Abstract
Human transcriptional positive cofactor 4 (PC4) is a novel marker for diagnosis and treatment of advanced human cancers metastasis. In human lung adenocarcinoma, tumor lymphangiogenesis, an important early event, can promotes lymphatic metastasis, while it has been reported that VEGF-C/VEGF-D/VEGFR-3 axis plays an important role in lymphangiogenesis. The proposed study aims to explore whether PC4 correlates with VEGF-C/VEGF-D/VEGFR-3 axis of lymphangiogenesis in the lymph node metastasis during lung adenocarcinoma. Here, small interfering RNA technique was employed to investigate the relationship of PC4 and the VEGF-C/VEGF-D/VEGFR-3 axis in lung adenocarcinoma cell lines as well as tumor xenografts of mice model. And then mRNA and protein levels of PC4, VEGF-C, VEGF-D and VEGFR-3 were analyzed. Moreover, the correlation between PC4 expression and lymphatic vessel density or the rate of metastatsis in vivo was also revealed. Down-regulating PC4 expression resulted in the lower expression of VEGFC, VEGF-D and VEGFR-3 in mRNA and protein levels, and PC4 expression was significantly related with the factor of VEGF-C/VEGF-D/VEGFR-3 axis expression (P<0.05). Meanwhile, high expression level of PC4 was accompanied by the higher density of tumor lymphatic vessels and the rate of metastatsis in vivo (P<0.05). PC4 expression correlated with the levels of VEGF-C, VEGF-D and VEGFR-3 during the development of lymphangiogenesis and lymphatic metastasis in lung adenocarcinoma in vitro and in vivo, which may be a novel marker in the development of lymphangiogenesis and lymphatic metastasis of tumors.
Keywords: PC4, lung adenocarcinoma, lymphangiogenesis, metastasis, VEGF-C/VEGF-D/VEGFR-3 axis
Introduction
Lymphatic metastasis is an important early event occurred during human lung carcinoma [1], but the molecular mechanism is not well known. Therefore, it is urgent to find a new target molecule for diagnosis and treatment of human cancers.
Human positive cofactor 4 (PC4), a nuclear protein and facilitates activator-dependent transcription, is a novel marker for cancer cell transformation and the diagnosis and treatment of lung carcinoma [2]. In 1994, PC4 was reported by Ge et al firstly [3], and then it was found to play an important role in various cellular processes such as transcription [4], replication [5], chromatin organization [6], and repair of oxidative DNA damage [7]. Additionally, PC4 is also activated in cell growth and differentiation through its interaction with p53, ras, bax, and so on [8]. Recently, Shi et al reported that PC4 expression was correlated to the development and progression of human lung cancer in clinical specimens [9], which can be a novel marker for adult multipotent stem cell transformation as well as the diagnosis and treatment of advanced human cancers.
VEGF-C and VEGF-D was reported to be the main factors of lymphangiogenesis [10], which has been a new research frontier in tumor metastasis. It was found VEGF-C overexpresion promoted the increasement of the lymphatic vessels density and spread of tumor cells to lymph nodes in animal model [11]. Another interesting study demonstrated that interaction of VEGF-C/D with the receptor VEGFR-3 is able to facilitate lymphangiogenesis through mitogen activated protein kinase and phosphatidylinositol 3-kinase signaling pathways [12]. Clinical evidence that VEGF-C or VEGF-D expression correlates with regional lymph nodes metastases in human lung carcinomas [13] verified the activation by VEGFR-3-VEGF-C/D to the lymphangiogenesis in tumor cells.
However, little is known about the relation of PC4 and the binding of VEGFR-3 and VEGF-C or VEGF-D signaling pathway in lymphangiogenesis and metastases in regional lymph nodes in human lung carcinomas. In this study, small interfering RNA technique was emplyed to investigate the relationship between PC4 and the combination of VEGF-C/D with the receptor VEGFR-3. We found PC4 is one of major upstream genes of VEGF-C and VEGF-D in lymphangiogenesis both in vivo and in vitro, and the PC4 correlates with VEGF-C, VEGF-D and VEGFR-3 both at the mRNA and protein levels. Moreover, PC4 correlates with lymph node metastasis in human lung adenocarcinoma.
Materials and methods
Cell culture
Human lung adenocarcinoma A549 cell lines were obtained from American Tissue Culture Colection. A549 cell lines were maintained with DMEM supplemented 10% fetal bovine serum (HyClone, Thermo scientific, USA) in a humidified atmosphere including 5% CO2 at 37°C.
RNA interference of PC4
The siRNA sequence targeting the PC4 gene corresponded to the nucleotides of the start codon (sense, 5’-r [ACAGAGCAGCAGCAGCAGA] dTT-3’; antisense, 5’-r [TCTGCTGCTGCTG CTCTGT] dTT-3’) were synthesized. PC4 cDNA were connected in a lentivirus expression plasmid vector VSVG components of GFP positive including pGCL-GFP, pHelper 1.0 and pHelper 2.0 (Shanghai GeneChem). The lentivirus packing cells 293T were transfected using siRNA and randomized control RNA with lipofectamine 2000 plus (Invitrogen) according to the manufacturer’s protocol, then a series of purification, concentrate, and virus titer detection, and active virus particles was acquired, and multiplicity of infection (MOI) was determined according to the manufacturer’s instructions. Lung adenocarcinoma cell A549 was infected by the above virus. The FCM (flow cytometry) Sorted green fluorescent protein cells, named the A549pGCL-siRNA PC4 cell lines and A549pGCL-siRNA control PC4 cell lines.
Quantitative real-time PCR (RT-PCR)
Total RNA was isolated from A549 cell, A549 siRNA PC4 cell, A549 siRNA control cell as well as fresh soft tumor xenografts tissues using Trizol reagent (Invitrogen, Rockville, MD, USA) according to the manufacturer’s instructions. One micrograms of total RNA was reverse-transcribed for cDNA synthesis using the PrimeScriptTMRT reagent Kit (TaKaRa, Japan). Quantitative real-time PCR (qRT-PCR) was carried out using SYBR PremixExTaq™ (Takara, Japan). SYBR Green qRT-PCR was performed on BIO-RAD IQ5 (USA). All primer sequences are listed in Table 1. The qRT-PCR results were analyzed and the relative CT (threshold cycle) values were converted into expression fold alterations.
Table 1.
Primer sequences of products expression
| Name (bp) | Sequence (5’-3’) | |
|---|---|---|
| PC4 (138 bp) | F | CACCTGCTCAGTTTTCACACA |
| R | TGGAAAGTCAGTGAAACTGTCA | |
| VEGF-C (178 bp) | F | ATTTACATGATGCTGCCATCAGTC |
| R | GGCAATAAAGCAATGGAGGAAGG | |
| VEGF-D (174 bp) | F | AGGTTTATTTGCATGTGCC |
| R | AGAGCAGGTCTTGATGTGTC | |
| VEGFR-3 (168 bp) | F | GAGAGAGAGAAGGCAGCATAC |
| R | GTGCTTCAGTGGTCACACTCC | |
| β-actin (285 bp) | F | AGCGAGCATCCCCCAAAGTT |
| R | GGGCACGAAGGCTCATCATT | |
Animal studies
Nude mice (Specific pathogen Free, BALB/c (nu/nu), female, 6-9 weeks of age, around 20 g of weight) were obtained from Laboratory Animal Center of Institute of Surgery Research, Daping Hospital, Third Military Medical University (Chongqing, China) and bred at constant temperature (23 ± 2°C) and humidity (50-70%). Nine mice were randomly divided into three groups, and 0.2 ml (1 × 107 cells/ml) of A549, A549siRNA PC4 or A549siRNA control cell suspension were respectively injected into the subcutaneous of the right back neck close to axilla of the mice, Tumor xenografts were staged for 7 days until the tumor volume reached to 0.5 × major axis × Minor axis2. We executed the mice when the tumor xenografts reached approximately 1 cm3 in size.
Immunohistochemistry
Mice were sacrificed when the tumor xenografts were staged for 60 days. The tumor xenografts and local lymph nodes were separated and applied for immunohistochemistry. Then the samples were incubated with rabbit polyclonal anti-PC4 (Abcam, USA, 1:100), rabbit polyclonal anti-VEGF-C (Abcam, USA, 1:50), rabbit polyclonal anti-VEGF-D (Abcam, USA, 1:50), mouse monoclonal anti-VEGFR-3 (Abcam, USA, 1:100), and rabbit polyclonal anti-LYVE-1 (Abcam, USA, 1:100). After overnight incubation at 4°C, slides were washed three times using PBS (pH 7.4-7.6) and then incubated with Real-envision Detection System (Dako, Shanghai, China). Negative controls were prepared using PBS instead of the primary antibody. The brown staining in cytoplasm and/or nucleus or membrane was considered positive. For determination of lymphatic vessel density, we identified the area containing the most LYVE-1 positive vessels (hot spot) by scanning the sections at low magnification (10 × 10). The number of LYVE-1 positive vessels from five areas of the highest vessel density/section at high magnification (10 × 40) was employed to determine the lymphatic vessel density.
Western blotting
Total protein was extracted from the A549 cell, A549siRNA PC4 cell, A549siRNA control cell and tumor xenograft tissues with Protein Extraction with RIPA Buffer (BBST, China). Protein concentration was determined using BCA Protein Assay Reagent Kit (Pierce, USA). Approximately 50 μg of the protein extraction was separated by 10% SDS-PAGE, then transferred to 0.22 mm nitrocellulose membranes (Sigma) and incubated with specific antibodies. The primary antibodies rabbit polyclonal anti-PC4, rabbit polyclonal anti-VEGF-C, rabbit polyclonal anti-VEGF-D, mouse monoclonal anti-VEGFR-3 were purchased from Abcam (Abcam, USA). Quantity One v4.4.0 software (Bio-Rad, USA) was used for assay optical density of the protein bands. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a control, and results were normalized to the expression of GAPDH.
Statistical analysis
Data are expressed as mean ± SEM. The difference among groups was determined by ANOVA analysis and comparison between two groups was analyzed by the Student’s t-test using GraphPad software version 5.0 (GraphPad Software, CA). P<0.05 was considered statistically significant.
Results
Establishment of the A549siRNA PC4 and A549siRNA control cell lines
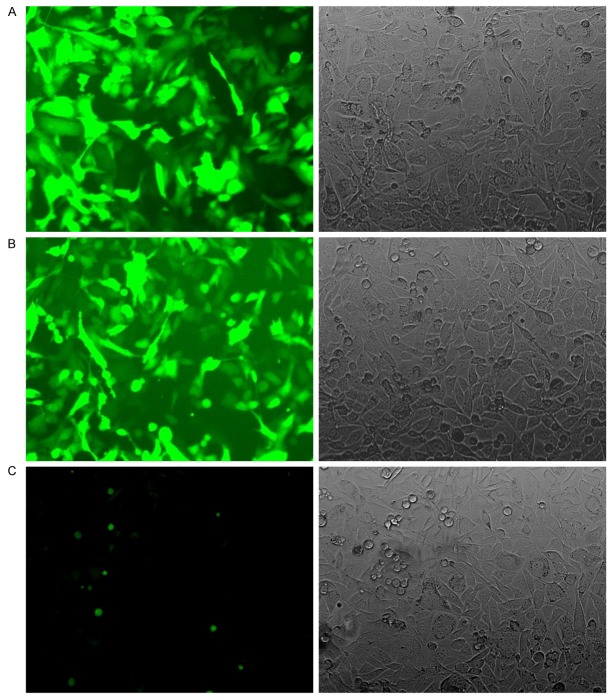
After the siRNA PC4 recombinant and control lentiviral vector, including green fluorecence protein, were infected into the lung adenocarcinoma cell A549, we observed the cell under the fluorescence microscope. It was at least 70% cell which expressed green fluorecence protein in each visual field (Figure 1). And then, we collected the A549 cell infected by virus and sorted the green fluorescent protein cells using the FCM (flow cytometry) (Figure 2). The cell were cultured and passaged for one or two generation to obtain the A549siRNA PC4 and A549siRNA control cell lines.
Figure 1.
The expression of green fluorecence protein in A549 cell. A. Infected the siRNA PC4 recombinant lentiviral vector (fluorescence microscope, × 200). B. Infected control recombinant lentiviral vector (fluorescence microscope, × 200). C. A549 cell (fluorescence microscope, × 200).
Figure 2.
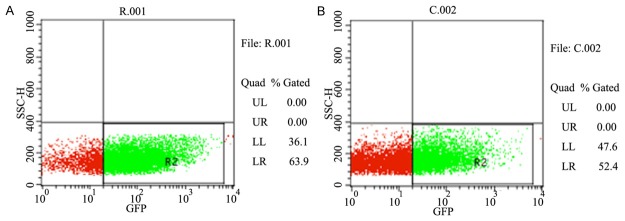
The results of fluorescence activated cell sorting. R001: the siRNA PC4 recombinant lentiviral vector group. C002: the control recombinant lentiviral vector group.
The mRNA expression of PC4, VEGF-C, VEGF-D and VEGFR-3 in vitro
To study the downstream effector gene of PC4 in human lung adenocarcinoma, the cell lines with or without siRNA PC4 gene were developed, which named A549siRNA PC4 and A549siRNA control, respectively. Figure 3 showed that lymphangiogenic factor VEGF-C, VEGF-D and VEGFR-3 genes were lower expressed with decreased downstream PC4 gene, which suggested the close link between PC4 VEGF-C, VEGF-D and VEGFR-3 at mRNA level.(tested by Spearman’s nonparametric correlation test, correlation coefficient 0.792, 0.592, 0.661, P<0.001; Figure 3).
Figure 3.
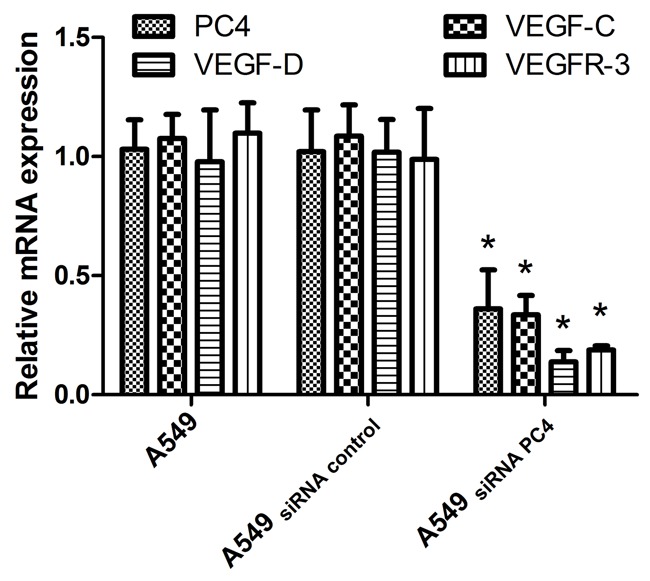
The mRNA expression of PC4, VEGF-C, VEGF-D and VEGFR-3 in vitro. There were the most significant knockdown effect on PC4 mRNA in the A549siRNA PC4 cell lines, with inhibition rates of 85.40%. The expression of PC4, VEGF-C, VEGF-D and VEGFR-3were lower in the A549siRNA PC4 cell lines than the A549cell lines and A549siRNA control cell lines (*P<0.05). The expression of mRNA levels were declined. The PC4 expression was significantly associated with the expression of VEGF-C, VEGF-D and VEGFR-3 (tested by Spearman’s nonparametric correlation test, correlation coefficient 0.792, 0.592, 0.661, P<0.001).
The most significant knockdown effect on PC4 mRNA in the A549siRNA PC4 cell lines was observed with inhibition rates of 85.40%. The expression of PC4, VEGF-C, VEGF-D and VEGFR-3 were lower in the A549siRNA PC4 cell lines than that in the A549 cell lines and A549siRNA control cell lines (*P<0.05). The expression of mRNA levels was declined. The PC4 expression was significantly associated with the expression of VEGF-C, VEGF-D and VEGFR-3 (tested by Spearman’s nonparametric correlation test, correlation coefficient 0.792, 0.592, 0.661, P<0.001).
The protein expression of PC4, VEGF-C, VEGF-D and VEGFR-3 in vitro
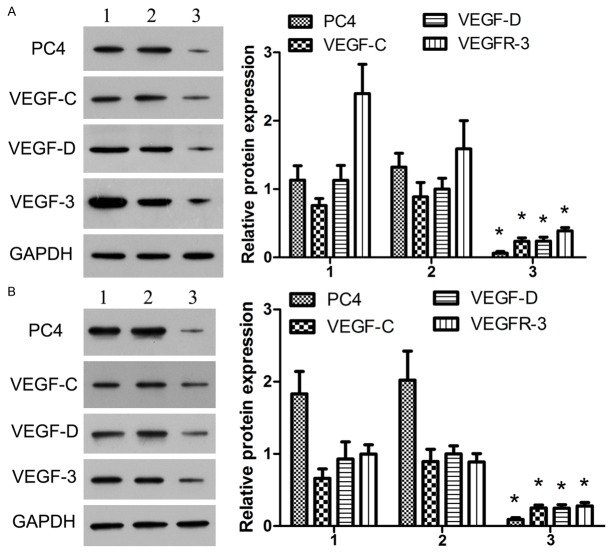
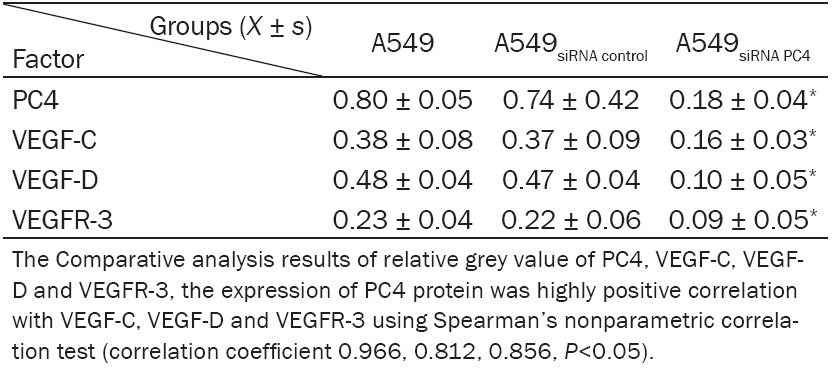
The A549siRNA PC4 cell lines with down-regulation of PC4 and the A549siRNA control cell lines were constructed with siRNA technology. The total protein were extracted and detected by western blotting. We found that the expression of PC4 in A549siRNA PC4 cell was much lower than that in A549 cell and A549siRNA control cell (P<0.05), the inhibition ratio reached to 77.5%. And similar result was observed for the expression level of VEGF-C, VEGF-D and VEGFR-3 (P<0.05, Figure 4), which suggested the highly positive correlation of PC4 protein VEGF-C, VEGF-D and VEGFR-3 using Spearman’s nonparametric correlation test (correlation coefficient 0.966, 0.812, 0.856, P<0.05, Table 2).
Figure 4.
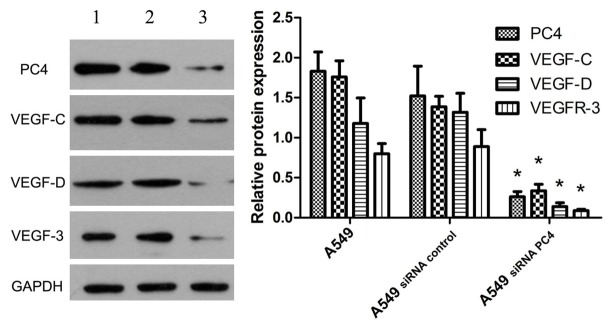
The protein expression of PC4, VEGF-C, VEGF-D and VEGFR-3 in vitro. We detected the total protein expression of PC4, VEGF-C, VEGF-D and VEGFR-3 in the A549 groups, the A549siRNA control groups and the A549siRNA PC4 groups. It was showed that the expression of PC4 in A549siRNA PC4 cell strictly lower than the A549 cell and the A549siRNA control cell (P<0.05), the inhibition ratio reached to 77.5%. However, the expression of VEGF-C, VEGF-D and VEGFR-3 also lower in A549siRNA PC4 cell than the A549 cell and the A549siRNA control cell (P<0.05). 1: The A549 group; 2: The A549siRNA control group; 3: The A549siRNA PC4 group.
Table 2.
The protein expression of PC4 was positive relation with VEGF-C, VEGF-D and VEGFR-3
|
The total protein expression of PC4, VEGF-C, VEGF-D and VEGFR-3 were detected in A549, A549siRNA control and the A549siRNA PC4 groups. It was showed that the expression of PC4 in A549siRNA PC4 cell was strictly lower than that in A549 cell and A549siRNA control cell (P<0.05), the inhibition ratio reached to 77.5%. Moreover, the expression of VEGF-C, VEGF-D and VEGFR-3 in A549siRNA PC4 cell was also evidently lower than that in A549 cell and the A549siRNA control cell (P<0.05).
Tumor volume and the number of metastatic lymph node
Being staged for 21 days, the mice were sacrificed, tumor xenografts and lymph node were isolated. The tumor size were measured, the average size of tumor xenografts was 85 ± 0.56 mm in A549nontransfection group, 70.6 ± 1.24 mm in A549siRNA PC4 transfection group and 84 ± 1.20 mm in A549siRNA control transfection group. The volume of A549siRNA PC4 transfection group was much smaller than the other two groups (P<0.05, Figure 5). Moreover, to confirm whether metastatsis occurred, lymph node sample was stained by hematoxylin and eosin (HE), and we found the rate of metastatsis (the number of metastatsis using htoxylin eosin (HE) staining/the total of lymph node) was 85% and 90% in A549nontransfection and A549siRNA control transfection group, while only 50% in A549siRNA PC4 transfection group, which is significantly lower (P<0.05, Figure 6).
Figure 5.
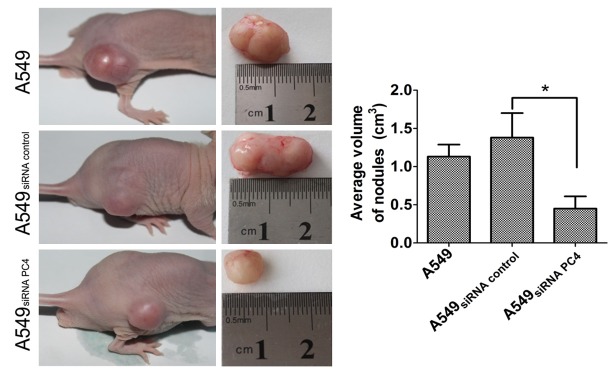
The average volumeof tumor xenografts in three groups. The volume of tomorxenografts before the A549 cell lines, A549siRNA control cell lines and A549siRNA PC4 cell lines injectedinto mice. The volume of A549siRNA PC4 transfection group significantly belower than A549nontransfection and A549siRNA control transfection group (*P<0.05, D).
Figure 6.
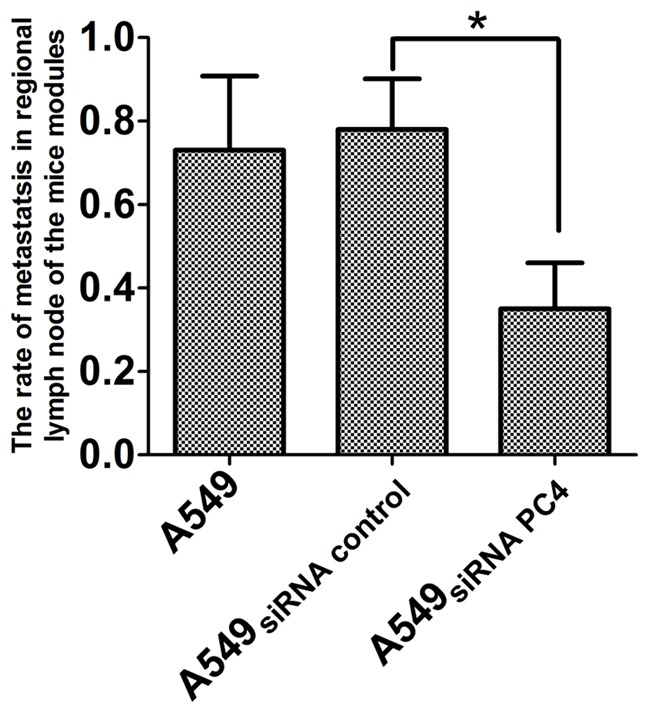
The rate of metastatsis in regional lymph node of the mice modules in the three groups. The rate of metastatsis in regional lymph node in A549nontransfection and A549siRNA control transfection groups were significantly highter than A549siRNA PC4 transfection groups (*P<0.05).
PC4 expression correlated with lymphatic vessel density
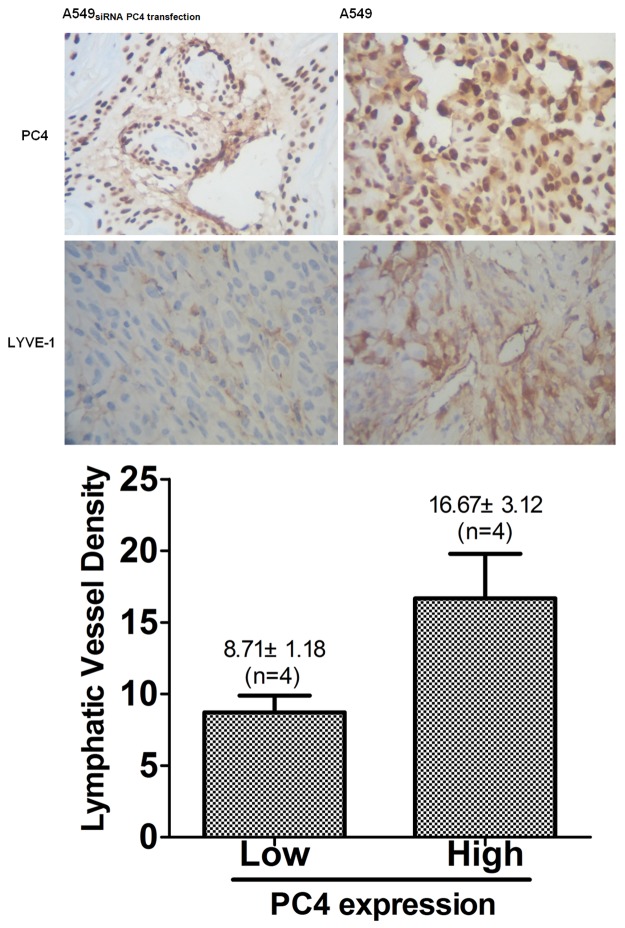
Histological staining with anti-LYVE-1 and VEGFR-3 antibodies was observed when the tumor xenografts were staged for 21 days, and we detected the expression of PC4 in three groups and with LYVE-1 and VEGFR-3 antibodies ,inspected the density of lymphatic vessels in tumor xenografts. As the results showed, in tumors with LYVE-1 positive vessels the tumor lymphatic vessels with high expression level of PC4 showed a higher density than tumor lymphatic vessels with low PC4 expression (16.67 ± 5.3 vessels/microscopic field for PC4 high expression tumors, n=4, and 8.7 ± 3.31 for PC4 low expression tumors, n=4, P<0.05, Student’s t test, Figure 7). And the similar results that the density of lymphatic vessels exhibited a positive correlation with PC4 expression was observed in tumors with VEGFR-3 positive vessels.
Figure 7.
PC4 expression correlates with lymphatic vessel density in vivo. A. Immunohistochemical staining of serial sections of tumor xenografts of lung cancer tissues shows identical expression and localization of PC4 down-regulates (× 400). B. Immunohistochemical staining of LYVE-1 and VEGFR-3in serial sections from lung adenocarcinoma of xenograftswith low or high PC4 expression (× 400). C. Quantitative analysis of LYVE-1 positive vessels (mean ± SD) by the standard methodof microvessel density determination.
The mRNA expression of PC4, VEGF-C, VEGF-D and VEGFR-3 in vivo
We also studied whether the injection of different cell suspension induce the change in mRNA levels of PC4, VEGF-C, VEGF-D and VEGFR-3 in the animal tumor tissues. The results showed that in animals treated with A549siRNA PC4, the mRNA levels of PC4, VEGF-C, VEGF-D and VEGFR-3 were lower than other two groups (P<0.01). The significant difference was obtained using Spearman’s nonparametric correlation test, and correlation coefficient were 0.852, 0.796, 0.915, respectively, (P<0.01; Figure 8).
Figure 8.
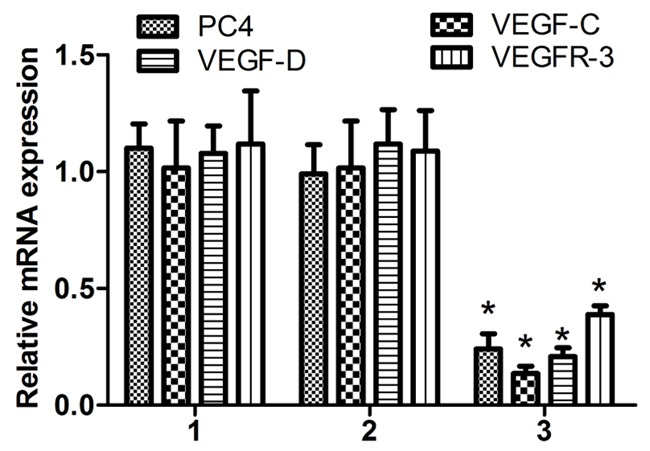
The mRNA expression of PC4, VEGF-C, VEGF-D and VEGFR-3 in vivo. The expression of PC4, VEGF-C, VEGF-D and VEGFR-3 mRNA levels significantly lower in the A549siRNA PC4 transfection groups than A549non transfection groups and the A549siRNA control transfection groups (P<0.01). The expression ofPC4 Mrna hightly correlation with the lymph angiogenic factor VEGF-C, VEGF-D and VEGFR-3 using Spearman’s nonparametric correlation test (correlation coefficient 0.852, 0.796, 0.915, P<0.01). 1: The A549non transfection group; 2: The A549siRNA control transfection group; 3: The A549siRNA PC4 transfection group.
The protein expression of PC4, VEGF-C, VEGF-D and VEGFR-3 in vivo
The protein expression levels of PC4, VEGF-C, VEGF-D and VEGFR-3 in the mice tissues were further investigated. The results revealed that compared with other two groups, the amount of PC4 was obviously lower in the tumor xenografts from the mice injected with A549siRNA PC4 cell suspension (P<0.05), meanwhile in the same group the much lower expression level of VEGF-C, VEGF-D and VEGFR-3 than other two groups was observed. Statistically significant difference obtained among the groups suggested that the protein expression level of PC4 positively correlated with VEGF-C, VEGF-D and VEGFR-3, and the correlation coefficient were 0.847, 0.809 and 0.951, respectively. (Spearman’s nonparametric correlation test, P<0.01; Figure 9A).
Figure 9.
The protein expression of PC4, VEGF-C, VEGF-D and VEGFR-3 invivo. A. In the tumor xenografts of lung cancer, the expression of PC4 significantly lower in the A549siRNA PC4 transfection groups than the A549nontransfection groups and A549siRNA control transfection groups (P<0.05), meanwhile, the expression of VEGF-C, VEGF-D and VEGFR-3 lower in the A549siRNA PC4 transfection groups than other. The PC4 positive correlation with the VEGF-C, VEGF-D and VEGFR-3 in protein levels (Spearman’s nonparametric correlation test, correlation coefficient 0.847, 0.809, 0.951, P<0.01). B. In the regional lymph node tissues, there were similar results by analysis of the expression of PC4, VEGF-C, VEGF-D and VEGFR-3 in three groups. Accompany the PC4lower expressing lymph node, the VEGF-C, VEGF-D and VEGFR-3 were lower. Analysis revealed that the PC4 expression inlymph node tissues hightly correlation with the lymph angiogenic factor VEGF-C, VEGF-D and VEGFR-3 using Spearman’s nonparametric correlation test (correlation coefficient 0.780, 0.885, 0.790, P<0.01). 1: the A549non transfection groups; 2: the A549siRNA control transfection groups; 3: the A549siRNA PC4 transfection groups.
In the meanwhile, similar results were observed by analyzing the expression of PC4, VEGF-C, VEGF-D and VEGFR-3 in the regional lymph node tissues from above three groups. Figure 9B obviously showed that PC4, relative to the other two groups VEGF-C, VEGF-D and VEGFR-3 expressions were all much lower in lymph node tissues dissected from the animals treated with A549siRNA PC4, and Spearman’s nonparametric correlation test analysis revealed that PC4 correlated with the lymphangiogenic factor VEGF-C, VEGF-D and VEGFR-3 in lymph node tissues (correlation coefficient 0.780, 0.885, 0.790, P<0.01).
Discussion
PC4 was reported to be a novel target molecule for the diagnosis and treatment of advanced human cancers [9]. This study demonstrated that PC4 was able to regulate lymphangiogenic factor VEGF-C and VEGF-D in a wide variety of non-small cell lung cancer, in which the receptor VEGFR-3 was involved for the first time in vitro and in vivo. The high expression level of PC4 showed a positive correlation with amount of VEGF-C, VEGF-D and VEGFR-3 and with lymphatic vessel density, which suggested a new role of PC4 in lymphangiogenesis of early lymph node metastasis in human lung adenocarcinoma.
PC4 is a highly conservatively multifunctional transcriptional coactivator, which plays an important role in transcription, replication, DNA repair, cell cycle progression, cellular transformation and chromatin organization [2]. And PC4 expression is reported to be associated with the development and progression of human prostate and breast, as well as lung cancer in clinical specimens [9,14]. More and more experimental evidence showed PC4 may be a promising molecule for the diagnosis, treatment and prediction of metastasis of advanced human cancers. Shi et al [9] found that PC4 was abundantly expressed in transformed cells, while much low amount of PC4 was detected in parental nontransformed cells, and moreover, using LNCaP/C4-2 human prostate cancer cell model they identified that PC4 over-expression led to the increased growth of advanced C4-2 cells on plastic plates and in matrigel. However, in this manuscript, we demonstrated that the expression of VEGF-C and/or VEGF-D was required for PC4 regulation. Although downstream effector of PC4 treatment in vitro and in vivo appears to slightly down-regulated VEGF-C, VEGF-D and VEGFR-3, level of PC4 was statistically significant correlated with VEGF-C, VEGF-D and VEGFR-3 in vitro by Spearman’s nonparametric correlation test analysis. Furthermore, the lower metastatsis rate was observed in lung adenocarcinoma xenografts of the mice with down-regulated PC4. In addition, the high expression level of PC4 inducing higher density of tumor lymphatic vessels was detected. Therefore our current results showed that PC4 correlated with VEGF-C, VEGF-D and VEGFR-3, suggested PC4 palys a role in lymphangiogenesis and metastasis.
Activation of VEFD-C and VEGF-D, the lymphangiogenic factors, is reported to be able to promote lymphangiogenesis significantly and resulted in the up-regulation of VEGFR-3 gene [15]. Weryńska and his group confirmed that the VEGF-C/VEGF-D-VEGFR-3 overexpression could promote greatly the lymphangiogenesis in lung cancer [16], which suggests that expression levels of VEGF-C, VEGF-D and VEGFR-3 are critical mediators during the tumor lymphangiogenesis. Su et al also found that the activation of the VEGFC/VEGF-D/VEGFR-3 axis increases the motility and invasiveness of neoplastic cells and promoted the development of neoplastic metastases in lung adenocarcinoma [17]. However, little is known about the activation mechanism of the axis. In this study, we proposed that PC4 is able to regulate the lymphangiogenic factor of VEFD-C and VEGF-D, leading to lymphangiogenesis and facilitates the metastases. The results suggested that high expression level of PC4 induced a higher density of tumor lymphatic vessels and higher rate of regional lymph node metastatsis. Moreover, when the LYVE-1 or VEGFR-3 was positive,the higher density of tumor lymphatic vessels had a highter expression level of VEGF-C and/or VEGF-D.
PC4 has a highly conservative sequence from yeast to human, and was originally identified as a transcription cofactor that plays both a negative or positive role [18]. One of the most important function is to mediate the response of RNA polymerase II and III to transcriptional activators [19]. PC4 may contribute to the transcription of VEGF-C and VEGF-D as a transcription cofactor. Salameh et al [20] reported that the VEGFC/VEGF-D/VEGFR-3 axis regulated lymphangiogenesis through the activation of AKT pathways, which suggested AKT pathways may work as a modulator in lymphangiogenesis regulation. This view supports our hypothesis here that PC4 also may mediated the VEGF-C/VEGF-D/VEGFR-3 axis through combined action with the AKT pathways as a transcription cofactor. However, no matter the PC4 directly activated the lymphangiogenic factor VEFD-C and VEGF-D or indirectly induced the AKT pathways in mediating the lymphangiogenesis, the expression of PC4 palyed an important role in the lymphangiogenesis and lymphatic metastasis. These findings have proved PC4 to be a novel target for lymphangiogenesis in lymphatic metastatic spread of lung adenocarcinoma.
In conclusion, the proposed work revealed that PC4 expression level was positively correlated with the VEGF-C, VEGF-D and VEGFR-3 during the development of lymphangiogenesis in lung adenocarcinoma in virto and in vivo. The down-regulation of PC4 induced the lower expression of VEGFC, VEGF-D and VEGFR-3, suggesting PC4 can be a novel molecule in the development of lymphangiogenesis and lymphatic metastasis.
Acknowledgements
The authors would like to thank Dr. Jiebin Kuang for helpful discussions and suggestions on the experimental design. This work was supported by the Research Foundation of the Third Military Medical University No. 2007XG60. We are grateful to Dr. Chunmeng Shi for help with designing this experiment.
Disclosure of conflict of interest
None.
References
- 1.Annema JT, van Meerbeeck JP, Rintoul RC, Dooms C, Deschepper E, Dekkers OM, De Leyn P, Braun J, Carroll NR, Praet M, de Ryck F, Vansteenkiste J, Vermassen F, Versteegh MI, Veseliç M, Nicholson AG, Rabe KF, Tournoy KG. Mediastinoscopy vs endosonography for mediastinal nodal staging of lung cancer: a randomized trial. JAMA. 2010;304:2245–2252. doi: 10.1001/jama.2010.1705. [DOI] [PubMed] [Google Scholar]
- 2.Conesa C, Acker J. Sub1/PC4 a chromatin associated protein with multiple functions in transcription. RNA Biol. 2010;7:287–290. doi: 10.4161/rna.7.3.11491. [DOI] [PubMed] [Google Scholar]
- 3.Ge H, Roeder RG. Purification, cloning, and characterization of a human coactivator, PC4, that mediates transcriptional activation of class II genes. Cell. 1994;78:513–523. doi: 10.1016/0092-8674(94)90428-6. [DOI] [PubMed] [Google Scholar]
- 4.Calvo O, Manley JL. The transcriptional coactivator PC4/Sub1 has multiple functions in RNA polymerase II transcription. EMBO J. 2005;24:1009–1020. doi: 10.1038/sj.emboj.7600575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brandsen J, Werten S, van der Vliet PC, Meisterernst M, Kroon J, Gros P. C-terminal domain of transcription cofactor PC4 reveals dimeric ssDNA binding site. Nat Struct Biol. 1997;4:900–903. doi: 10.1038/nsb1197-900. [DOI] [PubMed] [Google Scholar]
- 6.Das C, Hizume K, Batta K, Kumar BR, Gadad SS, Ganguly S, Lorain S, Verreault A, Sadhale PP, Takeyasu K, Kundu TK. Transcriptional coactivator PC4, a chromatin-associated protein, induces chromatin condensation. Mol Cell Biol. 2006;26:8303–8315. doi: 10.1128/MCB.00887-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Batta K, Yokokawa M, Takeyasu K, Kundu TK. Human transcriptional coactivator PC4 stimulates DNA end joining and activates DSB repair activity. J Mol Biol. 2009;385:788–799. doi: 10.1016/j.jmb.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 8.Rajagopalan S, Andreeva A, Teufel DP, Freund SM, Fersht AR. Interaction between the transactivation domain of p53 and PC4 exemplifies acidic activation domains as single-stranded DNA mimics. J Biol Chem. 2009;284:21728–21737. doi: 10.1074/jbc.M109.006429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi CM, Zhu Y, Zhau HE, Su YP, Hung LWK, Cheng TM. PC4, a novel marker for stem cell transformation and cancer progression. J Biotechnol. 2008;136S:187–197. [Google Scholar]
- 10.Albrecht I, Christofori G. Molecular mechanisms of lymphangiogenesis in development and cancer. Int J Dev Biol. 2011;55:483–494. doi: 10.1387/ijdb.103226ia. [DOI] [PubMed] [Google Scholar]
- 11.Skobe M, Dana R. Blocking the path of lymphatic vessels. Nat Med. 2009;15:993–994. doi: 10.1038/nm0909-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mäkinen T, Veikkola T, Mustjoki S, Karpanen T, Catimel B, Nice EC, Wise L, Mercer A, Kowalski H, Kerjaschki D, Stacker SA, Achen MG, Alitalo K. Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J. 2001;20:4762–4773. doi: 10.1093/emboj/20.17.4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng Y, Wang W, Hu J, Ma J, Zhang Y, Zhang J. Expression of VEGF-C and VEGF-D as significant markers for assessment of lymphangiogenesis and lymph node metastasis in nonsmall cell lung cancer. Anat Rec (Hoboken) 2010;293:802–812. doi: 10.1002/ar.21096. [DOI] [PubMed] [Google Scholar]
- 14.Peng Y, Su Y, Wang TM, Zhang C, Wang S, Tan X, Shi CM. Human transcriptional positive coactivator 4 participates in the proliferation and senescence of bone marrow-derived multipotent mesenchymal stromal cells. Exp Hematol. 2010;38:S55. [Google Scholar]
- 15.Su JL, Shih JY, Yen ML, Jeng YM, Chang CC, Hsieh CY, Wei LH, Yang PC, Kuo ML. Cyclooxygenase-2 induces EP1- and HER-2/Neu-dependent vascular endothelial growth factor-C up-regulation: a novel mechanism of lymphangiogenesis in lung adenocarcinoma. Cancer Res. 2004;64:554–564. doi: 10.1158/0008-5472.can-03-1301. [DOI] [PubMed] [Google Scholar]
- 16.Weryńska B, Dziegiel P, Jankowska R. Role of lymphangiogenesis in lung cancer. Folia Histochem Cytobiol. 2009;47:333–342. doi: 10.2478/v10042-009-0090-3. [DOI] [PubMed] [Google Scholar]
- 17.Su JL, Yen CJ, Chen PS, Chuang SE, Hong CC, Kuo IH, Chen HY, Hung MC, Kuo ML. The role of the VEGF-C/VEGFR-3 axis in cancer progression. Br J Cancer. 2007;96:541–545. doi: 10.1038/sj.bjc.6603487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malik S, Guermah M, Roeder RG. A dynamic model for PC4 coactivator function in RNA polymerase II transcription. Proc Natl Acad Sci U S A. 1998;95:2192–2197. doi: 10.1073/pnas.95.5.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schang LM, Hwang GJ, Dynlacht BD, Speicher DW, Bantly A, Schaffer PA, Shilatifard A, Ge H, Shiekhattar R. Human PC4 is a substrate-specific inhibitor of RNA polymerase II phosphorylation. J Biol Chem. 2000;275:6071–6074. doi: 10.1074/jbc.275.9.6071. [DOI] [PubMed] [Google Scholar]
- 20.Salameh A, Galvagni F, Bardelli M, Bussolino F, Oliviero S. Direct recruitment of CRK and GRB2 to VEGFR-3 induces proliferation, migration, and survival of endothelial cells through the activation of ERK, AKT, and JNK pathways. Blood. 2005;106:3423–3431. doi: 10.1182/blood-2005-04-1388. [DOI] [PubMed] [Google Scholar]