Introduction
Brimonidine is the prototype alpha agonist anti-glaucoma drug, with additional neuro-protective property. It is considered safe and has a favourable side effect profile.1,2 We report a case of hypertensive granulomatous uveitis in a female patient caused by the use of this drug, where prompt diagnosis and substitution of the drug led to resolution of this sight threatening condition. To our knowledge only eleven cases have been reported worldwide.3 Since brimonidine 0.15% eye drop is a commonly prescribed drug, this lesser known but serious side effect is considered notable.
Case report
A seventy year old female patient, who was a known case of primary open angle glaucoma in both eyes with neovascular glaucoma in the left eye consequent to central retinal vein occlusion (CRVO) presented to our centre with complaints of redness in the left eye for the past one week. The medical records revealed that she had been on treatment for glaucoma elsewhere for more than a year. She was using eye drops timolol 0.5% b.i.d in both eyes. In addition she was using eye drops dorzolamide 2% b.i.d and eye drop brimonidine 0.15% t.i.d in the left eye.
On initial evaluation, the best corrected visual acuity was 6/6 in right eye and 6/60 in left eye. The right eye was white and quiet with grade IV open angles (Shaffer's grading) on gonioscopy and deep cups with a cup disc ratio of 0.6 with inferior notch. The left eye had conjunctival congestion with mild chemosis. Cornea was clear with no staining, keratic precipitates (KP's), cells or flare. Gonioscopy showed grade IV open angles (Shaffer's grading). Fundus showed old retinal photocoagulation marks with cellophane maculopathy. Optic cup was deep with a cup disc ratio of 0.6 with a pale neuroretinal rim. There was no evidence of neovascularisation of retina. Intraocular pressure was 14 mm Hg in the right eye and 30 mm Hg by applanation tonometry.
A provisional diagnosis of drug induced allergic conjunctivitis was made. It was decided to discontinue eye drop dorzolamide 2%, since conjunctivitis is a known side effect of this drug and the same was substituted with tab acetazonamide 250 mg t.i.d. She was advised to continue timolol 0.5% and brimonidine 0.15% in left eye with. Citrus fruits and banana intake was advised to take care of potassium depletion.
She presented a week later with aggravation of her symptoms. On examination, the left eye had ciliary congestion with mutton fat keratic precipitates (Fig. 1) and 2+ cells and flare by SUN grading system. Intraocular pressure was 22 mm Hg. A diagnosis of brimonidine induced granulomatous uveitis was made. The drug was stopped and the patient was put on prednisolone acetate 1% q.i.d and homatropine 2% b.i.d. On day 5 of stopping brimonidine, the redness was markedly reduced. The anterior chamber reaction reduced considerably with 0.5 cells/flare with few KP's which resolved over the next 14 days. Oral acetazolamide was discontinued and the IOP remained well controlled in the left eye with eye drops dorzolamide 2% t.i.d and timolol 0.5% b.i.d. in the follow up visits over the next four months.
Fig. 1.
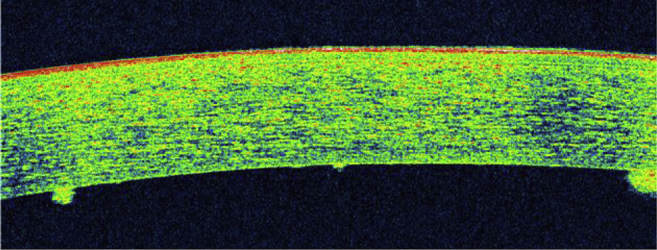
Multiple keratic precipitates as seen on OCT.
Discussion
Our patient was on topical brimonidine for more than a year. She presented with unexplained ocular congestion which progressed to granulomatous uveitis. The initial ophthalmic evaluation and base line investigations did not indicate any cause. Brimonidine has been reported in literature to cause uveitis on chronic use. Awareness of this possible side effect helped us to diagnose the entity promptly. As such, there was no obvious cause of granulomatous uveitis or involvement of the fellow eye and the disease resolved completely after discontinuation of the drops. In our patient, the IOP was well controlled after stopping brimonidine 0.15% by increasing the frequency of dorzolamide 2% to thrice a day from the previous dose of twice a day.
Delayed appearance of granulomatous uveitis following use of brimonidine eye drops has been reported previously.3 The concentration of drug used in all these cases was 0.2% but now brimonidine 0.15% eye drops is also available. This side effect appears to be a delayed hypersensitivity reaction which manifests on chronic use for more than a year. In some cases follicular conjunctivitis may precede the onset of severe uveitis.4 Naranjo's criteria5 can be used to establish the role of a drug suspected to have caused an adverse reaction (Table 1). In this case the rechallange could not be done due to advanced glaucoma and alternative medication could control IOP effectively.
Table 1.
Naranjo's criteria for adverse drug reactions.
| Criteria | Yes | No | Don't know | Score |
|---|---|---|---|---|
| Previous conclusive reports on this reaction | 1 | 0 | 0 | |
| Adverse reaction appeared after the drug was administered | 2 | −1 | 0 | |
| Adverse reaction improved when drug discontinued or specific antagonist administered | 1 | 0 | 0 | |
| Adverse reaction reappeared when the drug was readministered | 2 | −1 | 0 | |
| Alternative causes (other than the drug) caused the reaction | 0 | 2 | 0 | |
| Adverse reaction reappeared with placebo | −1 | 1 | 0 | |
| Toxic drug concentrations measured (systemic or local) | 1 | 0 | 0 | |
| Adverse reaction was dose-dependent | 1 | 0 | 0 | |
| Same adverse reaction to drugs in the same class | 1 | 0 | 0 | |
| Adverse event confirmed by any objective evidence | 1 | 0 | 0 | |
| 9–13 – Definite | ||||
| 5–8 – Probable | ||||
| 1–4 – Possible | ||||
| 0 – Doubtful |
This case highlights the need to have a high index of suspicion in patients presenting with uveitis who are on treatment with eye drop brimonidine 0.15% which is a frequently used drug. To the best of our knowledge this is the first case report of uveitis caused by 0.15% brimonidine eye drops. Clinicians should have close watch on patients using brimonidine who develop conjunctivitis for concurrent or subsequent development of uveitis.
References
- 1.Byles D.B., Frith P., Salmon J.F. Anterior uveitis as a side effect of topical brimonidine. Am J Ophthalmol. 2000;130:287–291. doi: 10.1016/s0002-9394(00)00491-8. [DOI] [PubMed] [Google Scholar]
- 2.Cantor L.B. The evolving pharmacotherapeutic profile of brimonidine, an alpha 2-adrenergic agonist, after four years of continuous use. Expert Opin Pharmacother. 2000:1815–1834. doi: 10.1517/14656566.1.4.815. [DOI] [PubMed] [Google Scholar]
- 3.Hondeghem K.A., Augustinus B., De Smet M.D. Bilateral granulomatous uveitis as a side effect of topical brimonidine: two case reports. Bull Soc Belge Ophtalmol. 2009;311:51–52. [PubMed] [Google Scholar]
- 4.Goyal R., Ram A.R. Brimonidine tartarate 0.2% (Alphagan) associated granulomatous anterior uveitis. Eye (Lond) 2000;14:908–910. doi: 10.1038/eye.2000.250. [DOI] [PubMed] [Google Scholar]
- 5.Naranjo C.A., Busto U., Sellers E.M. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]


