Abstract
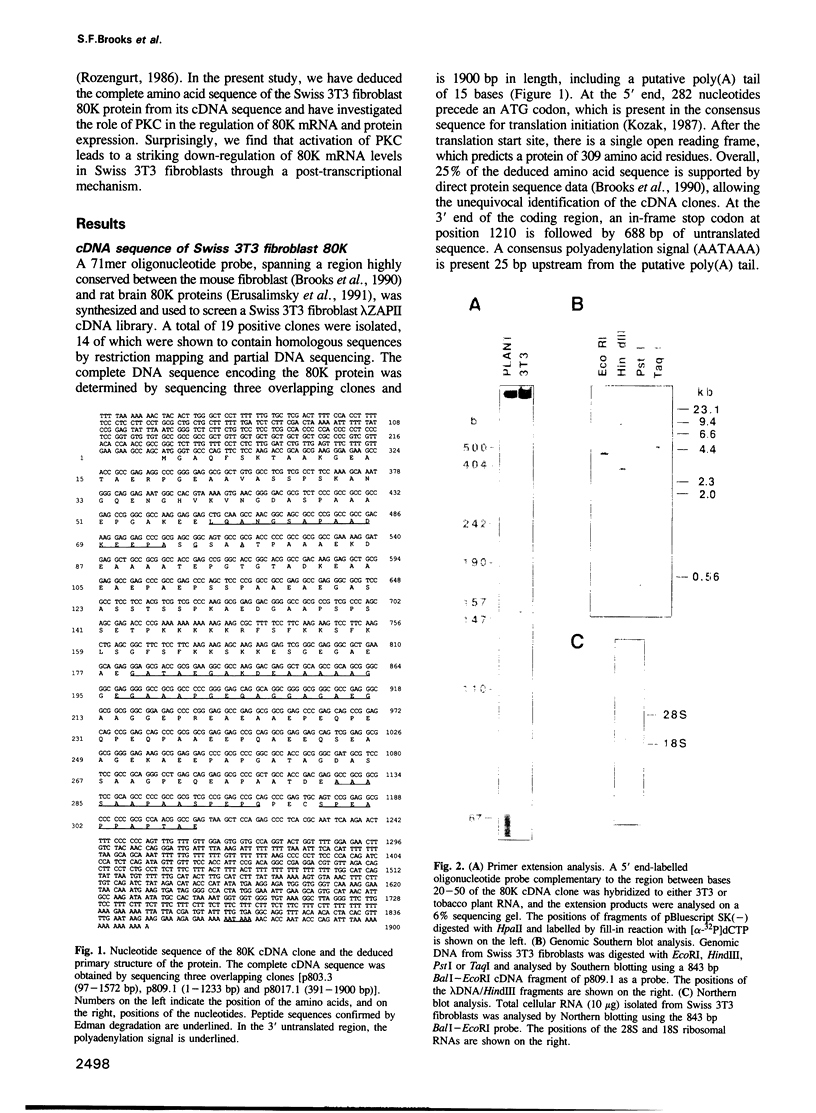
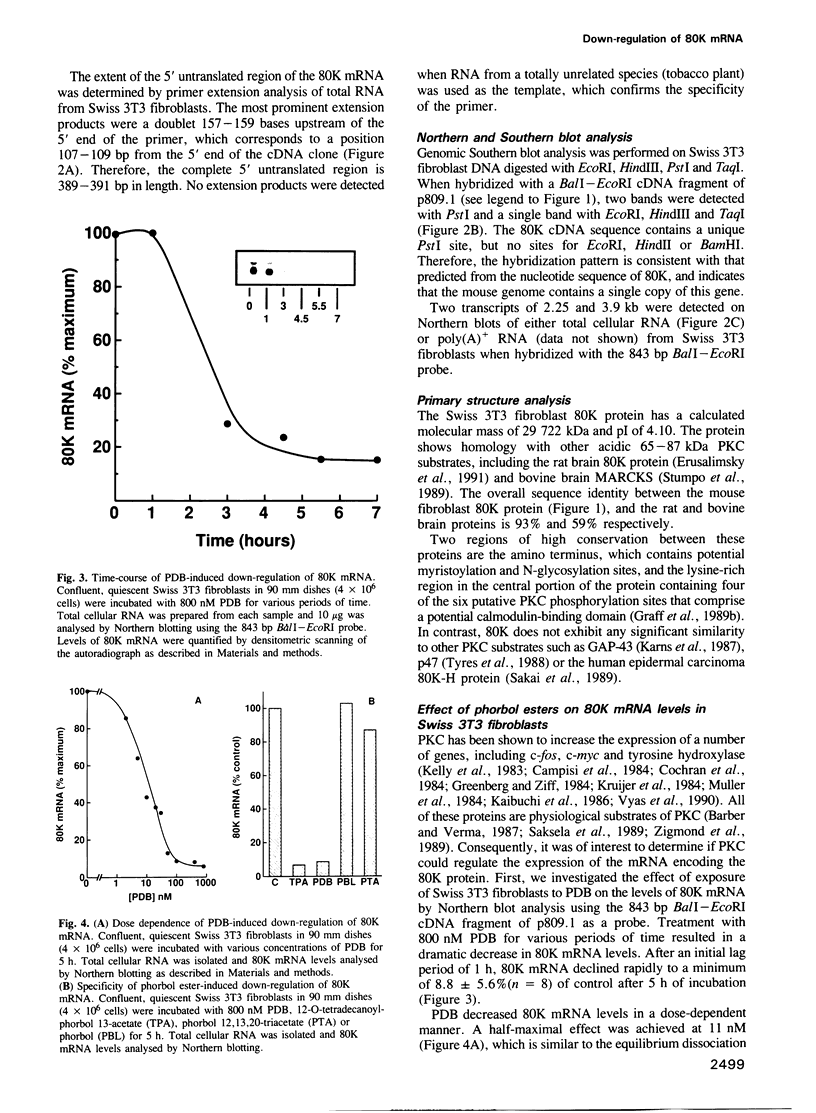
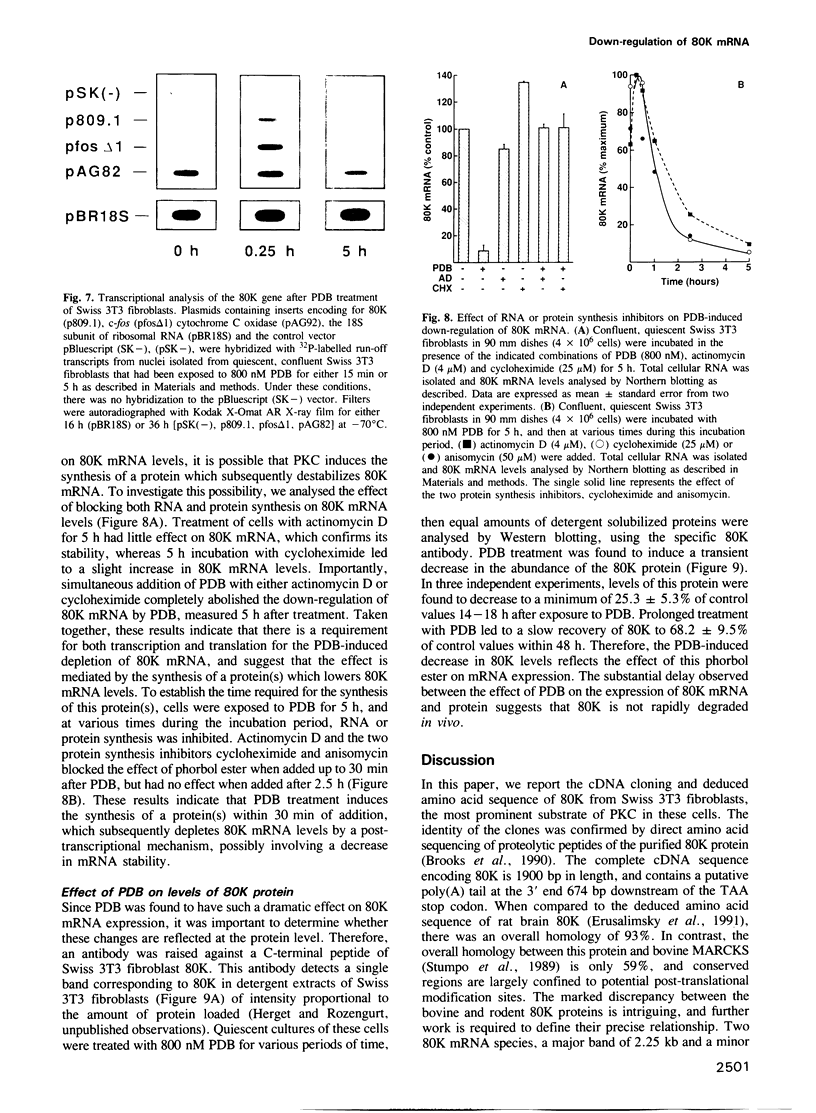
The amino acid sequence of 80K, the major acidic protein kinase C (PKC) substrate of Swiss 3T3 fibroblasts, was deduced from a cDNA nucleotide sequence. Overall, 25% of the predicted amino acid sequence is supported by direct protein sequence data. Southern blot analysis suggests that the mouse genome contains a single copy of this gene. Two 80K mRNA species, a major band of 2.25 kb and a minor band of 3.9 kb, were detected by Northern blot analysis. Stimulation of PKC by biologically active phorbol esters, including phorbol-12, 13-dibutyrate (PDB), reduced the steady state level of 80K mRNA to 8.8% of control within 5-7 h. This effect was dose-dependent, and was abolished by prior depletion of PKC. The PDB-induced down-regulation of 80K mRNA levels was transient, and recovery coincided with the disappearance of PKC activity. A similar transient decrease in 80K mRNA levels was also demonstrated in tertiary cultures of mouse embryo fibroblasts. The down-regulation of 80K mRNA levels was completely abolished by actinomycin D, cycloheximide or anisomycin if added up to 30 min after PDB addition. Since the rate of transcription of the 80K gene was unaltered by PDB treatment, we concluded that the PKC-induced down-regulation of 80K mRNA is mediated by a post-transcriptional mechanism. In addition, PDB transiently decreased the level of 80K protein within 14-18 h, thus reflecting the effects of this phorbol ester on mRNA expression.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aderem A. A., Albert K. A., Keum M. M., Wang J. K., Greengard P., Cohn Z. A. Stimulus-dependent myristoylation of a major substrate for protein kinase C. Nature. 1988 Mar 24;332(6162):362–364. doi: 10.1038/332362a0. [DOI] [PubMed] [Google Scholar]
- Albert K. A., Walaas S. I., Wang J. K., Greengard P. Widespread occurrence of "87 kDa," a major specific substrate for protein kinase C. Proc Natl Acad Sci U S A. 1986 May;83(9):2822–2826. doi: 10.1073/pnas.83.9.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel P., Imagawa M., Chiu R., Stein B., Imbra R. J., Rahmsdorf H. J., Jonat C., Herrlich P., Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987 Jun 19;49(6):729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- Barber J. R., Verma I. M. Modification of fos proteins: phosphorylation of c-fos, but not v-fos, is stimulated by 12-tetradecanoyl-phorbol-13-acetate and serum. Mol Cell Biol. 1987 Jun;7(6):2201–2211. doi: 10.1128/mcb.7.6.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biemann H. P., Erikson R. L. Abnormal protein kinase C down regulation and reduced substrate levels in non-phorbol ester-responsive 3T3-TNR9 cells. Mol Cell Biol. 1990 May;10(5):2122–2132. doi: 10.1128/mcb.10.5.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshear P. J., Wen L., Glynn B. P., Witters L. A. Protein kinase C-stimulated phosphorylation in vitro of a Mr 80,000 protein phosphorylated in response to phorbol esters and growth factors in intact fibroblasts. Distinction from protein kinase C and prominence in brain. J Biol Chem. 1986 Jan 25;261(3):1459–1469. [PubMed] [Google Scholar]
- Blackshear P. J., Witters L. A., Girard P. R., Kuo J. F., Quamo S. N. Growth factor-stimulated protein phosphorylation in 3T3-L1 cells. Evidence for protein kinase C-dependent and -independent pathways. J Biol Chem. 1985 Oct 25;260(24):13304–13315. [PubMed] [Google Scholar]
- Brooks S. F., Erusalimsky J. D., Totty N. F., Rozengurt E. Purification and internal amino acid sequence of the 80 kDa protein kinase C substrate from Swiss 3T3 fibroblasts. Homology with substrates from brain. FEBS Lett. 1990 Jul 30;268(1):291–295. doi: 10.1016/0014-5793(90)81030-r. [DOI] [PubMed] [Google Scholar]
- Campisi J., Gray H. E., Pardee A. B., Dean M., Sonenshein G. E. Cell-cycle control of c-myc but not c-ras expression is lost following chemical transformation. Cell. 1984 Feb;36(2):241–247. doi: 10.1016/0092-8674(84)90217-4. [DOI] [PubMed] [Google Scholar]
- Caput D., Beutler B., Hartog K., Thayer R., Brown-Shimer S., Cerami A. Identification of a common nucleotide sequence in the 3'-untranslated region of mRNA molecules specifying inflammatory mediators. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1670–1674. doi: 10.1073/pnas.83.6.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W. Gene regulation through messenger RNA stability. Curr Opin Cell Biol. 1989 Dec;1(6):1148–1153. doi: 10.1016/s0955-0674(89)80065-1. [DOI] [PubMed] [Google Scholar]
- Cochran B. H., Zullo J., Verma I. M., Stiles C. D. Expression of the c-fos gene and of an fos-related gene is stimulated by platelet-derived growth factor. Science. 1984 Nov 30;226(4678):1080–1082. doi: 10.1126/science.6093261. [DOI] [PubMed] [Google Scholar]
- Collins M. K., Rozengurt E. Binding of phorbol esters to high-affinity sites on murine fibroblastic cells elicits a mitogenic response. J Cell Physiol. 1982 Jul;112(1):42–50. doi: 10.1002/jcp.1041120108. [DOI] [PubMed] [Google Scholar]
- Coughlin S. R., Lee W. M., Williams P. W., Giels G. M., Williams L. T. c-myc gene expression is stimulated by agents that activate protein kinase C and does not account for the mitogenic effect of PDGF. Cell. 1985 Nov;43(1):243–251. doi: 10.1016/0092-8674(85)90029-7. [DOI] [PubMed] [Google Scholar]
- Dicker P., Rozengurt E. Phorbol esters and vasopressin stimulate DNA synthesis by a common mechanism. Nature. 1980 Oct 16;287(5783):607–612. doi: 10.1038/287607a0. [DOI] [PubMed] [Google Scholar]
- Edwards D. R., Murphy G., Reynolds J. J., Whitham S. E., Docherty A. J., Angel P., Heath J. K. Transforming growth factor beta modulates the expression of collagenase and metalloproteinase inhibitor. EMBO J. 1987 Jul;6(7):1899–1904. doi: 10.1002/j.1460-2075.1987.tb02449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erusalimsky J. D., Brooks S. F., Herget T., Morris C., Rozengurt E. Molecular cloning and characterization of the acidic 80-kDa protein kinase C substrate from rat brain. Identification as a glycoprotein. J Biol Chem. 1991 Apr 15;266(11):7073–7080. [PubMed] [Google Scholar]
- Erusalimsky J. D., Friedberg I., Rozengurt E. Bombesin, diacylglycerols, and phorbol esters rapidly stimulate the phosphorylation of an Mr = 80,000 protein kinase C substrate in permeabilized 3T3 cells. Effect of guanine nucleotides. J Biol Chem. 1988 Dec 15;263(35):19188–19194. [PubMed] [Google Scholar]
- Erusalimsky J. D., Rozengurt E. Vasopressin rapidly stimulates protein kinase C in digitonin-permeabilized Swiss 3T3 cells: involvement of a pertussis toxin-insensitive guanine nucleotide binding protein. J Cell Physiol. 1989 Nov;141(2):253–261. doi: 10.1002/jcp.1041410204. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Graff J. M., Stumpo D. J., Blackshear P. J. Molecular cloning, sequence, and expression of a cDNA encoding the chicken myristoylated alanine-rich C kinase substrate (MARCKS). Mol Endocrinol. 1989 Nov;3(11):1903–1906. doi: 10.1210/mend-3-11-1903. [DOI] [PubMed] [Google Scholar]
- Graff J. M., Young T. N., Johnson J. D., Blackshear P. J. Phosphorylation-regulated calmodulin binding to a prominent cellular substrate for protein kinase C. J Biol Chem. 1989 Dec 25;264(36):21818–21823. [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Herget T., Reich M., Stüber K., Starzinski-Powitz A. Regulated expression of repetitive sequences including the identifier sequence during myotube formation in culture. EMBO J. 1986 Apr;5(4):659–664. doi: 10.1002/j.1460-2075.1986.tb04264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herget T., Schell J., Schreier P. H. Elicitor-specific induction of one member of the chitinase gene family in Arachis hypogaea. Mol Gen Genet. 1990 Dec;224(3):469–476. doi: 10.1007/BF00262442. [DOI] [PubMed] [Google Scholar]
- Hirai M., Shimizu N. Stimulation of a MR 80,000 protein phosphorylation by EGF in EGF receptor-hyperproducing human tumor cells. J Cell Physiol. 1989 Apr;139(1):9–17. doi: 10.1002/jcp.1041390103. [DOI] [PubMed] [Google Scholar]
- Hornbeck P., Nakabayashi H., Fowlkes B. J., Paul W. E., Kligman D. A major myristylated substrate of protein kinase C and protein kinase C itself are differentially regulated during murine B- and T-lymphocyte development and activation. Mol Cell Biol. 1989 Sep;9(9):3727–3735. doi: 10.1128/mcb.9.9.3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornbeck P., Paul W. E. Anti-immunoglobulin and phorbol ester induce phosphorylation of proteins associated with the plasma membrane and cytoskeleton in murine B lymphocytes. J Biol Chem. 1986 Nov 5;261(31):14817–14824. [PubMed] [Google Scholar]
- Imagawa M., Chiu R., Karin M. Transcription factor AP-2 mediates induction by two different signal-transduction pathways: protein kinase C and cAMP. Cell. 1987 Oct 23;51(2):251–260. doi: 10.1016/0092-8674(87)90152-8. [DOI] [PubMed] [Google Scholar]
- Isacke C. M., Meisenhelder J., Brown K. D., Gould K. L., Gould S. J., Hunter T. Early phosphorylation events following the treatment of Swiss 3T3 cells with bombesin and the mammalian bombesin-related peptide, gastrin-releasing peptide. EMBO J. 1986 Nov;5(11):2889–2898. doi: 10.1002/j.1460-2075.1986.tb04584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issandou M., Rozengurt E. Bradykinin transiently activates protein kinase C in Swiss 3T3 cells. Distinction from activation by bombesin and vasopressin. J Biol Chem. 1990 Jul 15;265(20):11890–11896. [PubMed] [Google Scholar]
- Kaibuchi K., Tsuda T., Kikuchi A., Tanimoto T., Yamashita T., Takai Y. Possible involvement of protein kinase C and calcium ion in growth factor-induced expression of c-myc oncogene in Swiss 3T3 fibroblasts. J Biol Chem. 1986 Jan 25;261(3):1187–1192. [PubMed] [Google Scholar]
- Karns L. R., Ng S. C., Freeman J. A., Fishman M. C. Cloning of complementary DNA for GAP-43, a neuronal growth-related protein. Science. 1987 May 1;236(4801):597–600. doi: 10.1126/science.2437653. [DOI] [PubMed] [Google Scholar]
- Kelly K., Cochran B. H., Stiles C. D., Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Kikkawa U., Nishizuka Y. The role of protein kinase C in transmembrane signalling. Annu Rev Cell Biol. 1986;2:149–178. doi: 10.1146/annurev.cb.02.110186.001053. [DOI] [PubMed] [Google Scholar]
- Koeller D. M., Casey J. L., Hentze M. W., Gerhardt E. M., Chan L. N., Klausner R. D., Harford J. B. A cytosolic protein binds to structural elements within the iron regulatory region of the transferrin receptor mRNA. Proc Natl Acad Sci U S A. 1989 May;86(10):3574–3578. doi: 10.1073/pnas.86.10.3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruijer W., Cooper J. A., Hunter T., Verma I. M. Platelet-derived growth factor induces rapid but transient expression of the c-fos gene and protein. Nature. 1984 Dec 20;312(5996):711–716. doi: 10.1038/312711a0. [DOI] [PubMed] [Google Scholar]
- Marshall C. J. Tumor suppressor genes. Cell. 1991 Jan 25;64(2):313–326. doi: 10.1016/0092-8674(91)90641-b. [DOI] [PubMed] [Google Scholar]
- McIlroy B. K., Walters J. D., Blackshear P. J., Johnson J. D. Phosphorylation-dependent binding of a synthetic MARCKS peptide to calmodulin. J Biol Chem. 1991 Mar 15;266(8):4959–4964. [PubMed] [Google Scholar]
- Morris C., Rozengurt E. Purification of a phosphoprotein from rat brain closely related to the 80 kDa substrate of protein kinase C identified in Swiss 3T3 fibroblasts. FEBS Lett. 1988 Apr 25;231(2):311–316. doi: 10.1016/0014-5793(88)80840-8. [DOI] [PubMed] [Google Scholar]
- Müller R., Bravo R., Burckhardt J., Curran T. Induction of c-fos gene and protein by growth factors precedes activation of c-myc. Nature. 1984 Dec 20;312(5996):716–720. doi: 10.1038/312716a0. [DOI] [PubMed] [Google Scholar]
- Müllner E. W., Neupert B., Kühn L. C. A specific mRNA binding factor regulates the iron-dependent stability of cytoplasmic transferrin receptor mRNA. Cell. 1989 Jul 28;58(2):373–382. doi: 10.1016/0092-8674(89)90851-9. [DOI] [PubMed] [Google Scholar]
- Nelsen B., Hellman L., Sen R. The NF-kappa B-binding site mediates phorbol ester-inducible transcription in nonlymphoid cells. Mol Cell Biol. 1988 Aug;8(8):3526–3531. doi: 10.1128/mcb.8.8.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Pandey N. B., Marzluff W. F. The stem-loop structure at the 3' end of histone mRNA is necessary and sufficient for regulation of histone mRNA stability. Mol Cell Biol. 1987 Dec;7(12):4557–4559. doi: 10.1128/mcb.7.12.4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Pena A., Rozengurt E. Disappearance of Ca2+-sensitive, phospholipid-dependent protein kinase activity in phorbol ester-treated 3T3 cells. Biochem Biophys Res Commun. 1984 May 16;120(3):1053–1059. doi: 10.1016/s0006-291x(84)80213-2. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Pena A., Rozengurt E. Phosphorylation of an acidic mol. wt. 80 000 cellular protein in a cell-free system and intact Swiss 3T3 cells: a specific marker of protein kinase C activity. EMBO J. 1986 Jan;5(1):77–83. doi: 10.1002/j.1460-2075.1986.tb04180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Pena A., Rozengurt E. Serum, like phorbol esters, rapidly activates protein kinase C in intact quiescent fibroblasts. EMBO J. 1985 Jan;4(1):71–76. doi: 10.1002/j.1460-2075.1985.tb02319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Pena A., Rozengurt E. Vasopressin rapidly stimulates protein kinase C in quiescent Swiss 3T3 cells. J Cell Physiol. 1986 Oct;129(1):124–130. doi: 10.1002/jcp.1041290117. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Pena A., Zachary I., Rozengurt E. Rapid dephosphorylation of a Mr 80,000 protein, a specific substrate of protein kinase C upon removal of phorbol esters, bombesin and vasopressin. Biochem Biophys Res Commun. 1986 Oct 15;140(1):379–385. doi: 10.1016/0006-291x(86)91101-0. [DOI] [PubMed] [Google Scholar]
- Rozengurt E. Early signals in the mitogenic response. Science. 1986 Oct 10;234(4773):161–166. doi: 10.1126/science.3018928. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Rodriguez-Pena A., Coombs M., Sinnett-Smith J. Diacylglycerol stimulates DNA synthesis and cell division in mouse 3T3 cells: role of Ca2+-sensitive phospholipid-dependent protein kinase. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5748–5752. doi: 10.1073/pnas.81.18.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E., Rodriguez-Pena M., Smith K. A. Phorbol esters, phospholipase C, and growth factors rapidly stimulate the phosphorylation of a Mr 80,000 protein in intact quiescent 3T3 cells. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7244–7248. doi: 10.1073/pnas.80.23.7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E., Sinnett-Smith J. Early signals underlying the induction of the c-fos and c-myc genes in quiescent fibroblasts: studies with bombesin and other growth factors. Prog Nucleic Acid Res Mol Biol. 1988;35:261–295. doi: 10.1016/s0079-6603(08)60616-9. [DOI] [PubMed] [Google Scholar]
- Sager R. Tumor suppressor genes: the puzzle and the promise. Science. 1989 Dec 15;246(4936):1406–1412. doi: 10.1126/science.2574499. [DOI] [PubMed] [Google Scholar]
- Sakai K., Hirai M., Minoshima S., Kudoh J., Fukuyama R., Shimizu N. Isolation of cDNAs encoding a substrate for protein kinase C: nucleotide sequence and chromosomal mapping of the gene for a human 80K protein. Genomics. 1989 Aug;5(2):309–315. doi: 10.1016/0888-7543(89)90063-3. [DOI] [PubMed] [Google Scholar]
- Saksela K., Mäkelä T. P., Evan G., Alitalo K. Rapid phosphorylation of the L-myc protein induced by phorbol ester tumor promoters and serum. EMBO J. 1989 Jan;8(1):149–157. doi: 10.1002/j.1460-2075.1989.tb03359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C., King R. M., Philipson L. Genes specifically expressed at growth arrest of mammalian cells. Cell. 1988 Sep 9;54(6):787–793. doi: 10.1016/s0092-8674(88)91065-3. [DOI] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Sherman M. L., Stone R. M., Datta R., Bernstein S. H., Kufe D. W. Transcriptional and post-transcriptional regulation of c-jun expression during monocytic differentiation of human myeloid leukemic cells. J Biol Chem. 1990 Feb 25;265(6):3320–3323. [PubMed] [Google Scholar]
- Shirakawa F., Mizel S. B. In vitro activation and nuclear translocation of NF-kappa B catalyzed by cyclic AMP-dependent protein kinase and protein kinase C. Mol Cell Biol. 1989 Jun;9(6):2424–2430. doi: 10.1128/mcb.9.6.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpo D. J., Graff J. M., Albert K. A., Greengard P., Blackshear P. J. Molecular cloning, characterization, and expression of a cDNA encoding the "80- to 87-kDa" myristoylated alanine-rich C kinase substrate: a major cellular substrate for protein kinase C. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4012–4016. doi: 10.1073/pnas.86.11.4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TODARO G. J., GREEN H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963 May;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda T., Kaibuchi K., Kawahara Y., Fukuzaki H., Takai Y. Induction of protein kinase C activation and Ca2+ mobilization by fibroblast growth factor in Swiss 3T3 cells. FEBS Lett. 1985 Oct 28;191(2):205–210. doi: 10.1016/0014-5793(85)80009-0. [DOI] [PubMed] [Google Scholar]
- Tyers M., Rachubinski R. A., Stewart M. I., Varrichio A. M., Shorr R. G., Haslam R. J., Harley C. B. Molecular cloning and expression of the major protein kinase C substrate of platelets. Nature. 1988 Jun 2;333(6172):470–473. doi: 10.1038/333470a0. [DOI] [PubMed] [Google Scholar]
- Vyas S., Faucon Biguet N., Mallet J. Transcriptional and post-transcriptional regulation of tyrosine hydroxylase gene by protein kinase C. EMBO J. 1990 Nov;9(11):3707–3712. doi: 10.1002/j.1460-2075.1990.tb07583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisdom R., Lee W. Translation of c-myc mRNA is required for its post-transcriptional regulation during myogenesis. J Biol Chem. 1990 Nov 5;265(31):19015–19021. [PubMed] [Google Scholar]
- Wolfman A., Wingrove T. G., Blackshear P. J., Macara I. G. Down-regulation of protein kinase C and of an endogenous 80-kDa substrate in transformed fibroblasts. J Biol Chem. 1987 Dec 5;262(34):16546–16552. [PubMed] [Google Scholar]
- Zachary I., Sinnett-Smith J. W., Rozengurt E. Early events elicited by bombesin and structurally related peptides in quiescent Swiss 3T3 cells. I. Activation of protein kinase C and inhibition of epidermal growth factor binding. J Cell Biol. 1986 Jun;102(6):2211–2222. doi: 10.1083/jcb.102.6.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond R. E., Schwarzschild M. A., Rittenhouse A. R. Acute regulation of tyrosine hydroxylase by nerve activity and by neurotransmitters via phosphorylation. Annu Rev Neurosci. 1989;12:415–461. doi: 10.1146/annurev.ne.12.030189.002215. [DOI] [PubMed] [Google Scholar]







