Abstract
Image-guided drug delivery refers to the combination of drug targeting and imaging. Preclinically, image-guided drug delivery can be used for several different purposes, e.g. for monitoring biodistribution, target site accumulation, off-target localization, drug release and drug efficacy. Clinically, it holds significant potential for preselecting patients. In this perspective, we briefly summarize the main principles of image-guided drug delivery, and we describe its potential for facilitating, furthering and personalizing nanomedicine treatments.
The intravenous administration of chemotherapeutic drugs comes with many drawbacks, including suboptimal pharmacokinetics, low target site accumulation, low efficacy, high off-target localization and high toxicity, together limiting the utility of systemic anticancer therapy. To overcome these shortcomings, several different types of nanomedicine formulations have been designed and evaluated over the years. Nanomedicines, such as liposomes, polymers and micelles, are 1-100(0) nm-sized carrier materials which aim to improve the biodistribution and the target site accumulation of chemotherapeutic drugs, and to enable more effective therapies with less side effects (1-3). The Enhanced Permeability and Retention (EPR) effect is generally considered to be a key feature for nanomedicine formulations, enabling them to gradually accumulate at pathological sites characterized by enhanced vascular leakiness and non-functional lymphatic drainage (4). It has become more and more recognized, however, that EPR is a highly variable pathophysiological phenomenon, with large inter- and intra-individual differences not only in vascular leakiness and lymphatic drainage, but also in tumor vascularization, perfusion, interstitial fluid pressure and retention (5,6). Consequently, materials and methods (including new imaging instrumentation, such as hybrid PET-MRI) are needed which are able to visualize and quantify this variability in EPR, in order to pre-identify patients likely to benefit from nanomedicine therapy. In addition, at the preclinical level, such image-guided drug delivery approaches are useful to assess the pharmacokinetics and the biodistribution of drug delivery systems, as well as their target site accumulation and in vivo drug release characteristics. In the present perspective, we will briefly summarize the main concepts employed in image-guided drug delivery, and we will describe how the combination of drug targeting and imaging can be used to facilitate preclinical research and to individualize and improve nanomedicine treatments in the clinic.
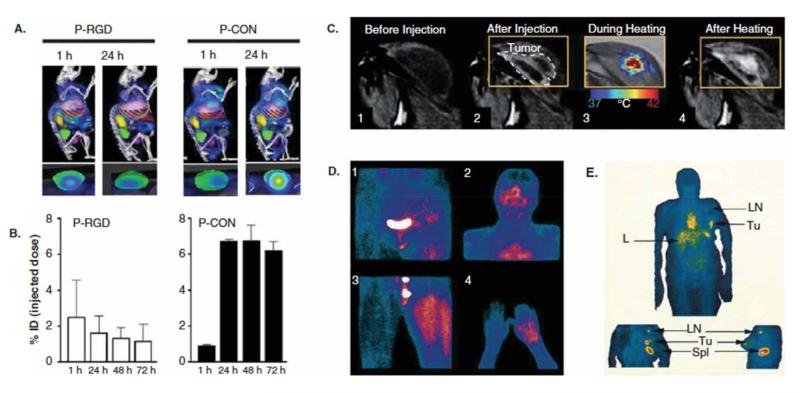
At the preclinical level, image-guided drug delivery is primarily used to non-invasively visualize and quantify the behavior of nanocarrier materials upon administration. This enables the longitudinal assessment of their accumulation at pathological sites (e.g. tumors, metastases, inflammatory lesions), as well as in potentially endangered off-target tissues. By including imaging, more extensive and more meaningful information can be obtained from a smaller group of animals. Imaging even enables the direct comparison of multiple carrier materials within a single animal. Such setups are considered to be useful for minimizing the variability in the target site accumulation of nanocarriers as a result of differences in e.g. tumor growth and tumor vascularization. Based on this reasoning, we have recently employed hybrid computed tomography - fluorescence-mediated tomography (CT-FMT) imaging (7) to evaluate the potential of active drug targeting to tumor blood vessels. Polymeric drug carriers based on N-(2-hydroxy-propyl)-methacrylamide (HPMA) were modified with peptide residues recognizing receptors overexpressed by angiogenic tumor blood vessels, and their biodistribution and tumor accumulation were compared to those of unmodified HPMA copolymers (8). Two different angiogenesis-specific peptides were used, i.e. RGD (which binds to integrins) and NGR (which binds to aminopeptidas-N). The peptide-modified actively targeted polymers were functionalized with a fluorophore which is excited at 680 nm (Dy676), and the peptide-free passively targeted polymers with a fluorophore which is excited at 750 nm (Dy750). Both polymers were co-injected into the same mouse, and their tumor accumulation and off-target localization were non-invasively monitored at several different time point up until 72 h post i.v. injection. Independent of the tumor model used (highly leaky CT26 colon carcinoma and poorly leaky BxPC3 pancreatic carcinoma), it was found that active targeting does work, resulting in significantly higher levels of polymer accumulation within tumors at early time points after i.v. injection (Figure 1A-B). At later time points, however, the concentrations of the peptide-free polymers in tumors were found to be higher. When using non-invasive imaging to calculate the areas-under-the-curve (AUC’s) over the first 72 h after i.v. administration, a 3-5-fold higher target site accumulation of the passively target polymers was detected. This was attributed to a much faster clearance of the actively targeted peptide-modified polymers from systemic circulation, as well as to a significantly higher degree of off-target localization (e.g. in liver and kidney). In this study, the use of imaging was instrumental, as it enabled the simultaneous assessment (and head-to-head comparison) of two different polymers within one mouse, and within one tumor (i.e. with minimal variability), at multiple time points. This study therefore nicely exemplifies the added value of using imaging investigate the principles of passive vs. active tumor targeting using nanomedicine formulations.
Figure 1. Image-guided drug delivery.
A-B: At the preclinical level, image-guided drug delivery can be used for several different purposes, including for the analysis of active vs. passive tumor targeting. Panels A and B show that at early time point after i.v. administration, RGD-modified tumor vasculature targeted polymeric drug carriers accumulate more efficiently within tumors than peptide-free passively targeted polymers. They also show, however, that over time, the latter are more efficient in achieving high tumor concentrations. C: MR imaging of gadolinium release from temperature-sensitive liposomes before and after heating, exemplifying that image-guided drug delivery can be used to tailor triggered drug release. D-E: Accumulation of radiolabeled liposomes in tumors in patients, showing that sarcomas tend to accumulate nanomedicine formulations relatively well (D), whereas breast carcinomas present with a relatively low degree of EPR-mediated tumor accumulation (E). Tu: tumor, LN: lymph node, L: liver, Spl: spleen. Images adapted, with permission, from [8,10,11,12].
Visualizing and quantifying (triggered) drug release from nanomedicine formulations at the target site is another highly useful application of image-guided drug delivery. This is particularly obvious in the case of temperature-sensitive liposomes, which can be triggered by moderate hyperthermia to release their contents (i.e. drugs and/or imaging agents), and for which it is almost impossible to assess drug release ex vivo, because of the instability of these lipid-based nanocarriers in case of tumor and cell lysis. In recent years, significant efforts have been made in employing magnetic-resonance-guided high-intensity focused ultrasound (MR-HIFU) for heating tumors (9). The advantage of this approach is that real-time MR-based temperature-mapping can be performed during the application of HIFU-mediated hyperthermia, to ensure efficient and homogenous heating. An additional advantage is that content release from temperature-sensitive liposomes can be visualized and quantified in real-time using MRI, provided that an MR contrast is (co-) entrapped within the liposomes. Several studies have recently been published providing proof-of-principle for MR imaging (and quantification) of content release from temperature-sensitive liposomes. As exemplified by Figure 1C, it has for instance been shown that it is possible, in a single experimental setup, to monitor tumors prior to liposome administration and heating, immediately after liposome administration (but before heating), during heating (to provide feedback on temperature), and after heating (to visualize and quantify drug release) (10). Such imaging-based analyses are useful for optimizing the composition of the temperature-sensitive liposomes under investigation, as well as for tailoring the intensity and the duration of the HIFU-based heating protocol.
From a clinical point-of-view, visualizing and quantifying drug targeting to pathological sites is arguably the most important application of image-guided drug delivery. By monitoring whether nanomedicine formulations are able to reach the pathological site, and by quantifying how efficiently they are able to do so, it would be possible to stratify potential responders from non-responders. Only very few studies have been published thus far in which nanomedicine formulations have been labeled with contrast agents, and in which their biodistribution and target site accumulation is visualized and quantified in patients. As exemplified by Figure 1D-E, Koukourakis and colleagues and Harrington and colleagues employed technetium- and indium-labeled PEGylated liposomes to monitor drug targeting to tumors (11,12). In case of sarcomas, relatively effective EPR-mediated passive tumor targeting was observed, whereas in case of breast cancer, relatively low amounts of radiolabeled liposomes accumulated in tumors. These accumulation patterns correspond quite well with the observed response rates in sarcoma vs. breast cancer patients. In Kaposi sarcoma, for instance, which is characterized by a high degree of EPR, PEGylated liposomal doxorubicin alone was twice as effective as a triple chemotherapy combination regimen based on doxorubicin, bleomycin and vincristine (13). In breast cancer, on the other hand, PEGylated liposomal doxorubicin was as (in-) effective as free doxorubicin, with similar response rates and survival times (but with better tolerability (14)). These findings indicate that the higher the degree of EPR-mediated tumor targeting is, the more effective nanomedicine treatment will be, and they therefore imply that imaging how well nanomedicine formulations are able to reach the target site can be used to preselect responders from non-responders.
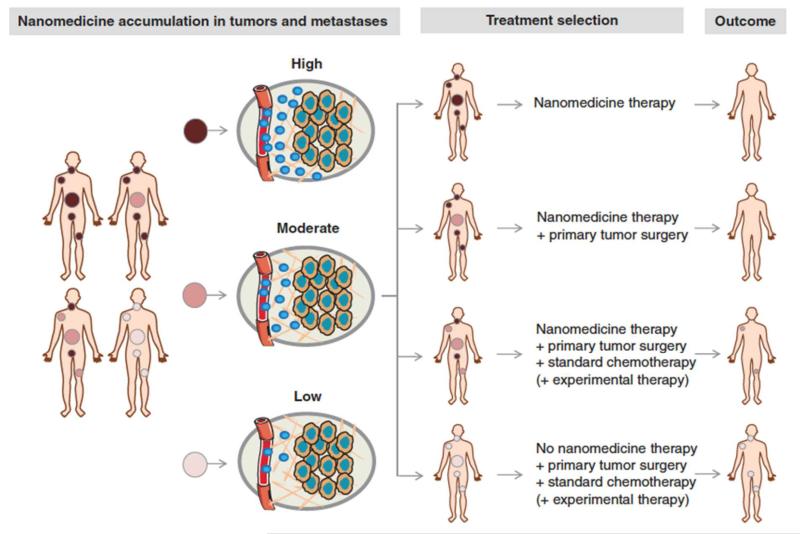
The clinical situation, however, is much less unidirectional and much more complex. This is particularly so because the vast majority of cancer patients do not succumb to a single solid tumor, but to the development of systemic metastases. Imaging and image-guided drug delivery can also be very helpful to treat metastatic cancer patients. If, for instance, on the basis of fluorodeoxyglucose-enhanced positron emission tomography (FDG-PET), it is known that patients present with one solid tumor and with several metastases, then nanomedicine accumulation in all of these lesions should be taken into account, and treatment planning should be performed accordingly. As exemplified by Figure 2, in such situations, it seems obvious that if all lesions are positive for nanomedicine uptake, that patients should then be treated with the nano-formulation in question. And vice versa, that if all lesions accumulate nanomedicines inefficiently, patients should be treated with alternative (non-nano) treatments. It seems likely, however, that the majority of clinical cases will be somewhere in-between, with some lesions being positive, some moderately positive, and some negative. In each individual situation, depending on the exact patient pattern of nanomedicine accumulation, decisions should be made with regard to whether or not to treat the patient in question, and whether or not to add alternative treatments (e.g. surgery, standard chemotherapy, or experimental chemo- or immunotherapy). Such considerations, as well as the development of novel nanotheranostics and nanomedicine-based companion diagnostics, are highly important for shaping the future fate of the (image-guided) drug delivery field.
Figure 2. Towards image-guided and personalized nanomedicine.
Personalized nanomedicine is based on the (pre-) selection of patients on the basis of non-invasive imaging information. Ideally, not only accumulation in primary tumors should be considered, but also localization in systemic metastases. Depending on the accumulation pattern of nanomedicines in tumors and metastases - which can vary quite substantially - optimized treatment regimens can be envisaged for each individual patient.
EXPERT OPINION.
Image-guided drug delivery is useful both at the preclinical and at the clinical level. At the preclinical level, it can be used to assess the pharmacokinetics, the biodistribution and the target site accumulation of nanocarrier materials, to evaluate the potential of active vs. passive tumor targeting, and to visualize and validate (triggered) drug release. At the clinical level, it can be used to pre-select patients likely to respond to nanomedicine therapies. Efforts should be invested in identifying as many cancerous lesions as possible, including both primary tumors and metastases, and in non-invasively quantifying nanomedicine accumulation in all of these lesions, in order to select the most optimal treatment regimen for each individual patient.
ACKNOWLEDGEMENTS
This work was supported by the German Research Foundation (DFG; LA 2937/1-2), by the European Research Council (ERC StG-309495: NeoNaNo), by the European Commission (COST-Action TD1004: Nanotheragnostics) and by the German Academic Exchange Service (DAAD; 290084/2011-3).
REFERENCES
- 1.Farokhzad OC, Langer R. Impact of nanotechnology on drug delivery. ACS Nano. 2009;3:16–20. doi: 10.1021/nn900002m. [DOI] [PubMed] [Google Scholar]
- 2.Moghimi SM, Peer D, Langer R. Reshaping the future of nanopharmaceuticals: Ad Iudicium. ACS Nano. 2011;5:8454–58. doi: 10.1021/nn2038252. [DOI] [PubMed] [Google Scholar]
- 3.Rizzo LY, Theek B, Storm G, et al. Recent progress in nanomedicine: therapeutic, diagnostic and theranostic applications. Curr Opin Biotechnol. 2013;24:1159–66. doi: 10.1016/j.copbio.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fang J, Nakamura H, Maeda H. The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev. 2011;63:136–51. doi: 10.1016/j.addr.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 5.Lammers T, Kiessling F, Hennink W, et al. Drug targeting to tumors: principles, pitfalls and (pre-) clinical progress. J Control Release. 2012;161:175–187. doi: 10.1016/j.jconrel.2011.09.063. [DOI] [PubMed] [Google Scholar]
- 6.Prabhakar U, Maeda H, Jain RK, Sevick-Muraca EM, et al. Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Caner Res. 2013;73:2412–7. doi: 10.1158/0008-5472.CAN-12-4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kunjachan S, Gremse F, Theek B, et al. Non-invasive optical imaging of nanomedicine biodistribution. ACS Nano. 2013;7:252–62. doi: 10.1021/nn303955n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kunjachan S, Pola R, Gremse F, et al. Passive vs. active tumor targeting using RGD-and NGR-modified polymeric nanomedicines. Nano Lett. 2014;14:972–98. doi: 10.1021/nl404391r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gruell H, Langereis S. Hyperthermia-triggered drug delivery from temperature-sensitive liposomes using MRI-guided high intensity focused ultrasound. J Control Release. 2012;161:317–27. doi: 10.1016/j.jconrel.2012.04.041. [DOI] [PubMed] [Google Scholar]
- 10.Negussie AH, Yarmolenko PS, Partanen A, et al. Formulation and characterisation of magnetic resonance-guided high intensity focused ultrasound. Int J Hyperthermia. 2011;27:140–155. doi: 10.3109/02656736.2010.528140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koukourakis MI, Koukourakis S, Giatromanolaki A, et al. High Intratumoral Accumulation of Stealth Liposomal Doxorubicin in Sarcomas. Acta Oncologica. 2000;39:207–211. doi: 10.1080/028418600430789. [DOI] [PubMed] [Google Scholar]
- 12.Harrington KJ, Mohammadtaghi S, Uster PS, et al. Effective targeting of solid tumors in patients with locally advanced cancers by radiolabled pegylated-liposomes. Clin Cancer Res. 2001;7:243–254. [PubMed] [Google Scholar]
- 13.Northfelt DW, Dezube BJ, Thommes JA, et al. Pegylated-liposomal doxorubicin versus doxorubicin, bleomycin, and vincristine in the treatment of AIDS-related Kaposi’s sarcoma: results of a randomized phase III clinical trial. J Clin Oncol. 1998;16:2445–51. doi: 10.1200/JCO.1998.16.7.2445. [DOI] [PubMed] [Google Scholar]
- 14.O’Brien ME, Wigler N, Inbar M, et al. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) vs. conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann Oncol. 2004;15:440–9. doi: 10.1093/annonc/mdh097. [DOI] [PubMed] [Google Scholar]