Introduction
Ebstein's anomaly accounts for < 1% of congenital heart diseases, the hallmark of which is the downward apical displacement of the tricuspid valve and cardinal albeit variable clinical symptoms of cyanosis, right-sided heart failure and arrhythmias.1,2 The typical echocardiographic feature of the disease is the apical displacement of the septal leaflet of tricuspid leaflet relative to the anterior mitral leaflet greater than 8.0 mm/m2.1 In Ebstein's anomaly manifest accessory pathways have been reported in 5–25% patients and half of these have multiple accessory pathways, mainly located on the right side. Paroxysmal atrioventricular reentrant tachycardia (AVRT) may occur in 25%–30% of patients on account of these manifest and additional concealed accessory pathways.3,4 We report a case of successful radiofrequency catheter ablation in a patient with Ebstein's anomaly who had earlier undergone two failed attempts.
Case report
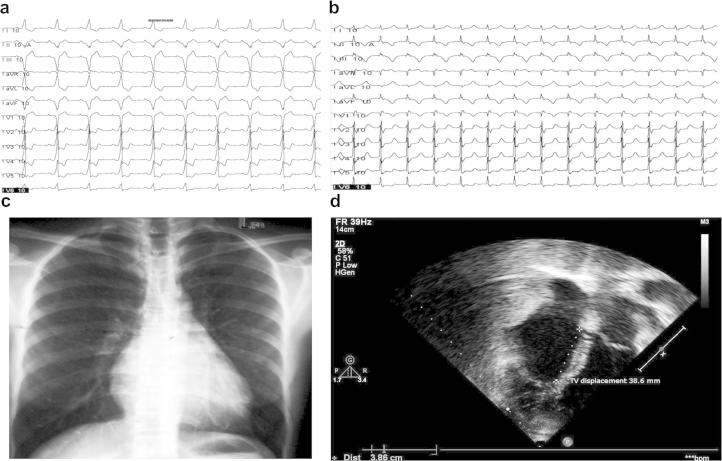
A 56 year old female patient presented with sudden onset of recurrent palpitations associated with dizziness and presyncope over the last 10 years. She was refractory to maximally tolerated drug therapy with β-blockers and calcium channel blockers. She had undergone two unsuccessful attempts at radiofrequency ablation (RFA) over the last 2 years. A 12-lead ECG during sinus rhythm showed ventricular pre-excitation of type B Wolff–Parkinson–White (WPW) syndrome (Fig. 1 – Panel (a)) and the ECG during tachycardia revealed a narrow QRS tachycardia (Fig. 1 – Panel (b)). Her chest X ray was normal (Fig. 1 – Panel (c)) and the echocardiogram revealed a sail like tricuspid leaflet displaced 38.6 mm (25.73 mm/m2)(Fig. 1 – Panel (d)).
Fig. 1.
a. 12 Lead ECG in sinus rhythm showing Type B WPW syndrome. b. 12 Lead ECG in AVRT. c. X ray chest PA view. d. Echocardiogram-showing apical displacement of TV by 38.6 mm.
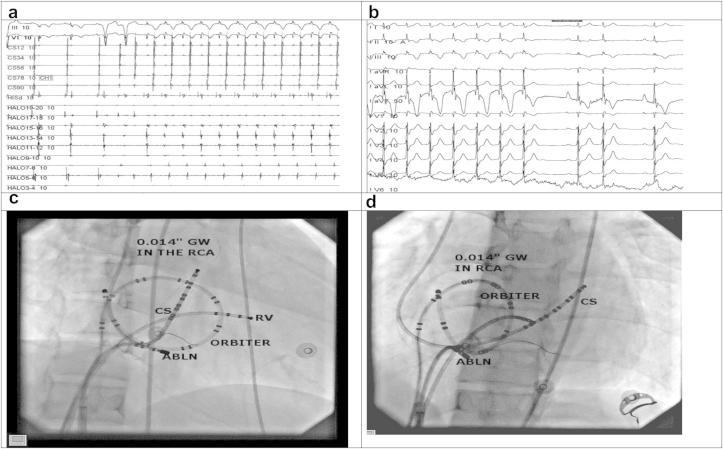
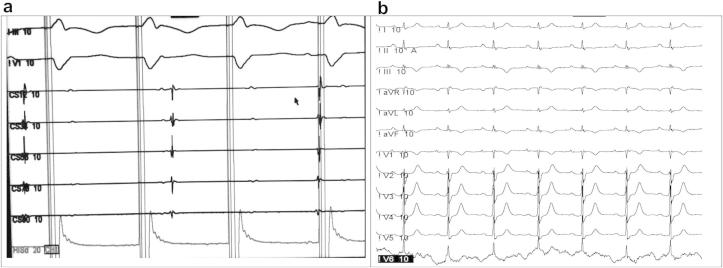
She was taken up for a repeat RFA. In order to mark the anatomic location of the tricuspid annulus (TA) we engaged the right coronary artery (RCA) using a JR 6.0 F coronary guiding catheter and performed a coronary angiogram in the LAO 40° and RAO 30° projections and then introduced a 0.014 inch Cougar® coronary guidewire (Medtronic Inc). Once the coronary guidewire was in place we disengaged the guiding catheter. Heparin was given intravenously to keep ACT between 250 and 300 s (secs) during the procedure. A Decapolar Steerable catheter (6.0 F) Livewire™ (St Jude Medical) was introduced into coronary sinus via the right internal jugular vein, a twenty-pole (7.0 F) Orbiter ST™ catheter (Bard Electrophysiology) was introduced via the left femoral vein at the TA mapped by the coronary guidewire, a Quadripolar Steerable (6.0 F) Livewire™ (St Jude Medical) was introduced via the right femoral vein at the His position and a St. Jude Medical Agilis™ EPI Steerable Introducer to improve catheter stability was introduced via the right femoral vein. Ablation catheter (Irvine Biomedical Inc. Medium curve 7.0 F) was introduced via the Agilis introducer (Fig. 2 – Panel (a) &(b)). An orthodromic reentrant tachycardia with a cycle length of 390 ms (ms) was easily inducible on coronary sinus pacing (Fig. 2 – Panel (c)). The ablation was performed during the tachycardia with anatomic localization to tricuspid annulus landmarked by the coronary guidewire and electrogram with earliest retrograde atrial activation in the Orbiter catheter. RFA was done at 40 W, 65 °C for 60 s using the Stockert (BWI) ablator. The tachycardia terminated after 3.584 s (s) with resumption of normal sinus rhythm with no evidence of pre-excitation (Fig. 2 – Panel (d)). After 30 min of observation there was no evidence of pre-excitation on atrial extrastimulus pacing and ventriculo-atrial (VA) dissociation was demonstrated on right ventricular pacing (Fig. 3). An ECG recorded after 24 h showed sinus rhythm with normal conduction which persisted on three months follow up.
Fig. 2.
a. Intracardiac electrograms during AVRT. b. Termination of AVRT with RFA. c. Fluoroscopy in RAO 30° showing successful ablation site. d. Fluoroscopy in LAO 30° showing successful ablation site.
Fig. 3.
a. VA dissociation post ablation. b. 12 Lead ECG 24 h post procedure.
Discussion
Catheter ablation of accessory pathways in Ebstein's anomaly is a challenging and demanding procedure with a generally low success rate fraught with recurrence and repeat procedures in almost up to half of the patients. The impediments are in that the atrialized portion of the right ventricle creates a discrepancy between the electrical and the anatomic atrioventricular (AV) junction hindering arrhythmia mapping and catheter stability.3–7 In our present case in a lady on whom radiofrequency ablation had failed on two previous occasions we improvised to facilitate electroanatomic mapping and impart catheter stability. We describe a novel method in which we innovated by identifying the anatomic location of the annulus with the coronary guidewire, used a twenty-pole catheter to identify the electrogram location at the (AV) junction and used the Agilis sheath to impart catheter stability.
Conclusion
In conclusion, radiofrequency catheter ablation is a modality of choice in drug resistant AVRT with Ebstein's anomaly. In comparison to conventional electrophysiological mapping the advent of 3D electroanatomic mapping profoundly assists in localization of the target ablation site. We have described a novel innovative technique as a surrogate for 3D mapping by improvising and use of improved hardware as a modality of ablation in the subset of elusive accessory pathways in Ebstein's anomaly.
Conflicts of interest
All authors have none to declare.
References
- 1.Attenhofer J.C.H., Connolly H.M., Dearani J.A. Ebstein’s anomaly. Circulation. 2007;115:277–285. doi: 10.1161/CIRCULATIONAHA.106.619338. [DOI] [PubMed] [Google Scholar]
- 2.Kumar A.E., Fyer D.C., Mietinen O.S. Ebstein’s anomaly: clinical profile and natural history. Am J Cardiol. 1971;28:84–95. doi: 10.1016/0002-9149(71)90038-5. [DOI] [PubMed] [Google Scholar]
- 3.Kastor J.A., Goldreyer B.N., Josephson M.E. Electrophysiologic characteristics of Ebstein’s anomaly of the tricuspid valve. Circulation. 1975;52:987–995. doi: 10.1161/01.cir.52.6.987. [DOI] [PubMed] [Google Scholar]
- 4.Cappato R., Schluter M., Weis C. Radiofrequency current catheter ablation of accessory atrioventricular pathways in Ebstein’s anomaly. Circulation. 1996;94:376–383. doi: 10.1161/01.cir.94.3.376. [DOI] [PubMed] [Google Scholar]
- 5.Hebe J. Ebstein’s anomaly in adults. Arrhythmias: diagnosis and therapeutic approach. Thorac Cardiovasc Surg. 2000;48:214–219. doi: 10.1055/s-2000-6897. [DOI] [PubMed] [Google Scholar]
- 6.Iturralde P., Nava S., Salica G. Electrocardiographic characteristics of patients with Ebstein’s anomaly before and after ablation of an accessory atrioventricular pathway. J Cardiovasc Electrophysiol. 2006;17:1332–1336. doi: 10.1111/j.1540-8167.2006.00617.x. [DOI] [PubMed] [Google Scholar]
- 7.Roten L., Lukac P., Groot N.D. Catheter ablation of arrhythmias in Ebstein’s anomaly: a multicenter study. J Cardiovasc Electrophysiol. 2011;22:1391–1396. doi: 10.1111/j.1540-8167.2011.02161.x. [DOI] [PubMed] [Google Scholar]