Abstract
Background: Fascin is an actin-bundling protein critical for tumor invasion. TGF-β could induce fascin expression in gastric cancer cells. In this study, we attempted to explore the role of p-smad3L in the expression of fascin induced by TGF-β in gastric cancer cells. Methods: Pseudopodia were evaluated by immunofluorescence. Fascin expression was detected by RT-PCR and western blot. Smad3 siRNA was used to repress the endogenous smad3. The phosphorylations of smad3 linker region at sites s204, s208 and s213 were detected by western blot. The fascin promoter reporter activity was measured by dual luciferase assay. Results: TGF-β could increase the formation of pseudopodia and the expression of fascin in gastric cancer cells. Smad3 depletion abrogated the expression of fascin induced by TGF-β. The phosphorylation of smad3 linker region at serine 204, 208 and 213 was enhanced in gastric cancer cells after TGF-β treatment. The fascin promoter reporter activity was significantly enhanced with TGF-β treatment in both wild-type Smad3 group and Smad3EPSM group (P<0.05). Furthermore, the fascin promoter reporter activity in the wild-type Smad3 transfectant cells was significantly higher than that in Smad3EPSM cells (P<0.05). Conclusions: fascin expression induced by TGF-β depends on smad3, at least in part, depends on smad3 linker phosphorylation.
Keywords: TGF-β, fascin, gastric cancer, smad3
Introduction
Gastric carcinoma (GC) ranks as the world’s second leading cause of cancer mortality behind lung cancer despite a sharp worldwide decline in both its incidence and mortality since the second half of the 20th century [1]. Most of patients with gastric cancer were in advanced stage when they were diagnosed.
Fascin is an actin-bundling protein critical for tumor invasion. Expression levels of fascin are almost undetectable or very low in normal epithelia, but are highly elevated in gastric cancer [2-4]. Overexpression of fascin protein is associated with poor prognosis in GC patients [2-4]. Knockdown of fascin expression inhibited tumor cell migration and invasion in vitro and decreased tumor metastasis in mouse models [5]. However, the molecular mechanisms underlying elevated fascin level in gastric cancer are not very clear.
TGFβ is a cytokine secreted by tumor microenvironment or tumor cells that regulates various tumor progressions. In early stage tumors, TGFβ is a potent proliferation inhibitor that deters tumor growth; however, late stage tumors are often able to evade the growth inhibition and secrete elevated levels of TGFβ to promote metastasis [6]. In our previous study, we found TGFβ could promote the invasion and metastasis of gastric cancer cells [7]. The canonical smad pathway was involved in the progression of malignant tumor. Moreover the phosphorylation of smad3 linker region was reported to participate in the invasion and metastasis of malignant tumor promoted by TGFβ [8].
In our previous report, we demonstrated that TGFβ could induce fascin expression in gastric cancer [9]. Here, we attempted to determine whether the phosphorylation of smad3 linker region was involved in the expression of fascin induced by TGF-β in gastric cancer cells.
In this study, we demonstrated that TGFβ elevated fascin protein expression and promoted filopodia formation in gastric cancer cells. We also showed that transcription of fascin mRNA induced by TGFβ was dependent on smad3, at least in part, on smad3 linker region phosphosrylation.
Material and method
Cell lines, cell culture and treatment
The gastric cancer cell lines MKN45, BGC823 and AGS were purchased from Center for Cell Resources of Shanghai Institutes for Life Sciences, Chinese Academy of Sciences/Cell bank of China Center for Type Culture Collection, Chinese Academy of Sciences (CTCCCAS). Cells were maintained in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS) in a humidified atmosphere of 95% air/5% CO2 at 37°C.
Plasmids
Smad3 (EPSM) [10], a smad3 linker region mutant (Addgene plasmid # 14963), and wild-type Smad3 plasmid [11] (Addgene plasmid # 27025) were gifts from Joan Massague, which were provided by AddGene.
TGFβ treatment
Unless indicated otherwise, 10 ng/ml TGFβ1 were added to cells (Peprotech, Rocky Hill, NJ) in growth medium for 48 h before being used for assays.
siRNA transfection
Smad3 siRNA (QIAGEN) was mixed with Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions and applied to each plate (cells at 30%-50% confluence). Transfection medium was removed and replaced with complete medium after 5 hrs of incubation.
Western blot analysis
Cells were washed twice with phosphate buffered saline (PBS) before being lysed on ice for 40 minutes with lysis buffer containing 50 mmol/L HEPES buffer, 150 mmol/L NaCl, 1 mmol/L EDTA (pH 8.0), 1 mmol/L EGTA (pH 8.0), 1% IGEPAL CA-630, 0.5% Triton X-100, 10 mmol/L NaF, 2 mmol/L Na3VO4, 10 mmol/L β-glycerophosphate, and 1% protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO). The lysate was centrifuged at 16,000 × g at 4°C for 10 minutes. Ten to fifty micrograms of total protein for each sample were separated by 8%~12% SDS-PAGE and transferred onto an Immobilon membrane (Millipore Inc, Billerica, MA.), and the desired proteins were probed with corresponding antibodies. Mouse anti-β-actin (1:1000 dilution), anti-fascin (1:1000), and rabbit anti-phospho smad3 linker region at serine 208 were purchased from Santa Cruz, rabbit anti-human phospho smad3 linker region at serine 204 and serine 213 (1:1000 dilution) from Abcam, rabbit anti-smad3 antibody from Epitomics. Horseradish peroxidase–conjugated secondary anti-mouse and anti-rabbit IgG (H+L) was obtained from Santa Cruz. Bound antibody was detected using the SuperSignal West Pico Chemoluminescence system (Pierce, Inc. Rockford, IL).
RT-PCR
Total cellular RNA was collected using Trizol according to the manufacturer’s instructions (Invitrogen). Two μg RNA were reverse-transcribed using SuperScript II reverse transcriptase. Two μl of the produced cDNA was used for PCR according to the manufacturer’s instructions (Invitrogen). The primers for fascin, smad3, and β-actin were listed in Table 1. The amplification profile consisted of 2 min at 94°C for denaturation, followed by 35 cycles of denaturation (94°C for 30 s), annealing (as indicated in Table 1 for 30 s), and extension (72°C for 60 s).
Table 1.
Sequences of primers used for PCR
| Gene name | Primer | Annealing temperature (°C) | Products size (bp) | |
|---|---|---|---|---|
| Fascin | Sense | 5’-CTGGCTACACGCTGGAGTTC-3’ | 62 | 492 |
| Antisense | 5’-CTGAGTCCCCTGCTGTCTCC-3’ | |||
| β-actin | Sense | 5’-TGACGTGGACATCCGCAAAG-3’ | 58 | 205 |
| Antisense | 5’-CTGGAAGGTGGACAGCGAGG-3’ | |||
| Smad3 | Sense | 5’-CGCTTGACTCACGCCTTCG-3’ | 62 | 422 |
| Antisense | 5’-TTCCTCTTGCGGCCACTT-3’ | |||
Immunofluorescence microscopy
Cells cultured on collagen-coated glass coverslips were fixed with 3.7% paraformaldehyde in PBS for 10 min at room temperature, permeabilized with 0.1% Triton X-100 for 5 min, and then washed with PBS three times. To block nonspecific binding, the cells were incubated with a solution of PBS containing 1% bovine serum albumin for 30 min and then incubated with mouse anti-fascin (1:200). Biotin-conjugated goat anti-mouse secondary antibody was applied to cells followed by SABC-FITC incubation. The coverslips were then fixed onto slides and imaged using an Olympus fluorescence microscope.
Constructs and luciferase assay
The promoter of the human fascin (-1375~+81) was PCR amplified and cloned into pMIR-reporter downstream of the firefly luciferase gene to generate the corresponding reporters. For fascin promoter activity analysis, cells were co-transfected with 0.2 μg of the reporter construct, 0.01 μg of pRL-TK vector, and 0.2 μg of wild-type smad3 plasmid or smad3EPSM plasmid, a smad3 linker region mutant. Cells were treated with or without TGFβ1 for 12 h after 48 h transfection, and then harvested and assayed with Dual Luciferase Assay (Promega, WI, USA) according to manufacturer’s instructions. All transfection assays were carried out in triplicates.
Statistical analyses
The comparison between TGFβ treatment group and control group was evaluated by Student’s T test (two-tailed). P≤0.05 was considered statistically significant.
Result
Elevated fascin expression after TGFβ treatment
To explore the kinetics of fascin mRNA expression induced by TGFβ, we used RT-PCR to determine fascin mRNA levels in MKN45 and AGS cells after treatment with TGFβ for different periods of time. As shown in Figure S1, there was a 2 h lag in TGFβ-induced fascin transcription. Fascin mRNA levels increased steadily after the lag, reaching the highest level after 4 h and then followed a little decrease, but still higher than without TGFβ treatment. Next, we further evaluated the effects of TGFβ on the protein expression kinetics of fascin. As shown in Figure 1, TGFβ significantly elevated the fascin protein level after 2 h. In agreement with our previous report, TGFβ could induce the expression of fascin protein in MKN45 [9].
Figure 1.

The kinetics of TGFβ-induced expression of fascin protein in gastric caner cell lines MKN45 and BGC823. Cells were treated with TGFβ and lysed at different time points as indicated. The levels of fascin protein were determined through western blot and β-actin was a loading control.
Increased pseudopodium after TGFβ treatment
The formation of lamellipodia is the key step for cancer cell migration [12]. To investigate the mechanisms underlying the enhanced cell migration in TGFβ-pretreated gastric cancer cells, we used immunofluorescence to study the membrane protrusion. As shown in Figures 2 and S2, TGFβ induced hyperactive membrane protrusion in MKN45 and AGS cells (P<0.05).
Figure 2.
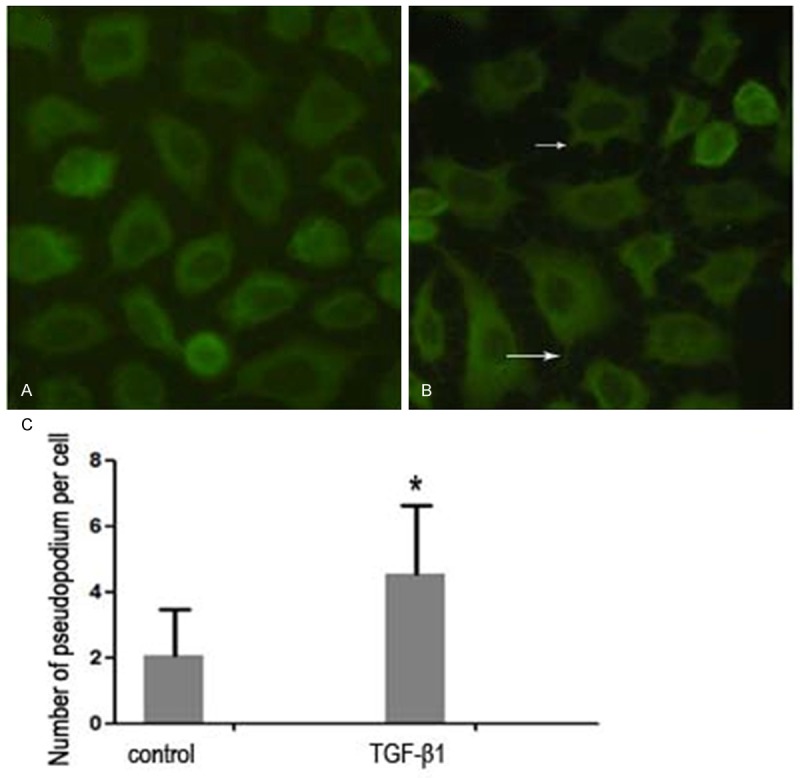
The formation of the pseudopodium in MKN45 cells without (A) or with (B) treatment of TGF-β for 48 h. The number of pseudopodium was counted as a histogram in (C). Asterisk: P<0.05.
Induction of fascin expression by TGFβ requires Smad3
To explore whether TGFβ-induced fascin expression is mediated through the TGFβ-Smad pathway, we employed small hairpin RNA (shRNA) to knock down the expression of Smad3. The knockdown of Smad3 was confirmed with RT-PCR and western blot. As shown in Figure 3, smad3 repression could attenuate the expression of fascin induced by TGFβ at both mRNA level (Figure 3A) and protein level (Figure 3B).
Figure 3.
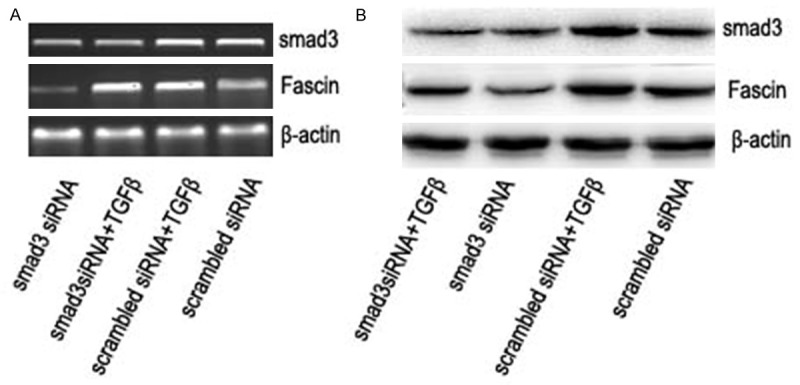
The mRNA level (A) and protein level (B) of smad3 and fascin with or without the treatment of TGF-β for 8 h after the transfection of smad3 siRNA or scrambled siRNA for 36 h. β-actin was a loading control.
TGFβ induces the phosphorylation of smad3 linker region
The phosphorylation of smad3 linker region was reported to be involved in TGFβ mediated tumor progression in colorectal cancer cells [8]. To investigate the effect of TGFβ on the phosphorylation of smad3 linker region in gastric cancer, we examined the kinetics of the phosphorylation of smad3 linker region at serines 204, 208 and 213 with the treatment of TGFβ for different periods of time. As shown in Figure 4A, TGFβ could induce the phosphorylation of smad3 at serine 204 and serine 208 after 0.5 h and the phosphorylation of smad3 at serine 213 in 1 h in MKN45 cells, followed by a steady increase to the highest level, then a little decrease. The similar results were also obtained in both AGS cells (Figure 4B) and BGC823 cells (Figure S3).
Figure 4.

Western blot analysis of phosphorylation level of smad3 linker region at serine 204, 208, and s213, respectively in MKN45 (A) and AGS (B). β -actin was a loading control.
TGFβ induces fascin promoter activity via phosphorylation of smad3 linker region
To further investigate the mechanisms smad3 involving fascin expression, fascin promoter was constructed, four smad3 binding sites were found at fascin promoter region. Fascin promoter was cloned from -1385 to +81 into pGL3. MKN45 cells were co-transfected with the fascin promoter luciferase reporter plasmid, wild-type smad3, or smad3 linker region mutant (smad3EPSM). As shown in Figure 5, no distinct changes were detected between wild-type smad3 group and smad3EPSM group without TGFβ treatment. TGFβ could result in a 2.5-fold activation of the fascin promoter reporter under physiological smad3 level. (versus control, P<0.05). Transfection of wild-type smad3 could result in 5.5-fold activation of the fascin promoter reporter with TGFβ treatment (versus control, P<0.01). However, Transfection of smad3EPSM could not further enhance the acitivity of fascin promoter reporter (versus control, P>0.05). Similar results were also obtained in BGC823 cells (Figure S4).
Figure 5.

The relative activity of fascin promoter reporter to Renilla luciferase activity after the co-transfection of p-RL (0.01 μg/well), fascin promoter reporter (0.2 μg/well), and smad3 WT plasmid (0.2 μg/well) or smad3 linker region mutant (smad3 EPSM) (0.2 μg/well) for 36 h with or without the treatment of TGF-β for 12 h in MKN45 cells, **: P<0.01; *: P<0.05.
Discussion
Fascin encodes a member of fascin family of actin-binding proteins. Fascin proteins organize F-actin into parallel bundles, and are required for the formation of actin-based cellular protrusions [13]. Fascin plays important roles in cell migration, motility, adhesion and cellular interactions and acts as an oncogene in multiple types of cancer by increasing cell motility [14-16]. Our previous study also revealed that knockdown of fascin1 expression could suppress the proliferation and metastasis of MKN45 gastric cancer cells [5]. TGFβ is a cytokine which regulates various tumor progressions in a context-dependent manner. In early stage tumors, TGFβ is a potent proliferation inhibitor that deters tumor growth; however, late stage tumors are often able to evade the growth inhibition and secrete elevated levels of TGFβ to promote metastasis [17]. Our previous study demonstrated that TGFβ could promote the invasion and metastasis of gastric cancer cells [7].
In breast and lung cancer cells, Sun J demonstrated TGFβ induced fascin expression in spindle-shaped tumor cells [18]. However, our present and previous studies [9] showed that TGFβ could induce fascin expression in gastric cancer cells, although they were polygonal, epithelial-like morphology. The difference between our reports and Sun J’s reports indicates that whether TGFβ could induce fascin expression in cancer cells is not just dependent on tumor cell morphology. Sun J further demonstrated that smad3 was involved in fascin expression regulated by TGFβ [18]. In agreement with this result, we also showed that TGFβ could induce fascin expression via smad3.
The phosphorylation of smad3 linker region was reported to involve in the TGFβ mediated tumor progression [8]. Our study also showed that TGFβ could induce the linker phosphorylation of smad3 in gastric cancer cells. Moreover, we further demonstrated that the phosphorylation of smad3 linker region was involved in TGFβ inducing fascin expression. To our knowledge, this is the first report revealing that TGFβ could induce fascin expression via the phosphorylation of smad3 linker region, at least in part. Besides smad pathway, our previous study also found that fascin expression was regulated by MAPK pathway [9].
Acknowledgements
This study was supported by the grant from National Natural Science Foundation of China (No. 81072036).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Kelley JR, Duggan JM. Gastric cancer epidemiology and risk factors. J Clin Epidemiol. 2003;56:1–9. doi: 10.1016/s0895-4356(02)00534-6. [DOI] [PubMed] [Google Scholar]
- 2.Hashimoto Y, Shimada Y, Kawamura J, Yamasaki S, Imamura M. The prognostic relevance of fascin expression in human gastric carcinoma. Oncology. 2004;67:262–270. doi: 10.1159/000081327. [DOI] [PubMed] [Google Scholar]
- 3.Kim SJ, Kim DC, Kim MC, Jung GJ, Kim KH, Jang JS, Kwon HC, Kim YM, Jeong JS. Fascin expression is related to poor survival in gastric cancer. Pathol Int. 2012;62:777–784. doi: 10.1111/pin.12012. [DOI] [PubMed] [Google Scholar]
- 4.Li X, Zheng H, Hara T, Takahashi H, Masuda S, Wang Z, Yang X, Guan Y, Takano Y. Aberrant expression of cortactin and fascin are effective markers for pathogenesis, invasion, metastasis and prognosis of gastric carcinomas. Int J Oncol. 2008;33:69–79. [PubMed] [Google Scholar]
- 5.Fu H, Wen JF, Hu ZL, Luo GQ, Ren HZ. Knockdown of fascin1 expression suppresses the proliferation and metastasis of gastric cancer cells. Pathology. 2009;41:655–660. doi: 10.3109/00313020903273100. [DOI] [PubMed] [Google Scholar]
- 6.Derynck R, Muthusamy BP, Saeteurn KY. Signaling pathway cooperation in TGF-betainduced epithelial-mesenchymal transition. Curr Opin Cell Biol. 2014;31:56–66. doi: 10.1016/j.ceb.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang KS, Hu ZL, Li JH, Xiao DS, Wen JF. Enhancement of metastatic and invasive capacity of gastric cancer cells by transforming growth factor-beta1. Acta Biochim Biophys Sin (Shanghai) 2006;38:179–186. doi: 10.1111/j.1745-7270.2006.00151.x. [DOI] [PubMed] [Google Scholar]
- 8.Matsuzaki K, Kitano C, Murata M, Sekimoto G, Yoshida K, Uemura Y, Seki T, Taketani S, Fujisawa J, Okazaki K. Smad2 and Smad3 phosphorylated at both linker and COOHterminal regions transmit malignant TGF-beta signal in later stages of human colorectal cancer. Cancer Res. 2009;69:5321–5330. doi: 10.1158/0008-5472.CAN-08-4203. [DOI] [PubMed] [Google Scholar]
- 9.Fu H, Hu Z, Wen J, Wang K, Liu Y. TGF-beta promotes invasion and metastasis of gastric cancer cells by increasing fascin1 expression via ERK and JNK signal pathways. Acta Biochim Biophys Sin (Shanghai) 2009;41:648–656. doi: 10.1093/abbs/gmp053. [DOI] [PubMed] [Google Scholar]
- 10.Kretzschmar M, Doody J, Timokhina I, Massague J. A mechanism of repression of TGFbeta/Smad signaling by oncogenic Ras. Genes Dev. 1999;13:804–816. doi: 10.1101/gad.13.7.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao S, Alarcon C, Sapkota G, Rahman S, Chen PY, Goerner N, Macias MJ, Erdjument-Bromage H, Tempst P, Massague J. Ubiquitin ligase Nedd4L targets activated Smad2/3 to limit TGF-beta signaling. Mol Cell. 2009;36:457–468. doi: 10.1016/j.molcel.2009.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 13.Kureishy N, Sapountzi V, Prag S, Anilkumar N, Adams JC. Fascins, and their roles in cell structure and function. Bioessays. 2002;24:350–361. doi: 10.1002/bies.10070. [DOI] [PubMed] [Google Scholar]
- 14.Papaspyrou K, Brochhausen C, Schmidtmann I, Fruth K, Gouveris H, Kirckpatrick J, Mann W, Brieger J. Fascin upregulation in primary head and neck squamous cell carcinoma is associated with lymphatic metastasis. Oncol Lett. 2014;7:2041–2046. doi: 10.3892/ol.2014.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma A, Badwal S, Dutta V, Basu A. Evaluation of fascin-1 expression as a marker of invasion in urothelial carcinomas. Med J Armed Forces India. 2014;70:139–143. doi: 10.1016/j.mjafi.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li A, Morton JP, Ma Y, Karim SA, Zhou Y, Faller WJ, Woodham EF, Morris HT, Stevenson RP, Juin A, Jamieson NB, MacKay CJ, Carter CR, Leung HY, Yamashiro S, Blyth K, Sansom OJ, Machesky LM. Fascin is regulated by slug, promotes progression of pancreatic cancer in mice, and is associated with patient outcomes. Gastroenterology. 2014;146:1386–1396. e1–17. doi: 10.1053/j.gastro.2014.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inman GJ. Switching TGFbeta from a tumor suppressor to a tumor promoter. Curr Opin Genet Dev. 2011;21:93–99. doi: 10.1016/j.gde.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Sun J, He H, Xiong Y, Lu S, Shen J, Cheng A, Chang WC, Hou MF, Lancaster JM, Kim M, Yang S. Fascin protein is critical for transforming growth factor beta protein-induced invasion and filopodia formation in spindle-shaped tumor cells. J Biol Chem. 2011;286:38865–38875. doi: 10.1074/jbc.M111.270413. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


