Abstract
Background & aims
Fibroblast growth factor 21 (FGF-21) is a liver-derived metabolic regulator induced by energy deprivation. However, its regulation in humans is incompletely understood. We addressed the origin and regulation of FGF-21 secretion in humans.
Methods
By determination of arterial-to-venous differences over the liver and the leg during exercise, we evaluated the organ-specific secretion of FGF-21 in humans. By four different infusion models manipulating circulating glucagon and insulin, we addressed the interaction of these hormones on FGF-21 secretion in humans.
Results
We demonstrate that the splanchnic circulation secretes FGF-21 at rest and that it is rapidly enhanced during exercise. In contrast, the leg does not contribute to the systemic levels of FGF-21. To unravel the mechanisms underlying the regulation of exercise-induced hepatic release of FGF-21, we manipulated circulating glucagon and insulin. These studies demonstrated that in humans glucagon stimulates splanchnic FGF-21 secretion whereas insulin has an inhibitory effect.
Conclusions
Collectively, our data reveal that 1) in humans, the splanchnic bed contributes to the systemic FGF-21 levels during rest and exercise; 2) under normo-physiological conditions FGF-21 is not released from the leg; 3) a dynamic interaction of glucagon-to-insulin ratio regulates FGF-21 secretion in humans.
Keywords: Liver, Hepatic, Hepatokine, Exercise
Abbreviations: FFA, free fatty acids; FGF-21, fibroblast growth factor-21; PPAR, peroxisome proliferator-activated receptor
Highlights
-
•
In humans, the splanchnic bed contributes to the systemic FGF-21 levels during rest and exercise.
-
•
Under normo-physiological conditions FGF-21 is not released from the leg in humans.
-
•
In humans, a dynamic interaction of glucagon-to-insulin ratio regulates FGF-21 secretion.
1. Introduction
Fibroblast growth factor-21 (FGF-21) is a circulating member of the FGF superfamily, which is primarily expressed in the liver [1]. FGF-21 is regarded as an important endocrine metabolic regulator [2,3] and holds promise as a therapeutic target in metabolic disorders such as type 2 diabetes [4]. However, the regulation of FGF-21 in humans remains incompletely understood.
In humans and particularly in mice, long-term energy deprivation such as fasting has been shown to increase circulating FGF-21 [5,6]. During fasting, several signals have been reported to be involved in mediating an increase in systemic FGF-21. In humans, free fatty acids (FFAs) [7], low protein intake independent of energy restriction [8] and glucagon [9,10] have been linked to increased plasma FGF-21. But whereas glucagon increases circulating FGF-21 [9–11] contrasting findings exist regarding the role of insulin [12,13]. Both animal and in vitro studies have demonstrated that FGF-21 is regulated in hepatocytes by peroxisome proliferator-activated receptor (PPAR) α agonism [14–16] and ketone bodies [14]. Collectively, prolonged conditions of energy deprivation induce hepatic FGF-21 secretion and several regulatory signals have been proposed.
During exercise, the body undergoes a state of acute energy deprivation. In order to maintain glucose homoeostasis, circulating levels of glucagon increase whereas insulin levels decrease [17–19]. In addition, free fatty acids (FFAs) increase, and, with prolonged exercise, circulating levels of ketone bodies increase [20,21]. Consequently, energy deprivation by fasting and acute exercise induces a similar pancreatic hormone response and similar metabolite signals [22–25]. Importantly, FGF-21 increases with acute exercise [11,26], suggesting that FGF-21 is regulated within a short time frame.
Many of the effects of FGF-21 signalling are similar to the beneficial effects of physical activity on metabolism, and it is likely that FGF-21 represents a key mediator of the exercise-induced metabolic improvements. FGF-21 signalling leads to metabolically beneficial effects including correction of hyperglycemia, lowering of plasma lipidemia and reduction of hepatic steatosis [2,14,27]. Most of these processes are believed to occur in the adipose tissue and the liver. However, skeletal muscle cells also secrete FGF-21 [28] during mitochondrial stress, and FGF-21 has been suggested to be a so-called “mitokine” [29,30]. Thus, the possibility existed that skeletal muscles could contribute to the circulating levels of FGF-21 during exercise.
The aim of this study was to identify the source of FGF-21 during exercise in humans and identify its regulation. By applying unique invasive techniques, we were able to study net-fluxes over the splanchnic bed as well as skeletal muscle at rest and during exercise, allowing us to determine if FGF-21 is secreted from the liver and/or skeletal muscles in humans. By experimentally mimicking the exercise-induced changes in glucagon/insulin levels in resting subjects, we evaluated hormonal regulatory mechanisms involved in FGF-21 secretion.
2. Materials and methods
2.1. Ethical committee approvals
The studies were approved by the Scientific Ethics Committee of the capital region of Denmark: The exercise study with hepatic vein and brachial artery catheterisation and the hormone infusion study were approved under the same ethical committee number: H-1-2012-129. The ethical committee approval for the exercise study with femoral vein and femoral artery catheterisation has previously been published [31]. All studies were executed in accordance with the Helsinki Declaration. All subjects provided written informed consent to participate.
2.2. Exercise study with hepatic vein and brachial artery catheterisation
In ten healthy males, catheters were placed in an antecubital vein, the right hepatic vein and the brachial artery of the non-dominant arm. The subjects exercised on an adjusted cycle ergometer in semi-supine position at 60% of VO2 max for 2 h and then rested for 4 h in the same position. Estimation of hepatic blood flow was performed by the indocyanine green (ICG) technique [32]. For further details on the experimental procedures, see Supplementary Materials and Methods.
2.3. Exercise study with femoral vein and femoral artery catheterisation
This study has previously been described [31]. Nine healthy males performed 2 h of one-legged knee extensor exercise at 50% of maximum workload with catheters inserted into the right and left femoral vein and the femoral artery of the resting leg. Femoral arterial blood flow was assessed by Doppler ultrasound (CFM-800, Wingmed A/S, Horten, Norway) [33]. None of the data presented here have been published previously. None of these subjects were included in the other studies presented here. For further details on the experimental procedures, see Supplementary Materials and Methods.
2.4. Animal experiments
Treadmill experiments have been described [34]. In brief, 12-week-old male C57Bl/6J mice ran after 5 min warm-up for 60 min at 14 m/min and 14° uphill slope. Immediately after the run, the mice were anesthetized by intraperitoneal injection of ketamine (150 μg/g body weight) and xylazine (10 μg/g body weight) and killed by decapitation. Tissues were immediately removed and frozen in liquid nitrogen. For further details on the experimental procedures, see Supplementary Materials and Methods. Animal data are presented as a Supplementary Figure (A4).
2.5. Hormone infusions
Ten healthy males went through four experimental protocols separated by at least 2 weeks (test days 1–4). Test day 1: glucagon (GlucaGen, Novo Nordisk Scandinavia, Copenhagen, Denmark) was infused for 1 h at 6 ng/kg/min. Test day 2: to identify the isolated effect of glucagon, an infusion of somatostatin (Octreotide, Hospira Nordic AB, Stockholm, Sweden) at 100 ng/kg/min was started 10 min prior to the glucagon infusion and was infused for additional 2 h. Glucagon was infused for 1 h at 6 ng/kg/min. Test day 3: somatostatin was infused at 100 ng/kg/min for 130 min. Test day 4: saline was infused for 1 h with same rate as the glucagon infusion rate. For further details on the experimental procedures, see Supplementary Materials and Methods. Of the included subjects, 3 subjects in the somatostatin infusion trial also participated in the exercise study with hepatic vein catheterisation and brachial artery catheterisation.
2.6. Statistics
Data are presented as means ± SEM. For analyses of hormone and blood glucose kinetics one-way ANOVAs with Dunnett's post hoc tests were applied. Where relevant, Student's t-tests were applied * significant by one-way ANOVA. # significant by one-way ANOVA and Dunnett's post hoc test. † significant by Student's t-test. p ≤ 0.05 was considered statistically significant. Analyses were performed by SAS 9.1, SAS Institute Inc., Cary, NC, USA and linear regression analyses by GraphPad Prism 4, GraphPad Software Inc, La Jolla, CA, USA.
3. Results
3.1. Splanchnic FGF-21 secretion and acute regulation during exercise in humans
Here, we quantified the hepatic FGF-21 production in healthy humans and evaluated the kinetics of the exercise-induced FGF-21 increase. First, we evaluated hepatic plasma flow (Supplementary Figure A1) and glucose and lactate flux over the splanchnic bed during exercise, which confirmed enhanced glucose production and lactate uptake by the liver (Supplementary Figure A2).
At rest we observed an arterial-hepatic vein (a-hv) difference of −30.2 ± 6.6 ng/l in plasma FGF-21 (p = 0.001) (Figure 1A). When taking hepatic blood flow into consideration (Supplementary Figure A1), this equals a hepatic FGF-21 production of 30.5 ± 7.4 ng/min (p = 0.002) at rest (Figure 1B). These data demonstrate a constant hepatic FGF-21 release in humans at rest after an overnight fast. During exercise, plasma FGF-21 increases in the hepatic vein from ∼200 ng/l at rest to a peak at ∼670 ng/l 30 min after exercise (p < 0.0001) (Figure 1C), an ∼5-fold increase (p < 0.0001) (individual data presented in Supplementary Figure A3). After exercise, plasma FGF-21 rapidly decreases and returns to baseline after 120 min. Thus, plasma FGF-21 is acutely regulated by exercise, and these data indicate a short plasma half-life. The a-hv difference for FGF-21 is negative at all time points (significant by Student's t-test) (Figure 1D), demonstrating a constant secretion of FGF-21 to the splanchnic circulation. During exercise, the a-hv difference of FGF-21 increases and thus net hepatic production peaks at 108 ng/min 30 min after the end of exercise (p = 0.03 and p = 0.04, resp.) (Figure 1E). Collectively, exercise increases the hepatic FGF-21 secretion ∼4-fold (p = 0.01).
Figure 1.
Splanchnic plasma FGF-21 kinetics in young men (n = 10) during exercise. At baseline, there is a significant arterial-venous difference (A) and net production of FGF-21 (B). During exercise, plasma FGF-21 increases (C) and the arterial-venous difference across the splanchnic circulation (D) and net hepatic production is significantly increased (E). * significant by one-way ANOVA. # significant by one-way ANOVA and Dunnett's post hoc test. † significant by Student's t-test. p < 0.05 was considered statistically significant. Data are presented as mean ± SEM. In (A) and (B) individual data are presented.
In mice, gene expression analysis confirmed the previous finding [26] that FGF-21 mRNA levels are up-regulated 3-fold in the liver immediately after exercise (p = 0.02) (Supplementary Figure A4), while they remained low in fat and soleus and tibialis muscles of sedentary as well as exercised mice.
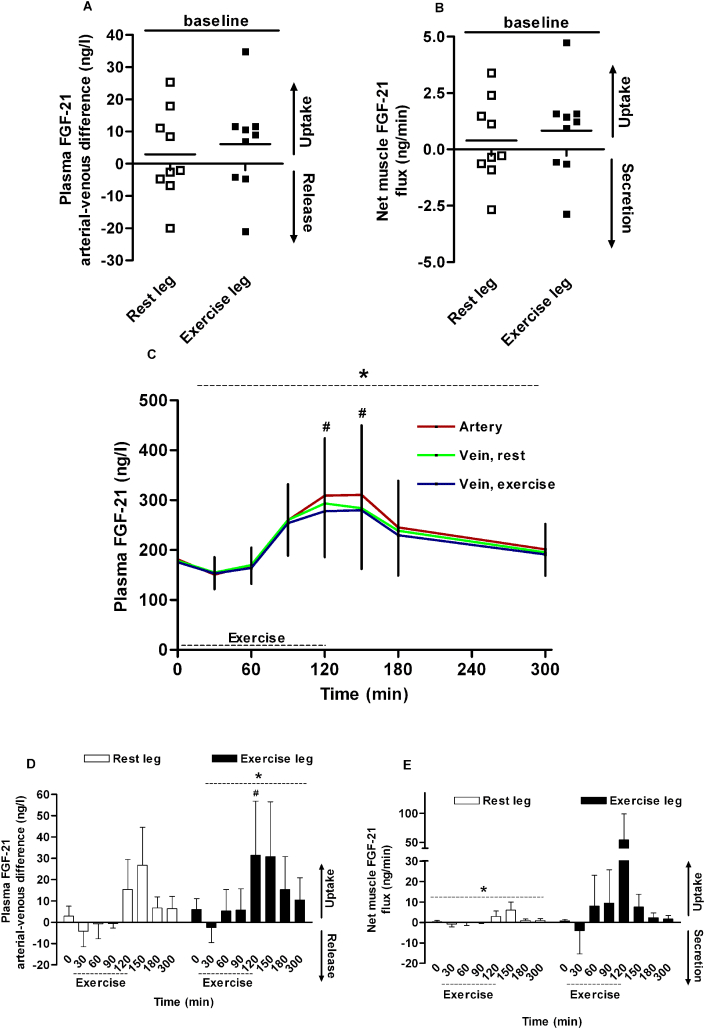
3.2. No FGF-21 release from the human leg
To investigate whether skeletal muscles contribute to circulating levels of FGF-21, we measured FGF-21 release from a resting and an exercising leg in humans. Again, we evaluated femoral plasma flow (Supplementary Figure A5), and glucose and lactate flux over the leg during exercise which confirmed enhanced glucose uptake and lactate release by the exercising leg (Supplementary Figure A6).
At rest, no release or uptake of FGF-21 over the legs could be demonstrated (resting leg, p = 0.55; exercising leg, p = 0.27) (Figure 2A). When femoral blood flow (Supplementary Figure A5) is taken into account, neither secretion nor uptake of FGF-21 across the leg is observed at rest (resting leg, p = 0.55; exercising leg, p = 0.27) (Figure 2B). During one-legged exercise, plasma FGF-21 increased although to a smaller extent than during two-legged exercise. In the artery and both veins plasma FGF-21 increased from ∼180 ng/l at rest to ∼300 ng/l at the end of exercise (p < 0.0001) (Figure 2C), corresponding to a ∼50–60% increase (p < 0.0001). Thus, one-legged exercise is less effective in stimulating secretion of FGF-21 than two-legged exercise, most likely due to a less pronounced systemic metabolic stress. The arterial-femoral vein (a-fv) differences of FGF-21 across both legs are not significantly different from zero at any time point; however, the positive a-fv differences could suggest a small uptake. One-way ANOVA analysis revealed an effect of time (Figure 2D, exercising leg p < 0.0001; Figure 2E, resting leg p = 0.049) that suggests an altered handling of FGF-21 in legs after exercise.
Figure 2.
Leg plasma FGF-21 kinetics in young men (n = 9) during one-legged exercise. At baseline, there is no arterial-venous difference (A) or net production/uptake of FGF-21 (B) over neither leg. During exercise, plasma FGF-21 increases (C) and the arterial-venous differences across the exercising leg (D) increases. Net muscle uptake is not significantly increased in the exercising leg (E). * significant by one-way ANOVA. # significant by one-way ANOVA and Dunnett's post hoc test. † significant by Student's t-test. p < 0.05 was considered statistically significant. Data are presented as mean ± SEM. In (A) and (B) individual data are presented.
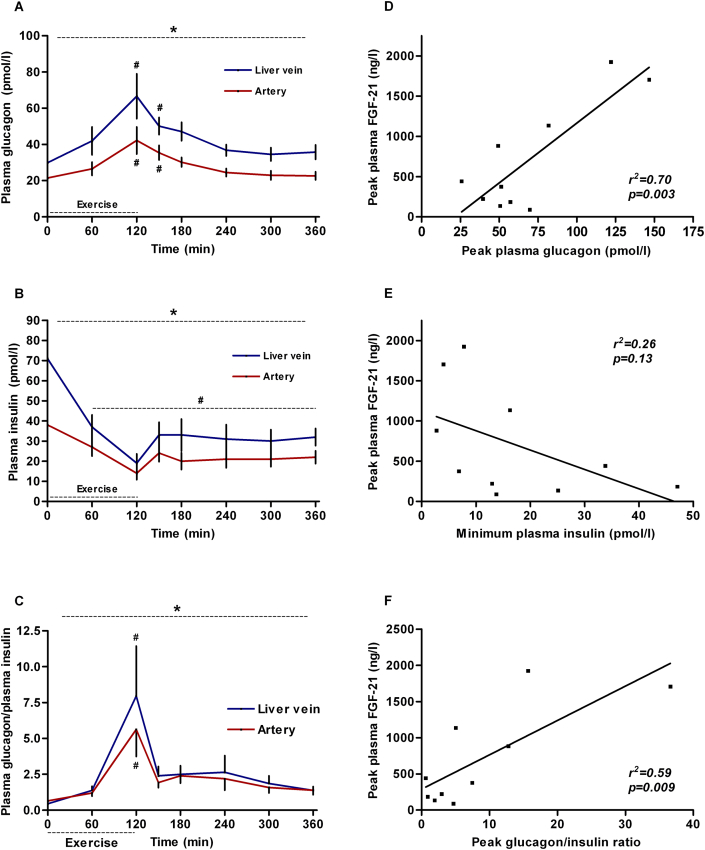
3.3. Exercise-induced FGF-21 correlates with changes in glucagon and insulin
During exercise, plasma glucagon increases whereas insulin decreases (both p < 0.0001) (Figure 3A–B). Consequently, the glucagon/insulin ratio increases by ∼7.5 fold (hepatic vein, p = 0.001 and artery, p < 0.0001) (Figure 3C). Both peak plasma glucagon and peak glucagon/insulin ratio correlates positively with the peak FGF-21 (r2 = 0.70, p = 0.003 and r2 = 0.59, p = 0.009, resp.) (Figure 3D,F), whereas there is no significant correlation between nadir plasma insulin and peak FGF-21 (r2 = 0.26, p = 0.13; Figure 3E). Of note, exercise-induced changes in splanchnic glucose production also correlate positively with peak FGF-21 (Supplementary Figure A7).
Figure 3.
Glucagon and insulin regulation during exercise in young men (n = 10). Glucagon and glucagon/insulin ratio increases in the hepatic vein (A, C, blue line) and artery (A, C, red line) with exercise. Insulin decreases in the hepatic vein (B, blue line) and artery (B, red line) with exercise. Peak glucagon (D) and peak glucagon/insulin ratio (F) correlate with peak FGF-21. No significant correlation between nadir insulin and peak FGF-21 (E). * significant by one-way ANOVA. # significant by one-way ANOVA and Dunnett's post hoc test. p < 0.05 was considered statistically significant. Data are presented as mean ± SEM.
3.4. Plasma FGF-21 is induced by changes in the circulating glucagon-to-insulin ratio
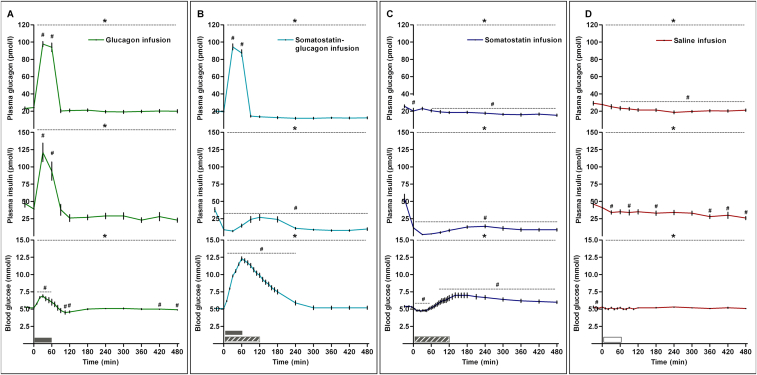
During 1 h of glucagon infusion, its plasma concentration is increased at 30–60 min to ∼100 pmol/l (p < 0.0001) and then returns to baseline level (Figure 4A). As a consequence of the glucagon-induced hyperglycaemia, plasma insulin increases rapidly during the initial part of the trial and is elevated at 30–60 min (p < 0.0001) and then returns to the baseline level (Figure 4A). Blood glucose increases initially and peaks at 6.8 mmol/l at 30 min (elevated at 10–60 min, p < 0.005) and then returns to baseline levels (Figure 4A).
Figure 4.
Hormone and glucose changes during glucagon or/and somatostatin infusion in young men (n = 10). Glucagon infusion increases glucagon, insulin and blood glucose (5A). Somatostatin-glucagon infusion increases glucagon, blood glucose and decreases insulin (5B). Somatostatin infusion decreases glucagon and insulin and induces moderate excursions in blood glucose (5C). Saline infusion induces small changes in glucagon, insulin and blood glucose (5D). Gray bar indicates glucagon infusion. Dashed gray bar indicates somatostatin infusion. White bar indicates saline infusion. * significant by one-way ANOVA. # significant by one-way ANOVA and Dunnett's post hoc test. p < 0.05 was considered statistically significant. Data are presented as mean ± SEM.
During the combined glucagon and somatostatin infusion, plasma glucagon increases to a similar level as in the glucagon infusion trial (p < 0.0001) (Figure 4B). After cessation of glucagon infusion, plasma glucagon decreases to baseline levels (Figure 4B). Plasma insulin decreases due to blockade of pancreatic insulin secretion by the somatostatin infusion (p < 0.0001) (Figure 4B). Blood glucose increases markedly (0–240 min) and peaks at a much higher concentration than in the glucagon infusion trial (all p < 0.0001) (Figure 4B), a phenomenon caused by the potent glucagon stimulus and the concomitant blockade of insulin secretion.
During infusion of somatostatin, both plasma glucagon and insulin decrease throughout the trial (both p < 0.0001) (Figure 4C). Blood glucose shows moderate excursions (decrease at 5–55 min, p < 0.0001, increase at 85–480 min, p < 0.05) (Figure 4C).
During 1 h saline infusion, both plasma glucagon and insulin decrease slowly throughout the trial (p = 0.04 and and p < 0.0001, resp.), and only small changes in blood glucose occur, which remains at ∼5 mmol/l (p < 0.0001) (Figure 4D).
During glucagon infusion, plasma FGF-21 decreases slowly from 114 ng/l at baseline to 40 ng/l at the end of the trial (p < 0.0001). Consequently, plasma FGF-21 is reduced to ∼40% of the baseline level at the end of the trial (Figure 5A). In contrast, plasma FGF-21 increases rapidly during the glucagon and somatostatin infusion from 130 ng/l at baseline to a peak at 370 ng/l at 90 min, which is 30 min after the end of the glucagon infusion (p < 0.0001) (Figure 5B). At 90 min, plasma FGF-21 has increased ∼3 fold and remains moderately elevated until 240 min; Plasma FGF-21 levels return to baseline after 360 min (Figure 5B). These data confirm glucagon as a potent stimulus for secretion of FGF-21 and, furthermore, demonstrate the role of insulin as suppressor of acute FGF-21 secretion. During somatostatin infusion, plasma FGF-21 increases from baseline to 120 min (p = 0.03), which corresponds to a 2-fold increase (p < 0.0001) (Figure 5C). We interpret this event as a consequence of withdrawal of insulin as an inhibitory signal rather than as consequence of the somatostatin infusion per se. When compared with the FGF-21 response to somatostatin-glucagon infusion, a two-way ANOVA reveal significant effect of time (p < 0.0001) and interaction (p = 0.05), but not of group (p = 0.13). During saline infusion, a small decrease in plasma FGF-21 is observed at the end of the trial, however only significant when analysed as fold change (p < 0.0001) (Figure 5D).
Figure 5.

Plasma FGF-21 is regulated by changes in glucagon and insulin in young men (n = 10). Glucagon infusion decreases FGF-21 (A), whereas somatostatin-glucagon infusion (B) and somatostatin (C) increases plasma FGF-21. Saline infusion does not change plasma FGF-21 (D). Gray bar indicates glucagon infusion. Dashed gray bar indicates somatostatin infusion. White bar indicates saline infusion. * significant by one-way ANOVA. # significant by one-way ANOVA and Dunnett's post hoc test. p < 0.05 was considered statistically significant. Data are presented as mean ± SEM.
In line with the positive correlation of peak FGF-21 with exercise-induced changes in splanchnic glucose production (Supplementary Figure A7), peak plasma FGF-21 levels during glucagon-somatostatin infusion correlate with the induction of glucose production (r2 = 0.49, p = 0.02, Supplementary Figure A8), supporting that induction of hepatic FGF-21 secretion and glucose production are tightly linked.
4. Discussion
Here, we demonstrate that FGF-21 is secreted from the splanchnic circulation in humans and is regulated by the glucagon/insulin ratio. In contrast, no FGF-21 secretion was demonstrated from either the exercising or the resting leg, leaving little evidence for FGF-21 as a circulating myokine with endocrine effects in humans. These observations were supported in mice where a marked increase in FGF-21 mRNA was observed in the liver in response to exercise whereas no regulation of FGF-21 mRNA was detected in skeletal muscles. The finding that FGF-21 is liver-derived is in line with the observation that liver-specific knockout of FGF-21 in mice removes FGF-21 from the circulation [3]. Collectively, the liver is the source of circulating FGF-21 both at rest and during exercise in man.
The present finding that FGF-21 is not released from the leg, i.e. skeletal muscle, during exercise is in line with mouse data obtained by Kim et al. [26]. Exercise increases hepatic FGF-21 mRNA in mice, whereas no changes are observed in skeletal muscle. Importantly, these data do not rule out a role for FGF-21 as a myokine with local paracrine or autocrine effects. In fact, it is clear that mitochondrial dysfunction in skeletal muscle leads to induction of FGF-21 gene expression [29]. Chronic hyperinsulinemia [13] and lipodystrophy-induced insulin resistance [35] led to elevated expression of FGF-21 mRNA in muscle, which could be associated with mitochondrial stress. Furthermore, muscle FGF-21 mRNA and plasma levels were induced in the skeletal muscle-specific UCP-1 transgenic mice [30], a model of mitochondrial stress.
These data suggest that under such circumstances (i.e. mitochondrial stress), FGF-21 acts as a secreted myokine with both para/autocrine and endocrine effects. Our data indicate that under normal resting (and exercise) conditions in humans, circulating FGF-21 levels are determined by the hepatic secretion.
Our data show that circulating FGF-21 is tightly regulated by the glucagon/insulin ratio in humans. An increase in plasma glucagon concomitant with a decrease in plasma insulin rapidly increases plasma FGF-21. These data are in line with reports showing that FGF-21 is secreted upon glucagon stimulation both in vivo [9,10] and in vitro [10]. In vivo, intramuscular administration of glucagon in humans in a supra-physiological dose stimulates FGF-21 secretion, and most likely overrules the inhibitory effect of insulin observed in the present study [9,10]. In mice, glucagon receptor knockout (gcgr−/−) blunts the induction of FGF-21 mRNA in response to exercise [11], supporting that exercise-induced FGF-21 is driven by glucagon. In vitro, glucagon receptor agonism stimulates FGF-21 in cultured primary murine hepatocytes, whereas this effect is lost in cultured hepatocytes from gcgr−/− mice [10]. We observe that insulin clearly inhibits FGF-21 secretion, and thus our data do not support that FGF-21 secretion in humans is regulated independently of insulin [9], whereas it supports that FGF-21 secretion is induced by insulin withdrawal [7]. In the liver specific insulin receptor knockout (LIRKO) mice, basal and fasted FGF-21 levels are unaffected compared with control mice [36], an observation that contrasts our finding. However, despite the lacking hepatic insulin signalling the LIRKO mice have normal hepatic glucose production in the basal state [37]. We observe a strong association between FGF-21 secretion and hepatic glucose production (Supplementary Figure A7). Accordingly, as the LIRKO mice have unaffected hepatic glucose production, the unaffected FGF-21 levels could be anticipated. Thus, the observations by Emanuelli et al. [36] are not in conflict with our findings. In summary, this classifies glucagon as an activator and insulin as an inhibitor of FGF-21 secretion.
The human in vivo measurements over the splanchnic circulation reveal that FGF-21 has a rapid turn-over. As seen in Figure 1E, the hepatic production of FGF-21 increases rapidly with exercise and returns to baseline production after 180 min. The arterial concentration of FGF-21 decreases over 60 min (180–240 min) to baseline when the hepatic FGF-21 contribution stops, indicating a short plasma half-life of FGF-21. In animals, the half-life of human recombinant FGF-21 is estimated to 30–120 min [38]. Our data indicate a more rapid clearance of FGF-21 in humans at least in the hour(s) after exercise.
The combination of increased glucagon and low insulin levels is present during exercise and prolonged fasting. Several studies demonstrate that FFAs are important regulatory signals for hepatic FGF-21 secretion via PPARα activation [7,15,39]. During exercise and upon infusion of glucagon, plasma FFAs increase [21,40], which could confound our conclusion. We observe that FGF-21 is induced by exercise and experimentally by high glucagon/low insulin with similar kinetics. In both experiments, we observe a rapid increase in circulating FGF-21 that peaks 30 min after the stimulus (i.e. exercise or high glucagon/low insulin) is stopped. Mai et al. observe a 1.3-fold increase after 240 min of continuously elevated FFA in humans [39], which differs from the kinetics observed during exercise. Berglund et al. demonstrate an additive effect of intralipid infusion on glucagon-stimulated hepatic FGF-21 gene expression, whereas intralipid infusion per se has no effect [11]. Thus, during exercise FFAs might act in concert with changes in glucagon-to-insulin ratio to stimulate hepatic FGF-21 secretion.
During the glucagon-somatostatin infusion blood glucose increases, whereas it decreases during exercise; however in both experiments plasma FGF-21 increases. During an oral glucose tolerance test, FGF-21 decreases initially (after 30 min) whereas there is a small increase after 180 min [41]. Based on these findings, it seems unlikely that glucose per se is responsible for the FGF-21 increase that we observe during glucagon-somatostatin infusion.
Plasma FGF-21 is paradoxically increased in metabolic disorders such as obesity, type 2 diabetes, and non-alcoholic fatty liver disease [42–44]. These pathophysiological conditions often go along with insulin resistance and hyperglucagonemia [45]. In addition, hepatic mitochondrial stress as observed in patients with non-alcoholic steatohepatitis is associated with increased plasma FGF-21 [46]. We observe an inhibitory effect of insulin on hepatic FGF-21 secretion, and our data consequently indicate that hepatic insulin resistance lead to increased circulating FGF-21. In combination with hyperglucagonemia, this could further add to the elevation of FGF-21 in these diseases.
The numerous beneficial metabolic actions mediated by FGF-21 are similar to the effects of exercise and FGF-21 could mediate at least a part of the exercise-induced metabolic improvements observed with regular physical activity. FGF-21 signals via the FGF receptor/β-klotho pathway [47], which is most abundantly expressed in liver, white and brown adipose tissue and, to a lesser extent, skeletal muscle [47,48]. FGF-21 was not released from the human leg; rather it appears to be taken up. In cultured primary human muscle cells, FGF-21 stimulates insulin-stimulated glucose uptake and protects against palmitate-induced insulin resistance [49,50]. Also FGF-21 directly stimulates glucose uptake in adipocytes and enhances whole-body insulin sensitivity [2,27,51]. FGF-21 administration improves hyperglycaemia in ob/ob mice and overexpression of FGF-21 leads to sustained glycemic control and protection against diet-induced obesity [2]. In addition, FGF-21 administration improves plasma lipid profile in mice and rhesus monkeys [2,52].
As the metabolically beneficial actions of FGF-21 are similar to those observed with exercise, it is likely that exercise-induced FGF-21 acts as an endocrine signal between the liver and, e.g. adipose tissue and skeletal muscle.
Hepatic glucagon signalling has gained attention as a potential therapeutic target to improve metabolic disorders. Two concepts are being pursued: glucagon antagonism and glucagon agonism [53]. As hyperglucagonemia is a contributing factor to dysglycemia in type 2 diabetes [53], blockade of its actions by glucagon receptor antagonism seems a rational therapeutic strategy. Indeed, glucagon receptor antagonism led to improved glycemic control, as recently reviewed [53]. Our data suggest that the advantage of FGF-21 mediated metabolic improvement is most likely lost in treatment with glucagon antagonists. However, development of glucagon and glucagon like peptide (GLP)-1 dual agonists emerge as interesting therapeutic alternatives [40,53,54]. These compounds improve glucose tolerance in combination with reducing food intake, increasing energy expenditure and promoting weight loss [40,54,55]. Based on our findings, the latter approach would likely lead to increased circulating FGF-21 which in turn could mediate improvement in whole-body energy homoeostasis. This idea is confirmed as a glucagon GLP-1 dual agonist led to increased circulating FGF-21 [55].
5. Conclusions
In conclusion, we provide evidence that the splanchnic bed contributes to the systemic levels of FGF-21 during rest and exercise in humans. In contrast, under normo-physiological conditions FGF-21 is not released from the leg. Finally, we demonstrate that the glucagon-to-insulin ratio is pivotal for the FGF-21 regulation in humans. Thus, these findings add important new knowledge with regard to identifying the source of FGF-21 secretion during exercise in humans and identify a hormonal mechanism explaining the regulation of FGF-21. These human integrative physiological studies contribute to understanding the role of FGF-21 in metabolic diseases.
6. Author contributions
JSH and PP designed and executed the human studies, analysed data, and wrote and revised manuscript. JOC and NHS executed the human studies, and wrote and revised manuscript. MH, AD and CW executed the mouse study, analysed data, and wrote and revised manuscript. BKP designed the study, and wrote and revised manuscript. All authors made substantial contributions to conception and design and revised the manuscript critically for important intellectual content. All authors have given their final approval of the manuscript to be published.
7. Transcript profiling
None.
8. Writing assistance
None.
Acknowledgements
The Centre of Inflammation and Metabolism (CIM) is supported by a grant from the Danish National Research Foundation (DNRF55). The Centre for Physical Activity Research (CFAS) is supported by Trygfonden. This study was further supported by grants from the Augustinus Fonden, Aase og Ejnar Danielsens Fond, A.P. Møller's, and Oda og Hans Svenningsen's Foundations. CIM is part of the UNIK Project: Food, Fitness & Pharma for Health and Disease, supported by the Danish Ministry of Science, Technology, and Innovation. CIM is a member of DD2 - the Danish Centre for Strategic Research in Type 2 Diabetes (the Danish Council for Strategic Research, grant no. 09-067009 and 09-075724). The Copenhagen Muscle Research Centre (CMRC) is supported by a grant from the Capital Region of Denmark. This work was further supported in part by grants from the German Federal Ministry of Education and Research (BMBF) to the German Centre for Diabetes Research (DZD e.V.; No. 01GI0925), by a grant from the Leibniz Gemeinschaft (SAW-FBN-2013-3) to C.W, and by the Deutsche Forschungsgemeinschaft to C.W. (GRK 1302/2).
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molmet.2015.06.001
Conflict of interest
The authors have declared that no conflict of interest exists.
Supplementary data
The following are the supplementary data related to this article:
References
- 1.Nishimura T., Nakatake Y., Konishi M., Itoh N. Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochimica et Biophysica Acta. 2000;1492:203–206. doi: 10.1016/s0167-4781(00)00067-1. [DOI] [PubMed] [Google Scholar]
- 2.Kharitonenkov A., Shiyanova T.L., Koester A., Ford A.M., Micanovic R., Galbreath E.J. FGF-21 as a novel metabolic regulator. Journal of Clinical Investigation. 2005;115:1627–1635. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Markan K.R., Naber M.C., Ameka M.K., Anderegg M.D., Mangelsdorf D.J., Kliewer S.A. Circulating FGF21 is liver derived and enhances glucose uptake during refeeding and overfeeding. Diabetes. 2014;63(12):4057–4063. doi: 10.2337/db14-0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaich G., Chien J.Y., Fu H., Glass L.C., Deeg M.A., Holland W.L. The effects of LY2405319, an FGF21 Analog, in obese human subjects with type 2 diabetes. Cell Metabolism. 2013;18:333–340. doi: 10.1016/j.cmet.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Galman C., Lundasen T., Kharitonenkov A., Bina H.A., Eriksson M., Hafstrom I. The circulating metabolic regulator FGF21 is induced by prolonged fasting and PPARalpha activation in man. Cell Metabolism. 2008;8:169–174. doi: 10.1016/j.cmet.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 6.Potthoff M.J., Inagaki T., Satapati S., Ding X., He T., Goetz R. FGF21 induces PGC-1alpha and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:10853–10858. doi: 10.1073/pnas.0904187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mai K., Bobbert T., Groth C., Assmann A., Meinus S., Kraatz J. Physiological modulation of circulating FGF21: relevance of free fatty acids and insulin. American Journal of Physiology. Endocrinology and Metabolism. 2010;299:E126–E130. doi: 10.1152/ajpendo.00020.2010. [DOI] [PubMed] [Google Scholar]
- 8.Laeger T., Henagan T.M., Albarado D.C., Redman L.M., Bray G.A., Noland R.C. FGF21 is an endocrine signal of protein restriction. Journal of Clinical Investigation. 2014;124:3913–3922. doi: 10.1172/JCI74915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arafat A.M., Kaczmarek P., Skrzypski M., Pruszynska-Oszmalek E., Kolodziejski P., Szczepankiewicz D. Glucagon increases circulating fibroblast growth factor 21 independently of endogenous insulin levels: a novel mechanism of glucagon-stimulated lipolysis? Diabetologia. 2013;56:588–597. doi: 10.1007/s00125-012-2803-y. [DOI] [PubMed] [Google Scholar]
- 10.Habegger K.M., Stemmer K., Cheng C., Muller T.D., Heppner K.M., Ottaway N. Fibroblast growth factor 21 mediates specific glucagon actions. Diabetes. 2013;62:1453–1463. doi: 10.2337/db12-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berglund E.D., Kang L., Lee-Young R.S., Hasenour C.M., Lustig D.G., Lynes S.E. Glucagon and lipid interactions in the regulation of hepatic AMPK signaling and expression of PPARalpha and FGF21 transcripts in vivo. American Journal of Physiology. Endocrinology and Metabolism. 2010;299:E607–E614. doi: 10.1152/ajpendo.00263.2010. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Lin Z., Gong Q., Wu C., Yu J., Lu T., Pan X. Dynamic change of serum FGF21 levels in response to glucose challenge in human. Journal of Clinical Endocrinology and Metabolism. 2012;97:E1224–E1228. doi: 10.1210/jc.2012-1132. [DOI] [PubMed] [Google Scholar]
- 13.Hojman P., Pedersen M., Nielsen A.R., Krogh-Madsen R., Yfanti C., Akerstrom T. Fibroblast growth factor-21 is induced in human skeletal muscles by hyperinsulinemia. Diabetes. 2009;58:2797–2801. doi: 10.2337/db09-0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Badman M.K., Pissios P., Kennedy A.R., Koukos G., Flier J.S., Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metabolism. 2007;5:426–437. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Inagaki T., Dutchak P., Zhao G., Ding X., Gautron L., Parameswara V. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metabolism. 2007;5:415–425. doi: 10.1016/j.cmet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Lundasen T., Hunt M.C., Nilsson L.M., Sanyal S., Angelin B., Alexson S.E. PPARalpha is a key regulator of hepatic FGF21. Biochemical and Biophysical Research. 2007;360:437–440. doi: 10.1016/j.bbrc.2007.06.068. [DOI] [PubMed] [Google Scholar]
- 17.Wasserman D.H., Spalding J.A., Lacy D.B., Colburn C.A., Goldstein R.E., Cherrington A.D. Glucagon is a primary controller of hepatic glycogenolysis and gluconeogenesis during muscular work. American Journal of Physiology. 1989;257:E108–E117. doi: 10.1152/ajpendo.1989.257.1.E108. [DOI] [PubMed] [Google Scholar]
- 18.Wasserman D.H., Williams P.E., Lacy D.B., Goldstein R.E., Cherrington A.D. Exercise-induced fall in insulin and hepatic carbohydrate metabolism during muscular work. American Journal of Physiology. 1989;256:E500–E509. doi: 10.1152/ajpendo.1989.256.4.E500. [DOI] [PubMed] [Google Scholar]
- 19.Emhoff C.A., Messonnier L.A., Horning M.A., Fattor J.A., Carlson T.J., Brooks G.A. Gluconeogenesis and hepatic glycogenolysis during exercise at the lactate threshold. Journal of Applied Physiology (1985) 2013;114:297–306. doi: 10.1152/japplphysiol.01202.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wasserman D.H., Spalding J.A., Bracy D., Lacy D.B., Cherrington A.D. Exercise-induced rise in glucagon and ketogenesis during prolonged muscular work. Diabetes. 1989;38:799–807. doi: 10.2337/diab.38.6.799. [DOI] [PubMed] [Google Scholar]
- 21.Wahren J., Sato Y., Ostman J., Hagenfeldt L., Felig P. Turnover and splanchnic metabolism of free fatty acids and ketones in insulin-dependent diabetics at rest and in response to exercise. Journal of Clinical Investigation. 1984;73:1367–1376. doi: 10.1172/JCI111340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Longuet C., Sinclair E.M., Maida A., Baggio L.L., Maziarz M., Charron M.J. The glucagon receptor is required for the adaptive metabolic response to fasting. Cell Metabolism. 2008;8:359–371. doi: 10.1016/j.cmet.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wasserman D.H., Cherrington A.D. Hepatic fuel metabolism during muscular work: role and regulation. American Journal of Physiology. 1991;260:E811–E824. doi: 10.1152/ajpendo.1991.260.6.E811. [DOI] [PubMed] [Google Scholar]
- 24.Frayn K.N. Third Edition. Wiley-Blackwell; 2010. Metabolic regulation - a human perspective. [Google Scholar]
- 25.Hoene M., Weigert C. The stress response of the liver to physical exercise. Exercise Immunology Review. 2010;16:163–183. [PubMed] [Google Scholar]
- 26.Kim K.H., Kim S.H., Min Y.K., Yang H.M., Lee J.B., Lee M.S. Acute exercise induces FGF21 expression in mice and in healthy humans. PLoS One. 2013;8:e63517. doi: 10.1371/journal.pone.0063517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu J., Lloyd D.J., Hale C., Stanislaus S., Chen M., Sivits G. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes. 2009;58:250–259. doi: 10.2337/db08-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Izumiya Y., Bina H.A., Ouchi N., Akasaki Y., Kharitonenkov A., Walsh K. FGF21 is an Akt-regulated myokine. FEBS Letters. 2008;582:3805–3810. doi: 10.1016/j.febslet.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim K.H., Jeong Y.T., Oh H., Kim S.H., Cho J.M., Kim Y.N. Autophagy deficiency leads to protection from obesity and insulin resistance by inducing Fgf21 as a mitokine. Nature Medicine. 2013;19:83–92. doi: 10.1038/nm.3014. [DOI] [PubMed] [Google Scholar]
- 30.Keipert S., Ost M., Johann K., Imber F., Jastroch M., van Schothorst E.M. Skeletal muscle mitochondrial uncoupling drives endocrine cross-talk through the induction of FGF21 as a myokine. American Journal of Physiology. Endocrinology and Metabolism. 2014;306:E469–E482. doi: 10.1152/ajpendo.00330.2013. [DOI] [PubMed] [Google Scholar]
- 31.Hansen J., Brandt C., Nielsen A.R., Hojman P., Whitham M., Febbraio M.A. Exercise induces a marked increase in plasma follistatin: evidence that follistatin is a contraction-induced hepatokine. Endocrinology. 2011;152:164–171. doi: 10.1210/en.2010-0868. [DOI] [PubMed] [Google Scholar]
- 32.Skak C., Keiding S. Methodological problems in the use of indocyanine green to estimate hepatic blood flow and ICG clearance in man. Liver. 1987;7:155–162. doi: 10.1111/j.1600-0676.1987.tb00336.x. [DOI] [PubMed] [Google Scholar]
- 33.Osada T., Radegran G. Femoral artery inflow in relation to external and total work rate at different knee extensor contraction rates. Journal of Applied Physiology (1985) 2002;92:1325–1330. doi: 10.1152/japplphysiol.00848.2001. [DOI] [PubMed] [Google Scholar]
- 34.Hoene M., Franken H., Fritsche L., Lehmann R., Pohl A.K., Haring H.U. Activation of the mitogen-activated protein kinase (MAPK) signalling pathway in the liver of mice is related to plasma glucose levels after acute exercise. Diabetologia. 2010;53:1131–1141. doi: 10.1007/s00125-010-1666-3. [DOI] [PubMed] [Google Scholar]
- 35.Lindegaard B., Hvid T., Grondahl T., Frosig C., Gerstoft J., Hojman P. Expression of fibroblast growth factor-21 in muscle is associated with lipodystrophy, insulin resistance and lipid disturbances in patients with HIV. PLoS One. 2013;8:e55632. doi: 10.1371/journal.pone.0055632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Emanuelli B., Vienberg S.G., Smyth G., Cheng C., Stanford K.I., Arumugam M. Interplay between FGF21 and insulin action in the liver regulates metabolism. Journal of Clinical Investigation. 2014;124:515–527. doi: 10.1172/JCI67353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fisher S.J., Kahn C.R. Insulin signaling is required for insulin's direct and indirect action on hepatic glucose production. Journal of Clinical Investigation. 2003;111:463–468. doi: 10.1172/JCI16426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kharitonenkov A., Wroblewski V.J., Koester A., Chen Y.F., Clutinger C.K., Tigno X.T. The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology. 2007;148:774–781. doi: 10.1210/en.2006-1168. [DOI] [PubMed] [Google Scholar]
- 39.Mai K., Andres J., Biedasek K., Weicht J., Bobbert T., Sabath M. Free fatty acids link metabolism and regulation of the insulin-sensitizing fibroblast growth factor-21. Diabetes. 2009;58:1532–1538. doi: 10.2337/db08-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Habegger K.M., Heppner K.M., Geary N., Bartness T.J., DiMarchi R., Tschop M.H. The metabolic actions of glucagon revisited. Nature Clinical Practice Endocrinology & Metabolism. 2010;6:689–697. doi: 10.1038/nrendo.2010.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dushay J.R., Toschi E., Mitten E.K., Fisher F.M., Herman M.A., Maratos-Flier E. Fructose ingestion acutely stimulates circulating FGF21 levels in humans. Mol Metab. 2015;4:51–57. doi: 10.1016/j.molmet.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chavez A.O., Molina-Carrion M., bdul-Ghani M.A., Folli F., Defronzo R.A., Tripathy D. Circulating fibroblast growth factor-21 is elevated in impaired glucose tolerance and type 2 diabetes and correlates with muscle and hepatic insulin resistance. Diabetes Care. 2009;32:1542–1546. doi: 10.2337/dc09-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dushay J., Chui P.C., Gopalakrishnan G.S., Varela-Rey M., Crawley M., Fisher F.M. Increased fibroblast growth factor 21 in obesity and nonalcoholic fatty liver disease. Gastroenterology. 2010;139:456–463. doi: 10.1053/j.gastro.2010.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang X., Yeung D.C., Karpisek M., Stejskal D., Zhou Z.G., Liu F. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes. 2008;57:1246–1253. doi: 10.2337/db07-1476. [DOI] [PubMed] [Google Scholar]
- 45.Unger R.H., Cherrington A.D. Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. Journal of Clinical Investigation. 2012;122:4–12. doi: 10.1172/JCI60016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koliaki C., Szendroedi J., Kaul K., Jelenik T., Nowotny P., Jankowiak F. Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metabolism. 2015;21:739–746. doi: 10.1016/j.cmet.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 47.Ding X., Boney-Montoya J., Owen B.M., Bookout A.L., Coate K.C., Mangelsdorf D.J. betaKlotho is required for fibroblast growth factor 21 effects on growth and metabolism. Cell Metabolism. 2012;16:387–393. doi: 10.1016/j.cmet.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fon T.K., Bookout A.L., Ding X., Kurosu H., John G.B., Wang L. Research resource: comprehensive expression atlas of the fibroblast growth factor system in adult mouse. Molecular Endocrinology. 2010;24:2050–2064. doi: 10.1210/me.2010-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mashili F.L., Austin R.L., Deshmukh A.S., Fritz T., Caidahl K., Bergdahl K. Direct effects of FGF21 on glucose uptake in human skeletal muscle: implications for type 2 diabetes and obesity. Diabetes-Metabolism Research and Reviews. 2011;27:286–297. doi: 10.1002/dmrr.1177. [DOI] [PubMed] [Google Scholar]
- 50.Lee M.S., Choi S.E., Ha E.S., An S.Y., Kim T.H., Han S.J. Fibroblast growth factor-21 protects human skeletal muscle myotubes from palmitate-induced insulin resistance by inhibiting stress kinase and NF-kappaB. Metabolism. 2012;61:1142–1151. doi: 10.1016/j.metabol.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 51.Berglund E.D., Li C.Y., Bina H.A., Lynes S.E., Michael M.D., Shanafelt A.B. Fibroblast growth factor 21 controls glycemia via regulation of hepatic glucose flux and insulin sensitivity. Endocrinology. 2009;150:4084–4093. doi: 10.1210/en.2009-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Adams A.C., Halstead C.A., Hansen B.C., Irizarry A.R., Martin J.A., Myers S.R. LY2405319, an engineered FGF21 variant, improves the metabolic status of diabetic monkeys. PLoS One. 2013;8:e65763. doi: 10.1371/journal.pone.0065763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cho Y.M., Merchant C.E., Kieffer T.J. Targeting the glucagon receptor family for diabetes and obesity therapy. Pharmacology & Therapeutics. 2012;135:247–278. doi: 10.1016/j.pharmthera.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 54.Day J.W., Ottaway N., Patterson J.T., Gelfanov V., Smiley D., Gidda J. A new glucagon and GLP-1 co-agonist eliminates obesity in rodents. Nature Chemical Biology. 2009;5:749–757. doi: 10.1038/nchembio.209. [DOI] [PubMed] [Google Scholar]
- 55.Patel V., Joharapurkar A., Dhanesha N., Kshirsagar S., Patel K., Bahekar R. Co-agonist of glucagon and GLP-1 reduces cholesterol and improves insulin sensitivity independent of its effect on appetite and body weight in diet-induced obese C57 mice. Canadian Journal of Physiology and Pharmacology. 2013;91:1009–1015. doi: 10.1139/cjpp-2013-0189. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.