Abstract
Purpose
Ebola virus (EBOV) is such kind of virus which is responsible for 23,825 cases and 9675 deaths worldwide only in 2014 and with an average diseases fatality rate between 25 % and 90 %. Although, medical technology has tried to handle the problems, there is no Food and Drug Administration (FDA)-approved therapeutics or vaccines available for the prevention, post exposure, or treatment of Ebola virus disease (EVD).
Methods
In the present study, we used the immunoinformatics approach to design a potential epitope-based vaccine against the RNA-dependent RNA polymerase-L of EBOV. BioEdit v7.2.3 sequence alignment editor, Jalview v2 and CLC Sequence Viewer v7.0.2 were used for the initial sequence analysis for securing the conservancy from the sequences. Later the Immune Epitope Database and Analysis Resource (IEDB-AR) was used for the identification of T-cell and B-cellepitopes associated with type I and II major histocompatibility complex molecules analysis. Finally, the population coverage analysis was employed.
Results
The core epitope “FRYEFTAPF” was found to be the most potential one, with 100 % conservancy among all the strains of EBOV. It also interacted with both type I and II major histocompatibility complex molecules and is considered as nonallergenic in nature. Finally, with impressive cumulative population coverage of 99.87 % for the both MHC-I and MHC-II class throughout the world population was found for the proposed epitope.
Conclusion
To end, the projected peptide gave us a solid stand to propose for vaccine consideration and that might be experimented for its potency in eliciting immunity through humoral and cell mediated immune responses in vitro and in vivo.
Electronic supplementary material
The online version of this article (doi:10.1186/s40203-015-0011-4) contains supplementary material, which is available to authorized users.
Keywords: Ebola, Computational approach, RNA polymerase, Epitope, Vaccine
Background
EVD, previously designated as Ebola haemorrhagic fever, is a fatal disease in humans and other mammals (monkeys, chimpanzees and gorillas) (Choi and Croyle 2013, Leroy et al. 2004, Sullivan et al. 2000). The fatality rate of EDV is varied from 25 to 90 % with an average of about 50 % (Peters and Peters 1999) and it is caused by a virus of the family Filoviridae, genus Ebolavirus. There are five separate Ebola virus species have been identified, four of which are disease causing to humans: Ebola virus (Zaire ebolavirus); Taï Forest virus (Taï Forest ebolavirus, formerly Côte d’Ivoire ebolavirus); Sudan virus (Sudan ebolavirus); and Bundibugyo virus (Bundibugyoebolavirus) (Hoenen et al. 2012). The fifth one, Reston virus (Reston ebolavirus), is harmful to nonhuman primates, but not to humans (Elisha and Adegboro 2014, Geisbert et al. 2009). Among the recognized species of ebolavirus, the notoriously deadly Zaire ebolavirus is responsible for epidemics which have been taken place mainly in African countries including Democratic Republic of Congo, Uganda, Sudan, the Ivory Coast, and Gabon (Baize et al. 2014, Chowell et al. 2004, Feldmann et al. 2003, Frieden et al. 2014, Hewlett and Hewlett 2005, Kuhn et al. 2010, Li and Chen 2014, Rouquet et al. 2005). This virus is passed on people from wild animals and through human-to-human contact transmits in the human population. Those are infected with this virus bear fearsome symptoms, including high fever, hemoptysis, impaired kidney and liver function and severe internal bleeding (Gatherer 2014, Goeijenbier et al. 2014, Keiser et al. 2004, Peters and Peters 1999). In the fall of 2014 the Ebola virus gained widespread attention when in West Africa the largest outbreak has been reported in history.
The EBOV genome is a single-stranded, negative-sense, non-segmented RNA approximately 19 kb long. It codes for seven tandemly arranged viral genes which order is 3′ leader- NP (nucleoprotein) - VP35 (virion protein 35)-VP40- GP (glycoprotein)-VP30-VP24- L (RNA-dependent RNA polymerase)-trailer −5′. Transcription and translation of this viral genome result in the synthesis of seven structural proteins and a single non-structural, secreted glycoprotein (Feldmann et al. 1999). Three of the structural proteins are membrane-associated proteins; GP is a type I transmembrane protein, while VP24 and VP40 are placed on the inner surface of the membrane. The remaining four, NP, VP30 (transcription factor), VP35 (polymerase cofactor), and L (RNA-dependent RNA polymerase), are essential to viral genomic RNA to form the ribonucleoprotein complex. These proteins have been shown to be necessary and sufficient for EBOV transcription and replication (Crary et al. 2003, Feldmann et al. 2001, Mühlberger et al. 1998; 1999, Takada et al. 1997).
To date, information regarding the processing, structure and functions of Ebola virus (EBOV) protein L (EBOL) demonstrates that it is an RNA-dependent RNA polymerase, with the assistance of VP35. It also shows mRNA (guanine-N (7)-)-methyltransferase, mRNA guanylyltransferase and poly (A) synthetase activities which are essential for the replication and transcription of EBOV (Poch et al. 1990). The viral mRNA guanylyltransferase serves either as transcriptase or as replicase. The transcriptase synthesizes subgenomic RNAs, assures their capping and polyadenylation. The transcriptase stutters on a specific sequence, leads to a co-transcriptional editing of the glycoprotein (GP) mRNA. In replicase mode, the polymerase replicates the viral genome without recognizing the transcriptional signals. These reports suggest that EBOL is an important cellular component for the transcription and replication of the EBOV genome and, as such, plays a key role in the EBOV life cycle.
Due to the emergence of Ebola virus outbreak, there is an immediate need to determine novel therapeutic targets against this pathogen. The identification of specific epitopes derived from infectious pathogens has significantly advanced the development of epitope-based vaccines (EVs). Bettered understanding of the molecular basis of antigen recognition and HLA binding motifs has resulted in the advancement of rationally designed vaccines depend on algorithms predicting the peptide’s binding to human HLA. In comparison to the conventional vaccines, peptide or epitope based vaccines are easy to develop, chemical stable, more specific, and free of any infectious or oncogenic potential hazard (Holland and Domingo 1998, Sette et al. 2002). Though EVs have varied advantages, the wet lab based discovery of candidate epitopes is expensive and time consuming. Furthermore, for the final selection of epitopes various immunological requirements are needed to be considered. As a result computational methods, an alternative in silico approaches (Germain 1994) have recently been attracting growing interest of the researchers for predicting epitopes with reduced cost and time. The application of bioinformatics in immunology is termed as immunoinformatics. Currently, numerous immunoinformatics tools are available for identifying B and T cell epitopes and human leukocyte antigen (HLA) ligands (Petrovsky and Brusic 2002, Poland et al. 2009, Sette and Fikes 2003) with high sensitivity and specificity. The ‘immunoinformatics’ approach has already proven its potency in the case of human immunodeficiency virus (Wilson et al. 2003), multiple sclerosis (Bourdette et al. 2005), tuberculosis (Robinson and Amara 2005) and malaria (López et al. 2001) with desired results. In the present study, we have followed immunoinformatics approaches for designing potential conserved epitope candidate for the utility of vaccine development against the deadly Ebola virus, with an expectation of further wet lab validation.
Methods
Sequence retrieval and conserved region identification
The protein sequences of the RNA-dependent RNA polymerase-L (Volchkov et al. 1999) of the EBOV were retrieved from the UniProtKB (Apweiler et al. 2004) database in the FASTA format. BioEdit v7.2.3 sequence alignment editor (Apweiler et al. 2004) was used for the identification of the conserved region among the sequences through multiple-sequence alignment (MSA) with ClustalW (Hall 1999). Finally, Jalview v2 tool (Thompson et al. 1994) was used to retrieve the alignment and the CLC Sequence Viewer v7.0.2 (http://www.clcbio.com) was used for analysis of the divergence among the different strains of the EBOV.
Antigenicity determination of the conserved peptides
VaxiJen v2. 0, a Web-based server (Waterhouse et al. 2009, Doytchinova and Flower 2007) was used for the determination of the antigenicity of the conserved sequences. Herein, we used the default parameters for the prediction, with a threshold value of 0.4.
T-cell epitope prediction
For this study, two online servers were used. Firstly, the NetCTL v1.2 server (Larsen et al. 2007) was used for predicting potential cytotoxic T lymphocyte (CTL) epitopes from the conserved peptides. Here for predicting the epitopes, we used a combined algorithm including major histocompatibility complex class I (MHC-I) binding, transporter of antigenic peptide (TAP) transport efficiency, and proteasomal C terminal cleavage prediction. Depending on the score, the best candidates were picked for further investigation. The epitope prediction was confined to 12 MHC-I supertypes. MHC-I binding and proteasomal cleavage were carried out through artificial neural networks and the weight matrix was used to estimate the TAP transport efficiency. The threshold value for epitope identification was set at 0.5 for maintaining sensitivity and specificity of 0.89 and 0.94, respectively during the analysis. This would support to assess the findings more decisively by developing more epitopes. Finally, for confirming the prediction with default parameters, CTLPred (Bhasin and Raghava 2004) was employed additionally.
MHC-I and MHC-II restriction analysis
Furthermore, from the Immune Epitope Database and Analysis Resource (IEDB-AR), T Cell Epitope Prediction Tools was implied for the identification of MHC-I (Hoof et al. 2009, Nielsen et al. 2007) and MHC-II (Wang et al. 2008; 2010) binding of the peptide. In order to calculate the half-maximal inhibitory concentration (IC50) values required for peptide binding to MHC-I molecules, Stabilized Matrix Method (Peters and Sette 2005) was applied with a preset 9.0-mer epitope.
In case of MHC-II binding analysis, the IEDB-recommended method was used for the specific HLA-DQ, HLA-DP, and HLA-DR loci. Herein, specific peptides were used to predict the MHC-II interaction on the basis of MHC-I analysis and antigenic conservancy.
Prediction of population coverage
Population coverage for epitope was assessed by the IEDB population coverage calculation tool (Bui et al. 2006). Here we used the allelic frequency of the interacting HLA alleles for the prediction of the population coverage for the corresponding epitope.
B-cell epitope prediction
Linear B cell epitopes are of different lengths of peptides from 2 to 85 in comparison to that of T cell epitopes. B-cell epitope produces immune response when it interacts with B lymphocytes. It then initiates the differentiation of B lymphocytes into plasma and memory cells (Nair et al. 2002). There are a number of Web-based tools are available for the prediction of B-cell epitope which are hosted by IEDB-AR. For the B-cell epitope prediction with high accuracy, multiple tools, including the Emini surface accessibility prediction (Emini et al. 1985), Kolaskar and Tongaonkar antigenicity scale (Kolaskar and Tongaonkar 1990), Parker hydrophilicity prediction, (Parker et al. 1986) and finally the Chou and Fasman beta turn prediction tool (Chou and Fasman 1979) were employed, because the antigenic parts of a protein belong to the beta turn regions (Rini et al. 1992).
Homology modeling and protein variability determination of the conserved region
The structure of the conserved region was constructed by homology modelling using the MODELLER v9 (Šali et al. 1995). MODELLER is a program that implements an automated approach to comparative protein structure modelling by satisfying spatial restraints (Fiser et al. 2000, Sali and Blundell 1993). Finally, the evaluation of the predicted model was verified by using two software tools, PROCHECK (Arnold et al. 2006, Laskowski et al. 1996) and QMEAN (Benkert et al. 2011). For predicting the disorder among the amino acid sequences, DISOPRED v3 (Ward et al. 2004) server was used. In order to calculate the protein variability index the Protein variability server was implied where Wu-Kabat variability coefficient (Garcia-Boronat et al. 2008) has been used.
Allergenicity and epitope conservancy analysis
The web-based AllerHunter server (Muh et al. 2009) was used to predict the allergenicity of our proposed epitope for vaccine development. This server predicts allergenicity through a combinational prediction, by using both integration of the Food and Agriculture Organization (FAO)/World Health Organization (WHO) allergenicity evaluation scheme and support vector machines (SVM)-pairwise sequence similarity. AllerHunter predicts allergens as well as nonallergens with high specificity. This makes AllerHunter is a very useful program for allergen cross-reactivity prediction (Liao and Noble 2003).
Epitope conservancy of the candidate epitopes was examined using a Web-based epitope conservancy tool available in IEDB analysis resource (Bui et al. 2007). The conservancy level of each potential epitope was calculated by looking for identities in all RNA-dependent RNA polymerase-L protein sequences of different strains retrieved from database.
Results
Analysis of the retrieved sequences with divergence and antigenicity
A total of 52 RNA-dependent RNA polymerase-L protein molecules from different variants of the EBOV were retrieved from the UniProt database. The MSA of the RNA-dependent polymerase-L proteins was retrieved from BioEdit tool through ClustalW with 1000 bootstrap replicates (Additional file 1: Figure S1). CLC Sequence Viewer was used to construct phylograms from the MSA obtained from BioEdit, in order to analyze the divergence among the retrieved sequences. Phylogram of RNA-dependent RNA polymerase-L is depicted in Fig. 1. Finally, the highly conserved region from the MSA was retrieved for the further analysis. The selected conserved region is depicted in the Fig. 2, from the MSA number 586 to 660. Then the VaxiJen v2.0 server calculate the antigenicity of the conserved sequences with a score 0.4888.
Fig. 1.
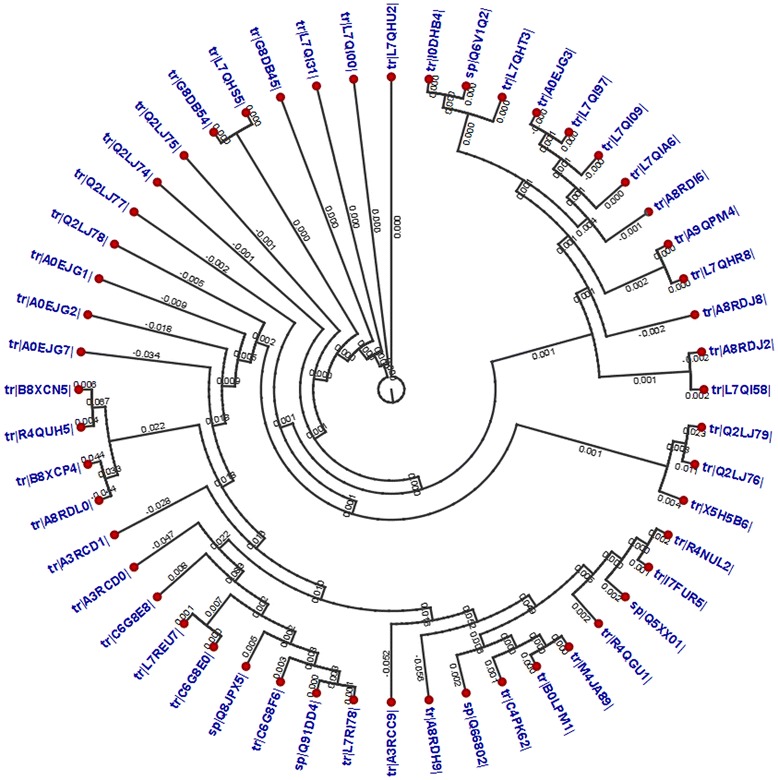
Phylogenetic tree showing the evolutionary divergence among the different RNA-dependent RNA polymerase-L proteins of the EBOV. Notes: Here, cladogram view is shown with appropriate distance among the different strains. The red dotted view indicates the node of the tree
Fig. 2.

MSA of the conserved region of RNA-dependent RNA polymerase-L. Only the conserved sequences containing the proposed epitope sequence are shown here. Notes: Clustalx color is used here. Different colors indicate different amino acid residues. The yellow bas at the bottom indicates the conservation of the amino acid residues
Identification of T-cell epitope and MHC interaction analysis
T-cell epitopes were selected firstly by using the NetCTL v1.2 server where the epitope prediction was confined to 12 MHC-I supertypes. Based on the combined score, the top five epitopes (Table 1) were listed for further analysis. T-cell epitopes were again predicted by the CTLPred server (Table 2). Here a combined approach of artificial neural networks and support vector machines was applied. Depending on the two analyses, the most common epitope—containing peptides, identified by both servers, was selected. The selected epitope was then used for the MHC-binding analysis.
Table 1.
Prediction of the T-cell epitope by NetCTL server on the basis of combined score
| Epitope | Start position | Combined score |
|---|---|---|
| FIEYCNHCY | 64 | 2.4978 |
| FRYEFTAPF | 56 | 2.0697 |
| RYEFTAPFI | 57 | 1.6395 |
| ESLLHQASW | 19 | 1.2675 |
| SFVTDLEKY | 44 | 1.1582 |
Table 2.
Prediction of the T-cell epitope by CTLPred server
| Epitope | Start position | Score(ANN/SVM) |
|---|---|---|
| KYNLAFRYE | 51 | 0.87/0.51591091 |
| RYEFTAPFI | 57 | 0.45/0.69332887 |
| FRYEFTAPF | 56 | 0.84/0.29033079 |
| KAFPSNMMV | 3 | 0.64/0.46418851 |
| LAKAFPSNM | 1 | 0.46/0.61842782 |
MHC-I-binding prediction, which was run through the Stabilized Matrix Method, predicted a wide range of MHC-I allele interactions for the proposed T-cell epitopes. The MHC-I alleles for which the epitope showed higher affinity (IC50 < 250 nM) are listed in Table 3. The output of the MHC-II interaction analysis is also shown in Table 3.
Table 3.
MHC-I and MHC-II interaction of the proposed sequence by IEDB analysis resource
| Epitope | MHC I interaction | Epitope | MHC II interaction |
|---|---|---|---|
| FRYEFTAPF | HLA-C*03:02,HLA-C*07:02, HLA-C*12:03,HLA-C*14:02, HLA-C*16:01,HLA-C*06:02, HLA-C*07:01,HLA-C*12:02, HLA-B*27:05, HLA-B*39:01 | NLAFRYEFTAPFIEY | HLA-DRB3*01:01, HLA-DQA1*04:01, HLA-DRB3*02:02, HLA-DRB1*03:01, HLA-DRB1*04:01, HLA-DRB1*04:05, HLA-DRB5*01:01, HLA-DPA1*02:01, HLA-DPA1*01:03, HLA-DQA1*03:01, HLA-DRB1*07:01, HLA-DRB1*08:02, HLA-DPA1*01, HLA-DRB1*11:01, HLA-DPA1*02:01, HLA-DQA1*05:01, HLA-DPA1*02:01, HLA-DRB1*09:01, HLA-DQA1*01:01, HLA-DPA1*03:01, HLA-DRB1*15:01, HLA-DRB1*13:02, HLA-DRB1*12:01, HLA-DRB4*01:01, HLA-DQA1*05:01, HLA-DQA1*01:02 |
Analysis of the population coverage
IEDB population coverage tool analyzed the Population coverage of the proposed epitope. The combined MHC-I and MHC-II class were assessed against the whole world population with the selected MHC-I and MHC-II interacted alleles (Fig. 3).
Fig. 3.
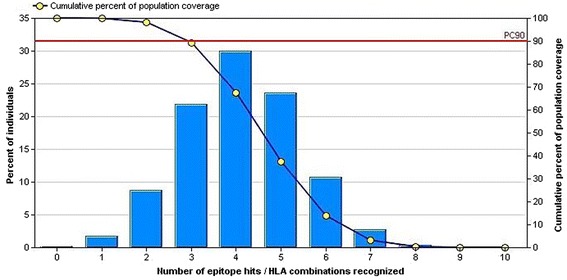
Population coverage based on MHC–I and MHC-II restriction data. The whole world populations are assessed for the proposed epitope. Notes: In the graphs, the line (−o-) represents the cumulative percentage of population coverage of the epitopes; the bars represent the population coverage for each epitope
Prediction B-cell epitope
Here, for predicting potential B-cell epitopes, we used amino acid–based methods. According to this procedure different analysis methods were applied for the identification of a continuous B cell epitope.
The Kolaskar and Tongaonkar antigenicity scale was used for assessing the antigenic property of the peptides. The average antigenic propensity of the protein was 1.014, with a maximum of 1.033 and a minimum of 1.002. For the protein the antigenic determination threshold value was 1.0, where all values equal or greater than 1.0 were potential antigenic determinants. The antigenic plot is depicted in the Fig. 4.
Fig. 4.
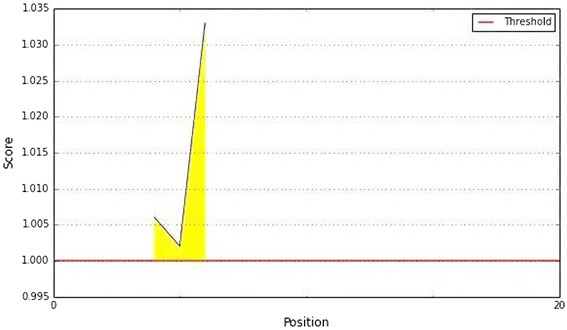
Kolaskar and Tongaonkar antigenicity prediction of the proposed epitope. Notes: The X- and Y-axes represent the sequence position and antigenic propensity score, respectively. The threshold value is 1.0. The regions above the threshold are antigenic, shown in yellow
To be a potent B cell epitope, it must be surface accessible. Hence, Emini surface accessibility prediction was employed, with a maximum propensity score of 1.297 at threshold 1.0 (Fig. 5). To strengthen our support for the prediction of the epitope to elicit B cell response the Parker hydrophilicity and the Chou and Fasman beta turn prediction were employed. Those are described in the Figs. 6 and 7.
Fig. 5.
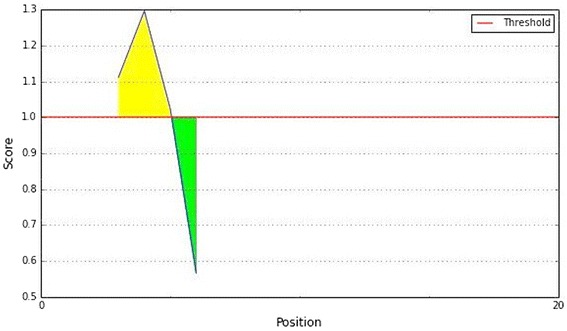
Emini surface accessibility prediction of the proposed epitope, with a minimum propensity score of 0.566 and maximum score of 1.297. Notes: The X- and Y-axes represent the sequence position and surface probability, respectively. The threshold value is 1.0. The regions above the threshold are antigenic, shown in yellow
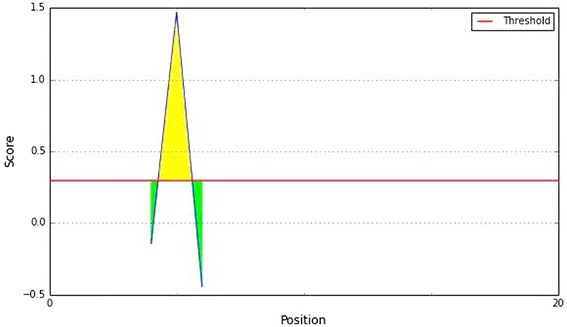
Fig. 6.
Parker hydrophilicity prediction of the epitope, with a minimum propensity score of −0.443 and maximum score of 1.471. Notes: The X- and Y-axes represent the sequence position and antigenic propensity score, respectively. The threshold value is 1.0. The regions above the threshold are antigenic, shown in yellow
Fig. 7.
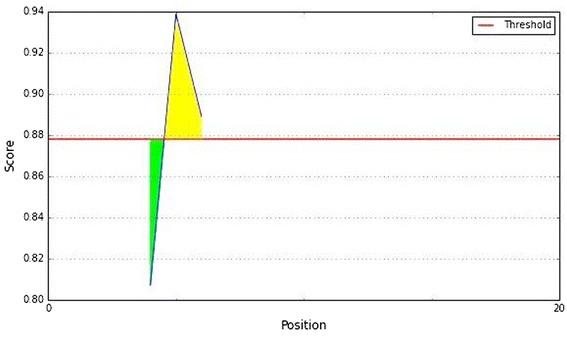
Chou and Fasman beta turn prediction of the epitope,with a minimum propensity score of 0.878 and maximum score of 0.939. Notes: The X- and Y-axes represent the sequence position and antigenic propensity score, respectively. The threshold value is 1.0. The regions above the threshold are antigenic, shown in yellow
Structure analysis and protein variability determination
Homology model of the conserved region was obtained by the MODELLER software, which is shown in Fig. 8a and b. PROCHECK server validated the stereochemical quality of the model through Ramachandran Plot (Fig. 8c), andQMEAN server also assessed the tertiary structure, with a Qmean6 score of 0.327. DISOPRED v3 server predicted the disorder of the conserved peptide in order to get insight about the disorder among the conserved sequences, which is depicted in Fig. 9. Protein variability server predicted the variability of the conserved region of the RNA-dependent RNA polymerase-L (Fig. 10) to ensure that the proposed epitope is within the invariable region.
Fig. 8.
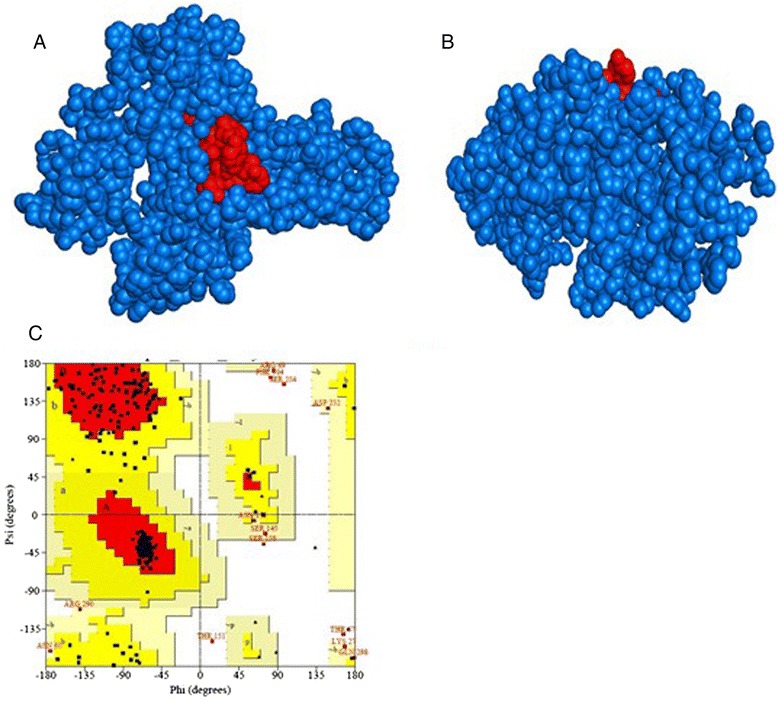
Three-dimensional model of the RNA-dependent RNA polymerase-L with the proposed epitope and validation. Notes: Two different view of the modeled protein (Blue spherical) with the predicted epitope (Red spherical). a Top view. b Side view. The outerside location of the epitope indicates its surface accessibility. c Ramachandran plot of the predicted model shows that most of the residues are in the allowed region of the plot, proving the validity of the model
Fig. 9.
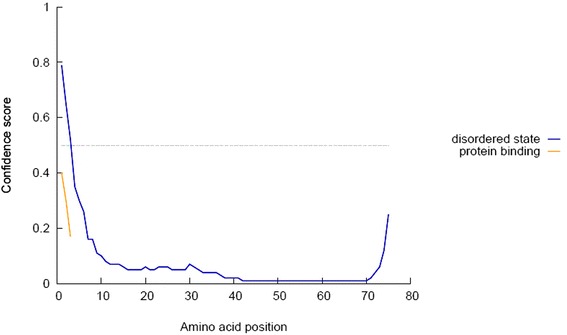
Disorder prediction of the conserved antigenic amino acid sequences. Here, our proposed epitope lies outside (56–64) of the disordered region to secure its potentiality as an effective epitope. Notes: Amino acids in the input sequence are considered disordered when the blue line is above the gray dashed line, that is, when the confidence score is 0.5. The orange line shows the confidence score of the disordered protein-binding residue predictions
Fig. 10.
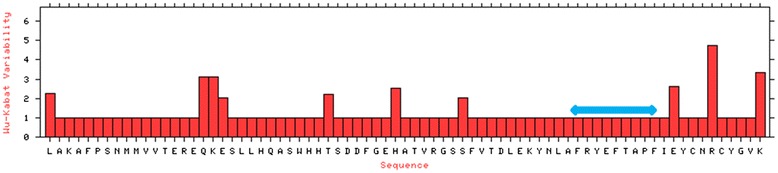
Protein variability index of the conserved peptides of all the sequences. The prediction suggests that our proposed epitope “FRYEFTAPF” falls in the invariable region (Blue line). Notes: The conservancy threshold was 1.0 in this analysis. The X-axis indicates the amino acid positions in the sequences and the Y-axis indicates the Shannon variability score
Epitope conservancy and allergenicity analysis
Conservation analyses of the proposed epitopes were analyzed by the IEDB conservancy analysis tool that is shown in Table 4. AllerHunter server predicted the allergenicity of the queried epitope with a score was 0.03 (sensitivity =94.4. %, specificity =70.3 %).
Table 4.
Epitope conservancy analysis
| Peptide sequence | Peptide length | Percentage of protein sequence match | Maximum identity |
|---|---|---|---|
| FRYEFTAPF | 9 | 100 % (52/52) | 100 % |
| NLAFRYEFTAPFIEY | 15 | 100 % (52/52) | 100 % |
Discussion
Our world is the habitation of more than seven billion people now. With the upgrade of medical science, new viruses along with their causing diseases are also emerging. Ebola virus is such kinds of virus with a deadly outrage of their endemic nature especially in Africa in recent time (Evans and Popova 2015). Till now there is no potential treatment for this virus to combat its deadly effects.
Recent time, the immunoinformatics approach give us some sort of hope for the design of an effective therapeutics, like vaccine, in association with the advancement of sequence based technology. Similar approaches have been used successfully for identifying vaccine candidates in several pathogens viz. human corona virus (Oany et al. 2008), Saint Louis encephalitis virus (Hasan et al. 2013), Crimean–Congo hemorrhagic fever virus (Oany et al. 2015), Chikungunya virus (Hasan et al. 2015) and some others. The in vitro validation of this type of work has also been proven in recent time (Khan et al. 2014).
Though epitope-based vaccine designing has become a familiar approach, in the case of EBOV no significant work yet has been done. EBOV is an RNA virus which has genetic blueprints made of RNA instead of DNA. Creating vaccines is particularly difficult for RNA viruses as they can quickly mutate their different exposed proteins (Twiddy et al. 2003). Therefore the most potential way to create stable antiviral therapies against RNA viruses including EBOV is to target the transcription or replication machinery. Scientists revealed that RNA-dependent RNA polymerase-L (EBOL) is an important cellular component for the transcription and replication of the EBOV genome. When an EBOV infects a cell, its RNA genetic blueprint enters the cell along with RNA-dependent RNA polymerase-L. This polymerase normally “read” the RNA genetic blueprint in order to synthesize mRNA, which then leads to the formation of viral proteins as well as viral replication and more viral particles are produced. For these two vital involvements at the gateway, this protein was targeted to design most potential epitopes using in silico computational approaches.
In the current study, firstly all the available sequences of RNA-dependent RNA polymerase-Lwere retrieved from database. Then antigenicity of the conserved peptides, generated by multiple sequence alignment was predicted by VaxiJen, which suggested their ability to elicit potential immune response. Sequence based bioinformatics approaches were applied to predict both B cell and T cell epitopes for conferring immunity in different ways. Though at present, most of the vaccines are based on B cell immunity; vaccines based on T cell epitope have been encouraged recently. It is because, with time humoral response from memory B cells can be overcome easily by antigenic drift, while cell mediated immunity often provides long lasting immunity (Bacchetta et al. 2005, Igietseme et al. 2004). Cytotoxic CD8+T lymphocytes (CTL) inhibit the spread of infectious agents by recognizing and killing infected cells or secreting specific antiviral cytokines (Garcia et al. 1999, Shrestha and Diamond 2004). Thus, vaccination based on T cell epitope is a unique approach to obtain strong immune response against infectious agents, such as, viruses (Klein et al. 2005).
Both NetCTL and CTLPred server were used to find epitopes for the activation of T-cell immunity with potential antigenicity. By examining the output it was predicted that FRYEFTAPF would be the best epitope candidate and was further subjected for binding proficiency analysis.
Length is an important factor to consider for peptide antigen binding with MHC or TCR or both. T cell epitopes presented by MHC class I molecules are generally peptides between 8 and 11 amino acids in length. We therefore set peptide lengths at 9 before making software based MHC class I T cell epitope identification using immune epitope database (IEDB). Analysis revealed that the core epitope “FRYEFTAPF” would interact with ten different MHC class I alleles. On the other hand, the complete peptide “NLAFRYEFTAPFIEY” interacts with the highest numbers of MHC class II alleles (as many as 26 alleles).
Along with the T-cell epitope, in our study, attention was also given to the B-cell epitope, which can induce both primary and secondary humoral immunity (Trainor et al. 2007). Multiple prediction methods were applied to determine the B-cell epitope considering several criteria of antigenicity, hydrophilicity, surface accessibility, and beta-turn. Our proposed epitope has met all the criteria of the above B-cell prediction methods.
The three-dimensional model of the conserved protein ensured the exact location of the epitope outside of the protein (Fig. 8a and b) surface and the model validity was assessed by Ramachandran Plot (Fig. 8c), whereby 87.8 % amino acid residues were found within the favored region. The epitope was also treated as suitable candidate for vaccine through tenabled its position in the conserved sequence, by the Discopred and protein variability server (Figs. 9 and 10).
Conservancy is the most important criterion of an epitope to consider it for vaccine development. Conservancy analysis of our proposed epitope showed 100 % conservancy among all the available sequences. Another important feature of the peptide vaccine is its allergenicity (McKeever et al. 2004). In silico analysis revealed that the proposed epitope is nonallergenic in nature.
Wide range population coverage must be needed for a potential vaccine aspirant. At this point, our proposed epitope covers a remarkable population of 99.87 % for both types of MHC allele throughout the world population. That makes the epitope as a supreme candidate for vaccine consideration.
Finally, from the above in silico analysis, we are really optimistic that our proposed epitope would trigger an immune response in vitro and in vivo.
Conclusion
A number of approaches exist for new vaccine development, such as recombinant vaccines, sub-unit protein and DNA vaccines, auxotrophic organisms to deliver genes and so on. Current study is an attempt to identify potential epitope targets against EBOV using different computational tools. It is quite obvious that in order to minimize the deadly effects of EBOV, highly potential drugs are immediately required and these in silico approaches will reduce the wet lab efforts with higher probability of success. Therefore, it is concluded that the identified epitope may be exploited further for developing epitope-based vaccine against EBOV. Nevertheless, the initial hints we obtained will help to prioritize potential therapeutics for EBOV.
Additional file
MSA of the RNA-dependent polymerase-L proteins of the different EBOV. (PNG 1540 kb)
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
ARO has made substantial contributions to conception and design, acquisition of data, analysis and interpretation of data. TS and ASC carried out the molecular genetic studies, participated in the sequence alignment and drafted the manuscript. TPJ worked for computational analysis. MAH conceived of the study, and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
References
- Apweiler R, Bairoch A, Wu CH, et al. UniProt: the universal protein knowledgebase. Nucleic Acids Res. 2004;32(1):115–119. doi: 10.1093/nar/gkh131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold K, Bordoli L, Kopp J, et al. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics. 2006;22(2):195–201. doi: 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]
- Bacchetta R, Gregori S, Roncarolo M-G. CD4+ regulatory T cells: mechanisms of induction and effector function. Autoimmun Rev. 2005;4(8):491–496. doi: 10.1016/j.autrev.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Baize S, Pannetier D, Oestereich L, et al. Emergence of Zaire Ebola virus disease in Guinea. N Engl J Med. 2014;371(15):1418–1425. doi: 10.1056/NEJMoa1404505. [DOI] [PubMed] [Google Scholar]
- Benkert P, Biasini M, Schwede T. Toward the estimation of the absolute quality of individual protein structure models. Bioinformatics. 2011;27(3):343–350. doi: 10.1093/bioinformatics/btq662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhasin M, Raghava G. Prediction of CTL epitopes using QM, SVM and ANN techniques. Vaccine. 2004;22(23):3195–3204. doi: 10.1016/j.vaccine.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Bourdette DN, Edmonds E, Smith C, et al. A highly immunogenic trivalent T cell receptor peptide vaccine for multiple sclerosis. Mult Scler. 2005;11(5):552–561. doi: 10.1191/1352458505ms1225oa. [DOI] [PubMed] [Google Scholar]
- Bui HH, Sidney J, Dinh K, et al. Predicting population coverage of T-cell epitope-based diagnostics and vaccines. BMC Bioinformatics. 2006;17(7):153. doi: 10.1186/1471-2105-7-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui HH, Sidney J, Li W, et al. Development of an epitope conservancy analysis tool to facilitate the design of epitope-based diagnostics and vaccines. BMC Bioinformatics. 2007;8(1):361. doi: 10.1186/1471-2105-8-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JH, Croyle MA. Emerging targets and novel approaches to Ebola virus prophylaxis and treatment. BioDrugs. 2013;27(6):565–583. doi: 10.1007/s40259-013-0046-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou PY, Fasman GD. Empirical predictions of protein conformation. Annu Rev Biochem. 1979;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Chowell G, Hengartner NW, Castillo-Chavez C, et al. The basic reproductive number of Ebola and the effects of public health measures: the cases of Congo and Uganda. J Theor Biol. 2004;229(1):119–126. doi: 10.1016/j.jtbi.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Crary S, Towner J, Honig J, et al. Analysis of the role of predicted RNA secondary structures in Ebola virus replication. Virology. 2003;306:210–218. doi: 10.1016/S0042-6822(02)00014-4. [DOI] [PubMed] [Google Scholar]
- Doytchinova IA, Flower DR. VaxiJen: a server for prediction of protective antigens, tumour antigens and subunit vaccines. BMC Bioinformatics. 2007;8(1):4. doi: 10.1186/1471-2105-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elisha A, Adegboro B. Ebola virus diseases. Afr J Clin Exp Microbiol. 2014;15(3):117–121. doi: 10.4314/ajcem.v15i3.1. [DOI] [Google Scholar]
- Emini EA, Hughes JV, Perlow DS, et al. Induction of hepatitis A virus-neutralizing antibody by a virus-specific synthetic peptide. J Virol. 1985;55(3):836–839. doi: 10.1128/jvi.55.3.836-839.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DK, Popova A. West African Ebola crisis and orphans. Lancet. 2015;385(9972):945–946. doi: 10.1016/S0140-6736(15)60179-9. [DOI] [PubMed] [Google Scholar]
- Feldmann H, Volchkov VE, Volchkova VA, et al. The glycoproteins of Marburg and Ebola virus and their potential roles in pathogenesis. Arch Virol. 1999;15:159–169. doi: 10.1007/978-3-7091-6425-9_11. [DOI] [PubMed] [Google Scholar]
- Feldmann H, Volchkov V, Ströher U, et al. Biosynthesis and role of filovirus glycoprotein. J Gen Virol. 2001;82:2839–2848. doi: 10.1099/0022-1317-82-12-2839. [DOI] [PubMed] [Google Scholar]
- Feldmann H, Jones S, Klenk HD, et al. Ebola virus: from discovery to vaccine. Nat Rev Immunol. 2003;3(8):677–685. doi: 10.1038/nri1154. [DOI] [PubMed] [Google Scholar]
- Fiser A, Do RK, Sali A. Modeling of loops in protein structures. Protein Sci. 2000;9:1753–1773. doi: 10.1110/ps.9.9.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frieden TR, Damon I, Bell BP, et al. Ebola 2014—new challenges, new global response and responsibility. N Engl J Med. 2014;371(13):1177–1180. doi: 10.1056/NEJMp1409903. [DOI] [PubMed] [Google Scholar]
- Garcia KC, Teyton L, Wilson IA. Structural basis of T cell recognition. Annu Rev Immunol. 1999;17:369–397. doi: 10.1146/annurev.immunol.17.1.369. [DOI] [PubMed] [Google Scholar]
- Garcia-Boronat M, Diez-Rivero CM, Reinherz EL, et al. PVS: a web server for protein sequence variability analysis tuned to facilitate conserved epitope discovery. Nucleic Acids Res. 2008;36(2):35–41. doi: 10.1093/nar/gkn211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatherer D. The 2014 Ebola virus disease outbreak in West Africa. J Gen Virol. 2014;95(8):1619–1624. doi: 10.1099/vir.0.067199-0. [DOI] [PubMed] [Google Scholar]
- Geisbert TW, Geisbert JB, Leung A, et al. Single-injection vaccine protects nonhuman primates against infection with marburg virus and three species of ebola virus. J Virol. 2009;83(14):7296–7304. doi: 10.1128/JVI.00561-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain RN. MHC-dependent antigen processing and peptide presentation: providing ligands for T lymphocyte activation. Cell. 1994;76(2):287–299. doi: 10.1016/0092-8674(94)90336-0. [DOI] [PubMed] [Google Scholar]
- Goeijenbier M, van Kampen JJ, Reusken CB. Ebola virus disease: a review on epidemiology, symptoms, treatment and pathogenesis. Neth J Med. 2014;72(9):442–448. [PubMed] [Google Scholar]
- Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- Hasan MA, Hossain M, Alam MJ. A computational assay to design an epitope-based Peptide vaccine against Saint Louis encephalitis virus. Bioinform Biol Insights. 2013;7:347–355. doi: 10.4137/BBI.S13402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan MA, Khan MA, Datta A, et al. A comprehensive immunoinformatics and target site study revealedthe corner-stone toward Chikungunya virus treatment. Mol Immunol. 2015;65:189–204. doi: 10.1016/j.molimm.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewlett BL, Hewlett BS. Providing care and facing death: nursing during Ebola outbreaks in central Africa. J Transcult Nurs. 2005;16(4):289–297. doi: 10.1177/1043659605278935. [DOI] [PubMed] [Google Scholar]
- Hoenen T, Groseth A, Feldmann H. Current Ebola vaccines. Expert Opin Biol Ther. 2012;12(7):859–872. doi: 10.1517/14712598.2012.685152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland J, Domingo E. Origin and evolution of viruses. Virus Genes. 1998;16(1):13–21. doi: 10.1023/A:1007989407305. [DOI] [PubMed] [Google Scholar]
- Hoof I, Peters B, Sidney J, et al. NetMHCpan, a method for MHC class I binding prediction beyond humans. Immunogenetics. 2009;61:1–13. doi: 10.1007/s00251-008-0341-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igietseme JU, Eko FO, He Q, Black CM. Antibody regulation of T-cell immunity: implications for vaccine strategies against intracellular pathogens. Expert Rev Vaccines. 2004;3(1):23–34. doi: 10.1586/14760584.3.1.23. [DOI] [PubMed] [Google Scholar]
- Keiser J, Utzinger J, Tanner M, Singer BH. Representation of authors and editors from countries with different human development indexes in the leading literature on tropical medicine: survey of current evidence. BMJ. 2004;328(7450):1229–1232. doi: 10.1136/bmj.38069.518137.F6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MK, Zaman S, Chakraborty S, et al. In silico predicted mycobacterial epitope elicits in vitro T-cell responses. Mol Immunol. 2014;61(1):16–22. doi: 10.1016/j.molimm.2014.04.009. [DOI] [PubMed] [Google Scholar]
- Klein RS, Lin E, Zhang B, et al. Neuronal CXCL10 directs CD8+ T-cell recruitment and control of West Nile virus encephalitis. J Virol. 2005;79(17):11457–11466. doi: 10.1128/JVI.79.17.11457-11466.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolaskar AS, Tongaonkar PC. A semi-empirical method for prediction of antigenic determinants on protein antigens. FEBS Lett. 1990;276(1–2):172–174. doi: 10.1016/0014-5793(90)80535-Q. [DOI] [PubMed] [Google Scholar]
- Kuhn JH, Becker S, Ebihara H, et al. Proposal for a revised taxonomy of the family Filoviridae: classification, names of taxa and viruses, and virus abbreviations. Arch Virol. 2010;155(12):2083–2103. doi: 10.1007/s00705-010-0814-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen MV, Lundegaard C, Lamberth K, et al. Large-scale validation of methods for cytotoxic T-lymphocyte epitope prediction. BMC Bioinformatics. 2007;8(1):424. doi: 10.1186/1471-2105-8-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowski RA, Rullmann JAC, MacArthur MW, et al. AQUA and PROCHECK-NMR: programs for checking the quality of protein structures solved by NMR. J Biomol NMR. 1996;8(4):477–486. doi: 10.1007/BF00228148. [DOI] [PubMed] [Google Scholar]
- Leroy EM, Rouquet P, Formenty P, et al. Multiple Ebola virus transmission events and rapid decline of central African wildlife. Science. 2004;303(5656):387–390. doi: 10.1126/science.1092528. [DOI] [PubMed] [Google Scholar]
- Li YH, Chen SP. Evolutionary history of Ebola virus. Epidemiol Infect. 2014;142(06):1138–1145. doi: 10.1017/S0950268813002215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao L, Noble WS. Combining pairwise sequence similarity and support vector machines for detecting remote protein evolutionary and structural relationships. J Comput Biol. 2003;10(6):857–868. doi: 10.1089/106652703322756113. [DOI] [PubMed] [Google Scholar]
- López JA, Weilenman C, Audran R, et al. A synthetic malaria vaccine elicits a potent CD8+ and CD4+ T lymphocyte immune response in humans. Implications for vaccination strategies. Eur J Immunol. 2001;31(7):1989–1998. doi: 10.1002/1521-4141(200107)31:7<1989::AID-IMMU1989>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- McKeever TM, Lewis SA, Smith C, et al. Vaccination and allergic disease: a birth cohort study. Am J Public Health. 2004;94(6):985. doi: 10.2105/AJPH.94.6.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muh HC, Tong JC, Tammi MT. AllerHunter: a SVM-pairwise system for assessment of allergenicity and allergic cross-reactivity in proteins. PLoS One. 2009;4(6):5861. doi: 10.1371/journal.pone.0005861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlberger E, Lotfering B, Klenk H-D, et al. Three of the four nucleocapsid proteins of Marburg virus, NP, VP35, and L, are sufficient to mediate replication and transcription of Marburg virus-specific monocistronic minigenomes. J Virol. 1998;72:8756–8764. doi: 10.1128/jvi.72.11.8756-8764.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlberger E, Weik M, Volchkov V, et al. Comparison of the transcription and replication strategies of Marburg virus and Ebola virus by using artificial replication systems. J Virol. 1999;73:2333–2342. doi: 10.1128/jvi.73.3.2333-2342.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair DT, Singh K, Siddiqui Z, et al. Epitope recognition by diverse antibodies suggests conformational convergence in an antibody response. J Immunol. 2002;168(5):2371–2382. doi: 10.4049/jimmunol.168.5.2371. [DOI] [PubMed] [Google Scholar]
- Nielsen M, Lundegaard C, Blicher T, et al. NetMHCpan, a method for quantitative predictions of peptide binding to any HLA-A and -B locus protein of known sequence. PLoS One. 2007;2:796. doi: 10.1371/journal.pone.0000796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oany AR, Emran AA, Jyoti TP. Design of an epitope-based peptide vaccine against spike protein of human corona virus: an in silico approach. Drug Des Devel Ther. 2008;8:1139–1149. doi: 10.2147/DDDT.S67861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oany AR, Ahmad SAI, Hossain MU, Jyoti TP. Identification of highly conserved regions in L-segment of Crimean–Congo hemorrhagic fever virus and immunoinformatic prediction about potential novel vaccine. Adv Appl Bioinformatics Chem. 2015;8:1–10. doi: 10.2147/AABC.S75250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JM, Guo D, Hodges RS. New hydrophilicity scale derived from high-performance liquid chromatography peptide retention data: correlation of predicted surface residues with antigenicity and X-ray-derived accessible sites. Biochemistry. 1986;25:5425–5432. doi: 10.1021/bi00367a013. [DOI] [PubMed] [Google Scholar]
- Peters CJ, Peters JW. An introduction to Ebola: the virus and the disease. J Infect Dis. 1999;179(1):ix–xvi. doi: 10.1086/514322. [DOI] [PubMed] [Google Scholar]
- Peters B, Sette A. Generating quantitative models describing the sequence specificity of biological processes with the stabilized matrix method. BMC Bioinformatics. 2005;6(1):132. doi: 10.1186/1471-2105-6-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovsky N, Brusic V. Computational immunology: the coming of age. Immunol Cell Biol. 2002;80(3):248–254. doi: 10.1046/j.1440-1711.2002.01093.x. [DOI] [PubMed] [Google Scholar]
- Poch O, Blumberg BM, Bougueleret L, et al. Sequence comparison of five polymerases (L proteins) of unsegmented negative-strand RNA viruses: theoretical assignment of functional domains. J Gen Virol. 1990;71:1153–1162. doi: 10.1099/0022-1317-71-5-1153. [DOI] [PubMed] [Google Scholar]
- Poland GA, Ovsyannikova IG, Jacobson RM. Application of pharmacogenomics to vaccines. Pharmacogenomics. 2009;10(5):837–852. doi: 10.2217/pgs.09.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rini JM, Schulze-Gahmen U, Wilson IA. Structural evidence for induced fit as a mechanism for antibody-antigen recognition. Science. 1992;255(5047):959–965. doi: 10.1126/science.1546293. [DOI] [PubMed] [Google Scholar]
- Robinson HL, Amara RR. T cell vaccines for microbial infections. Nat Med. 2005;11:25–32. doi: 10.1038/nm1212. [DOI] [PubMed] [Google Scholar]
- Rouquet P, Froment JM, Bermejo M. Wild animal mortality monitoring and human Ebola outbreaks, Gabon and Republic of Congo, 2001–2003. Emerg Infect Dis. 2005;11(2):283–290. doi: 10.3201/eid1102.040533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sali A, Blundell TL. Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol. 1993;234:779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- Šali A, Potterton L, Yuan F, et al. Evaluation of comparative protein modeling by MODELLER. Proteins. 1995;23(3):318–326. doi: 10.1002/prot.340230306. [DOI] [PubMed] [Google Scholar]
- Sette A, Fikes J. Epitope-based vaccines: an update on epitope identification, vaccine design and delivery. Curr Opin Immunol. 2003;15(4):461–470. doi: 10.1016/S0952-7915(03)00083-9. [DOI] [PubMed] [Google Scholar]
- Sette A, Newman M, Livingston B, et al. Optimizing vaccine design for cellular processing, MHC binding and TCR recognition. Tissue Antigens. 2002;59(6):443–451. doi: 10.1034/j.1399-0039.2002.590601.x. [DOI] [PubMed] [Google Scholar]
- Shrestha B, Diamond MS. Role of CD8+ T cells in control of West Nile virus infection. J Virol. 2004;78(15):8312–8321. doi: 10.1128/JVI.78.15.8312-8321.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan NJ, Sanchez A, Rollin PE, Yang ZY, Nabel GJ. Development of a preventive vaccine for Ebola virus infection in primates. Nature. 2000;408:605–609. doi: 10.1038/35046108. [DOI] [PubMed] [Google Scholar]
- Takada A, Robison C, Goto H, et al. A system for functional analysis of Ebola virus glycoprotein. Proc Natl Acad Sci. 1997;94(26):14764–14769. doi: 10.1073/pnas.94.26.14764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22(22):4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor NB, Crill WD, Roberson JA, et al. Mutation analysis of the fusion domain region of St. Louis encephalitis virus envelope protein. Virology. 2007;360(2):398–406. doi: 10.1016/j.virol.2006.10.033. [DOI] [PubMed] [Google Scholar]
- Twiddy SS, Holmes EC, Rambaut A. Inferring the rate and time-scale of dengue virus evolution. Mol Biol Evol. 2003;20(1):122–129. doi: 10.1093/molbev/msg010. [DOI] [PubMed] [Google Scholar]
- Volchkov VE, et al. Characterization of the L gene and 5′trailer region of Ebola virus. J Gen Virol. 1999;80(2):355–362. doi: 10.1099/0022-1317-80-2-355. [DOI] [PubMed] [Google Scholar]
- Wang P, Sidney J, Dow C, et al. A systematic assessment of MHC class II peptide binding predictions and evaluation of a consensus approach. PLoS Comput Biol. 2008;4(4):e1000048. doi: 10.1371/journal.pcbi.1000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Sidney J, Kim Y, et al. Peptide binding predictions for HLA DR, DP and DQ molecules. BMC Bioinformatics. 2010;11(1):568. doi: 10.1186/1471-2105-11-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JJ, McGuffin LJ, Bryson K, et al. The DISOPRED server for the prediction of protein disorder. Bioinformatics. 2004;20(13):2138–2139. doi: 10.1093/bioinformatics/bth195. [DOI] [PubMed] [Google Scholar]
- Waterhouse AM, Procter JB, Martin DM, et al. Jalview version 2 – a multiple sequence alignment editor and analysis workbench. Bioinformatics. 2009;25(9):1189–1191. doi: 10.1093/bioinformatics/btp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CC, McKinney D, Anders M, et al. Development of a DNA vaccine designed to induce cytotoxic T lymphocyte responses to multiple conserved epitopes in HIV-1. J Immunol. 2003;171(10):5611–5623. doi: 10.4049/jimmunol.171.10.5611. [DOI] [PubMed] [Google Scholar]


