Abstract
The purpose of this study was to develop and validate low dose 18F-FDG-PET acquisition protocols for detection of inflamed carotid plaques specifically for simultaneous PET/MR imaging. The hypothesis was that increasing the duration of the PET acquisition to match that of the MR acquisition might allow for the use of lower levels of the radiotracer, while preserving quantification and image quality. Seven subjects were scanned twice at least one week apart on a simultaneous PET/MR scanner using either the standard clinical dose of 18F-FDG (373 ± 63 MBq) for 8 minutes or a low dose (93 ± 17 MBq) for 75 minutes. A maximum absolute percent difference of only 4.17% and 7.49% in the left and right carotid TBR was found between the standard dose and four time points of the low dose acquisitions (8, 24, 45, 75 minutes). Only the 8-minute low dose PET data was significantly different in terms of SNR (P = 0.009; % difference = -51%) and qualitative image quality evaluation (P = 0.0005; % difference = -45%). Our preliminary findings indicate that up to 75% reduction of the clinical standard 18F-FDG dose could be achieved using the proposed acquisition scheme while maintaining accurate quantification and SNR.
Keywords: PET/MR, dose reduction, FDG, atherosclerosis
Introduction
Atherosclerosis is responsible for the majority of disabilities and deaths in developed countries and is often not diagnosed before the onset of clinical events [1]. Positron emission tomography (PET) using 18F-fluorodeoxyglucose (18F-FDG) has been successfully used to characterize and quantify vascular plaque inflammation, an important hallmark of high-risk atherosclerotic plaques. 18F-FDG-PET has also become a marker of atherosclerotic disease for clinical trials that use imaging as an endpoint to monitor vascular disease [2,3].
Clinical PET scanners are typically available as hybrid systems that are combined with either computed tomography (CT) or magnetic resonance (MR) [4]. Recently introduced PET/MR scanners offer the benefit of delivering less ionizing radiation compared to that from PET/CT. Moreover, because MR exams are generally longer in duration than CT, further reduction in radiation exposure could be achieved by administering lower doses of the radioactive tracer used in PET imaging and matching the duration of the PET acquisition to that of the MR exam. This strategy could result in high quality PET data, even when a lower level of radio-tracer is injected. Previous studies conducted on phantoms have shown the feasibility of such acquisition approach, however, clinical data has not yet been shown [5].
18F-FDG-PET/MR carotid imaging of atherosclerotic plaques is well suited for low dose, long duration protocols. Firstly, carotid exams are generally performed using only one bed position centered on the carotid bifurcation in the neck making it feasible to perform the needed MR exams simultaneously during the PET data collection without the need to move the patient table [6]. More importantly, recent studies have shown that PET quantification of atherosclerotic plaques does not change significantly over a period of 1 to 3 hours and thus tracer redistribution due to longer exams should not affect the measured tracer uptake [7,8].
In this study, preliminary clinical evaluation of low dose 18F-FDG-PET imaging of the carotids was performed using a simultaneous PET/MR scanner. Subjects were scanned with both the standard clinical dose and acquisition time or longer acquisition time with low dose of 18F-FDG. The resulting low dose data was compared quantitatively and qualitatively to show the feasibility of low dose 18F-FDG-PET protocols in the quantification of vascular inflammation in the carotid arteries.
Methods
PET/MR imaging
This study was approved by the local institutional review board. All participating subjects signed written informed consent. Seven subjects (mean weight = 76.1 ± 12.2 kg; mean age = 31.6 ± 12.8 years) with two or more risk factors for cardiovascular disease were recruited for this study. All subjects underwent two PET/MR scans using a clinical standard and a low dose of 18F-FDG at least one week apart in randomized order on the Siemens Biograph mMR (Erlangen, Germany). The clinical standard scan was conducted for 8 minutes after 69±13 minutes of circulation time using a weight adjusted dose of 18F-FDG (~5 MBq per Kg) of 373 ± 63 MBq. The long duration, low dose scan was conducted for 75 minutes after 68 ± 13 minutes of circulation time using a dose approximately 75% lower than that from the clinical standard scan (93 ± 17 MBq). The listmode PET data from the low dose scan was reconstructed using the first 8, 24, 45, and 75 minutes of the data to be compared to the standard dose scan. PET images were reconstructed using the point spread function compensated ordinary Poisson ordered subsets expectation maximization algorithm (PSF-OP-OSEM) using 3 iterations and 21 subsets. The size of the reconstructed PET image was 344 x 344 x 127 voxels with a matrix size of 2.08 x 2.08 x 2.03 mm3. The final images were smoothed using a 4 mm Gaussian filter. Attenuation correction for the patient was done using the clinical standard MR segmentation based attenuation correction that is standard on the scanner. MR imaging was performed using the head and neck coil, which was corrected for in the PET reconstruction using a CT based attenuation map included on the scanner. For all PET/MRI exams a volumetric high-resolution MR image (TR = 1000 ms; TE = 4.89 ms; 1.67 mm isotropic resolution) was simultaneously acquired within the same imaging session for anatomical localization.
Data processing and analysis
Standard dose PET images were compared to the low dose data using the target-to-background ratio (TBR). The TBR was calculated by normalizing the mean uptake in the carotid artery by that of the blood pool from the corresponding jugular vein. The TBR is a standard quantitative measure of PET images used in clinical trials evaluating vascular inflammation [9]. In addition to the use of the TBR, the standard uptake value (SUV) of the carotid artery and jugular vein was also quantified.
The TBR was calculated using regions-of-interest (ROI) that were manually traced on the simultaneously acquired high resolution MR images (standard or low dose acquisitions) and transferred to the corresponding PET image using Osirix (Osirix Imaging software, Pixmeo, Geneva, Switzerland). The ROIs that were defined were the left and right carotid arteries (LC; RC) and left and right jugular veins (LJ; RJ). At least 20 ROIs per subjected per vessel were traced in the trans-axial MR images starting from the carotid bifurcation. In a second data analysis approach, the MR image collected in the standard dose imaging session was co-registered to the MR image of the low dose scan. Then, the measured transformation was used to warp the standard dose PET image so that both the low and standard dose PET images were aligned. The ROIs that were previously defined on the low dose MR image were then transferred the co-registered standard dose PET image to measure the TBR. The registration procedure was initialized by rigid registration that maximized the normalized mutual information over six degrees of freedom (3 translation and 3 rotation) between the MR images. Subsequently, non-rigid registration was performed using the diffeomorphic demons algorithm as implemented in the National Library of Medicine Insight Segmentation and Registration Toolkit (ITK).
Quantitative image quality evaluation was performed using the signal-to-noise (SNR) metric. SNR was calculated as the mean activity divided by the standard deviation within ROIs that were defined on large homogenous regions on the corresponding MR images and transferred to the PET images. Blinded qualitative Image quality scores were determined by an experienced clinician (OL, MD, PhD) from 1 to 4 with 1 = non diagnostic, 2 = poor, 3 = moderate and 4 = good. The analysis was performed on the registered standard dose PET image.
Statistical analysis to compare quantification of standard dose and low dose 18F-FDG-PET was conducted using a one-way ANOVA with Bonferroni post hoc correction. P < 0.05 was considered statistically significant.
Results
Results of the registration procedure are shown in Figure 1. Excellent overlap is observed following co-registration, particularly in the carotids, as shown in the insets of the figure.
Figure 1.
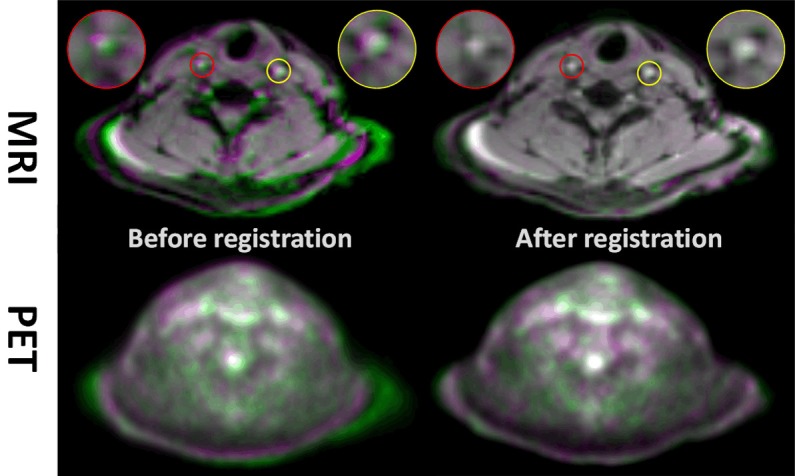
Data analysis was performed by registering the standard dose acquisition MR image to the low dose acquisition MR image. The measured transformation field is subsequently applied to the standard dose PET image before tracing of the ROIs. Top Panel: Low dose acquisition (green colormap) MR image overlapping the standard clinical dose acquisition MR image (purple colormap). Inserts are zoom-in of the left and right carotid to show accuracy of registration. When images are well registered, the overlap images turn to grayscale colormap (e.g. white signal indicates excellent overlap) Bottom Panel: Corresponding low and standard dose PET images.
Comparing the two different methods for analysis of the standard dose data (i.e. direct ROI tracing vs. registration to the low dose data) on the left side of Table 1 shows an insignificant difference of only 3.47% and 2.47% in the LC and RC respectively.
Table 1.
Summary of target to background ratio (TBR) for both the LC and RC
| Vessel/TBR | Standard dose | Low dose | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| 8 minutes | 8 minutesŦ | 8 minutes | 24 minutes | 45 minutes | 75 minutes | |
| Right carotid (RC) | 1.35 ± 0.21 | 1.32 ± 0.19 | 1.34 ± 0.32 | 1.37 ± 0.27 | 1.36 ± 0.26 | 1.36 ± 0.24 |
| Left carotid (LC) | 1.37 ± 0.20 | 1.41 ± 0.30 | 1.35 ± 0.25 | 1.35 ± 0.21 | 1.33 ± 0.20 | 1.31 ± 0.20 |
Data are presented as mean ± standard deviation.
Indicates the standard dose data analyzed using the proposed registration approach.
A representative standard dose PET image compared to the low dose PET images is shown in Figure 2. Low dose images appear noisier at short durations, but image quality improves with increased acquisition durations. Quantitative results are summarized in Table 1. Insignificant absolute percent difference in the TBR was found between the standard dose and low dose data of only 4.16 ± 2.23 (max = 7.49%) in the LC and 2.07 ± 1.36 (max = 4.17%) in the RC.
Figure 2.
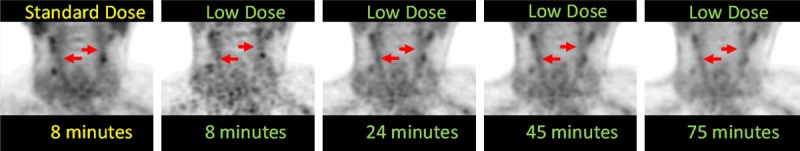
Representative coronal PET images in the same subject using the clinical standard dose of 18F-FDG acquired for 8 minutes compared to the low dose images acquired for 8, 25, 45 and 75 minutes.
The SUV mean for all subjects using the standard clinical dose was 1.31 ± 0.30 for the RC and 1.34 ± 0.26 for the LC. The SUV from the low dose studies in the RC was 1.07 ± 0.18, 1.06 ± 0.20, 1.05 ± 0.20, 1.02 ± 0.20 for the 8, 24, 45 and 75 minutes respectively. For the LC, the SUV was 1.10 ± 0.0.36, 1.17 ± 0.26, 1.14 ± 0.27, 1.10 ± 0.25 for the same time points as the RC. In the case of Jugular vein, the SUV mean was 0.97 ± 0.13 for the RJ and 0.98 ± 0.18 for the LJ when the standard clinical dose was used. In the case of the low dose studies, the SUV in the RJ was 0.80 ± 0.18, 0.77 ± 0.12, 0.78 ± 0.10, and 0.75 ± 0.15 for the 8, 24, 45, and 75 minutes scans respectively. For the LJ, the SUV for the low dose studies was 0.82 ± 0.17, 0.87 ± 0.15, 0.86 ± 0.12, and 0.84 ± 0.10 for the same time points as the RJ.
SNR in the standard dose and low dose PET images is shown in Figure 3A. A significant difference of -51% was found between the standard dose and the 8-minute low dose data, but not the 24, 45, and 75 minutes low dose scans. Similar trend was also observed in the image quality score analysis in Figure 3B where significant differences were measured only between the standard dose and the 8-minute low dose data of -45.4%.
Figure 3.
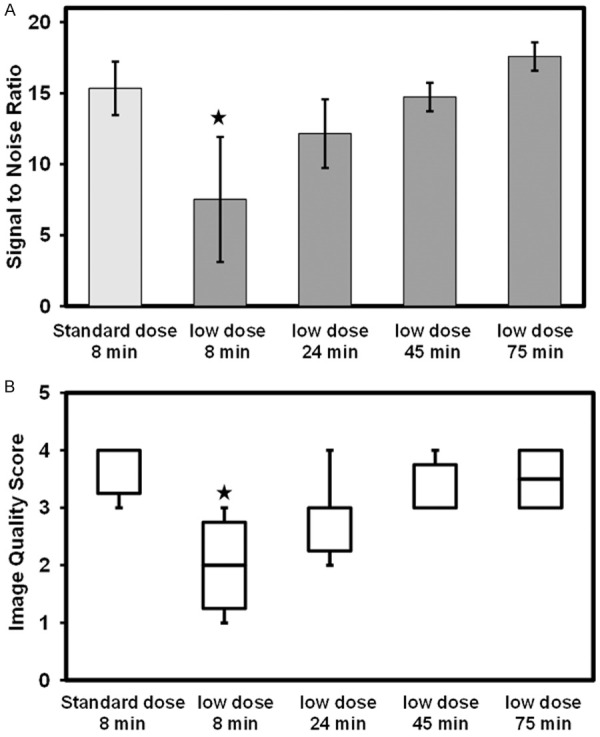
Quantitative comparisons of standard dose to low dose carotid 18F-FDG-PET imaging. A. Bar plot showing the SNR comparing clinical standard dose and duration to low dose acquisition at different durations. B. Box plot plot showing differences in image quality score. Indicates significant difference.
Discussion
In this study, the feasibility of a low dose protocol was evaluated in vivo for 18F-FDG-PET imaging of the carotids vasculature. We found that up to 75% reduction in 18F-FDG dose can be achieved by increasing the duration of the acquisition (which was necessary for the simultaneous MR imaging) from 8 minutes to 24 minutes at minimum. Such a large decrease in dose with increased acquisition time still allowed for the detection of increased uptake in the carotids as shown in Figure 2. Comparable TBR and SNR were measured with the low dose scan when evaluated against the standard clinical dose (Table 1; Figure 3). The recent introduction of simultaneous clinical PET/MR scanners has prompted the development of new acquisition protocols that are different from that of sequential PET/CT. We have exploited the fact that MR exams are longer in duration than that of CT and thus, using simultaneous acquisitions, reduction in the administered radiotracer dose could be achieved by matching the duration of the PET acquisition to that of MR, while maintaining quantification and image quality.
We used the TBR metric as a measure of carotid vessel wall inflammation. Several recent studies have indicated that the TBR is a more reliable metric for uptake quantification and thus its use was extended to this study [8-10].
Two methods for data analysis were used in our study. In the first approach, ROIs were traced on MR images that were simultaneously acquired with the standard or low dose acquisition. A drawback to this method is that it requires a re-trace of the ROIs by the image analyst, which could result in quantitative error due to the difficulty of reproducible ROI definitions and is time consuming [11]. As an alternative approach, the images from both acquisitions were co-registered non-rigidly and the ROI definition was only performed once. The limitation of this approach is that it is sensitive to errors in registration. This error, however, is expected to be small given the accuracy of the non-rigid transformation that was measured from the high-resolution MR images and was applied to the relatively low resolution of the PET image [12]. Results in this study shown in the left side of Table 1 show, however, that there were no significant differences between the two data analysis methods. This may indicate that the registration based data analysis approach presented here could be a useful method for data analysis of studies that scan the same subject over several time points, as is the case during therapeutic drug trials, for example [9].
A limitation to long duration PET scans is that they could be corrupted by motion resulting in a blurred image that is quantitatively inaccurate. Motion correction was not addressed in this study; however, several studies have shown the feasibility of data driven motion correction approaches [13]. This is an active area of research and we expect several possible solutions in the near future.
Another limitation to long duration exams is that the tracer could be redistributed during the scan resulting in a dynamic measurement. Our results in this pilot study indicate that the TBR from a long duration exam is similar to that from a short one. These results are consistent with findings from a recent study that found that the TBR is not sensitive to circulation time differences [7]. It is important to point out that the duration of MR exams are generally at least 30 minutes and thus the PET exam in a simultaneous scanner should be adjusted to match the duration of the MR exam with the benefit of reduction of the administered radio-active tracer. This kind of acquisition scheme, however, might not be appropriate for all kind of PET exams as some targets might be sensitive to differences in circulation time.
For robust carotid MRI, a dedicated carotid receiver coil is typically used for MR imaging. Such coils are known to induce quantitative errors if used in PET/MR exams without accounting for their attenuation. Several approaches, however, have been proposed to localize such coils and account for their attenuation in the PET reconstruction using a template coil attenuation map [14,15].
The TBR values obtained in this study are slightly lower compared to previous studies where a TBR of 1.6 or greater reflects vascular inflammation [8]. The age of the patient cohort used in this study, however, was lower compared to a typical population with advanced cardiovascular disease, which could account for this difference. Moreover, in this pilot study a small number of subjects were enrolled and thus, such low dose acquisition protocols should be validated in larger clinical study.
Conclusions
In this study, the feasibility of low dose 18F-FDG PET for the detection of inflammation in the carotids was evaluated. By matching the duration of the low dose PET acquisition to that of the MR scan in a simultaneous PET/MR scanner, significant reduction in the dose can be achieved, while maintaining excellent image quality. The advent of simultaneous PET/MRI may allow significantly lower radiation exposure to patients making it a more desirable tool for longitudinal studies of disease evaluation and for monitoring therapeutic interventions compared to PET/CT.
Acknowledgements
The authors would like to thank Siemens Healthcare for its technical support. This work is supported in part by a grant from the National Institute of Health, National Heart Lung and Blood Institute, and National Center for Advancing Translational Science (NIH/NHLBI R01 HL071021, R01 HL078667 and NIH/NCATS CTSA UL1TR000067) (ZAF).
Disclosure of conflict of interest
None.
References
- 1.Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat Med. 2011;17:1410–22. doi: 10.1038/nm.2538. [DOI] [PubMed] [Google Scholar]
- 2.Sanz J, Fayad ZA. Imaging of atherosclerotic cardiovascular disease. Nature. 2008;451:953–957. doi: 10.1038/nature06803. [DOI] [PubMed] [Google Scholar]
- 3.Calcagno C, Ramachandran S, Izquierdo-Garcia D, Mani V, Millon A, Rosenbaum D, Tawakol A, Woodward M, Bucerius J, Moshier E, Godbold J, Kallend D, Farkouh ME, Fuster V, Rudd JH, Fayad ZA. The complementary roles of dynamic contrast-enhanced MRI and 18F-fluorodeoxyglucose PET/CT for imaging of carotid atherosclerosis. Eur J Nucl Med Mol Imaging. 2013;40:1884–1893. doi: 10.1007/s00259-013-2518-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delso G, Furst S, Jakoby B, Ladebeck R, Ganter C, Nekolla SG, Schwaiger M, Ziegler SI. Performance measurements of the Siemens mMR integrated whole-body PET/MR scanner. J Nucl Med. 2011;52:1914–1922. doi: 10.2967/jnumed.111.092726. [DOI] [PubMed] [Google Scholar]
- 5.Oehmigen M, Ziegler S, Jakoby BW, Georgi JC, Paulus DH, Quick HH. Radiotracer Dose Reduction in Integrated PET/MR: Implications from National Electrical Manufacturers Association Phantom Studies. J Nucl Med. 2014;55:1361–1367. doi: 10.2967/jnumed.114.139147. [DOI] [PubMed] [Google Scholar]
- 6.Huet P, Burg S, Le Guludec D, Hyafil F, Buvat I. Variability and Uncertainty of 18F-FDG PET Imaging Protocols for Assessing Inflammation in Atherosclerosis: Suggestions for Improvement. J Nucl Med. 2015;56:552–559. doi: 10.2967/jnumed.114.142596. [DOI] [PubMed] [Google Scholar]
- 7.Bucerius J, Mani V, Moncrieff C, Machac J, Fuster V, Farkouh ME, Tawakol A, Rudd JH, Fayad ZA. Optimizing 18F-FDG PET/CT imaging of vessel wall inflammation: the impact of 18F-FDG circulation time, injected dose, uptake parameters, and fasting blood glucose levels. Eur J Nucl Med Mol Imaging. 2014;41:369–383. doi: 10.1007/s00259-013-2569-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rudd JH, Myers KS, Bansilal S, Machac J, Pinto CA, Tong C, Rafique A, Hargeaves R, Farkouh M, Fuster V, Fayad ZA. Atherosclerosis inflammation imaging with 18F-FDG PET: carotid, iliac, and femoral uptake reproducibility, quantification methods, and recommendations. J Nucl Med. 2008;49:871–878. doi: 10.2967/jnumed.107.050294. [DOI] [PubMed] [Google Scholar]
- 9.Fayad ZA, Mani V, Woodward M, Kallend D, Abt M, Burgess T, Fuster V, Ballantyne CM, Stein EA, Tardif JC, Rudd JH, Farkouh ME, Tawakol A, dal PI. Safety and efficacy of dalcetrapib on atherosclerotic disease using novel non-invasive multimodality imaging (dal-PLAQUE): a randomised clinical trial. Lancet. 2011;378:1547–1559. doi: 10.1016/S0140-6736(11)61383-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niccoli Asabella A, Ciccone MM, Cortese F, Scicchitano P, Gesualdo M, Zito A, Di Palo A, Angiletta D, Regina G, Marzullo A, Rubini G. Higher reliability of 18F-FDG target background ratio compared to standardized uptake value in vulnerable carotid plaque detection: a pilot study. Ann Nucl Med. 2014;28:571–579. doi: 10.1007/s12149-014-0850-9. [DOI] [PubMed] [Google Scholar]
- 11.Krak NC, Boellaard R, Hoekstra OS, Twisk JW, Hoekstra CJ, Lammertsma AA. Effects of ROI definition and reconstruction method on quantitative outcome and applicability in a response monitoring trial. Eur J Nucl Med Mol Imaging. 2005;32:294–301. doi: 10.1007/s00259-004-1566-1. [DOI] [PubMed] [Google Scholar]
- 12.Vercauteren T, Pennec X, Perchant A, Ayache N. Diffeomorphic demons: efficient non-parametric image registration. Neuroimage. 2009;45:S61–72. doi: 10.1016/j.neuroimage.2008.10.040. [DOI] [PubMed] [Google Scholar]
- 13.Rahmim A, Dinelle K, Cheng JC, Shilov MA, Segars WP, Lidstone SC, Blinder S, Rousset OG, Vajihollahi H, Tsui BM, Wong DF, Sossi V. Accurate event-driven motion compensation in high-resolution PET incorporating scattered and random events. IEEE Trans Med Imaging. 2008;27:1018–1033. doi: 10.1109/TMI.2008.917248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eldib M, Bini J, Calcagno C, Robson PM, Mani V, Fayad ZA. Attenuation correction for flexible magnetic resonance coils in combined magnetic resonance/positron emission tomography imaging. Invest Radiol. 2014;49:63–69. doi: 10.1097/RLI.0b013e3182a530f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kartmann R, Paulus DH, Braun H, Aklan B, Ziegler S, Navalpakkam BK, Lentschig M, Quick HH. Integrated PET/MR imaging: automatic attenuation correction of flexible RF coils. Med Phys. 2013;40:082301. doi: 10.1118/1.4812685. [DOI] [PubMed] [Google Scholar]


