Abstract
Radiation therapy (RT) induces vascular changes that increase the risk of cardiovascular diseases in some patients. The objective was to determine if in vivo positron emission tomography (PET) with fluorodeoxyglucose (18F-FDG) can identify increased vascular inflammation in patients without changes in vascular intima media thickness (IMT). Patients previously receiving unilateral RT due to lymphoma were prospectively recruited (N=10). The untreated contralateral artery functioned as control. All patients underwent a dedicated vascular PET/CT. Vascular tracer uptake was quantified by drawing regions of interests around the carotid artery or the iliac arteries. The IMT of the carotid arteries was measured using ultrasound. Eight patients (25% male, 42-83 years old) that had received RT involving unilateral carotid arteries and 2 patients (both male, 38 and 58 years old) that had received radiotherapy involving the unilateral iliac artery were included. The patients had completed their RT 2-7 years before. Eight patients showed increased uptake of 18F-FDG in the irradiated side compared to the non-irradiated side, 1 showed no difference, while 1 patient showed highest uptake in the non- irradiated side (P=0.04). Measurement of IMT showed that 4 patients had the highest thickness in the irradiated side, while the other 4 patients had the highest thickness in the non-irradiated side (P=0.8). In conclusion, we found that 18F-FDG PET imaging may be used to detect vascular changes induced by RT. Larger prospective follow-up studies are needed to determine the prognostic value of increased vascular FDG-uptake.
Keywords: Molecular imaging, vascular inflammation, positron emission tomography, fluorodeoxyglucose, radiation therapy
Introduction
Radiation therapy (RT) is an integrated part of cancer treatment in many patients. Accordingly, it is estimated, that more than half of all long-term cancer survivors have received RT. Radiation treatment plans that include irradiation of the large arteries enhance the risk of atherosclerosis. In line with this, long term cancer survivors have an increased risk of cardiovascular disease associated with irradiation [1-4].
The precise mechanism behind radiation-induced cardiovascular disease remains to be determined. The initial event is thought to be endothelial cell damage leading to endothelial dysfunction [5], progressive cell proliferation and subsequently intima media thickening of the vessel [6]. However, human studies with diverse designs have shown conflicting results regarding the time frame from RT to a significant increase in carotid artery intima media thickness [7-9].
Several non-invasive imaging modalities can be used to characterize atherosclerotic changes. Most of these methods (like ultrasound, CT-angiography and magnetic resonance imaging) rely on morphological changes in the arterial wall (e.g. intima media thickness). In comparison, positron emission tomography (PET) using the glucose analog 18F-fluorodeoxyglucose (FDG) is a molecular imaging technique that identifies cells with high metabolic activity. A very early event in atherogenesis is macrophage invasion and proliferation. These activated macrophages have a high metabolic rate and are therefore readily identified by FDG-PET. A number of previous studies have used FDG uptake as marker of the arterial inflammation that precedes the morphological changes seen with other non-invasive imaging techniques [10-15]. Studies of this very early atherosclerosis with FDG-PET may offer ways to monitor early atherosclerosis and guide future interventions against cardiovascular disease in RT treated patients and may also provide increased knowledge of the pathogenesis behind the increased risk of cardiovascular disease following RT.
The aim of this study was to determine if in vivo FDG PET imaging can identify increased vascular inflammation following RT. To do this, RT effects were studied less than 10 years after unilateral involved node radiation therapy (INRT) of lymphoma with the patient’s contra-lateral non-irradiated artery serving as intra-subject reference.
Materials and methods
Design
Patients previously treated according to INRT guidelines [16] with RT for unilateral lymphoma near the carotid artery (N=8) or the internal iliac artery (N=2) were prospectively included in the trial. Exclusion criteria were age < 18 years, diabetes, pregnancy, renal insufficiency, or active infection. The initial INRT was delivered as 3-dimensional conformal radiation therapy (3D-CRT) from 2005 to 2010. The dose delivered to the carotid artery or the internal iliac artery was assessed bilaterally from the CT-fused radiation treatment plans.
All patients underwent a dedicated vascular FDG-PET/CT and carotid ultrasound on the same day. The study was approved by the Regional Scientific Ethical Committee (protocol H-1-2010-064) and all subjects received oral and written information about the study and signed an informed consent before inclusion.
FDG-PET/CT
Patients fasted 6 hours prior to injection of 400 MBq 18F-FDG (range 393-454 MBq) in a cubital vein. Plasma glucose measured before injection ranged from 4.9 to 7.1 mmol/l. To reduce tracer uptake in neck musculature the patients were not allowed to talk, and to avoid uptake in brown fat they rested in calm and warm surroundings from 15 min before injection until 30 min after injection.
Three hours after 18F-FDG injection (range 169 to 204 minutes), the patients were scanned using a combined PET/CT-scanner (Siemens Biograph mCT64, Siemens, Berlin, Germany). This prolonged circulation time from injection to PET leads to a low FDG content in the blood and is preferred for imaging of vascular inflammation [17,18]. PET was acquired in three-dimensional list mode for 3 min over one field of view centered at the carotid bifurcation or the internal iliac artery. Two CT examinations were performed in each patient; one CT scan for attenuation correction and one contrast enhanced CT scan (120 kV, reference mAs 225 (care dose)) was performed just after the PET acquisition. Contrast was injected by pump (100 ml of Optiray 300 mg/ml at 2.5 ml/s followed by 100 ml of saline at 2.5 ml/s) and the CT was automatically initiated using bolus tracking in the descending aorta.
A routinely used, optimized clinical reconstruction setting using CT based attenuation correction was employed, with both resolution-recovery (point spread function, TrueX) and time-of-flight (2 iterations, 21 subsets, zoom 1.0) giving 400×400 image slices (voxel size 2.00×2.04×2.04). A 2 mm full width at half maximum Gaussian filter was then applied to all images post-reconstruction.
Data analysis
A free-hand 3D ROI was drawn around the common carotid artery and the internal carotid artery or the internal iliac artery slice by slice on the axial contrast-enhanced CT images including both the vessel wall and the vessel lumen. The ROI started 20 mm caudal to the bifurcation and extended distally to 10 mm cranial to the bifurcation of the carotid. Likewise a 3D ROI was drawn to include the proximal 10 mm of the internal iliac artery bilaterally.
Anatomical co-registration of CT and PET was carefully checked by matching anatomical landmarks such as the salivary glands. The ROI including the relevant vascular segment was copied from the CT to the FDG-PET. The vascular FDG-uptake was assessed in the irradiated artery segment (carotid artery or internal iliac artery) and the contralateral non-irradiated artery by maximum standardized-uptake-values (SUVmax) that corrects for injected dose, patient weight and time to acquisition.
Vascular ultrasound
Before tracer injection, real-time intima media thickness was measured along a 10 mm segment of the common carotid artery 1 cm caudally of the sinus caroticus by ultrasound (Mylab25Gold, Esaote, Italy) using a 6.5 MHz transducer and automatic software (RF-QIMT, Esaote, Italy). QIMT uses radiofrequency signal tracking - a ‘raw’ ultrasound signal from the ultrasound scanner that is not yet processed - to measure the thickness of the intima media layer with high spatial resolution and with independency of the investigator and device settings. Figure 1 shows a case sample from our lab of the automated intima media thickness detection.
Figure 1.
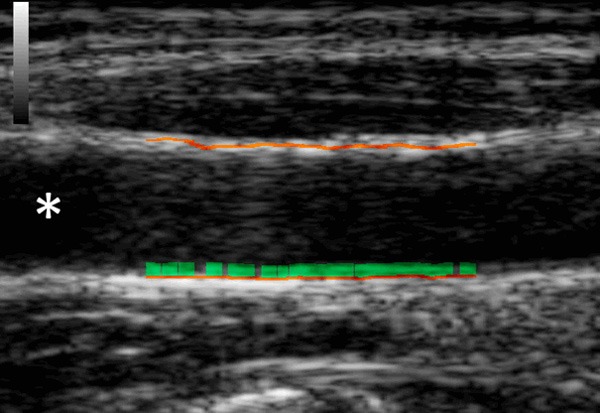
A sample case of automated radiofrequency based intima media evaluation. The distal lumen of the common carotid artery is marked with an asterisk.
Statistics
The inclusion of patients with unilateral radiation therapy allowed for a paired design where each patient served as their own control (irradiated vs. non-irradiated carotid artery or irradiated vs. non-irradiated internal iliac artery). In addition to increased statistical power, this design spared the need for a background correction like calculating target-to-background ratio.
The pre-trial power calculation showed that a sample size of 11 would give a power of 0.7 to detect a proportion of 0.8 using the sign-test.
Data were logarithmically transformed when appropriate. The paired analyses of FDG-uptake in the irradiated versus non-irradiated vascular segment were tested using both the sign-test (binominal distribution) and the paired sample t-test. Correlation between vascular FDG uptake and adjacent FDG uptake, or intima media thickness was tested using Spearman’s rho. Analyses were performed with IBM SPSS Statistics (version 22, IBM).
Results
Eight patients previously treated with unilateral RT in the carotid region and 2 patients with previous unilateral RT to the iliac artery region were included. All were patients with a unilateral lymphoma. The median time from RT to the PET examination was 2 years (range 2-7 years). The total estimated radiation dose to the artery (derived from the CT-fused radiation treatment plans) ranged from 11 to 35 Gy. The individual time from RT to PET, histological diagnosis of lymphoma type as well as chemotherapy regimens are shown in Table 1. The contralateral vascular segment that served as control in this study received in all cases less than 3 Gy assessed from the original radiation plan. All included patients had few traditional cardiovascular risk factors and none had symptoms from vascular disease (Table 2).
Table 1.
Details of all included patients
| pt ID | Diagnosis | Location of radiation exposed vessel | Time from RT to PET (years) | Estimated Gy to the vessel | Chemotherapy |
|---|---|---|---|---|---|
| 1 | Diffuse large B-cell lymphoma | Right carotid artery | 2 | 31-35 | R-CHOP |
| 2 | Lymphoplasmacytic lymphoma | Right carotid artery | 2 | 16-20 | No chemotherapy |
| 3 | Diffuse large B-cell lymphoma | Right carotid artery | 3 | 16-20 | R-CHOP |
| 4 | Diffuse large B-cell lymphoma | Left carotid artery | 2 | 31-35 | R-CHOP |
| 5 | Nodular sclerosis Hodgkin lymphoma | Left carotid artery | 3 | 11-15 | ABVD |
| 6 | Diffuse large B-cell lymphoma | Left carotid artery | 2 | 31-35 | R-CHOP |
| 7 | Diffuse large B-cell lymphoma | Right carotid artery | 2 | 31-35 | R-CHOP |
| 8 | Diffuse large B-cell lymphoma | Right carotid artery | 2 | 26-30 | R-CHOEP |
| 9 | Nodular sclerosis Hodgkin lymphoma | Right internal iliac artery | 7 | 21-25 | ABVD |
| 10 | Mixed cellularity Hodgkin lymphoma | Left internal iliac artery | 2 | 31-35 | ABVD |
ABVD = Adriamycin, bleomycin, vinblastine, dacarbazine; R-CHOP = Rituximab, cyklofosfamide, adriamycine, vincristine, prednisone; R-CHOEP = Rituximab, cyklofosfamide, adriamycine, vincristine, etoposid, prednisone.
Table 2.
Characteristics of study population
| Male gender | 4 (40%) |
| Median age (range) | 58 (38-83) |
| Median body mass index (range) | 27.7 (16.9-43.6) |
| Known cardiovascular disease | 0 |
| Current smokers | 3 (30%) |
| Family history of heart disease | 2 (20%) |
| Medical treatment for hypertension | 2 (20%) |
| Medical treatment for hypercholesterolemia | 1 (10%) |
| Diabetes* | 0 |
Exclusion criteria.
All patients had unilateral RT and thus served as their own control when assessing vascular FDG uptake. The carotid artery was examined in eight patients and the internal iliac artery in 2 patients (Figures 2 and 3). Of the 10 included patients, only 1 had highest vascular FDG uptake in the non-irradiated side, one patient had equal uptake in the two sides, and 8 patients had highest uptake in the irradiated vascular segment (P=0.04). Both patients with iliac artery RT had highest uptake in the irradiated side (Figure 4). The average uptake in the irradiated side was 2.3 (s.d. 1.1) compared to 1.9 (s.d. 0.7) in the non-irradiated side. The mean difference between FDG uptake (SUVmax) in the irradiated and contralateral non-irradiated vascular segments was 0.42 (P<0.05, Figure 4).
Figure 2.
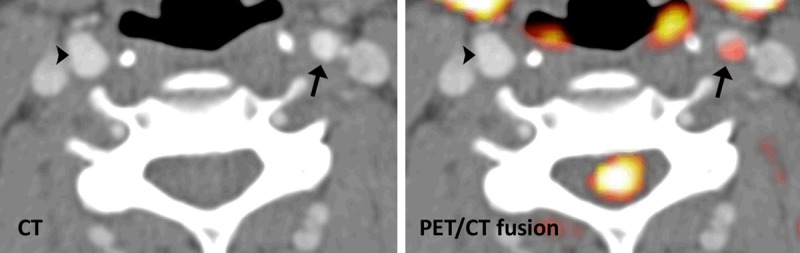
Representative FDG-PET/CT from irradiated (arrow) and non-irradiated (arrowhead) carotid artery. The left panel shows the contrast enhanced CT and the right panel the FDG-PET fused with the CT.
Figure 3.
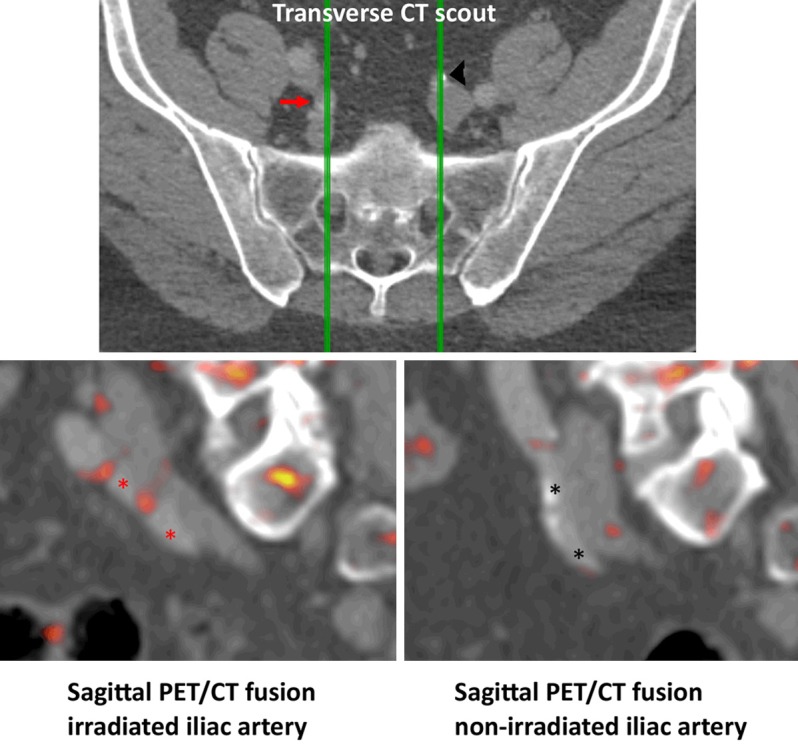
Representative FDG-PET/CT from irradiated (red arrow and asterisks) and non-irradiated (black arrowhead and asterisks) internal iliac artery. The top-row show the contrast enhanced CT with green lines representing the sagittal planes used for the bottom-row FDG-PET fused with CT.
Figure 4.
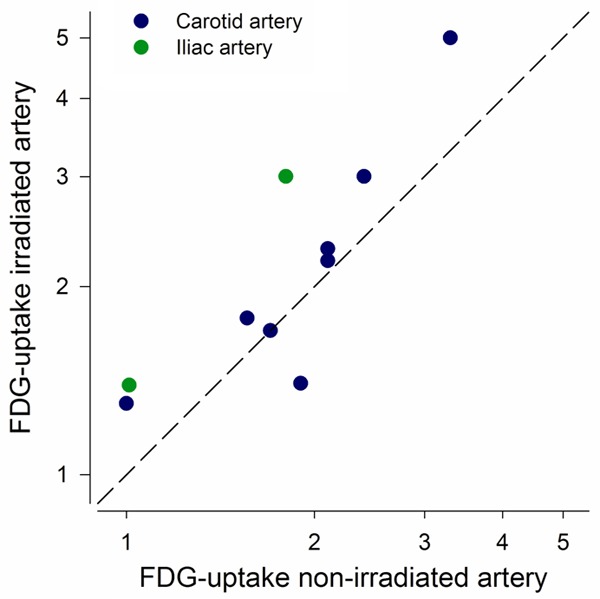
Correlation between FDG-uptake (SUVmax) in the irradiated and contralateral non-irradiated artery from the 10 included patients. The dashed identity-line is shown in black. The carotid artery was compared in 8 patients (blue circle) and the internal iliac artery in another 2 patients (green circle). Axes are in logarithmic scale.
The average FDG uptake (SUVmax) in the adjacent soft tissue was 1.6 (s.d. 0.8). As expected we did not find a significant correlation between SUVmax in the arteries and SUVmax in the adjacent soft tissue (r=-0.08, P=0.7), indicating that the high vascular uptake is not caused by spill over from adjacent tissue.
Intima media thickness in the 8 patients with unilateral carotid irradiation did not differ in the irradiated versus the non-irradiated side (mean difference 0.02 mm; P=0.8, Figure 5). The average intima media thickness in the irradiated side was 837 µm (s.d. 326 µm) compared to 856 µm (s.d. 248 µm) in the non-irradiated side. Also, intima media thickness did not correlate with carotid FDG uptake (r=-0.2; P=0.9).
Figure 5.
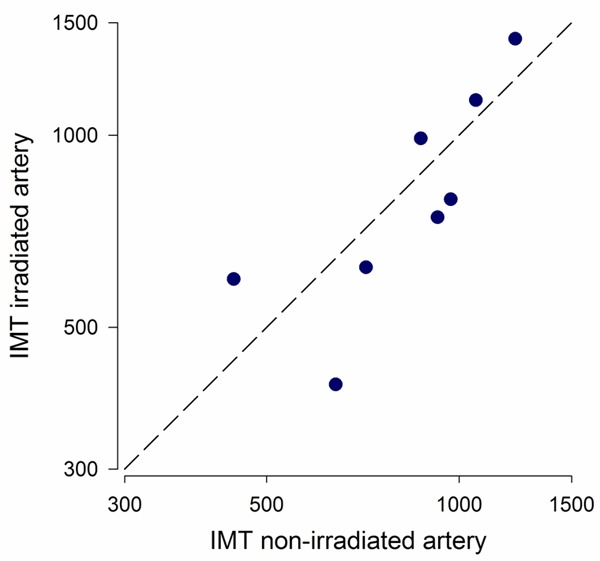
Correlation between intima media thickness (IMT) in the irradiated and contralateral non-irradiated carotid artery from the included patients. The dashed identity-line is shown in black. Axes are in logarithmic scale.
Discussion
This is the first study to investigate the influence of RT on vascular uptake of FDG in humans. Our data suggest that FDG uptake is increased in vascular segments less than 10 years after RT. It is our hypothesis that the increased FDG uptake indicates increased vascular inflammation and this could be a determinant of increased risk of cerebro-cardiovascular disease. The clinical perspective of our trial is to stratify at-risk individuals based on the level of FDG-uptake to offer individualized primary prevention. Some experimental studies have indicated that e.g. HMG-CoA reductase inhibitor (statin) therapy may reduce vascular lesions following RT [19,20].
Risk of cerebrovascular disease following RT
Numerous clinical studies have established RT as a risk factor for cerebrovascular disease in diseases like head-and-neck cancer [21], lung cancer [22], and breast cancer [23]. The effect of RT is however not easily assessed since the patients typically also have classic cardiovascular risk factors, and cancer as well as several chemotherapeutic regimens may affect the risk. These confounders may also influence the wide range of reported median times from RT to cardiovascular event [2,4]. Moreover, most of the clinical data stem from a time when extended field RT was used. A recent study [24] used dose-response curves from published Hodgkin lymphoma data to compare stroke risk estimates following INRT or extensive mantle field RT. This study indicates that INRT reduces the stroke risk substantially as compared to previous mantle field RT. To overcome some of these confounding issues we designed a study with unilateral INRT treated patients where each patient could serve as their own vascular control. A potential limitation of this design is that a few studies have found impaired peripheral endothelial function indicating a systemic effect of RT [25]. Such an effect would be masked in our paired study.
Vascular inflammation preceding morphological changes
Based on our results, we suggest that irradiation damages the vessels and this leads to long term inflammation identified by FDG uptake years after the radiation. We found no correlation between carotid intima media thickness and FDG-uptake, and intima media thickness did not differ between the irradiated and non-irradiated side. This result was not unexpected since we included patients less than 7 years after RT (most only 3 years after) and similar trials have found that intima media thickness is not significantly changed this early after RT below 50 Gy [7,9]. Also, our study was not powered to identify minor intima media thickness differences.
Our results are in agreement with the general belief that macrophage infiltration and inflammation are early events in the atherogenesis that precedes morphological changes like increased intima media thickness [26]. Vascular FDG uptake is known to correlate with macrophage infiltration in human atherosclerosis [10,27] and a few retrospective studies have indicated that vascular FDG uptake is a risk factor for future cerebrovascular events [28,29]. We can only speculate if this is also the case in our patients since our case-control study cannot determine the prognostic impact of increased vascular uptake in these patients.
In vivo imaging of vascular FDG-uptake using PET is potentially influenced by the small diameter of the carotid and iliac arties close to the spatial resolution of the PET system. Therefore, vascular SUVmax is potentially affected by spillover from adjacent FDG avid tissue. We tested this potential mechanism by assessing the FDG uptake (SUVmax) in adjacent soft tissue, and this did not correlate to the FDG uptake in the arteries. We thus find it most likely that our vascular SUVmax does represent FDG uptake in the vessel wall.
Even though vascular FDG-uptake is a recognized molecular marker of inflammation our patient-population does not allow us to get ex vivo confirmation of an ongoing inflammation in the irradiated versus the non-irradiated artery. It is impossible to get an intravascular biopsy, and the patients are not candidates for vascular surgery since they do not have any stenotic lesions. We do however have indirect evidence of a causal relationship between FDG-uptake and macrophage infiltration from both animal [30] and human [10] studies of more advanced atherosclerotic plaques. In this light an animal study of vascular FDG-uptake after RT would be if interest since this would allow for ex vivo confirmation of inflammation following RT.
Our study is limited by the small sample size of only 10 patients. However, we were not able to recruit any more eligible patients. The few patients prohibited any meaningful subgroup analyses. Our finding of equal intima media thickness was not part of the power-calculation and could represent a type 2 error. However, our results are supported by another study showing equal intima media thickness less than 10 years after RT [7]. Also, a larger population would allow for a meaningful testing of dose-response between radiation dose and FDG-uptake. Finally, our follow-up studies are needed to determine if the vascular inflammation detected with FDG-PET will predict future vascular complications as described in other populations [28,29].
In conclusion, we have found a significant increase in vascular FDG uptake 2-7 years after RT, without any morphologic indication of unilateral accelerated atherosclerosis. Future prospective follow-up trials will have to show if these changes predict vascular morbidity and if classic risk factor reduction can diminish the vascular changes and thus vascular morbidity.
Acknowledgements
The staff in the PET center is thanked for their skillful assistance. This work was supported by unrestricted grants from the John & Birthe Meyer Foundation; the National Advanced Technology Foundation; Danish Medical Research Council; Rigshospitalets Research Foundation; Svend Andersen Foundation; AP Møller Foundation; Novo Nordisk Foundation; and Lundbeck Foundation.
Disclosure of conflict of interest
None.
References
- 1.Plummer C, Henderson RD, O’Sullivan JD, Read SJ. Ischemic stroke and transient ischemic attack after head and neck radiotherapy: a review. Stroke. 2011;42:2410–2418. doi: 10.1161/STROKEAHA.111.615203. [DOI] [PubMed] [Google Scholar]
- 2.Bowers DC, McNeil DE, Liu Y, Yasui Y, Stovall M, Gurney JG, Hudson MM, Donaldson SS, Packer RJ, Mitby PA, Kasper CE, Robison LL, Oeffinger KC. Stroke as a late treatment effect of Hodgkin’s Disease: a report from the Childhood Cancer Survivor Study. J. Clin. Oncol. 2005;23:6508–6515. doi: 10.1200/JCO.2005.15.107. [DOI] [PubMed] [Google Scholar]
- 3.De Bruin ML, Dorresteijn LD, van’t Veer MB, Krol AD, van der Pal HJ, Kappelle AC, Boogerd W, Aleman BM, van Leeuwen FE. Increased risk of stroke and transient ischemic attack in 5-year survivors of Hodgkin lymphoma. J Natl Cancer Inst. 2009;101:928–937. doi: 10.1093/jnci/djp147. [DOI] [PubMed] [Google Scholar]
- 4.Dorresteijn LD, Kappelle AC, Boogerd W, Klokman WJ, Balm AJ, Keus RB, van Leeuwen FE, Bartelink H. Increased risk of ischemic stroke after radiotherapy on the neck in patients younger than 60 years. J. Clin. Oncol. 2002;20:282–288. doi: 10.1200/JCO.2002.20.1.282. [DOI] [PubMed] [Google Scholar]
- 5.Sugihara T, Hattori Y, Yamamoto Y, Qi F, Ichikawa R, Sato A, Liu MY, Abe K, Kanno M. Preferential impairment of nitric oxide-mediated endothelium-dependent relaxation in human cervical arteries after irradiation. Circulation. 1999;100:635–641. doi: 10.1161/01.cir.100.6.635. [DOI] [PubMed] [Google Scholar]
- 6.Fonkalsrud EW, Sanchez M, Zerubavel R, Mahoney A. Serial changes in arterial structure following radiation therapy. Surg Gynecol Obstet. 1977;145:395–400. [PubMed] [Google Scholar]
- 7.Dorresteijn LD, Kappelle AC, Scholz NM, Munneke M, Scholma JT, Balm AJ, Bartelink H, Boogerd W. Increased carotid wall thickening after radiotherapy on the neck. Eur J Cancer. 2005;41:1026–1030. doi: 10.1016/j.ejca.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 8.Bilora F, Pietrogrande F, Campagnolo E, Rossato A, Polato G, Pomerri F, Muzzio PC. Are Hodgkin and non-Hodgkin patients at a greater risk of atherosclerosis? A follow-up of 3 years. Eur J Cancer Care (Engl) 2010;19:417–419. doi: 10.1111/j.1365-2354.2008.01048.x. [DOI] [PubMed] [Google Scholar]
- 9.Martin JD, Buckley AR, Graeb D, Walman B, Salvian A, Hay JH. Carotid artery stenosis in asymptomatic patients who have received unilateral head-and-neck irradiation. Int J Radiat Oncol Biol Phys. 2005;63:1197–1205. doi: 10.1016/j.ijrobp.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 10.Pedersen SF, Graebe M, Fisker Hag AM, Hojgaard L, Sillesen H, Kjaer A. Gene expression and 18FDG uptake in atherosclerotic carotid plaques. Nucl Med Commun. 2010;31:423–429. doi: 10.1097/MNM.0b013e32833767e0. [DOI] [PubMed] [Google Scholar]
- 11.Knudsen A, Hag AM, Loft A, von BE, Keller SH, Moller HJ, Lebech AM, Ripa RS, Kjaer A. HIV infection and arterial inflammation assessed by (18)F-fluorodeoxyglucose (FDG) positron emission tomography (PET): A prospective cross-sectional study. J Nucl Cardiol. 2015;22:372–380. doi: 10.1007/s12350-014-0032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bucerius J, Mani V, Wong S, Moncrieff C, Izquierdo-Garcia D, Machac J, Fuster V, Farkouh ME, Rudd JH, Fayad ZA. Arterial and fat tissue inflammation are highly correlated: a prospective 18F-FDG PET/CT study. Eur J Nucl Med Mol Imaging. 2014;41:934–945. doi: 10.1007/s00259-013-2653-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Wijk DF, Sjouke B, Figueroa A, Emami H, van der Valk FM, MacNabb MH, Hemphill LC, Schulte DM, Koopman MG, Lobatto ME, Verberne HJ, Fayad ZA, Kastelein JJ, Mulder WJ, Hovingh GK, Tawakol A, Stroes ES. Nonpharmacological Lipoprotein Apheresis Reduces Arterial Inflammation in Familial Hypercholesterolemia. J Am Coll Cardiol. 2014;64:1418–1426. doi: 10.1016/j.jacc.2014.01.088. [DOI] [PubMed] [Google Scholar]
- 14.Graebe M, Pedersen SF, Borgwardt L, Hojgaard L, Sillesen H, Kjaer A. Molecular pathology in vulnerable carotid plaques: correlation with [18]-fluorodeoxyglucose positron emission tomography (FDG-PET) Eur J Vasc Endovasc Surg. 2009;37:714–721. doi: 10.1016/j.ejvs.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 15.Pedersen SF, Sandholt BV, Keller SH, Hansen AE, Clemmensen AE, Sillesen H, Hojgaard L, Ripa RS, Kjaer A. 64Cu-DOTATATE PET/MRI for Detection of Activated Macrophages in Carotid Atherosclerotic Plaques: Studies in Patients Undergoing Endarterectomy. Arterioscler Thromb Vasc Biol. 2015;35:1696–703. doi: 10.1161/ATVBAHA.114.305067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Girinsky T, van der Maazen R, Specht L, Aleman B, Poortmans P, Lievens Y, Meijnders P, Ghalibafian M, Meerwaldt J, Noordijk E. Involved-node radiotherapy (INRT) in patients with early Hodgkin lymphoma: concepts and guidelines. Radiother Oncol. 2006;79:270–277. doi: 10.1016/j.radonc.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 17.Ripa RS, Knudsen A, Hag AM, Lebech AM, Loft A, Keller SH, Hansen AE, von BE, Hojgaard L, Kjaer A. Feasibility of simultaneous PET/MR of the carotid artery: first clinical experience and comparison to PET/CT. Am J Nucl Med Mol Imaging. 2013;3:361–371. [PMC free article] [PubMed] [Google Scholar]
- 18.Graebe M, Borgwardt L, Hojgaard L, Sillesen H, Kjaer A. When to image carotid plaque inflammation with FDG PET/CT. Nucl Med Commun. 2010;31:773–779. doi: 10.1097/MNM.0b013e32833c365e. [DOI] [PubMed] [Google Scholar]
- 19.Gaugler MH, Vereycken-Holler V, Squiban C, Vandamme M, Vozenin-Brotons MC, Benderitter M. Pravastatin limits endothelial activation after irradiation and decreases the resulting inflammatory and thrombotic responses. Radiat Res. 2005;163:479–487. doi: 10.1667/rr3302. [DOI] [PubMed] [Google Scholar]
- 20.Nubel T, Damrot J, Roos WP, Kaina B, Fritz G. Lovastatin protects human endothelial cells from killing by ionizing radiation without impairing induction and repair of DNA doublestrand breaks. Clin Cancer Res. 2006;12:933–939. doi: 10.1158/1078-0432.CCR-05-1903. [DOI] [PubMed] [Google Scholar]
- 21.Huang YS, Lee CC, Chang TS, Ho HC, Su YC, Hung SK, Lee MS, Chou P, Chang YH, Lee CC. Increased risk of stroke in young head and neck cancer patients treated with radiotherapy or chemotherapy. Oral Oncol. 2011;47:1092–1097. doi: 10.1016/j.oraloncology.2011.07.024. [DOI] [PubMed] [Google Scholar]
- 22.Hung SK, Lee MS, Chiou WY, Lee CC, Chen YC, Lai CL, Chien NC, Hsu WL, Liu DW, Su YC, Li SC, Lai HC, Tsai SJ, Hsu FC, Lin HY. High incidence of ischemic stroke occurrence in irradiated lung cancer patients: a population-based surgical cohort study. PLoS One. 2014;9:e94377. doi: 10.1371/journal.pone.0094377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stokes EL, Tyldesley S, Woods R, Wai E, Olivotto IA. Effect of nodal irradiation and fraction size on cardiac and cerebrovascular mortality in women with breast cancer treated with local and locoregional radiotherapy. Int J Radiat Oncol Biol Phys. 2011;80:403–409. doi: 10.1016/j.ijrobp.2010.02.041. [DOI] [PubMed] [Google Scholar]
- 24.Maraldo MV, Brodin P, Aznar MC, Vogelius IR, Munck af Rosenschöld P, Petersen PM, Specht L. Doses to carotid arteries after modern radiation therapy for Hodgkin lymphoma: is stroke still a late effect of treatment? Int J Radiat Oncol Biol Phys. 2013;87:297–303. doi: 10.1016/j.ijrobp.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Zelcer S, Chen B, Mangel J, Vujovic O, Thiessen-Philbrook HR, Reider M, Mahmud FH. Impaired vascular function in asymptomatic young adult survivors of Hodgkin Lymphoma following mediastinal radiation. J Cancer Surviv. 2010;4:218–224. doi: 10.1007/s11764-010-0138-6. [DOI] [PubMed] [Google Scholar]
- 26.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 27.Rudd JH, Warburton EA, Fryer TD, Jones HA, Clark JC, Antoun N, Johnstrom P, Davenport AP, Kirkpatrick PJ, Arch BN, Pickard JD, Weissberg PL. Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation. 2002;105:2708–2711. doi: 10.1161/01.cir.0000020548.60110.76. [DOI] [PubMed] [Google Scholar]
- 28.Grandpierre S, Desandes E, Meneroux B, Djaballah W, Mandry D, Netter F, Wahl D, Fay R, Karcher G, Marie PY. Arterial foci of F-18 fluorodeoxyglucose are associated with an enhanced risk of subsequent ischemic stroke in cancer patients: a case-control pilot study. Clin Nucl Med. 2011;36:85–90. doi: 10.1097/RLU.0b013e318203bb42. [DOI] [PubMed] [Google Scholar]
- 29.Rominger A, Saam T, Wolpers S, Cyran CC, Schmidt M, Foerster S, Nikolaou K, Reiser MF, Bartenstein P, Hacker M. 18F-FDG PET/CT identifies patients at risk for future vascular events in an otherwise asymptomatic cohort with neoplastic disease. J Nucl Med. 2009;50:1611–1620. doi: 10.2967/jnumed.109.065151. [DOI] [PubMed] [Google Scholar]
- 30.Hag AM, Pedersen SF, Christoffersen C, Binderup T, Jensen MM, Jorgensen JT, Skovgaard D, Ripa RS, Kjaer A. (18)F-FDG PET imaging of murine atherosclerosis: association with gene expression of key molecular markers. PLoS One. 2012;7:e50908. doi: 10.1371/journal.pone.0050908. [DOI] [PMC free article] [PubMed] [Google Scholar]


