Abstract
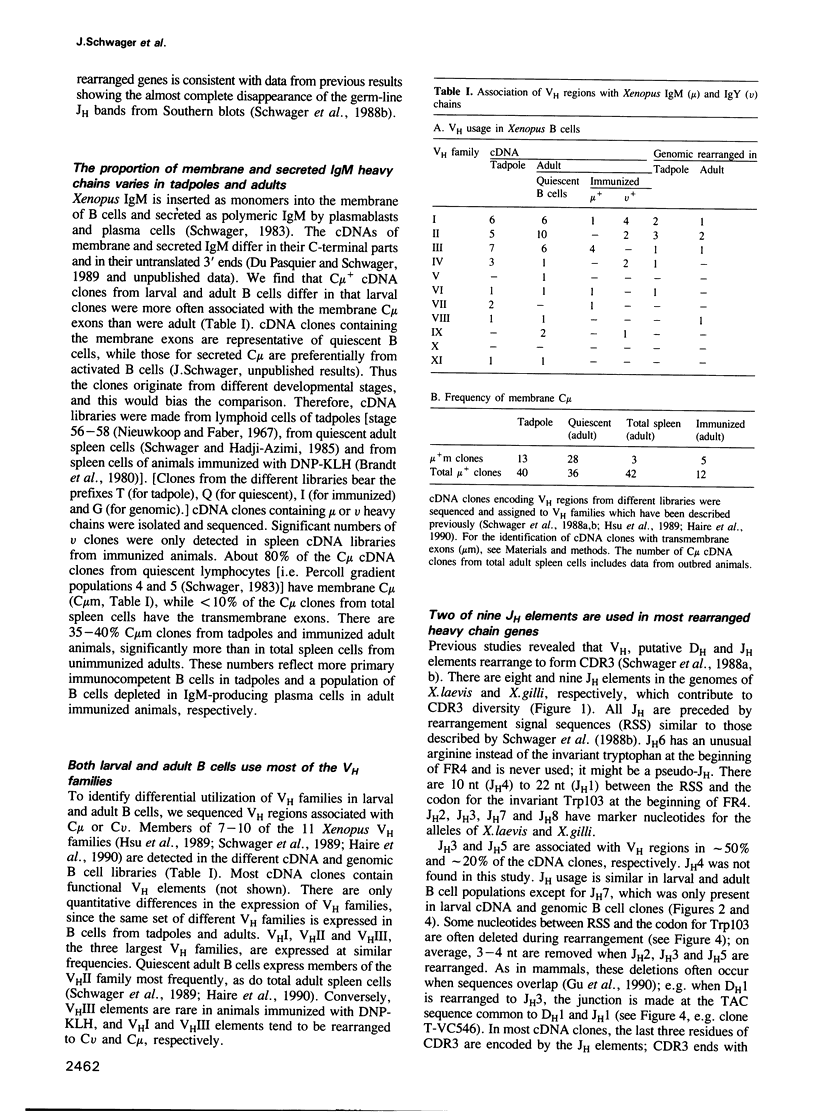
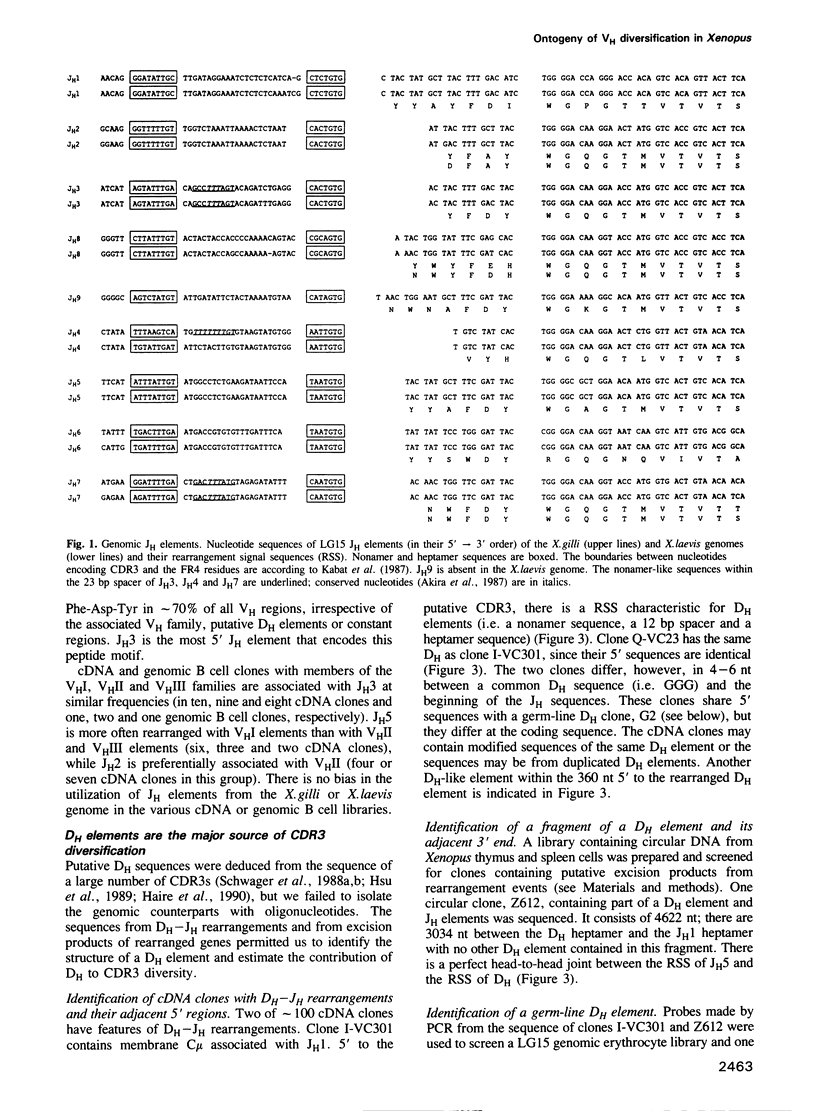
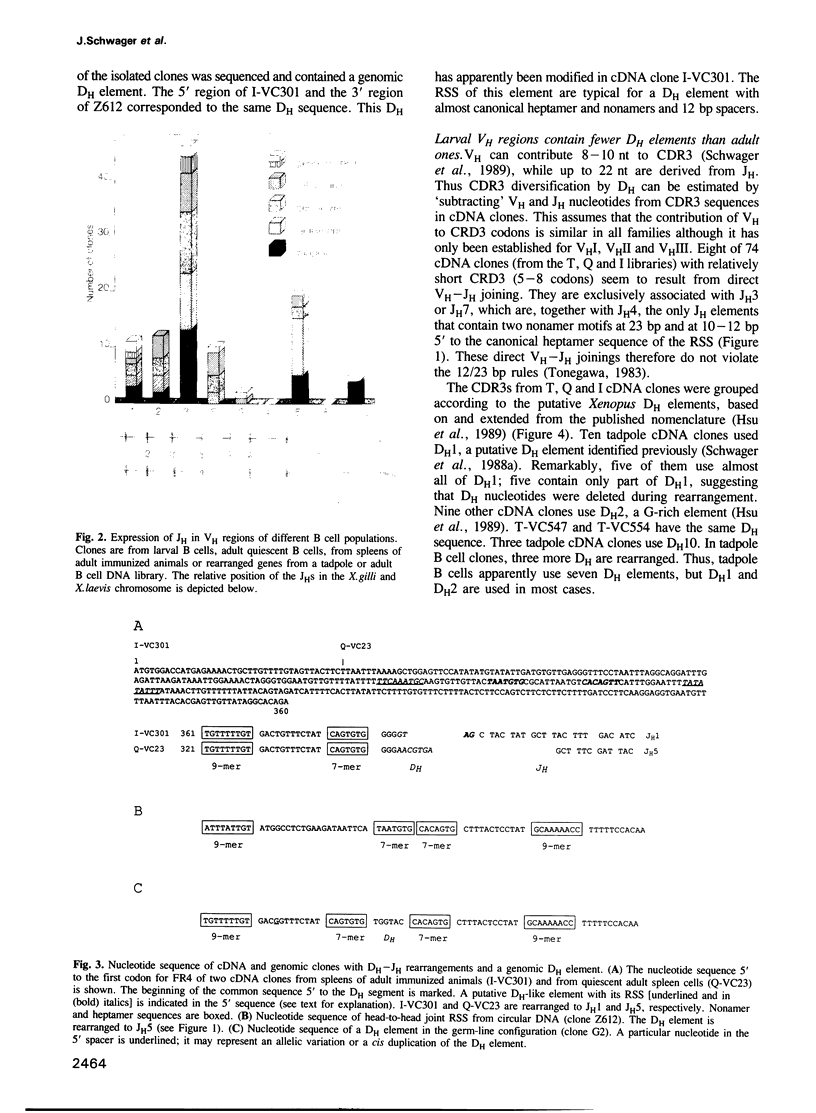
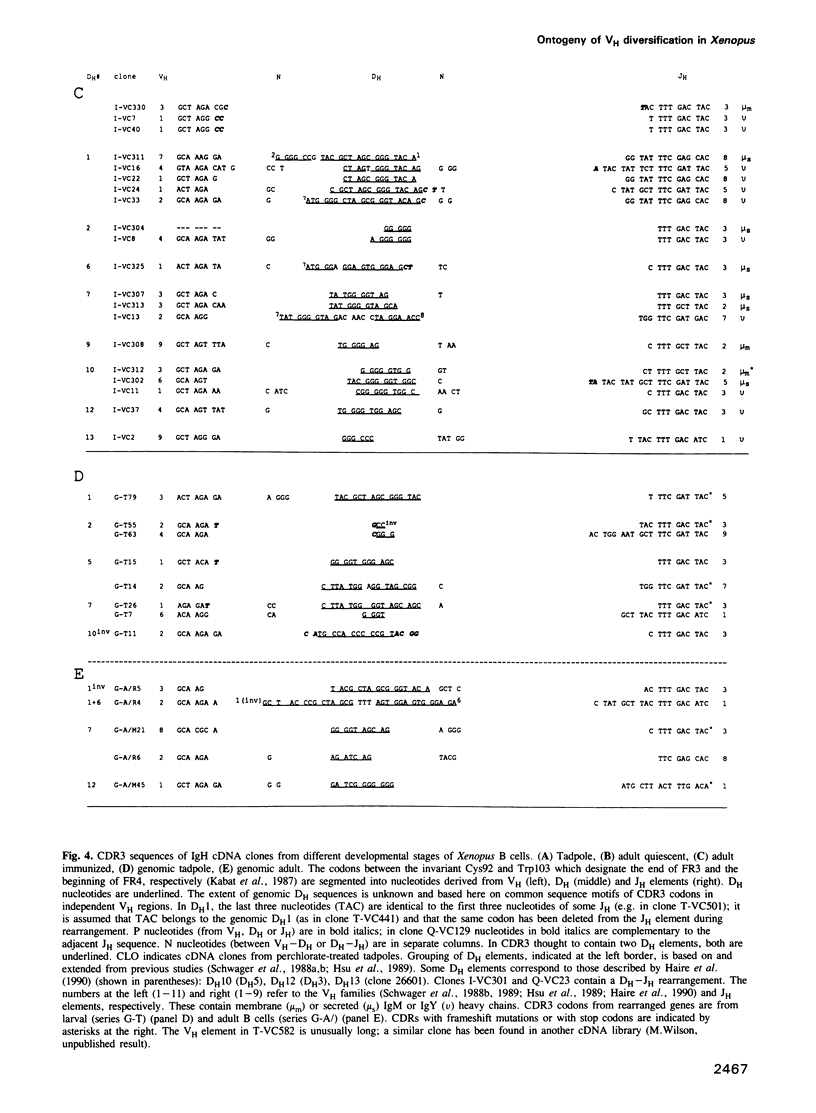
Since the larval and adult antibody responses are distinct and restricted in the clawed toad Xenopus, it offers a near ideal model for studying the ontogeny of antibody repertoires and the mechanisms involved. Immunoglobulin heavy chain (IgH) cDNA clones and B cell IgH DNA clones from various larval and adult libraries have been analysed in isogenic Xenopus. Some features are similar in adults and tadpoles, while others differ and explain the particularities observed previously at the protein level. Among the similarities we found are: (i) the mode of rearrangements (there are approximately 50% abortive events in B cells from both stages), (ii) VH family usage (10 of 11 known VH families are expressed proportionally to the number of VH elements per family), and (iii) JH usage (of the eight to nine Xenopus JH elements, two are used in approximately 70% of the VH regions in both stages of development). We found that there is relatively higher membrane exon expression in tadpoles compared with adults; and that most of the differences come from the diversification of CDR3 through DH usage and N diversification. Unlike in mammals, Xenopus DH elements are used with a remarkable flexibility with inversion, fusions and usage in different reading frames, but tadpoles show a strong bias for the usage of only a few DH elements and of a preferred reading frame. There is N diversification, which further increases CDR3 heterogeneity, in adult Xenopus but virtually none in tadpoles. These observations can account for the fact that larval antibody responses are less heterogeneous than those of adults.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akira S., Okazaki K., Sakano H. Two pairs of recombination signals are sufficient to cause immunoglobulin V-(D)-J joining. Science. 1987 Nov 20;238(4830):1134–1138. doi: 10.1126/science.3120312. [DOI] [PubMed] [Google Scholar]
- Bleicher P. A., Cohen N. Monoclonal anti-IgM can separate T cell from B cell proliferative responses in the frog, Xenopus laevis. J Immunol. 1981 Oct;127(4):1549–1555. [PubMed] [Google Scholar]
- Brandt D. C., Griessen M., Du Pasquier L., Jaton J. C. Antibody diversity in amphibians: evidence for the inheritance of idiotypic specificities in isogenic Xenopus. Eur J Immunol. 1980 Oct;10(10):731–736. doi: 10.1002/eji.1830101002. [DOI] [PubMed] [Google Scholar]
- Carlsson L., Holmberg D. Genetic basis of the neonatal antibody repertoire: germline V-gene expression and limited N-region diversity. Int Immunol. 1990;2(7):639–643. doi: 10.1093/intimm/2.7.639. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Du Pasquier L., Blomberg B., Bernard C. C. Ontogeny of immunity in amphibians: changes in antibody repertoires and appearance of adult major histocompatibility antigens in Xenopus. Eur J Immunol. 1979 Nov;9(11):900–906. doi: 10.1002/eji.1830091112. [DOI] [PubMed] [Google Scholar]
- Du Pasquier L., Hsu E. Immunoglobulin expression in diploid and polyploid interspecies hybrid of Xenopus: evidence for allelic exclusion. Eur J Immunol. 1983 Jul;13(7):585–590. doi: 10.1002/eji.1830130714. [DOI] [PubMed] [Google Scholar]
- Du Pasquier L., Wabl M. R. Antibody diversity in amphibians: inheritance of isoelectric focusing antibody patterns in isogenic frogs. Eur J Immunol. 1978 Jun;8(6):428–433. doi: 10.1002/eji.1830080611. [DOI] [PubMed] [Google Scholar]
- Feeney A. J. Lack of N regions in fetal and neonatal mouse immunoglobulin V-D-J junctional sequences. J Exp Med. 1990 Nov 1;172(5):1377–1390. doi: 10.1084/jem.172.5.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoire K. E., Goldschneider I., Barton R. W., Bollum F. J. Ontogeny of terminal deoxynucleotidyl transferase-positive cells in lymphohemopoietic tissues of rat and mouse. J Immunol. 1979 Sep;123(3):1347–1352. [PubMed] [Google Scholar]
- Gu H., Förster I., Rajewsky K. Sequence homologies, N sequence insertion and JH gene utilization in VHDJH joining: implications for the joining mechanism and the ontogenetic timing of Ly1 B cell and B-CLL progenitor generation. EMBO J. 1990 Jul;9(7):2133–2140. doi: 10.1002/j.1460-2075.1990.tb07382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haire R. N., Amemiya C. T., Suzuki D., Litman G. W. Eleven distinct VH gene families and additional patterns of sequence variation suggest a high degree of immunoglobulin gene complexity in a lower vertebrate, Xenopus laevis. J Exp Med. 1990 May 1;171(5):1721–1737. doi: 10.1084/jem.171.5.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu E., Schwager J., Alt F. W. Evolution of immunoglobulin genes: VH families in the amphibian Xenopus. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8010–8014. doi: 10.1073/pnas.86.20.8010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokubu F., Litman R., Shamblott M. J., Hinds K., Litman G. W. Diverse organization of immunoglobulin VH gene loci in a primitive vertebrate. EMBO J. 1988 Nov;7(11):3413–3422. doi: 10.1002/j.1460-2075.1988.tb03215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafaille J. J., DeCloux A., Bonneville M., Takagaki Y., Tonegawa S. Junctional sequences of T cell receptor gamma delta genes: implications for gamma delta T cell lineages and for a novel intermediate of V-(D)-J joining. Cell. 1989 Dec 1;59(5):859–870. doi: 10.1016/0092-8674(89)90609-0. [DOI] [PubMed] [Google Scholar]
- Meek K. Analysis of junctional diversity during B lymphocyte development. Science. 1990 Nov 9;250(4982):820–823. doi: 10.1126/science.2237433. [DOI] [PubMed] [Google Scholar]
- Schwager J., Bürckert N., Courtet M., Du Pasquier L. Genetic basis of the antibody repertoire in Xenopus: analysis of the Vh diversity. EMBO J. 1989 Oct;8(10):2989–3001. doi: 10.1002/j.1460-2075.1989.tb08449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwager J., Bürckert N., Schwager M., Wilson M. Evolution of immunoglobulin light chain genes: analysis of Xenopus IgL isotypes and their contribution to antibody diversity. EMBO J. 1991 Mar;10(3):505–511. doi: 10.1002/j.1460-2075.1991.tb07976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwager J., Grossberger D., Du Pasquier L. Organization and rearrangement of immunoglobulin M genes in the amphibian Xenopus. EMBO J. 1988 Aug;7(8):2409–2415. doi: 10.1002/j.1460-2075.1988.tb03086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwager J., Hadji-Azimi I. Anti-immunoglobulin M induces both B-lymphocyte proliferation and differentiation in Xenopus laevis. Differentiation. 1985;30(1):29–34. doi: 10.1111/j.1432-0436.1985.tb00509.x. [DOI] [PubMed] [Google Scholar]
- Schwager J., Mikoryak C. A., Steiner L. A. Amino acid sequence of heavy chain from Xenopus laevis IgM deduced from cDNA sequence: implications for evolution of immunoglobulin domains. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2245–2249. doi: 10.1073/pnas.85.7.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz M., Altenburger W., Zachau H. G. A rearranged DNA sequence possibly related to the translocation of immunoglobulin gene segments. Nucleic Acids Res. 1980 Apr 25;8(8):1709–1720. doi: 10.1093/nar/8.8.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Yancopoulos G. D., Desiderio S. V., Paskind M., Kearney J. F., Baltimore D., Alt F. W. Preferential utilization of the most JH-proximal VH gene segments in pre-B-cell lines. Nature. 1984 Oct 25;311(5988):727–733. doi: 10.1038/311727a0. [DOI] [PubMed] [Google Scholar]



