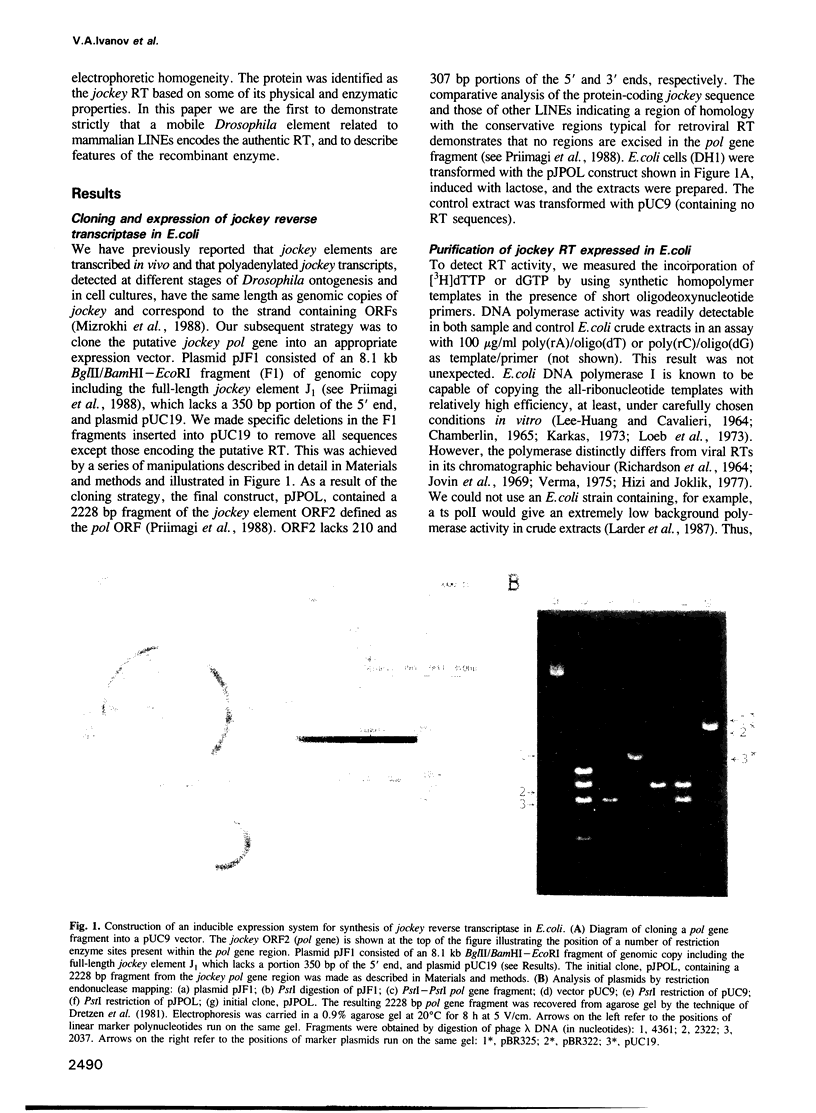
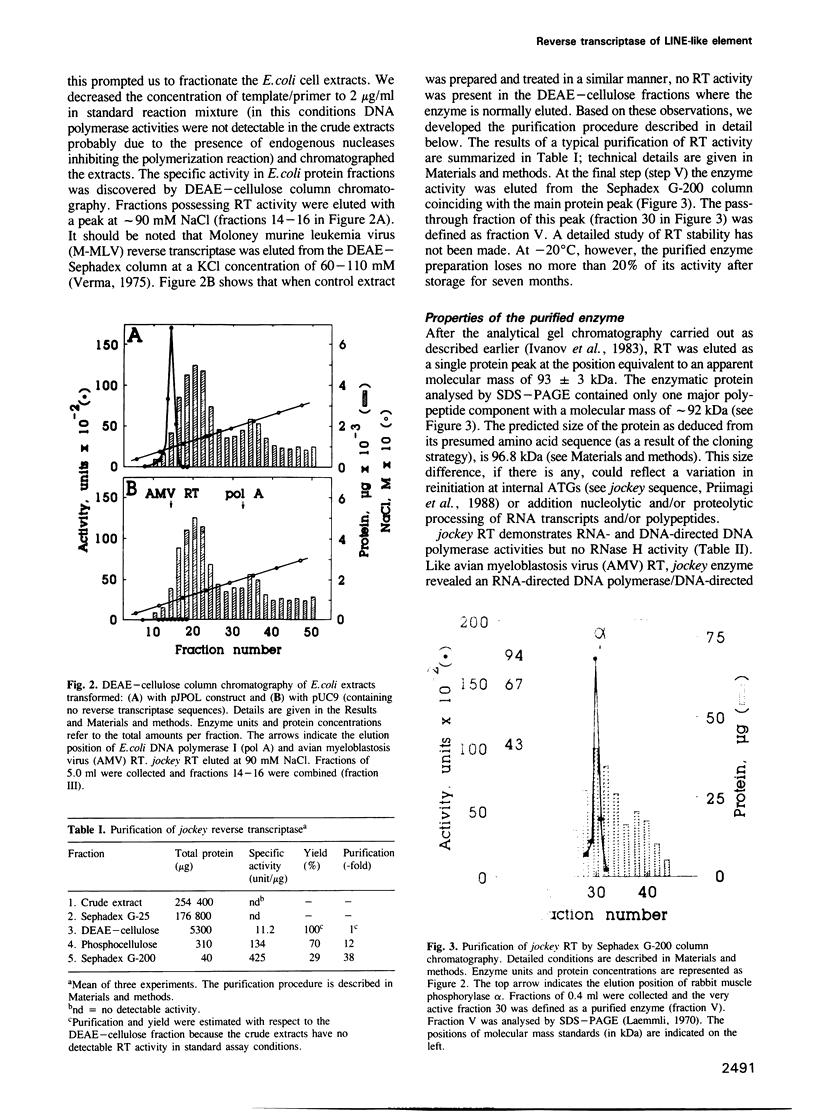
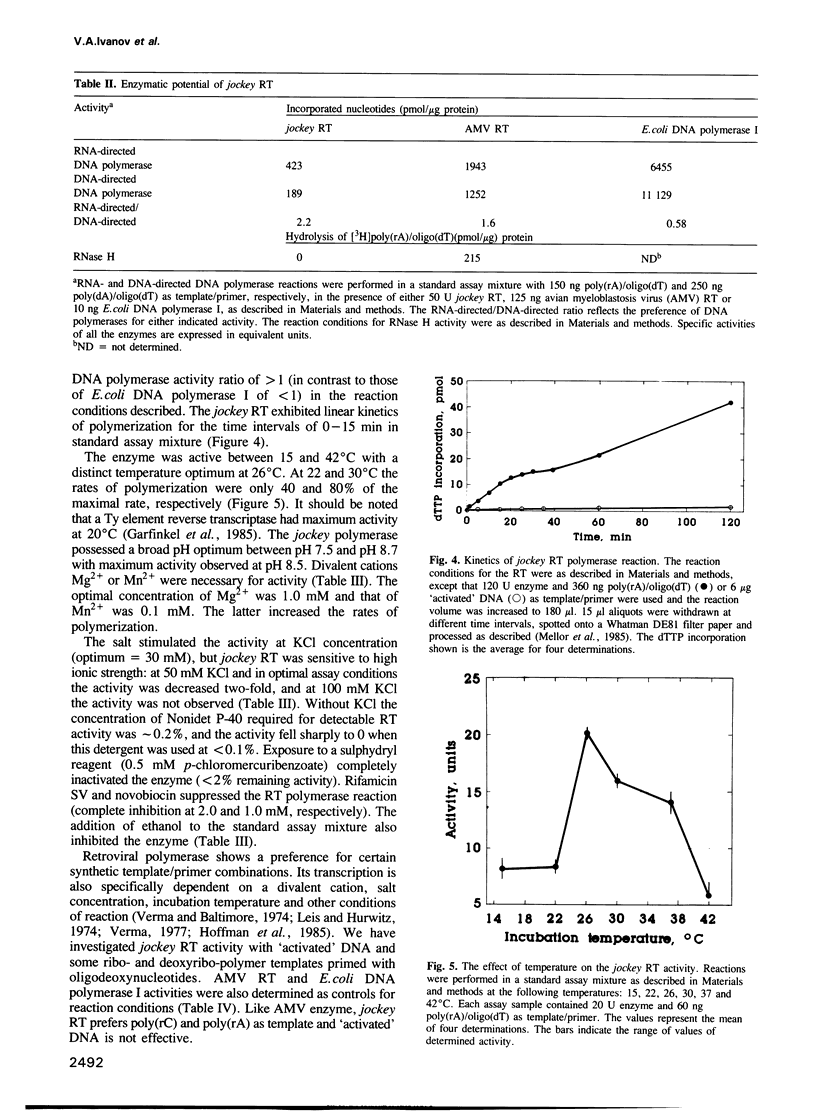
Abstract
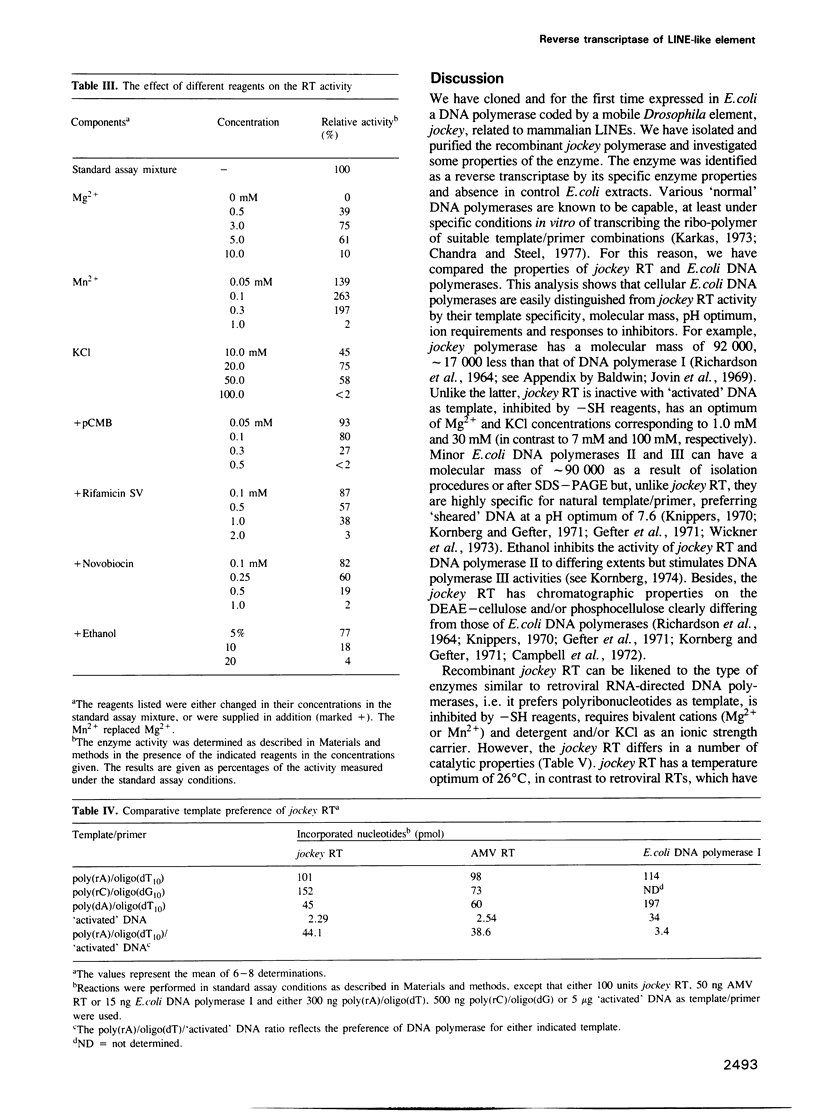
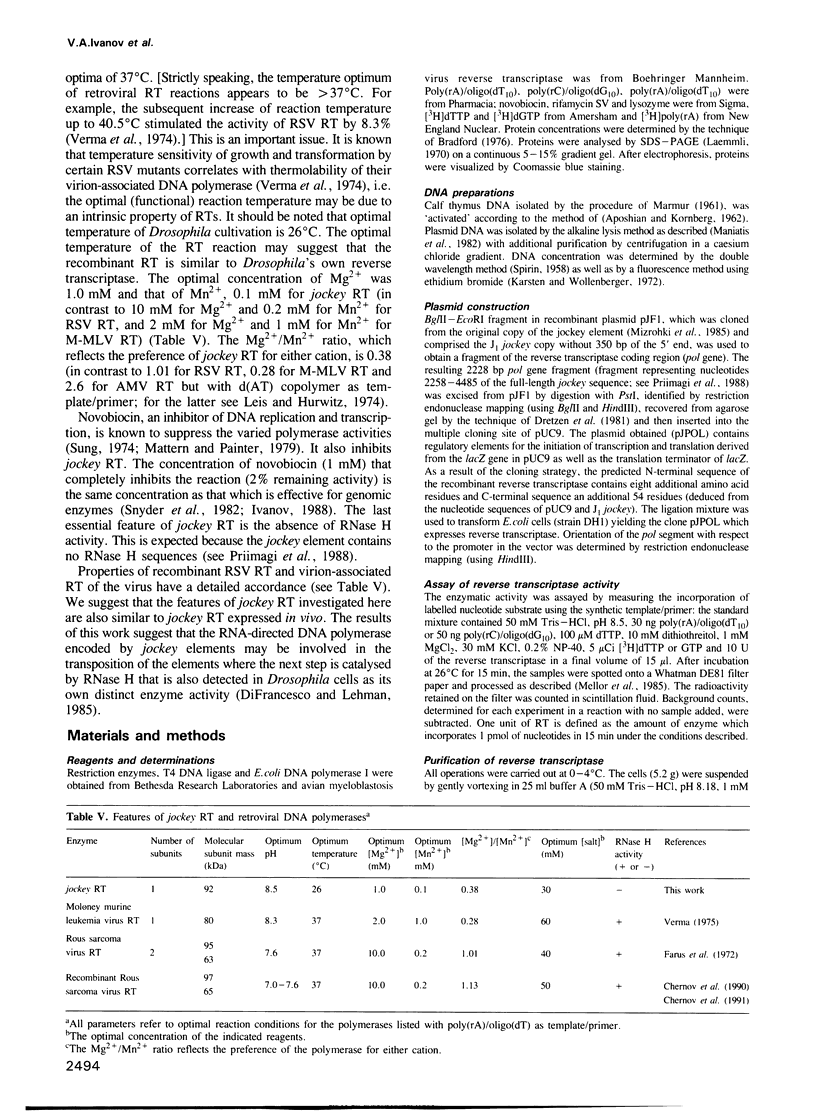
The mobile element jockey is similar in structural organization and coding potential to the LINEs of various organisms. It is transcribed at different stages of Drosophila ontogenesis. The Drosophila LINE family includes active transposable elements. Current models for the mechanism of transposition involve reverse transcription of an RNA intermediate and utilization of element-encoded proteins. As demonstrated here, a 2.23 kb DNA fragment from the region of jockey encoding the putative reverse transcriptase was stably introduced into an expression system under inducible control of the Escherichia coli lac regulatory elements. We describe the expression of the 92 kDa protein and identify this polypeptide alone as the authentic jockey reverse transcriptase based on some of its physical and enzymic properties. The jockey polymerase demonstrates RNA and DNA-directed DNA polymerase activities but lacks detectable RNase H, has a temperature optimum at 26 degrees C, requires Mg2+ or Mn2+ as a cofactor and is inactivated by sulphydryl reagent. The enzyme prefers poly(rC) and poly(rA) as template and 'activated' DNA is not effective.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APOSHIAN H. V., KORNBERG A. Enzymatic synthesis of deoxyribonucleic acid. IX. The polymerase formed after T2 bacteriophage infection of Escherichia coli: a new enzyme. J Biol Chem. 1962 Feb;237:519–525. [PubMed] [Google Scholar]
- Alexander F., Leis J., Soltis D. A., Crowl R. M., Danho W., Poonian M. S., Pan Y. C., Skalka A. M. Proteolytic processing of avian sarcoma and leukosis viruses pol-endo recombinant proteins reveals another pol gene domain. J Virol. 1987 Feb;61(2):534–542. doi: 10.1128/jvi.61.2.534-542.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Campbell J. L., Soll L., Richardson C. C. Isolation and partial characterization of a mutant of Escherichia coli deficient in DNA polymerase II. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2090–2094. doi: 10.1073/pnas.69.8.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaboissier M. C., Busseau I., Prosser J., Finnegan D. J., Bucheton A. Identification of a potential RNA intermediate for transposition of the LINE-like element I factor in Drosophila melanogaster. EMBO J. 1990 Nov;9(11):3557–3563. doi: 10.1002/j.1460-2075.1990.tb07566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlin M. J. Comparative properties of DNA, RNA, and hybrid homopolymer pairs. Fed Proc. 1965 Nov-Dec;24(6):1446–1457. [PubMed] [Google Scholar]
- Chandra P., Steel L. K. Purification, biochemical characterization and serological analysis of cellular deoxyribonucleic acid polymerases and a reverse transcriptase from spleen of a patient with myelofibrotic syndrome. Biochem J. 1977 Dec 1;167(3):513–524. doi: 10.1042/bj1670513f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernov A. P., Mel'nikov A. A., Shmatchenko V. V., Fodor I. Rekombinantnaia RNK-zavisimaia DNK-polimeraza RSV. Vydelenie i obshchaia kharakteristika. Biokhimiia. 1990 Apr;55(4):586–594. [PubMed] [Google Scholar]
- Deragon J. M., Sinnett D., Labuda D. Reverse transcriptase activity from human embryonal carcinoma cells NTera2D1. EMBO J. 1990 Oct;9(10):3363–3368. doi: 10.1002/j.1460-2075.1990.tb07537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco R. A., Lehman I. R. Interaction of ribonuclease H from Drosophila melanogaster embryos with DNA polymerase-primase. J Biol Chem. 1985 Nov 25;260(27):14764–14770. [PubMed] [Google Scholar]
- Doolittle R. F., Feng D. F., Johnson M. S., McClure M. A. Origins and evolutionary relationships of retroviruses. Q Rev Biol. 1989 Mar;64(1):1–30. doi: 10.1086/416128. [DOI] [PubMed] [Google Scholar]
- Dretzen G., Bellard M., Sassone-Corsi P., Chambon P. A reliable method for the recovery of DNA fragments from agarose and acrylamide gels. Anal Biochem. 1981 Apr;112(2):295–298. doi: 10.1016/0003-2697(81)90296-7. [DOI] [PubMed] [Google Scholar]
- Faras A. J., Taylor J. M., McDonnell J. P., Levinson W. E., Bishop J. M. Purification and characterization of the deoxyribonucleic acid polymerase associated with Rous sarcoma virus. Biochemistry. 1972 Jun 6;11(12):2334–2342. doi: 10.1021/bi00762a020. [DOI] [PubMed] [Google Scholar]
- Garfinkel D. J., Boeke J. D., Fink G. R. Ty element transposition: reverse transcriptase and virus-like particles. Cell. 1985 Sep;42(2):507–517. doi: 10.1016/0092-8674(85)90108-4. [DOI] [PubMed] [Google Scholar]
- Gefter M. L., Hirota Y., Kornberg T., Wechsler J. A., Barnoux C. Analysis of DNA polymerases II and 3 in mutants of Escherichia coli thermosensitive for DNA synthesis. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3150–3153. doi: 10.1073/pnas.68.12.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hizi A., Joklik W. K. RNA-dependent DNA polymerase of avian sarcoma virus B77. I. Isolation and partial characterization of the alpha, beta2, and alphabeta forms of the enzyme. J Biol Chem. 1977 Apr 10;252(7):2281–2289. [PubMed] [Google Scholar]
- Hoffman A. D., Banapour B., Levy J. A. Characterization of the AIDS-associated retrovirus reverse transcriptase and optimal conditions for its detection in virions. Virology. 1985 Dec;147(2):326–335. doi: 10.1016/0042-6822(85)90135-7. [DOI] [PubMed] [Google Scholar]
- Ivanov V. A., Gaziev A. I., Tretyak T. M. Exodeoxyribonuclease from rat brain specific for single-stranded DNA. Eur J Biochem. 1983 Dec 15;137(3):517–522. doi: 10.1111/j.1432-1033.1983.tb07856.x. [DOI] [PubMed] [Google Scholar]
- Ivanov V. A. Variations in DNA topoisomerase I activity after gamma irradiation of mature neurons. Neurosci Lett. 1988 Nov 22;94(1-2):99–103. doi: 10.1016/0304-3940(88)90277-7. [DOI] [PubMed] [Google Scholar]
- Jovin T. M., Englund P. T., Bertsch L. L. Enzymatic synthesis of deoxyribonucleic acid. XXVI. Physical and chemical studies of a homogeneous deoxyribonucleic acid polymerase. J Biol Chem. 1969 Jun 10;244(11):2996–3008. [PubMed] [Google Scholar]
- Karkas J. D. Reverse transcription by Escherichia coli DNA polymerase I. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3834–3838. doi: 10.1073/pnas.70.12.3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsten U., Wollenberger A. Determination of DNA and RNA in homogenized cells and tissues by surface fluorometry. Anal Biochem. 1972 Mar;46(1):135–148. doi: 10.1016/0003-2697(72)90405-8. [DOI] [PubMed] [Google Scholar]
- Knippers R. DNA polymerase II. Nature. 1970 Dec 12;228(5276):1050–1053. doi: 10.1038/2281050a0. [DOI] [PubMed] [Google Scholar]
- Kornberg T., Gefter M. L. Purification and DNA synthesis in cell-free extracts: properties of DNA polymerase II. Proc Natl Acad Sci U S A. 1971 Apr;68(4):761–764. doi: 10.1073/pnas.68.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE-HUNG S., CAVALIERI L. F. ISOLATION AND PROPERTIES OF A NUCLEIC ACID HYBRID POLYMERASE. Proc Natl Acad Sci U S A. 1964 Jun;51:1022–1028. doi: 10.1073/pnas.51.6.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larder B., Purifoy D., Powell K., Darby G. AIDS virus reverse transcriptase defined by high level expression in Escherichia coli. EMBO J. 1987 Oct;6(10):3133–3137. doi: 10.1002/j.1460-2075.1987.tb02623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leis J., Hurwitz J. RNA-dependent DNA polymerase from avian myeloblastosis virus. Methods Enzymol. 1974;29:143–150. doi: 10.1016/0076-6879(74)29017-7. [DOI] [PubMed] [Google Scholar]
- Leis J., Hurwitz J. RNA-dependent DNA polymerase from avian myeloblastosis virus. Methods Enzymol. 1974;29:143–150. doi: 10.1016/0076-6879(74)29017-7. [DOI] [PubMed] [Google Scholar]
- Loeb L. A., Tartof K. D., Travaglini E. C. Copying natural RNAs with E. coli DNA polymerase I. Nat New Biol. 1973 Mar 21;242(116):66–69. doi: 10.1038/newbio242066a0. [DOI] [PubMed] [Google Scholar]
- Mattern M. R., Painter R. B. Dependence of mammalian DNA replication on DNA supercoiling. II. Effects of novobiocin on DNA synthesis in Chinese hamster ovary cells. Biochim Biophys Acta. 1979 Jul 26;563(2):306–312. doi: 10.1016/0005-2787(79)90049-2. [DOI] [PubMed] [Google Scholar]
- Mel'nikov A. A., Molnar J., Horvath P., Fodor I. Klonirovanie i ékspressiia v Escherichia coli obratnoi transkriptazy virusa sarkomy Rausa. Dokl Akad Nauk SSSR. 1988;299(2):486–489. [PubMed] [Google Scholar]
- Mellor J., Malim M. H., Gull K., Tuite M. F., McCready S., Dibbayawan T., Kingsman S. M., Kingsman A. J. Reverse transcriptase activity and Ty RNA are associated with virus-like particles in yeast. Nature. 1985 Dec 12;318(6046):583–586. doi: 10.1038/318583a0. [DOI] [PubMed] [Google Scholar]
- Mizrokhi L. J., Georgieva S. G., Ilyin Y. V. jockey, a mobile Drosophila element similar to mammalian LINEs, is transcribed from the internal promoter by RNA polymerase II. Cell. 1988 Aug 26;54(5):685–691. doi: 10.1016/s0092-8674(88)80013-8. [DOI] [PubMed] [Google Scholar]
- Mizrokhi L. J., Obolenkova L. A., Priimägi A. F., Ilyin Y. V., Gerasimova T. I., Georgiev G. P. The nature of unstable insertion mutations and reversions in the locus cut of Drosophila melanogaster: molecular mechanism of transposition memory. EMBO J. 1985 Dec 30;4(13B):3781–3787. doi: 10.1002/j.1460-2075.1985.tb04148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priimägi A. F., Mizrokhi L. J., Ilyin Y. V. The Drosophila mobile element jockey belongs to LINEs and contains coding sequences homologous to some retroviral proteins. Gene. 1988 Oct 30;70(2):253–262. doi: 10.1016/0378-1119(88)90197-7. [DOI] [PubMed] [Google Scholar]
- Pritchard M. A., Dura J. M., Pélisson A., Bucheton A., Finnegan D. J. A cloned I-factor is fully functional in Drosophila melanogaster. Mol Gen Genet. 1988 Nov;214(3):533–540. doi: 10.1007/BF00330491. [DOI] [PubMed] [Google Scholar]
- RICHARDSON C. C., SCHILDKRAUT C. L., APOSHIAN H. V., KORNBERG A. ENZYMATIC SYNTHESIS OF DEOXYRIBONUCLEIC ACID. XIV. FURTHER PURIFICATION AND PROPERTIES OF DEOXYRIBONUCLEIC ACID POLYMERASE OF ESCHERICHIA COLI. J Biol Chem. 1964 Jan;239:222–232. [PubMed] [Google Scholar]
- SPIRIN A. S. Spektrofotometricheskoe opredelenie summarnogo kolichestva nukleinovykh kislot. Biokhimiia. 1958;23(5):656–662. [PubMed] [Google Scholar]
- Snyder R. D., Van Houten B., Regan J. D. Studies on the inhibition of repair of ultraviolet- and methyl methanesulfonate-induced damage in the DNA of human fibroblasts by novobiocin. Nucleic Acids Res. 1982 Oct 11;10(19):6207–6219. doi: 10.1093/nar/10.19.6207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung S. C. Effect of novobiocin on DNA-dependent DNA polymerases from developing rat brain. Biochim Biophys Acta. 1974 Aug 15;361(1):115–117. doi: 10.1016/0005-2787(74)90214-7. [DOI] [PubMed] [Google Scholar]
- Verma I. M., Mason W. S., Drost S. D., Baltimore D. DNA polymerase activity from two temperature-sensitive mutants of Rous sarcoma virus is thermolabile. Nature. 1974 Sep 6;251(5470):27–31. doi: 10.1038/251027a0. [DOI] [PubMed] [Google Scholar]
- Verma I. M. Studies on reverse transcriptase of RNA tumor viruses III. Properties of purified Moloney murine leukemia virus DNA polymerase and associated RNase H. J Virol. 1975 Apr;15(4):843–854. doi: 10.1128/jvi.15.4.843-854.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma I. M. The reverse transcriptase. Biochim Biophys Acta. 1977 Mar 21;473(1):1–38. doi: 10.1016/0304-419x(77)90005-1. [DOI] [PubMed] [Google Scholar]
- Wickner W., Schekman R., Geider K., Kornberg A. A new form of DNA polymerase 3 and a copolymerase replicate a long, single-stranded primer-template. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1764–1767. doi: 10.1073/pnas.70.6.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y., Eickbush T. H. Similarity of reverse transcriptase-like sequences of viruses, transposable elements, and mitochondrial introns. Mol Biol Evol. 1988 Nov;5(6):675–690. doi: 10.1093/oxfordjournals.molbev.a040521. [DOI] [PubMed] [Google Scholar]