Abstract
Objective
Promotion of endogenous β-cell mass expansion could facilitate regeneration in patients with diabetes. We discovered that the secreted protein CTGF (aka CCN2) promotes adult β-cell replication and mass regeneration after injury via increasing β-cell immaturity and shortening the replicative refractory period. However, the mechanism of CTGF-mediated β-cell proliferation is unknown. Here we focused on whether CTGF alters cells of the immune system to enhance β-cell replication.
Methods
Using mouse models for 50% β-cell ablation and conditional, β-cell-specific CTGF induction, we assessed changes in immune cell populations by performing immunolabeling and gene expression analyses. We tested the requirement for macrophages in CTGF-mediated β-cell proliferation via clodronate-based macrophage depletion.
Results
CTGF induction after 50% β-cell ablation increased both macrophages and T-cells in islets. An upregulation in the expression of several macrophage and T-cell chemoattractant genes was also observed in islets. Gene expression analyses suggest an increase in M1 and a decrease in M2 macrophage markers. Depletion of macrophages (without changes in T cell number) blocked CTGF-mediated β-cell proliferation and prevented the increase in β-cell immaturity.
Conclusions
Our data show that macrophages are critical for CTGF-mediated adult β-cell proliferation in the setting of partial β-cell ablation. This is the first study to link a specific β-cell proliferative factor with immune-mediated β-cell proliferation in a β-cell injury model.
Keywords: CTGF, β-cell, Proliferation, Regeneration, Macrophages, T-cells
Highlights
-
•
Following partial beta cell ablation, induction of CTGF in beta cells increases the number of pancreatic macrophages and T cells.
-
•
Increased macrophages are found preferentially within islets.
-
•
Expression of genes involved in macrophage and T cell chemoattraction is increased in islets following CTGF induction.
-
•
Depletion of macrophages completely abrogates CTGF-mediated increases in beta cell proliferation after injury.
1. Introduction
Connective Tissue Growth Factor (CTGF/CCN2), a member of the CCN family of secreted extracellular matrix (ECM)-associated proteins, is a β-cell proliferative factor [1], [2], [3]. Although CTGF induction in adult β-cells does not increase proliferation [4], CTGF treatment after 50% β-cell ablation promotes β-cell proliferation and regeneration by modifying β-cell intrinsic characteristics, including maturity state and replication refractory period [2]. We hypothesized that CTGF induction might also elicit β-cell mass regeneration by altering extrinsic factors of the islet micro-environment, in particular immune cell populations. In other models of tissue damage, CTGF promotes wound repair via immune cell modulation [5]. For example, in the kidney, CTGF induction promoted infiltration of both macrophages and T cells [6], suggesting that CTGF serves as an immune cell chemoattractant. Further, in an ethanol model of pancreatic injury, CTGF over-expression increased recruitment of neutrophils and T-cells to the pancreas [7]. CTGF may thus promote the recruitment of particular immune cell populations and/or alter their characteristics, resulting in increased β-cell proliferation and regeneration.
The role of the immune system in β-cell regeneration is well-appreciated [8], [9], [10]. Specifically, macrophages are critical for proper β-cell mass regeneration after injury by partial duct ligation [10] and VEGF-mediated islet endothelial cell expansion [9]. Additionally, several groups have proposed that T cells promote β-cell mass regeneration in the setting of diabetes mellitus [8], [11]. Following 50% β-cell ablation, we observed an increase in macrophages, which was further heightened by CTGF induction after β-cell destruction. In addition, we observed an increase in T cells in the parenchyma only in pancreata from animals with CTGF induction after 50% β-cell ablation. Whole islet gene expression analysis revealed that CTGF induction after β-cell ablation increased the expression of several markers of M1 macrophages and T cells as well as chemoattractant genes. Thus, it appears that CTGF induction after 50% β-cell ablation promotes an increase in both T cells and macrophages.
We assessed the requirement for macrophages in CTGF-mediated β-cell regeneration via macrophage depletion. We observed that a reduction in macrophages (with no changes in T cell number) in this model of β-cell destruction inhibits the proliferative effects elicited by CTGF induction. Also, we propose that macrophage depletion, promotes a more mature β-cell phenotype, via an unknown mechanism, contributing to the inability of β-cells to proliferate in response to CTGF. Together these data suggest a critical role for macrophages during CTGF-mediated β-cell proliferation and regeneration.
2. Materials and methods
2.1. Animals
Generation of RIP-rtTA [12], TetO-CTGF [1], and RIP-DTR [13] transgenic mice has been described previously. Primers are available upon request. Female mice were administered 2 mg/ml of doxycycline (DOX) in 2% Splenda (to avoid taste aversion) in drinking water after diphtheria toxin (DT) administration. Mice were treated with DOX for 2 days. DT (126 ng; Sigma) was given I.P. three times at 8 weeks of age. PBS- or clodronate-liposomes (250 μL; clodronateliposomes.com) were given I.P. once a day for 8 days. Liposomes (artificially prepared lipid vesicles) contained either PBS or clodrinate, a non-toxic bisphosphonate. After injection, liposomes are ingested and digested by macrophages. Clodronate is released and accumulates intracellularly, where at high intracellular concentrations, it induces apoptosis [14]. These studies were all approved by the Vanderbilt University Institutional Animal Care and Use Committee.
2.2. Immunolabeling
Pancreata were dissected and fixed for 1 h in 4% paraformaldehyde at 4°C, and placed in a 30% sucrose solution overnight at 4°C. Pancreata were embedded in O.C.T. (Tissue-Tek) and serial sectioned on a cryostat at 7 μM. Indirect protein localization was obtained by incubations with primary antibodies overnight at 4°C: Guinea Pig α-Insulin (DAKO; 1:500), Rabbit α-Ki67 (AbCam; 1:500), Rabbit α-MafA (Bethyl Laboratories; 1:400), Rat α-CD45 (BD Pharmingen; 1:100), Rat α-F4/80 (Invitrogen; 1:100), Rat α-B220 (BD Pharmingen; 1:100), Rat α-CD3 (BD Pharmingen; 1:00). Nuclei were visualized with DAPI (Molecular Probes). Immunohistochemistry for Neutrophil Marker (Sigma) was completed by Vanderbilt University's Translational Pathology Shared Resource. Imaging was with a ScanScope FL scanner (Aperio Technologies, Inc.) and quantified using Metamorph 6.1 (Molecular Devices). Unless otherwise noted, 4 sections more than 250 μM apart were selected and immunolabeled, with ≥4,000 cells per animal quantified.
2.3. β-cell proliferation
Five slides (at least 250 μm apart) per animal were immunolabeled for insulin and Ki67. A minimum of 4,000 cells were counted using Metamorph 6.1 software (Molecular Devices). The percentage of proliferating cells was determined by dividing the number of Ki67/insulin double-positive cells by the total number of insulin+ cells.
2.4. Analysis of β-cell maturity
Sections were immunolabeled for insulin, and MafA or MafB. Percentage of mature and immature β-cells was determined by dividing number of MafA/insulin double-positive cells or MafB/insulin double positive cells by total number of insulin+ cells, respectively.
2.5. Gene expression analysis
Islets were isolated from 10 week old females and immediately prepared for RNA isolation by dissolving in Trizol reagent. RNA was isolated using the RNeasy Mini kits (Qiagen). Greater than 250 ng cDNA was prepared from islet RNA using the SuperScript III First Stand Synthesis System (Invitrogen). TLDAs were conducted on a 7900HT Fast Real-Time PCR system. Data was analyzed with SDS RQ Study software (Applied Biosystems, Life Technologies). cDNA for qRT-PCR was generated using the iScript cDNA synthesis kit (BioRad). Relative gene expression was assayed by the FAM-conjugated TaqMan Gene Expression Assay (Life Technologies) on a BioRad CFX Real Time PCR Instrument (BioRad). Gene expression for qRT-PCR analysis is relative to Emr1, after first normalizing to GAPDH. Statistical comparisons were analyzed by the Pfaffl method. Primer sequences available upon request.
2.6. Quantification of immune cell populations
Sections were immunolabeled for insulin and immune cell markers; CD45 (pan-immune), B220 (B cells), CD3 (T cells), Neutrophil Marker (neutrophils), and F4/80 (macrophages) as described earlier. One pancreatic section from each slide was imaged via a ScanScope FL slide scanner (Aperio Technologies, Inc.). Five random insulin+ areas (40002 pixels) were extracted per slide. Through Metamorph 6.1 software (Molecular Devices) immune cells were binned as either islet associated or within the exocrine compartment. Macrophage proliferation was quantified by dual labeling for F4/80 and Ki67. Sections were imaged via a ScanScope FL slide scanner. Five random pancreatic areas (40002 pixels) were extracted per slide and the percentage of Ki67 positive F4/80 positive cells out of the total number of F4/80 positive cells was quantified using Metamorph 6.1
2.7. Statistics
Results are expressed as mean ± SEM. Statistical significance was calculated by Student's T test, One-way, or Two-way ANOVA analysis where applicable. p ≤ 0.05 was considered significant.
3. Results
3.1. CTGF induction increases islet-associated macrophages and T cells at the peak of β-cell proliferation
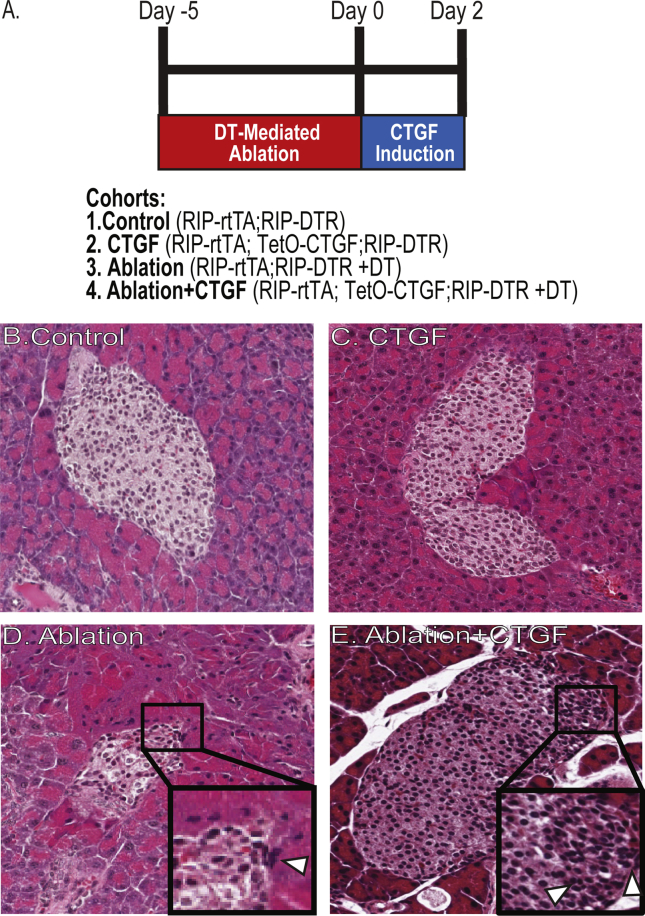
We previously showed that CTGF promotes β-cell mass regeneration, without altering α-cell proliferation or number [2]. This was achieved by using a diphtheria toxin (DT)-mediated mouse model (RIP-DTR) of 50% β-cell ablation paired with our previously described β-cell specific doxycycline (Dox)-inducible CTGF bi-transgenic model (RIP-rtTA; TetO-CTGF) [1], [4], [13]. This model of β-cell ablation does not result in alterations in glucose homeostasis [2]. At 8 weeks of age, DT was administered to RIP-DTR; RIP-rtTA controls (“Ablation”) and RIP-DTR; RIP-rtTA; TetO-CTGF experimental animals (“Ablation + CTGF”) and CTGF induced for 2 days (Figure 1A). This time was chosen as it is the peak of β-cell proliferation in Ablation + CTGF pancreata [2]. Non-DT injected animals included controls (“Control”) and those in which CTGF was induced without ablation (“CTGF”). β-cell regeneration occurs only in the Ablation + CTGF animals, neither CTGF induction under normal conditions nor 50% β-cell ablation alone elicits β-cell proliferation or mass expansion [2].
Figure 1.
Experimental design for β-cell ablation and CTGF overexpression. (A) Experimental outline and cohorts. (B–E) H&E staining for immune cell detection adjacent to islets. Representative images of Control (B), CTGF (C), Ablation (D), and Ablation + CTGF (E) islets after 2 days of CTGF induction. Insets highlight small, dark and closely clustered nuclei that are indicative of immune cells (white arrows).
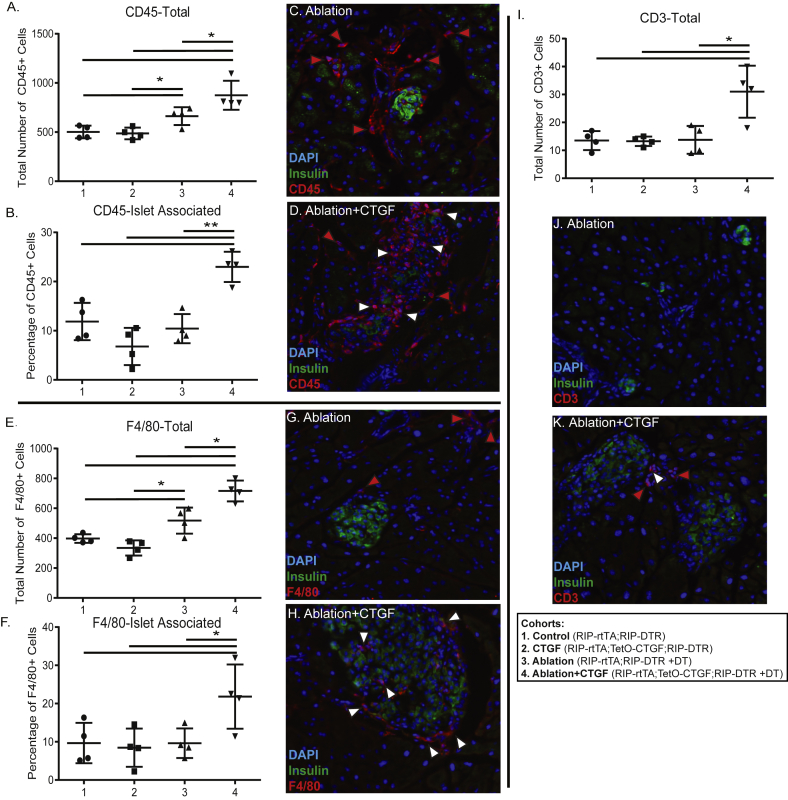
To assess whether CTGF induction increased the population of immune cells in the pancreas, Hematoxylin and Eosin (H&E) staining was conducted (Figure 1B–E). In both the Ablation and Ablation + CTGF cohorts, analysis of H&E staining indicated the presence of inflammation and increased immune cells, in the absence of fibrosis (Figure 1D,E [2]). In order to confirm the increase in immune cells in the pancreas parenchyma, immunohistochemistry with the pan-leukocyte marker, CD45, was conducted at the 2 day time point (Figure 2A–D). CTGF induction under normal conditions in adult islets elicited no increase in leukocyte number (Figure 2A). However, 50% β-cell ablation alone did promote an increase in the number of leukocytes in the pancreas (Figure 2A) although these cells were not targeted preferentially to the islets (Figure 2B). The increase in immune cell number in response to ablation was further heightened upon CTGF induction after injury (Figure 2A,D), and there was a greater proportion of CD45+ cells localized to islets (Figure 2B,D).
Figure 2.
Alterations to immune cell populations by β-cell ablation and CTGF induction. (A) Total number of pancreatic CD45 + cells and (B) Proportion of islet-localized CD45-positive cells. (C–D) Representative images of Ablation (C) and Ablation + CTGF (D) islets after 2 days CTGF. Insulin (green), CD45 (red). (E) Total number of F4/80-positive cells and (F) Proportion of islet-localized macrophages. (G–H) Representative images of Ablation (G) and Ablation + CTGF (H) islets after 2 days CTGF. Insulin (green), F4/80 (red). (I) Total number of CD3-positive cells. (J–K) Representative images of Ablation (J) and Ablation + CTGF (K) islets after 2 days CTGF. Insulin (green), CD3 (red). Immune cells within the exocrine or endocrine compartments are demarked by red and white arrows, respectively. n = 4. *p < 0.05, **p < 0.01.
To determine which specific immune populations were increasing following ablation and CTGF, immunohistochemistry for immune cell populations was conducted. The largest proportion of immune cells in any of the cohorts was macrophages, as detected by immunolabeling against F4/80 (Figure 2E–H). 50% β-cell ablation did elicit an increase in pancreatic macrophages (Figure 2E); CTGF induction further enhanced this increase (Figure 2E,H). Additionally, a greater proportion of macrophages were islet associated in the Ablation + CTGF cohort as compared to all other groups (Figure 2F,H). Interestingly, only CTGF induction after β-cell ablation elicited a modest increase in T cells (Figure 21,K), as assessed by CD3 immunolabeling (Figure 2J–K). The increased T cells were not specifically targeted to islets (not shown). Very few B cells, as detected by B220 immunolabeling (Supplemental Figure 1A,C–F), were observed within the pancreatic parenchyma of any cohort, and rarely were they observed close to islets (Supplemental Figure 1B,C-F) Additionally, as CTGF has been shown to recruit neutrophils in other models of pancreatic injury, we assessed this immune population via immunohistochemistry (Supplemental Figure 1I–L). However, in our model of β-cell ablation, no significant increase of neutrophils was observed (Supplemental Figure 1G–L). Finally, we observed no presence of eosinophils, as assessed by H&E staining, in any cohort (not shown).
3.2. Gene expression analyses reveal changes associated with increased macrophages and T cells
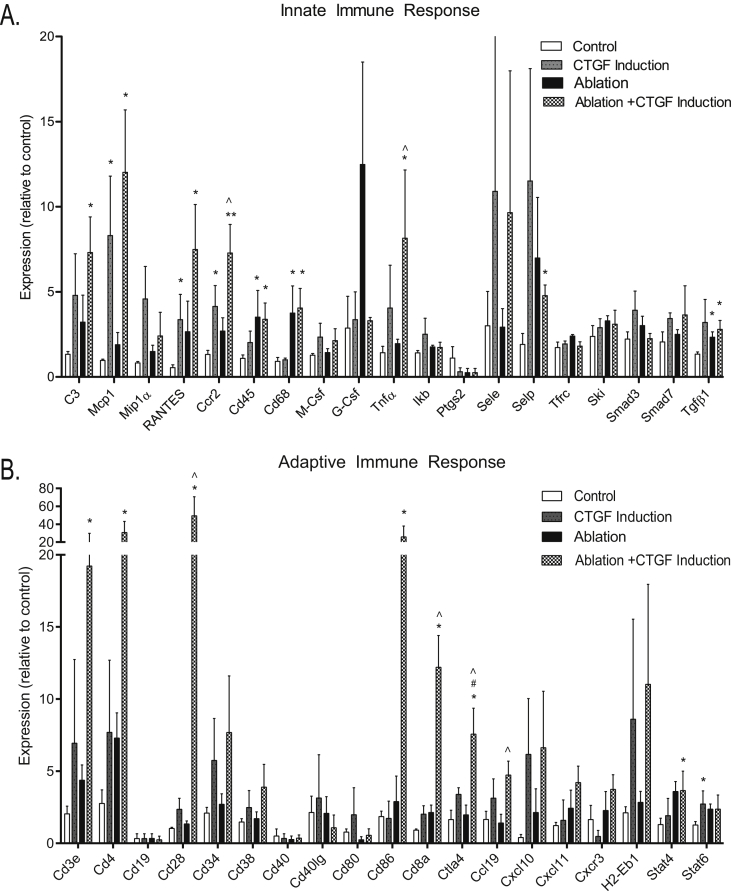
To gain further insight into which immune cell populations and associated signaling pathways are altered by CTGF with or without β-cell ablation, gene expression analysis was conducted on islets isolated from animals following 2 days of CTGF induction in vivo. We specifically assessed changes in expression of genes associated with the innate and adaptive immune cell response, including cytokine expression changes (Figure 3; Supplemental Figure 2A). In addition, gene expression alterations in ECM components, vascular markers, and the stress response were determined (Supplemental Figure 2B,C).
Figure 3.
CTGF induces expression of genes involved in the innate (A) and adaptive (B) immune response. Gene expression analysis on whole islets using Taqman Universal PCR Mastermix. Islets isolated from animals with/without β-cell ablation ± CTGF induction for 2 days. All samples were run in duplicate. n = 4. * compared to Control, # compared to CTGF induction, ˆ compared to Ablation. *, #, ˆp < 0.05, **p < 0.01.
There were several changes in key innate and adaptive immune response genes following ablation and/or CTGF induction (Figure 3A,B). CTGF induction under normal conditions or after β-cell ablation elicited an increase in Mcp1 (Macrophage Chemoattractant Protein 1), RANTES (Regulated on Activation, Normal T cell Expressed and Secreted/CCL5), and Ccr2 (C–C chemokine receptor type 2). MCP1 and its receptor, Ccr2, serve as chemoattractants for macrophages [15], [16], in agreement with the immunolabeling results showing increased macrophages in islets. In addition, RANTES promotes macrophage activation along with T cell recruitment [17], further corroborating the observed increase in T cells in our Ablation + CTGF cohort. β-cell ablation alone and in conjunction with CTGF induction increased expression of CD45, a pan-leukocyte marker, and CD68, a macrophage marker, once again aligning with immunolabeling findings. These results were highly suggestive of a critical role for macrophages in CTGF-mediated β-cell proliferation. The unique setting of CTGF induction after β-cell ablation resulted in the specific upregulation of C3 (Complement Component 3), TNFα (Tissue Necrosis Factor α), and Selp (Selectin P). These genes are all associated with inflammation [18], [19], while Selp also serves as a leukocyte chemoattractant [20].
Alterations in expression of genes associated with the adaptive immune response focused primarily on T cells (Figure 3B). CTGF induction under normal conditions did not promote the expression of any genes associated with the adaptive immune response (Figure 3B). However, β-cell ablation alone or with CTGF induction increased the expression of CD3e, a marker of T cells, and Stat4, a promoter of Th1 development (Figure 3B; Ablation; [21]). Expression of several genes was increased only in the Ablation + CTGF cohort (Figure 3B; Ablation + CTGF). These included several additional markers of T cells, including CD4 (T helper cells), CD28 (costimulator necessary for T cell activation), and CD8a (Cytotoxic T cells). Additionally, CTGF induction after β-cell ablation elicited the increased expression of macrophage-expressed genes that promote T cell activation (CD86) and trafficking (Ccl19) [22], [23]. Finally, CTGF induction alone promoted the expression of Ctla4 (Cytotoxic T Lymphocyte Associated protein 4), which downregulates T cell activation [24] (Figure 3A). As predicted by immunolabeling, we did not observe changes in expression of genes associated with B cells (Figure 3B, CD19, CD40, CD38). We also assessed changes in the expression of several cytokines (Supplemental Figure 2A). However, the only observed alteration was with IL-12b (Interluekin-12b), which was induced by CTGF expression after β-cell ablation and under normal settings (Supplemental Figure 2A). IL-12b is expressed by macrophages and aids T helper cell development [25]. Overall, these findings align well with our observed increase in T cells in the Ablation + CTGF cohort (Figure 2I), suggesting that CTGF induction promotes β-cell regeneration through macrophages and/or T cells.
Finally, we assessed alterations to genes associated with the ECM and vasculature, which play key roles in immune cell trafficking (Supplemental Figure 2B). In our model Vcam1 (Vascular Cell Adhesion Molecule 1) was the sole gene significantly upregulated, and only with CTGF induction after β-cell ablation (Supplemental Figure 2B). Vcam1 is critical for adhesion of leukocytes to endothelial cells and subsequent signal transduction, leading to extravasation [26]. Increased Vcam1 expression, suggested to us that the increase in macrophages was due to increased extravasation from the pancreatic vasculature. As an alternative, we examined whether CTGF increased macrophage proliferation, but failed to detect any proliferating macrophages (Supplemental Figure 3). Thus, increased macrophage recruitment, rather than proliferation of resident pancreatic macrophages in response to CTGF, appears to cause the increase in islet-associated macrophages in our model.
We also assessed whether our model of CTGF mediated β-cell regeneration involved induction or alterations to the cellular stress response (Supplemental Figure 2C). However, no alterations were observed. Thus, it appears that in CTGF-mediated β-cell mass expansion after β-cell ablation, CTGF induction promotes an increase in and activation of primarily macrophages and T cells.
3.3. Macrophages are required for CTGF-mediated β-cell proliferation
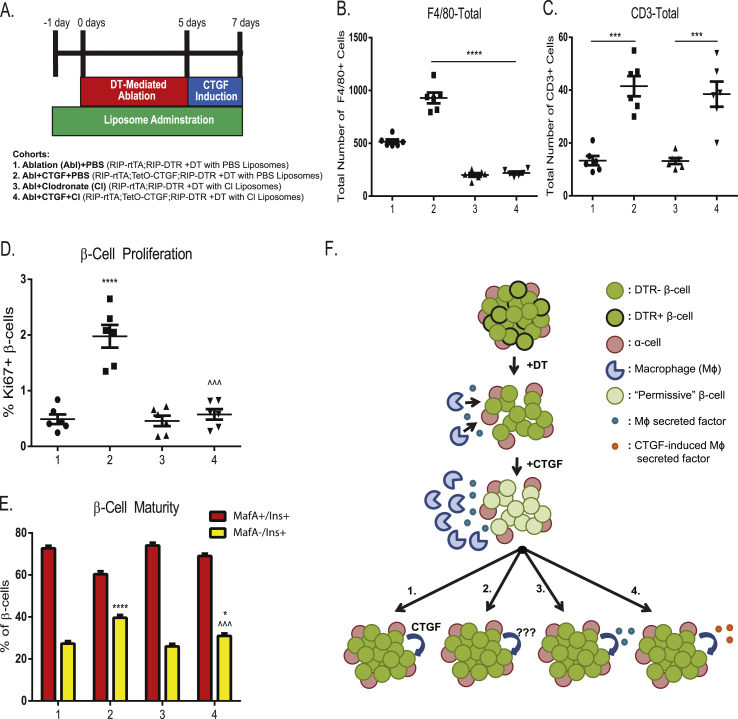
In order to assess whether infiltrating macrophages are involved in CTGF-mediated β-cell proliferation, we conducted macrophage depletion using liposomes containing clodronate. Clodronate liposomes were administered, one day prior to, during, and for 2 days following DT injections in 8 week old RIP-DTR; RIP-rtTA controls (“Ablation + Clodronate”) and RIP-DTR; RIP-rtTA; TetO-CTGF experimental animals (“Ablation + CTGF + Clodronate”) and CTGF induced by Dox induction for 2 days after DT injection. Additional controls included RIP-DTR; RIP-rtTA animals injected with PBS-containing liposomes (“Ablation + PBS”) and DTR; RIP-rtTA; TetO-CTGF animals injected with PBS-containing liposomes in which CTGF was induced (“Ablation + CTGF + PBS”) (Figure 4A).
Figure 4.
CTGF-mediated β-cell regeneration is dependent on macrophages. (A) Experimental outline and cohorts. Total number of (B) F4/80- and (C) CD3-positive cells. (D) β-cell proliferation. (E) Proportion of MafA+ (red bars) or MafA− (yellow bars) β-cells. (F) Model of potential mechanisms of CTGF mediated β-cell mass regeneration. After 50% β-cell ablation, macrophages enter the islet to remove dead β-cells. Upon CTGF induction, more macrophages are recruited to the islet, elevating the levels of a secreted factor that promotes β-cell permissiveness to the proliferative factor. The β-cell proliferative factor is: 1. CTGF itself, 2. other CTGF-induced proliferative factors, such as 5-HT, Integrin β1, or HGF, 3. elevated levels of a macrophage-derived factor, or 4. upon CTGF induction, the character of recruited macrophages is altered by CTGF (i.e. polarization, chemokine profile etc.) resulting in the secretion of CTGF-induced macrophage factor. n = 6 (B–C) ***p < 0.001, ****p < 0.0001. (D) ****p < 0.0001 comparing 2 vs 1,3. ###p < 0.0001 comparing 4 vs 2. (E) *p < 0.05 comparing 4 vs 1,3. ****p < 0.0001 comparing 2 vs 1,3. ˆ ˆ ˆ p < 0.001 comparing 4 vs 2.
F4/80 immunolabeling confirmed clodronate induction-specific depletion of macrophages (Figure 4B). Additionally, since macrophages can serve as T cell chemoattractors [27], we assessed whether removal of macrophages also decreased the observed increase in T cells in our “Ablation + CTGF” cohort (Figure 2I). However, clodronate liposome treatment does not result in a decrease in pancreatic T cells (Figure 4C). Thus, we were able to assess the macrophage-specific effects on CTGF-mediated β-cell proliferation.
CTGF induction elicits β-cell regeneration by increasing β-cell proliferation [2]. Thus, we assessed whether the increase of macrophages after β-cell ablation was required for increased β-cell proliferation. Depletion of macrophages during CTGF-mediated β-cell regeneration decreased the percentage of proliferating β-cells to control cohort levels (Figure 4D; 2 vs. 4). Thus, macrophages are essential for CTGF-mediated increases in β-cell proliferation.
Since 50% β-cell ablation promotes a more immature β-cell phenotype, which is further heightened upon CTGF induction [2], we also assessed the requirement of macrophages for this effect. Macrophage depletion decreased the percentage of immature (MafA−) β-cells as compared to the Ablation + CTGF control cohort (Figure 4E; 2 vs 4). However, it must be noted that “Ablation + CTGF + Clodrinate” islets are still more phenotypically immature as compared to control, non-ablated islets (Figure 4E 1,3 vs 4), [2]). We also assessed β-cell immaturity via MafB immunolabeling. We observed no significant difference in the percentage of immature (MafB+) β-cells in our Ablation + CTGF + Clodrinate cohort as compared to the Ablation cohorts (Data not shown). Thus, macrophages are required for both the β-cell proliferative and maturity state alterations mediated by CTGF after 50% β-cell ablation.
4. Discussion
In other pancreatic injury models, CTGF recruits immune cells to the site of injury [7]. Here we investigated the potential role of the immune system in CTGF-mediated β-cell proliferation after partial β-cell destruction. Importantly, blood glucose homeostasis is unaffected by partial β-cell ablation or CTGF induction. β-cell destruction and CTGF induction in the setting of DT-mediated β-cell ablation increased the total number of macrophages and T cells in the pancreas and heightened the number of macrophages specifically within the endocrine compartment. In fact, macrophages comprised the vast majority of immune cells within the pancreas. Our findings are consistent with studies from other groups who have shown that macrophages are involved in β-cell mass regeneration following injury [9], [10]. However, our study is the first to our knowledge, to utilize a β-cell-specific injury model and is the first to link a specific β-cell proliferative factor to increased macrophage numbers and enhanced β-cell proliferation.
We considered the possibility that a fraction of proliferating insulin-positive cells were actually proliferating macrophages that had engulfed β-cells. However, we failed to detect any proliferating macrophages nor did we observe any cells co-labeled with insulin and F4/80, arguing against this idea. Additionally, gene expression analysis showed that CTGF induction specifically increased expression of several macrophage markers and macrophage chemoattractant genes. Several genes (CD86, Ccl19, IL-12b) associated with the pro-inflammatory M1 macrophage polarization phenotype were increased in the setting of CTGF induction after β-cell destruction. Likewise, preliminary qRT-PCR data showed a highly significant decrease in Arg1 expression, a gene associated with M2 macrophage polarization, in islets from Ablation + CTGF mice compared to Control islets (not shown). Mgl1 and Chil3, two other genes associated with M2 macrophage polarization, also displayed a trend towards decreased expression in Ablation + CTGF samples compared to Ablation alone (not shown). Further studies are needed to fully characterize the macrophage polarization phenotype in this model of β-cell regeneration.
The increase in T cells specifically in the setting of CTGF induction after β-cell destruction was not unexpected, as studies have proposed that T cells promote β-cell mass regeneration in the setting of diabetes mellitus [8], [11]. Also, CTGF promotes T cell recruitment in the kidney [6]. Gene expression analysis on whole islets corroborated our immunofluorescence findings as upregulation of several T cell markers and chemoattractant genes was observed in the Ablation + CTGF cohort. While several of the T cell marker genes pointed towards a T helper cell population, further studies are needed to determine the specific T cell sub-population(s) involved in CTGF-mediated β-cell proliferation. Our current analysis cannot distinguish whether CTGF induction after β-cell ablation recruits T cells from outside the pancreas to the pancreas/islets or if CTGF promotes proliferation of pre-existing resident pancreatic T cells. Regardless, our data demonstrate that T cells are not required for CTGF-mediated β-cell proliferation in this model.
Through clodronate liposome-based macrophage depletion techniques, we observed that macrophages are absolutely essential for CTGF-mediated increases in β-cell proliferation in the setting of β-cell destruction. Additionally, macrophages may play a role in β-cells adapting a more immature phenotype after β-cell ablation, as removal of macrophages in the presence of Ablation + CTGF appears to prevent the decline in MafA+ β-cells we observed in Ablation + CTGF alone.
We propose a potential model on how CTGF promotes β-cell mass expansion (Figure 4F). An increase in macrophages is observed following β-cell destruction. However, these macrophages alone are unable to promote β-cell proliferation. We hypothesize that CTGF induction increases the number of islet-associated macrophages, thus elevating the levels of an as yet unidentified factor above a required threshold to allow for β-cell responsiveness (Figure 4F). CTGF-mediated macrophage recruitment alters the islet microenvironment to become more responsive to proliferative stimuli. We propose four potential candidates for this proliferative stimulus (Figure 4): 1) CTGF itself, 2) other proliferative factors induced by CTGF (i.e. Hepatocyte Growth Factor (HGF), Serotonin (5-HT), Integrin β1, [2]), 3) an endogenous macrophage-derived factor or 4) a novel macrophage-derived factor induced by CTGF. In other words, CTGF could affect the concentration of a factor or the type of factor being produced.
The relevance of CTGF to human patients with diabetes and diabetic animal models remains unknown. A gene expression analysis reported by Keller et al., found a reduction in Ctgf expression in islets from 4 week old but not 10 week old non-diabetic ob/ob animals compared with controls [28]. The relationship between the difference in CTGF expression and the impact it had on β-cell proliferation is not clear.
CTGF mediated β-cell mass proliferation involves the modification of several β-cell intrinsic characteristics. We now show that the immune system, specifically macrophages, is also required for effective CTGF-mediated β-cell mass expansion following injury. We have identified a novel interaction between CTGF and the immune system to promote β-cell proliferation and thus, regeneration. As all forms of diabetes are demarked by both inflammation and insufficient functional β-cell mass, our studies highlight the significance of understanding the role of the immune system in promoting β-cell regeneration.
Acknowledgments
We thank Anastasia Coldren for islet isolations; and Dr. Alvin C. Powers (Vanderbilt) and Dr. Pedro Herrera (Geneva) for reagents. We thank Dr. Mark Keller (University of Wisconsin) for helpful discussions. This research involved use of the Islet Procurement and Analysis Core of the Vanderbilt Diabetes Research and Training Center supported by NIH grant DK20593. The VANTAGE facility was used for TLDA experiments (supported by the Vanderbilt-Ingram Cancer Center P30 CA58648, the Vanderbilt Vision Center P30 EY08126, and NIH/NCRR G20 RR030956). Finally, neutrophil immunohistochemistry involved the use of the Translational Pathology Share Resource funded by the Mouse Metabolic Phenotyping Center (5U24 DK059637). This work was supported by the Juvenile Diabetes Research Foundation International (1-2011-592 & 1-INO-2014-177-A-V), a Department of Veteran's Affairs Merit Review Award (1BX00090-01A1, to M.G.), a Department of Veteran's Affairs Merit Review Award (5I01BX002195, to A.H.H.), and an American Heart Association Postdoctoral Fellowship (14POST20380262, to R.C.P).
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molmet.2015.05.002
Conflicts of interest
The authors have no conflicts of interest to declare.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Guney M.A., Petersen C.P., Boustani A., Duncan M.R., Gunasekaran U., Menon R. Connective tissue growth factor acts within both endothelial cells and beta cells to promote proliferation of developing beta cells. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(37):15242–15247. doi: 10.1073/pnas.1100072108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riley K.G., Pasek R.C., Maulis M.F., Peek J., Thorel F., Brigstock D.R. CTGF modulates adult beta-cell maturity and proliferation to promote beta-cell regeneration in mice. Diabetes. 2015;64(4):1284–1296. doi: 10.2337/db14-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crawford L.A., Guney M.A., Oh Y.A., Deyoung R.A., Valenzuela D.M., Murphy A.J. Connective tissue growth factor (CTGF) inactivation leads to defects in islet cell lineage allocation and beta-cell proliferation during embryogenesis. Molecular Endocrinology. 2009;23(3):324–336. doi: 10.1210/me.2008-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gunasekaran U., Hudgens C.W., Wright B.T., Maulis M.F., Gannon M. Differential regulation of embryonic and adult beta cell replication. Cell Cycle. 2012;11(13):2431–2442. doi: 10.4161/cc.20545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alfaro M.P., Deskins D.L., Wallus M., DasGupta J., Davidson J.M., Nanney L.B. A physiological role for connective tissue growth factor in early wound healing. Laboratory Investigation; A Journal of Technical Methods and Pathology. 2013;93(1):81–95. doi: 10.1038/labinvest.2012.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanchez-Lopez E., Rayego S., Rodrigues-Diaz R., Rodriguez J.S., Rodrifues-Diaz R., Rodriguez-Vita J. Connective tissue growth factor (CTGF): a key factor in the onset and progression of kidney damage. Nefrologia. 2009;29(5):382–391. doi: 10.3265/Nefrologia.2009.29.5.5429.en.full. [DOI] [PubMed] [Google Scholar]
- 7.Charrier A., Chen R., Kemper S., Brigstock D.R. Regulation of pancreatic inflammation by connective tissue growth factor (CTGF/CCN2) Immunology. 2014;141(4):564–576. doi: 10.1111/imm.12215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nir T., Melton D.A., Dor Y. Recovery from diabetes in mice by beta cell regeneration. The Journal of Clinical Investigation. 2007;117(9):2553–2561. doi: 10.1172/JCI32959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brissova M., Aamodt K., Brahmachary P., Prasad N., Hong J.Y., Dai C. Islet microenvironment, modulated by vascular endothelial growth factor-A signaling, promotes beta cell regeneration. Cell Metabolism. 2014;19(3):498–511. doi: 10.1016/j.cmet.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiao X., Gaffar I., Guo P., Wiersch J., Fischbach S., Perrish L. M2 macrophages promote beta-cell proliferation by up-regulation of SMAD7. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(13):E1211–E1220. doi: 10.1073/pnas.1321347111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dirice E., Kahraman S., Jiang W., El Ouaamari A., De Jesus D.F., Teo A.K. Soluble factors secreted by T cells promote beta-cell proliferation. Diabetes. 2014;63(1):188–202. doi: 10.2337/db13-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Milo-Landesman D., Surana M., Berkovich I., Compagni A., Christofori G., Fleischer N. Correction of hyperglycemia in diabetic mice transplanted with reversibly immortalized pancreatic beta cells controlled by the tet-on regulatory system. Cell Transplant. 2001;10(7):645–650. [PubMed] [Google Scholar]
- 13.Thorel F., Nepote V., Avril I., Kohno K., Desgraz R., Chera S. Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature. 2010;464(7292):1149–1154. doi: 10.1038/nature08894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Rooijen N., Hendrikx E. Liposomes for specific depletion of macrophages from organs and tissues. Methods in Molecular Biology. 2010;605:189–203. doi: 10.1007/978-1-60327-360-2_13. [DOI] [PubMed] [Google Scholar]
- 15.Carr M.W., Roth S.J., Luther E., Rose S.S., Springer T.A. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(9):3652–3656. doi: 10.1073/pnas.91.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aragay A.M., Mellado M., Frade J.M., Martin A.M., Jimenez-Sainz M.C., Martinez-A C. Monocyte chemoattractant protein-1-induced CCR2B receptor desensitization mediated by the G protein-coupled receptor kinase 2. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(6):2985–2990. doi: 10.1073/pnas.95.6.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schall T.J., Bacon K., Toy K.J., Goeddel D.V. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature. 1990;347(6294):669–671. doi: 10.1038/347669a0. [DOI] [PubMed] [Google Scholar]
- 18.Sahu A., Lambris J.D. Structure and biology of complement protein C3, a connecting link between innate and acquired immunity. Immunological Reviews. 2001;180:35–48. doi: 10.1034/j.1600-065x.2001.1800103.x. [DOI] [PubMed] [Google Scholar]
- 19.Wajant H., Pfizenmaier K., Scheurich P. Tumor necrosis factor signaling. Cell Death & Differentiation. 2003;10(1):45–65. doi: 10.1038/sj.cdd.4401189. [DOI] [PubMed] [Google Scholar]
- 20.Lorenzon P., Vecile E., Nardon E., Ferrero E., Harlan J.M., Tedesco F. Endothelial cell E- and P-selectin and vascular cell adhesion molecule-1 function as signaling receptors. Journal of Cell Biology. 1998;142(5):1381–1391. doi: 10.1083/jcb.142.5.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wurster A.L., Tanaka T., Grusby M.J. The biology of Stat4 and Stat6. Oncogene. 2000;19(21):2577–2584. doi: 10.1038/sj.onc.1203485. [DOI] [PubMed] [Google Scholar]
- 22.Cohen J. New protein steals the show as ‘costimulator’ of T cells. Science. 1993;262(5135):844–845. doi: 10.1126/science.7694360. [DOI] [PubMed] [Google Scholar]
- 23.Rossi D.L., Vicari A.P., Franz-Bacon K., McClanahan T.K., Zlotnik A. Identification through bioinformatics of two new macrophage proinflammatory human chemokines: MIP-3alpha and MIP-3beta. Journal of Immunology. 1997;158(3):1033–1036. [PubMed] [Google Scholar]
- 24.Walunas T.L., Lenschow D.L., Bakker C.Y., Linsley P.S., Freeman G.J., Green J.M. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1(5):405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 25.Li L., Hsu H.C., Stockard C.R., Yang P., Zhou J., Wu Q. IL-12 inhibits thymic involution by enhancing IL-7- and IL-2-induced thymocyte proliferation. Journal of Immunology. 2004;172(5):2909–2916. doi: 10.4049/jimmunol.172.5.2909. [DOI] [PubMed] [Google Scholar]
- 26.Barreiro O., Yarnez-Mo M., Serrador J.M., Montoya M.C., Vicente-Manzanares M., Tejedor R. Dynamic interaction of VCAM-1 and ICAM-1 with moesin and ezrin in a novel endothelial docking structure for adherent leukocytes. The Journal of Cell Biology. 2002;157(7):1233–1245. doi: 10.1083/jcb.200112126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lyons C.R., Lipscomb M.F. Alveolar macrophages in pulmonary immune responses. I. Role in the initiation of primary immune responses and in the selective recruitment of T lymphocytes to the lung. Journal of Immunology. 1983;130(3):1113–1119. [PubMed] [Google Scholar]
- 28.Keller M.P., Choi Y., Wang P., Davis D.B., Rabaglia M.E., Oler A.T. A gene expression network model of type 2 diabetes links cell cycle regulation in islets with diabetes susceptibility. Genome Research. 2008;18(5):706–716. doi: 10.1101/gr.074914.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.