Introduction
Percutaneous transluminal angioplasty and stent placement of carotid arteries has become widely accepted as an alternative to endarterectomy for symptomatic patients with internal carotid artery stenosis more than 70%.1
The potential complications of carotid artery stenting (CAS) are acute neurological deficits, access-site vessel injuries, stent malfunction, stent restenosis, and baroreflex responses such as bradycardia, hypotension, and vasovagal reactions.1,2 Among these the most common and life threatening early complication is periprocedural stroke which occurs in approximately 5% cases.2 Though this is commonly due to athero-embolism, cerebral hypoperfusion, cerebral hyperperfusion and intracranial haemorrhage also contribute to it.2
Cerebral Hyperperfusion Syndrome (HPS) is an uncommon complication with a high morbidity and mortality, necessitating early recognition and aggressive management.2–5 It has bee observed after both Carotid Endarterectomy (CEA) and CAS, with an overall incidence of 0.2–0.7% in CEA and a relatively higher rate of up to 5% in CAS.3 It presents in the immediate and early post-procedural period with ipsilateral frontotemporal or retroorbital headache, nausea, vomiting and features of raised intracaranial pressure. Though intracerebral haemorrhage (ICH) is seen in over 85% cases it is not a mandatory feature of HPS. ICH occurs predominantly in the region of the basal ganglia.3–5 We present one such case.
Case report
A 77 years hypertensive male patient presented with history of recurrent transient ischemic attacks (TIA) with left sided hemiplegia. Detailed clinical examination revealed primary hypertension (BP: 140/90 mmHg) without any secondary cause or target organ damage. There was no focal neurological deficit and examination of the other systems was essentially normal. An MRI of the brain showed only a small focal hyperintensity on FLAIR imaging in the right temporo-parieto-occipital region without any restricted diffusion. An MR Angiography (MRA) of the neck and brain showed a 95% tubular right proximal Internal Carotid Artery (ICA) stenosis with reduced distal flow. The contralateral ICA, and its intracranial branches were normal. The vertebral arteries, the basilar artery and the posterior cerebral arteries were normal with a complete Circle of Willis.
Eight weeks after the last TIA the patient was started on a combination of Aspirin and Clopidogrel and taken up for Right Internal Carotid Artery (RICA) angioplasty. Cerebral angiogram confirmed the MRA findings, revealing a 95% stenosis (NASCET criteria) of RICA (Fig. 1). Circle of Willis was complete and the other cerebral vessels were normal. Heparin (5000 IU) was administered and right common carotid artery (RCCA) was cannulated with an 8F Judkins right guiding catheter (Cordis Corporation, NJ, USA). A 7 mm filter-protection device (AngioGuard XP, Cordis Corporation, NJ, USA) was placed distal to RICA lesion for embolic protection. A 9 × 40 mm Nitinol self deploying carotid stent (Cristallo Ideale Meditronic Invatec Corporation, Brescia, Italy) was placed across the lesion. The stent was post dilated with 5 × 20 mm coronary balloon at 8 atm. The morphological result was excellent with only 10–15% residual stenosis (Fig. 2). There was hypotension and bradycardia which was managed with Injection Atropine (0.6 mg IV) and Injection Dopamine (5 μg/Kg/Min).
Fig. 1.
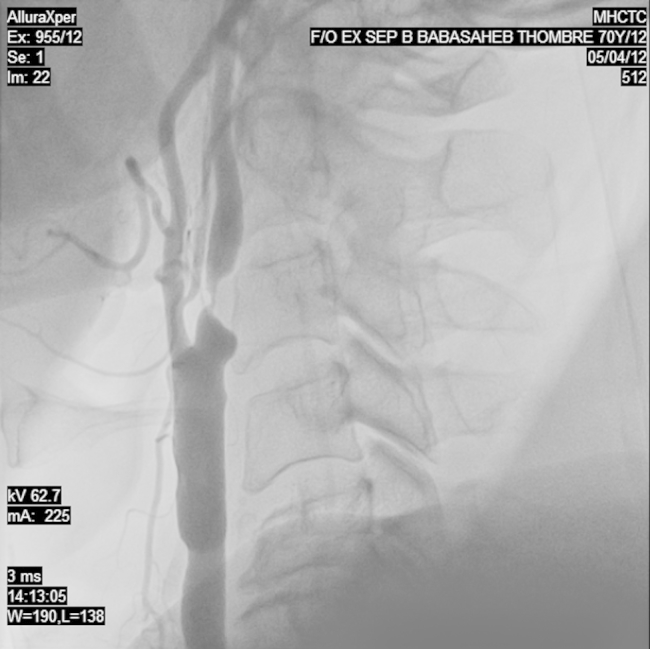
Carotid angiography showing a 95% eccentric occlusion of the proximal right internal carotid artery.
Fig. 2.
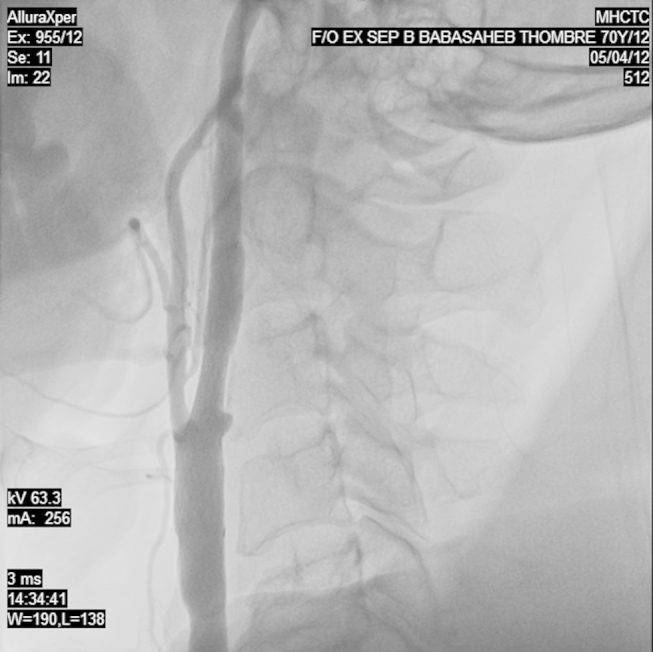
Carotid angiography of the right internal carotid artery after the adequate deployment of the stent showing the good resolution of the stenosis.
Four hours post-intervention patient developed headache. The blood pressure being 140/90 mmHg the dopamine infusion was stopped. However, over the next 1 h the patient became agitated and unresponsive. Neurological examination showed a Glasgow Coma Scale (GCS) of E1M2V1, anisocoria with a right sided dilated pupil with no reaction to light. An immediate non-contrast enhanced cranial CT (NCCT) scan showed a large right-sided ICH affecting the basal ganglia, thalamus and the surrounding parenchyma. There was a subarachnoid and subdural extension with a marked midline shift causing uncal herniation of the right temporal lobe with brainstem compression (Fig. 3). Immediate decongestive measures with Inj Mannitol, and Fursemide were begun. The patient was electively intubated and taken up for an emergency craniotomy and drainage of the intracranial hematoma. The pre and post-procedural hematological and coagulation profile were normal.
Fig. 3.
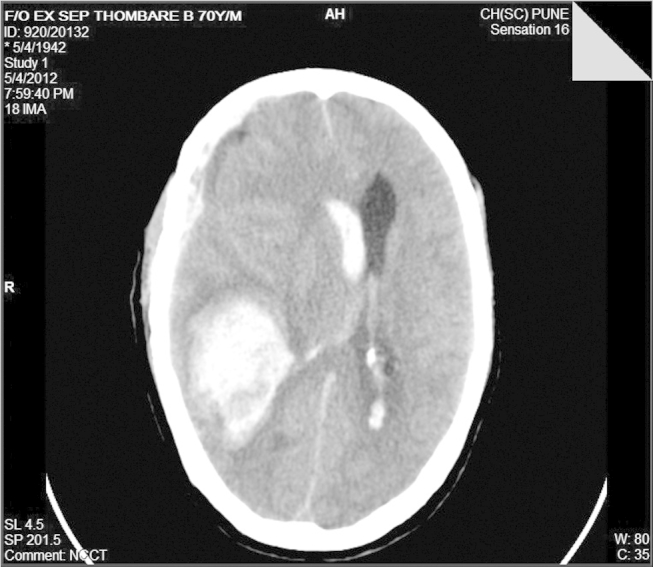
A non-contrast enhanced CT scan of the head showing a large bleed in the right basal ganglia with and an associated right intraventricular and subarachnoid extension. There is also a marked midline shift visible.
Post-surgery he was ventilated and continued on cerebral decongestive measures. His pupils normalized but he continued to be unresponsive to any vocal or painful stimuli. Over the next 14 days his neurological state gradually improved to a GCS of E2M2V1. He developed a ventilator associated pneumonia which was adequately treated with broad-spectrum antibiotics and a tracheostomy. An NCCT showed good resolution of the ICH and midline shift (Fig. 4). 4 weeks later he was discharged from hospital for domiciliary care. At discharge though conscious (GCS E2M2V1), he was aphasic, with dense left sided hemiplegia. He was bedbound, on nasogastric feeding, with complete dependency for activities of daily living.
Fig. 4.
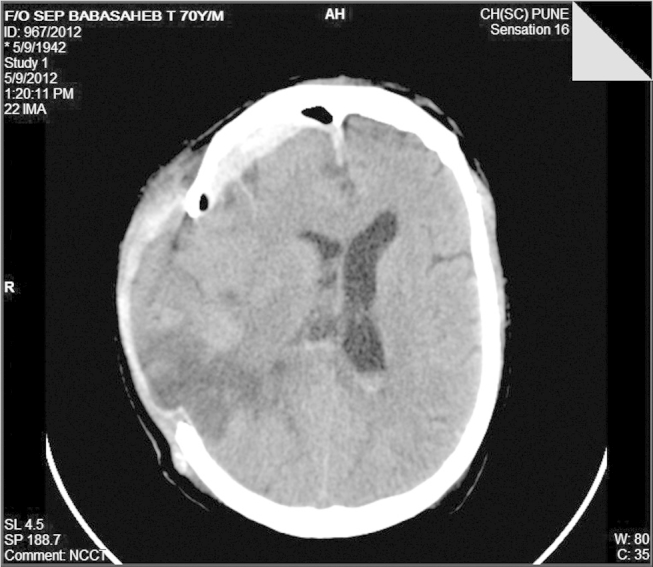
Post-hemicraniotomy there is good resolution of the midline shift and the evacuation of the intracerebral hematoma.
Discussion
HPS has been defined as the occurrence, either singly or in combination, of ipsilateral (to the treated artery) temporal, frontal or retro-orbital throbbing headache with or without nausea, vomiting, ipsilateral focal seizures, or focal neurological deficit without radiographic evidence of infarction.4 Various retrospective studies have shown the incidence of HPS to range between 1.1% and 5%.5–7 About 50% cases of HPS are associated with varying degrees of intracranial hemorrhage (ICH) which markedly increase the morbidity and mortality.6 ICH in HPS occurs characteristically in the ipsilateral basal ganglia with varying degrees of ventricular or subarachnoid extension.
Impaired autoregulation of intracerebral blood flow is the most accepted cause of HPS.3–5 Severe carotid stenosis produces a chronic low-flow state distal to the stenosis. This causes compensatory dilatation of cerebral vessels beyond the restriction which over time lose their ability to autoregulate the vascular resistance in response to changes in blood pressure. Recanalization leads to a ‘flash flood’ like increased cerebral blood flow which is termed as “hyperperfusion”.
The risk factors for HPS include intraparenchymal microvascular changes, hypertension, recent stroke or ischemia, severe ipsilateral ICA stenosis, and the presence of contralateral stenosis or occlusion.4,7 The presence of cerebral microangiopathy with insufficient intracranial collateralization indicates a high risk for HPS.3,6 Pre and Post-procedural hypertension is a critical (though not essential), finding associated with HPS and is seen in majority of patients who developed ICH.6,7 This could be further supported by the fact that most bleeds occurred in the basal ganglia, which itself is the most common site for hypertension associated ICH. Critical ICA stenosis >90% is also a major risk factor7 with most patients of HPS who developed ICH having a high-grade stenoses in the treated vessel.6–8 Though antiplatelets and anticoagulation have been considered as a cause, no direct causal relation has been found,4,9 and at best may contribute in the progression of the bleed in cases of HPS with ICH.
Our patient was over 70 years, a known hypertensive with recent history of TIA and a critical stenosis of ICA. He was thus likely to have microangiopathy in the parenchymal vessels. With these multiple risk factors our patient was indeed at a very high risk for developing HPS. The blood pressure was adequately controlled before and after the procedure. The dosage of anticoagulants was deliberately kept low to prevent any anticoagulation induced ICH. An isolated anticoagulation induced bleeding was not likely as the MRI was suggestive of only a small area of affection in the right temporo-parietal region while the ICH occurred in the ipsilateral basal ganglion that is not in the region of ischemic features. Though the blood pressure was adequately lowered before the procedure, post-procedural hypertension could have precipitated the HPS.
HPS being a rare complication a high index of suspicion is necessary to diagnose it. Once suspected an urgent CT scan should be done to ascertain any ICH and its complications. Immediate antihypertensive and decongestive therapy should be started. Good blood pressure control is mandatory and the blood pressure should be kept below 130/85 mmHg. Prevention of HPS requires a strict perioperative blood pressure control for the initial 2 weeks. Beta-blockers are preferred over direct vasodilators like hydralazine or nitrates to control the blood pressure.
Conclusion
HPS is a rare complication of carotid artery stenting or endarterectomy associated with high incidence of morbidity and mortality. It is mandatory to screen and identify patients predisposed to HPS prior to carotid endartrectomy or CAS. Post procedure a high index of suspicion for early detection and energetic multi disciplinary management is required for optimal outcomes.
Conflicts of interest
All authors have none to declare.
References
- 1.Brott T.G., Halperin J.L., Abbara S. JASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary. Circulation. 2011;124:e54–e130. [Google Scholar]
- 2.Gray W.A., Hopkins L.N., Yadav S. Protected carotid stenting in high-surgical-risk patients: the ARCHeR results. J Vasc Surg. 2006;44:258–268. doi: 10.1016/j.jvs.2006.03.044. [DOI] [PubMed] [Google Scholar]
- 3.Buhk J.H., Cepek L., Knauth M. Hyperacute intracerebral hemorrhage complicating carotid stenting should be distinguished from hyperperfusion syndrome. AJNR Am J Neuroradiol. 2006;27:1508–1513. [PMC free article] [PubMed] [Google Scholar]
- 4.Abou-Chebl A., Yadav J.S., Reginelli J.P. Intracranial hemorrhage and hyperperfusion syndrome following carotid artery stenting: risk factors, prevention, and treatment. J Am Coll Cardiol. 2004;43:1596–1601. doi: 10.1016/j.jacc.2003.12.039. [DOI] [PubMed] [Google Scholar]
- 5.Lieb M., Shah U., Hines G.L. Cerebral hyperperfusion syndrome after carotid intervention: a review. Cardiol Rev. 2012 Mar-Apr;20(2):84–89. doi: 10.1097/CRD.0b013e318237eef8. [DOI] [PubMed] [Google Scholar]
- 6.Mori T., Fukuoka M., Kazita K. Intraventricular hemorrhage after carotid stenting. J Endovasc Surg. 1999;6:337–341. doi: 10.1177/152660289900600407. [DOI] [PubMed] [Google Scholar]
- 7.Masuo O., Terada T., Matsumoto H. Haemorrhagic complication following percutaneous transluminal angioplasty for carotid stenosis. Acta Neurochir (Wien) 2000;142:1365–1368. doi: 10.1007/s007010070006. [DOI] [PubMed] [Google Scholar]
- 8.Coutts S.B., Hill M.D., Hu W.Y. Hyperperfusion syndrome: toward a stricter definition. Neurosurgery. 2003;53:1053–1058. doi: 10.1227/01.neu.0000088738.80838.74. [DOI] [PubMed] [Google Scholar]
- 9.Meyers P.M., Higashida R.T., Phatouros C.C. Cerebral hyperperfusion syndrome after percutaneous transluminal stenting of the craniocervical arteries. Neurosurgery. 2000;47:335–343. doi: 10.1097/00006123-200008000-00013. [DOI] [PubMed] [Google Scholar]


