indian J Radiol Imag. 2008;18(1):66–68. doi: 10.4103/0971-3026.37111. [DOI] [Google Scholar]
- 16.Rastogi R, Meena GL, Rastogi N, Rastogi V. Interstitial ectopic pregnancy: a rare and difficult clinicosonographic diagnosis. J Hum Reprod Sci. 2008;1(2):81–82. doi: 10.4103/0974-1208.44116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grivell R, Reid K, Mellor A. Uterine arteriovenous malformations: a review of the current literature. Obstet Gynaecol Survey. 2005;60(11):761–767. doi: 10.1097/01.ogx.0000183684.67656.ba. [DOI] [PubMed] [Google Scholar]
- 18.Polat P, Suma S, Kantarcy M, Alper F, Levent A. Colour doppler ultrasound in the evaluation of uterine vascular abnormalities. Radiographics. 2002;22:47–53. doi: 10.1148/radiographics.22.1.g02ja0947. [DOI] [PubMed] [Google Scholar]
- 19.M Nori, J Venkateswarlu (2013) Pseudoaneurysm of the uterine artery presenting as a cystic sol in fundus: diagnosis and non surgical management. Internet J Radiol. vol 16 Number 1
- 20.Nogales FF, Isaac MA, Hardisson D, Bosincu L, Palacios J, Ordi J, et al. Adenomatoid tumors of the uterus: an analysis of 60 cases. Inter JGynecol Pathol. 2002;21:34–40. doi: 10.1097/00004347-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Hendrickson MR, Tavassoli FA, Kempson RL, et al. (2003) Mesenchymal tumours and related lesions. In: Tavassoli FA, Devilee P, editors. Tumours of the breast and female genital organs. Lyon: IARC Press-World Health Organization Classification of Tumours; pp 243-4
- 22.Furui T, Imai A, Yokoyama Y, Sato E, Tamaya T. Cavernous lymphangioma arising from uterine corpus. Gynecol Oncol. 2003;90(1):195–199. doi: 10.1016/S0090-8258(03)00197-5. [DOI] [PubMed] [Google Scholar]
- 23.Bazot M, Cortez A, Darai E, Rouger J, Chopier J, et al. Ultrasonography compared with magnetic resonance imaging for the diagnosis of adenomyosis: correlation with histopathology. Hum Reprod. 2001;16(11):2427–2433. doi: 10.1093/humrep/16.11.2427. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi K, Okada S, Okada M, Kitao M, Kaji Y, Sugimura K. Magnetic resonance relaxation time in evaluating the cyst fluid characteristics of endometrioma. Hum Reprod. 1996;11(4):857–860. doi: 10.1093/oxfordjournals.humrep.a019266. [DOI] [PubMed] [Google Scholar]
- 25.Tamai K, Koyama T, Togashi K. MR features of ectopic pregnancy. Eur Radiol. 2007;17(12):3236–3246. doi: 10.1007/s00330-007-0751-6. [DOI] [PubMed] [Google Scholar]
- 26.Filhastre M, Dechaud H, Lesnik A, Taourel P. Interstitial pregnancy: role of MRI. Eur Radiol. 2005;15(1):93–95. doi: 10.1007/s00330-004-2306-4. [DOI] [PubMed] [Google Scholar]
- 27.Weinreb JC, Barkoff ND, Megibow A, et al. The value of MRI imaging in distinguishing leiomyomas from other solid masses when sonography is indetermine. AJR. 1993;154:295–299. doi: 10.2214/ajr.154.2.2105017. [DOI] [PubMed] [Google Scholar]