Abstract
Dysregulation of Wnt-mediated β-catenin signaling is associated with carcinogenesis and progression of hepatocellular carcinoma (HCC). Our previous studies showed that the Wnt10B gene, a member of Wnt gene family, over-activated in HCC tissues and cells. Here we demonstrate that stable silencing of Wnt10B reduces the viability of HCC cells in culture. HepG2, a human HCC cell line, was cultured in vitro and Wnt10B gene in the cells stably silenced, as showed in Western blotting analysis, by the shRNA interference with lentivirus plasmid transfection. Compared to the control (HepG2 cells without Wnt10B silencing), the Wnt10B-silencing cells showed significant reductions in proliferation, colony formation, migration and invasion. Furthermore, serum deprivation-induced apoptotic death, assessed by Hoechst 33342 staining and fluorescent microscopy, increased significantly in the Wnt10B-silencing cells. FACScan analysis indicated an arrest of the cell cycle in the Wnt10B-silencing HCC cells, with significant increases in the number of cells in G0-G1 and S phases. Thus, we hypothesize that Wnt10B plays an oncogenic role in HCC and is a potential therapeutic target.
Keywords: Wnt10B, hepatocellular carcinoma cells, proliferation, invasion, apoptosis
Introduction
Hepatocellular carcinoma (HCC) is a most malignant tumor with very poor prognosis. It is the sixth most common cancer in the world and leads cancer-related death (>500,000 deaths annually) worldwide [1]. More than 80% of HCC cases have occurred in developing countries, with 55% in China alone. In Guangxi province of China, HCC is the first most common cancer among the man and its mortality rate is about 55.3/100,000 [2]. The incidence of HCC continues to rise. Although previous studies have contributed considerably to the knowledge of HCC, to date there are limitations associated with effective prevention and treatment available for this disease.
Wnt-mediated β-catenin signaling regulates cell proliferation, differentiation, and fate decisions; dysregulation of β-catenin signaling due to aberrant expression of Wnt genes links to human tumorigenesis [3]. Nineteen members of the Wnt gene family, which encode secretory cystein-enriched glycoprotein ligands, have been identified in human or mouse genomes. As a canonical (β-catenin-dependent) pathway, Wnt ligands bind to Frizzled (Fz) receptors and LRP6 co-receptors, subsequently leading to Dishevelled (Dvl) activation and then inhibiting β-catenin phosphorylation, resulting in intracellular accumulation of β-catenin that enters into the nucleus and thereby activating the Wnt transcriptional programme. Activation of Wnt genes results in increased cell proliferation and differentiation, reduces cell-to-cell adhesion, enhances cell migration, and promotion of tumor formation [3]. Wnt10B (named as Wnt10b in mouse), a 389-amino acid protein that is most closely related to zebrafish Wnt10a, was originally identified in the Mouse Mammary Tumor Virus (MMTV), and like other identified members of Wnt gene family, is involved in up-regulation of the oncogenic β-catenin pathway [4].
Previous studies have shown that an over expression of Wnt10B exists in many human malignant tumors such as osteosarcoma, breast cancer, endometrial cancer, cervical cancer, esophageal cancer, gastric cancer, colorectal cancer, and pancreatic cancer [5-8]. Overexpression of this gene may also occur in situation of the tumor/cancer distant metastasis [5,6,8]. Wnt10B has been minimally studied in HCC. Research in liver cells demonstrated that the β-catenin signaling takes part in regulation of hepatic mitochondrial function and energy balance [9] and that Wnt10B aberrantly expresses in HCC tissues and cells [10-12]. These studies implicate a particular relationship between Wnt10B and HCC, which, however, has still been poorly characterized [12]. In the previous study [11], we showed that several human HCC cell lines including HepG2, Huh7 and Bel7402 over-expressed Wnt10B. In this study, we used RNA interference techniques to knockdown Wnt10B expression in HepG2 cell line and examined the impact of targeting to silence this gene on the viability and function of HCC cells.
Materials and methods
Cell culture
HepG2, a perpetual cell line derived from the liver tissue of a 15-year-old male with a well-differentiated HCC, was obtained from the American Type Culture Collection (ATCC No. HB-8065). The cells were cultured in a complete medium, Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS) (GIBCO), 100 U/mL penicillin, and 100 ìg/mL streptomycin, at 37°C in humidified 5% CO2 atmosphere. Media were changed every other day and cells subcultured when reached to ~90% confluence (usually in 4 days). For the subculture, cells were harvested by a trypsin (0.25%) digestion, seeded (1:3) into new tissue culture plates with complete DMEM, and continually cultured in the CO2 incubator.
Transfection of pLV-shwnt10b plasmids
For silencing Wnt10B expression, two oligo DNAs (TCCTGATCGATCTGCCCAC; GAGTCCCTATAGTTTCACC) which generated the small hairpin RNA (shRNA) targeting to region 1 and 2, respectively, of Wnt10B were synthesized and cloned into lentivirus vectors (pLVTHM) to form constructed pLVTHM-shRNA plasmids, according to the method reported previously [13]. To establish stably Wnt10B-silent HepG2 cells, the cells were seeded into 6-well plates 24 hours prior to the transfection, then incubated with pLVTHM (vector control), pLVTHM containing the sequence 1 (pLV-shwnt10b1) or pLVTHM containing the sequence 2 (pLV-shwnt10b2) in serum-free transfection medium (GIBCO) for 24 hours, and replaced with fresh complete DMEM and cultured additionally for 48 hours.
Western blotting analysis
HepG2 cells were washed (3×) with ice-cold PBS, lysed on ice (30 min) in RIPA lysis buffer (50 mM Tris-HCL, pH7.4, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS) with protease inhibitors (per mL containing 10 μL of 0.1 mM PMSF and 1 mL of 0.1% protease inhibitor solution; Beyotime), and scraped into microfuge tubes. Total proteins in the supernatant were isolated by centrifugation (12,000 rpm/min, 4°C) for 10-20 minutes and quantitatively analysed by using a BCA kit (Beyotime). Equal amount (25 μg) of the protein were separated on 5-10% gradient SDS polyacrylamide gels and transferred to PVDF membranes (Millipore). After blocking with 5% non-fat milk, the membrane (blot) was incubated with the primary antibody against Wnt10B (ProMab, 1:1000 dilution) or GAPDH (Bioworld, 1:2000 dilution) for 1 hour, then peroxidase-conjugated second antibody (Zsbio, 1:1000 dilution) for 1 hour. The blot was incubated with an enhanced chemiluminescent substrate (Thermo Scientific) and exposed to Hyperfilm (Kodak Image Station 440CF; Kodak).
Cell proliferation assay
Cell proliferation was determined by using a Cell Counting Kit (CCK)-8 (Dojindo, Japan), according to the manufacturer’s instructions. In brief, HepG2 cells were seeded in 96-well plates (2,000 cells/well), 100 μL per well of CCK-8 solution added at different time points (2, 48, 72 or 96 hours) after the seeding, and incubated for 2 hours at 37ºC. The absorbance at 450 nm was measured by using a microplate reader (Thermo Multiskan GO) with DMEM as a blank.
Colony formation assay
HepG2 cells were seeded in 6-well plates (1,200 cell/well) and cultured in complete DMEM for up to 3 weeks. When macroscopic clone seen (by naked eyes) in the plate, the culture was stop and cells were treated as follows. After washing with PBS, the cells were fixed with 4% paraformaldehyde (2 mL/well) for 15-20 minutes, stained with 0.1% crystal violet (1 mL/well) for 15-20 minutes, and after washing with distilled water and drying, the clones were observed and counted. For quantitative analysis, 33% acetic acid (1 mL/well) were added, then the plates shaken in a table concentrator at the speed of 80-90 r/min for 30 minutes, and the absorbance at 560 nm was measured in the microplate reader (Thermo Multiskan GO).
Cell migration assay
The migration capacity of cells was accessed by using the wound-healing testing. HepG2 cells were seeded into 6-well plates at a density of 5×105 cells per well and cultured in complete DMEM up to 24 hours to reach confluence. The confluent cells were scratched linearly in multiple areas with a plastic 200ìl-pipette tip, then allowed to migrate toward the wound for 48, 72, or 96 hours, and the “wounded” areas were photographed by an Olympus inverted microscope coupled to a CCD-SPOT digital camera (Olympus DP71; Olympus, Japan).
Cell invasion assay
Cell invasion was assessed by a Transwell assay of the cells cultured in matrigel (100 ìg/ml; BD Bioscience)-coated 24-hole chambers with chemoattractants (Corning Incorporated). A total of 2×105 HepG2 cells in 200ìL serum-free DMEM were seeded into the upper chamber, with 600 ìl 10% FBS DMEM in the lower chamber, and cultured in the CO2 incubator at 37ºC for 48, 72, or 96 hours. Cells in the upper chamber were removed by scrapping gently the matrigel coated on the inner surface. The invaded cells remaining in the chamber were fixed in 600 ìL 4% paraformaldehyde for 15 minutes at room temperature, washed (3×) in PBS, and stained with 600 ìL 0.1% crystal violet for 10 minutes; all reagents were directly added into the lower chamber. The stained cells were observed and counted under light microscope (×400), then washed with PBS, wiped out, and shaken in a concentrator (80-90 rpm, 30 min) for quantitative analysis by measuring the absorbance at 560 nm using a microplate reader (Thermo Multiskan GO).
Apoptosis analysis
Cell apoptosis was assessed by Hoechst 33342 staining and fluorescent microscopy.
HepG2 cells were seeded in 24-well plates (2×105 cells/well) with a cover glass in each well and cultured in complete DMEM overnight, then cultured in serum-free DMEM for 48, 72, or 96 hours. The cells were incubated with 1 mL/well Hoechst 33342 staining solution (Fanbo Biochemicals, China) at 37°C for 15-20 minutes in the CO2 incubator. The cover glasses were taken out from plates and cells observed under fluorescence microscope.
Cell cycle analysis
HepG2 cells seeded in 70 mm cell culture dishes (1×106 cells/dish) were cultured in complete DMEM for 48, 72 or 96 hours, harvested by 0.25% Trypsin digestion, washed in PBS, and fixed in ice-cold 70% ethanol-phosphate-buffered saline at -20°C overnight. After washing to remove the ethanol, cells were treated with 0.01% RNase (10 mg/mL; Sigma) at 37°C for 10 minutes, then stained with 0.05% propidium iodide at 4°C in dark for 20 minutes. The cell cycle distribution was determined using a FACScan (Becton Dickinson) and analysed with Modfit software (Phoenix).
Statistical analysis
Data were expressed as mean ± standard deviation (SD) of three and more independent experiments. Statistically significant difference between groups was determined by the Least Significant Difference t-test using SPSS version 18.0 for windows (SPSS Inc.). A value of P<0.05 was considered statistically significant.
Results
Expression analysis of the Wnt10B gene in HepG2 cells
To silence the Wnt10B gene, transfection of lentiviral plasmids constructed with the sequences targeting to distinctive coding regions of the gene was initially developed in HEK293T cells with the infection efficiency satisfied (Data not shown). Two of these Wnt10B-specific shRNA plasmids were applied to establish stable Wnt10B-silencing HepG2 cells, as described in the method section. Relatively high efficiency of the transfection was shown in the fluorescent staining assay (Figure 1) and Wnt10B silencing confirmed by Western blotting analysis of its protein expression (Figure 2) in the cells following the transfection. The efficiency of transfection of pLV-shwnt10b2 appeared a little higher than that of pLV-shwnt10b1 and consistently Wnt10B protein was less in the shWnt10b#2 than shWnt10b#1 cells.
Figure 1.
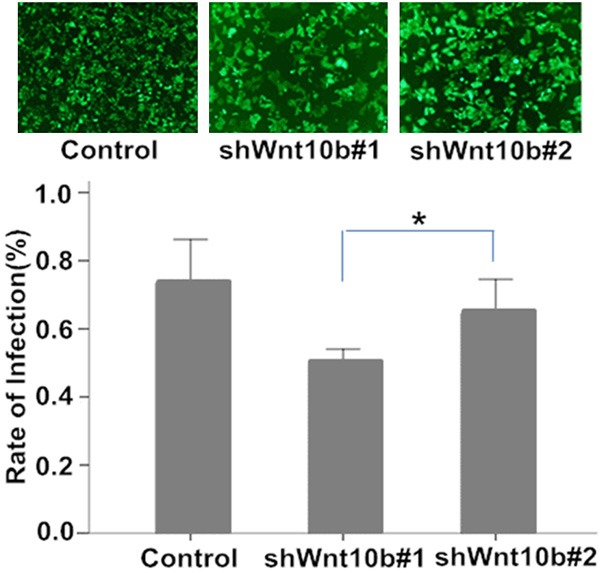
Fluorescent staining analysis of HepG2 cells in culture with lentivirus vector transfection. HepG2 cells infected by lentivirus vectors pLVTHM (control), pLV-shwnt10b1 (shWnt10b#1) or pLV-shwnt10b2 (shWnt10b#2) were under fluorescent staining and microscopy (photos), with a relatively high efficiency of the transfection (the rate of infection) achieved (bar graph, *P<0.05, n=3).
Figure 2.
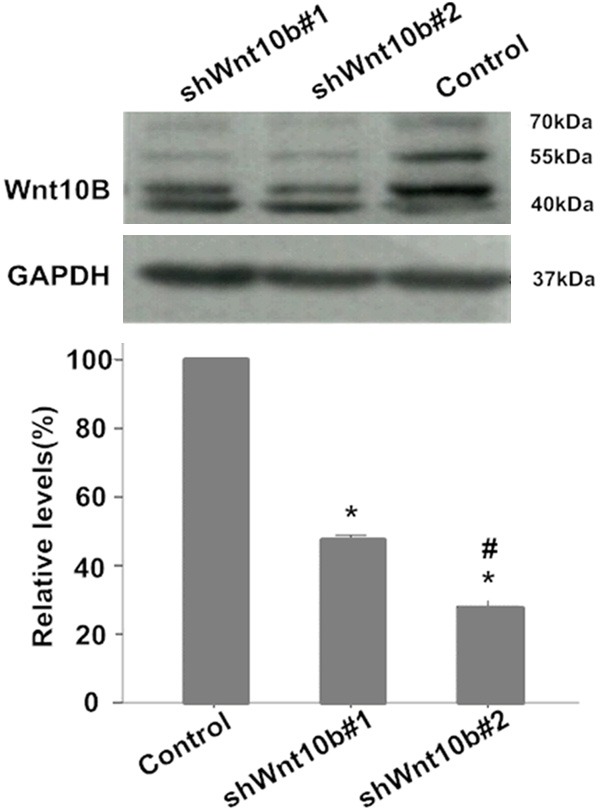
Expression of Wnt10B protein in HepG2 cells in culture. Western blotting analysis of protein expression of Wnt10B in HepG2 cells transfected with pLVTHM (control), pLV-shwnt10b1 (shWnt10b#1), or pLV-shwnt10b2 (shWnt10b#2). Photos: Protein signals of Wnt10B (bands at ~42-55 kDa) presented on Western blots in a representative result from three independent experiments. Bar graph: relative levels of Wnt10B proteins are showed as mean ± SD (n=3); *P<0.01, vs. control; #P<0.05, vs. shWnt10b#1.
Silencing of Wnt10B inhibits proliferation and colony formation in HepG2 cells
Compared to the control (cells with mock-vehicle infection), serum-induced proliferation slowed in Wnt10B silencing cells (shWnt10b#1 and shWnt10b#2) in a time-dependent manner (Figure 3) and similarly, the colony formation significantly delayed as well in the Wnt10B silencing cells (Figure 4), indicating an effect of the gene silencing. More delays of the proliferation and colony formation were shown in shWnt10b#2 than shWnt10b#1 cells, further indicating the effect dependent on Wnt10B expression since it was more silent in shWnt10b#2 than shWnt10b#1 cells (Figure 2).
Figure 3.
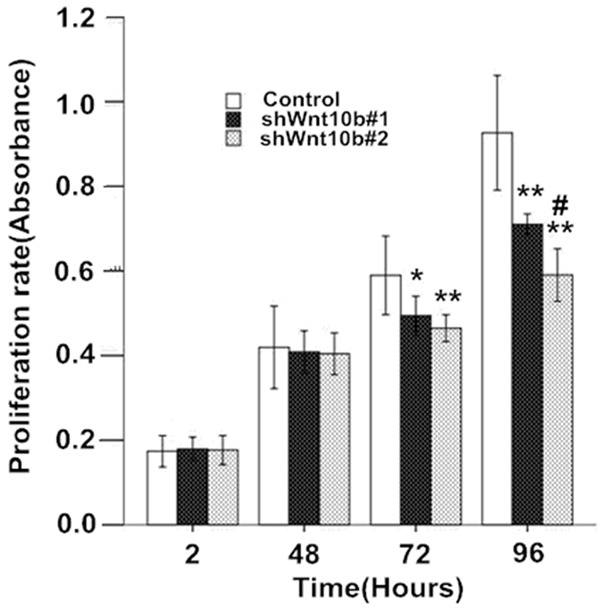
Silencing of Wnt10B attenuates the proliferation in HepG2 cells in culture. HepG2 cells transfected with pLVTHM (control), pLV-shwnt10b1 (shWnt10b#1), or pLV-shwnt10b2 (shWnt10b#2) were cultured for 2 to 92 hours and at different time points assessed with a CCK-assay to determine cell proliferation rate (absorbance at 450 nm). Results are expressed as the mean ± SD (n=3); *P<0.05, **P<0.01, vs. control; #P<0.05, vs. shWnt10b#1.
Figure 4.
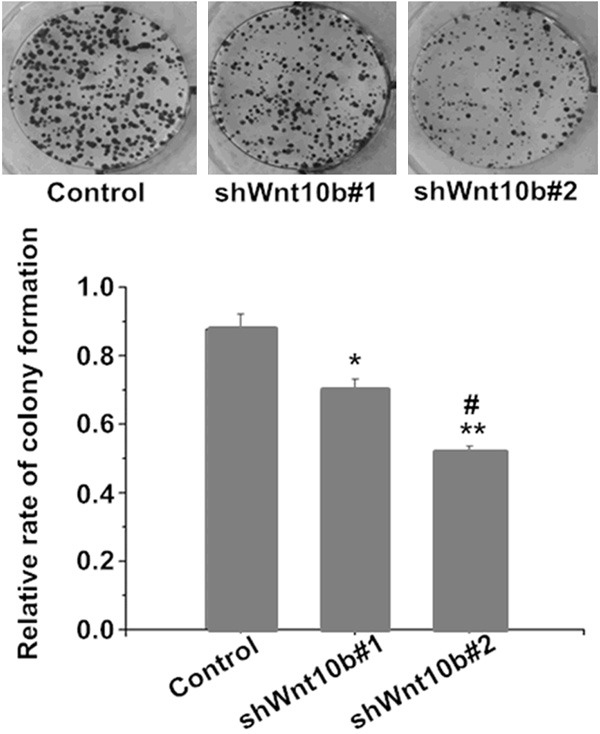
Silencing of Wnt10B delays the colony formation in HepG2 cells in culture. HepG2 cells transfected with pLVTHM (control), pLV-shwnt10b1 (shWnt10b#1), or pLV-shwnt10b2 (shWnt10b#2) were cultured for 2-3 weeks, then cell growth and colony formation determined. Photos: a representative result from three independent experiments. Bar graph: data are shown as mean ± SD (n=3). *P<0.05, **P<0.01 vs. control; #P<0.05, vs. shWnt10b#1.
Silencing of Wnt10B reduces migration and invasion in HepG2 cells
In the wound healing assay, the motility and wound healing process were significantly reduced in the Wnt10B-silencing cells, with the rate of reduction at ~34-40% in shWnt10b#1 and ~55-67% in shWnt10b#2 cells in comparison with the control (Figure 5). Transwell matrigel assay showed that the in vitro cell invasion was significantly inhibited in the Wnt10B-silencing cells in a time-dependent manner, with the rate of inhibition at ~32-45% in shWnt10b#1 and ~44-62% in shWnt10b#2 cells (Figure 6).
Figure 5.
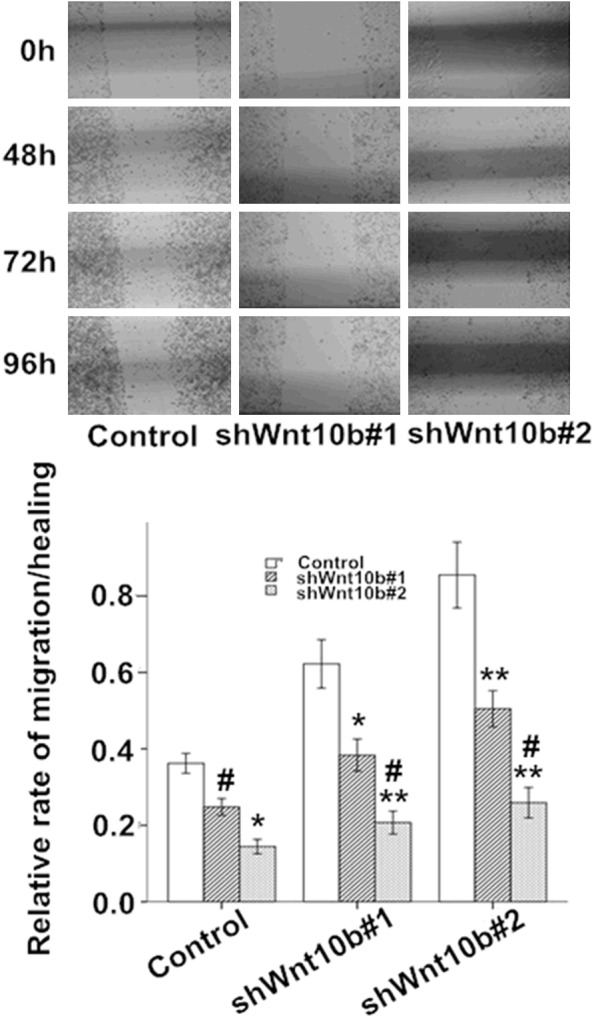
Silencing of Wnt10B obstructs migration in HepG2 cells in culture. HepG2 cells transfected with pLVTHM (control), pLV-shwnt10b1 (shWnt10b#1), or pLV-shwnt10b2 (shWnt10b#2) were cultured to reach to confluence, scratched and cultured continually for 48-96 hours, then the cell migration and wound healing assessed by microscopy at different time points. Photos: a representative result from three independent experiments. Bar graph: data are shown as mean ± SD (n=3). *P<0.05, **P<0.01 vs. control; #P<0.05, vs. shWnt10b#1.
Figure 6.
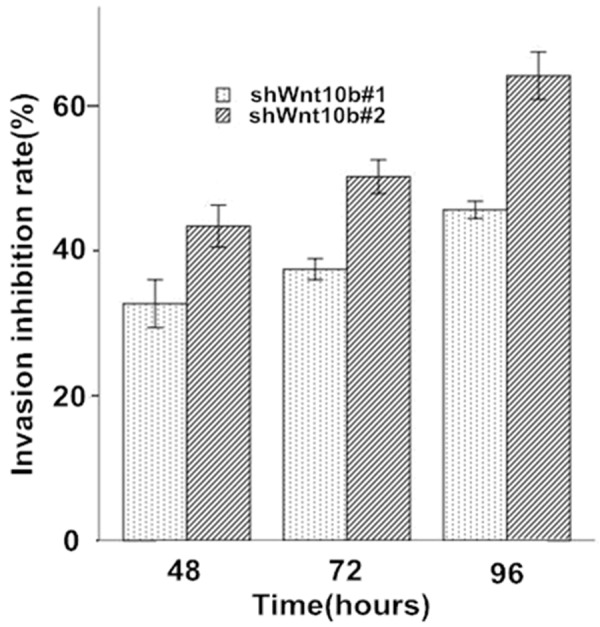
Silencing of Wnt10B reduces invasion of HepG2 cells in culture. HepG2 cells transfected with pLVTHM (control), pLV-shwnt10b1 (shWnt10b#1), or pLV-shwnt10b2 (shWnt10b#2) were cultured in Matrigel chamber for 48-96 hours and analyzed at different time points as described in Materials and Methods. The rate of invasion in control cells was as 100% and the inhibitory rate of invasion in Wnt10B silencing cells compared to the control. Data are shown as mean ± SD (n=3). *P<0.05, **P<0.01 vs. control; #P<0.05, vs. shWnt10b#1.
Silencing of Wnt10B induces apoptosis in HepG2 cells
In Hoechst 33342 staining, typical apoptotic cells appeared a high concentration of nucleus chromatins, siding and fragmentation of the nuclear, and apoptotic bodies observed under microscopy (photos in Figure 7). During the periods of serum-free culture for 48-96 hours, few apoptotic cells (<5%) were present in the control HepG2 cells but significant increasing in the apoptotic damage was found in the Wnt10B-silencing cells, with ~15-28% in shWnt10b#1 and ~26-41% in shWnt10b#2 cells as apoptosis at 48-72 hours (bar graph in Figure 7).
Figure 7.
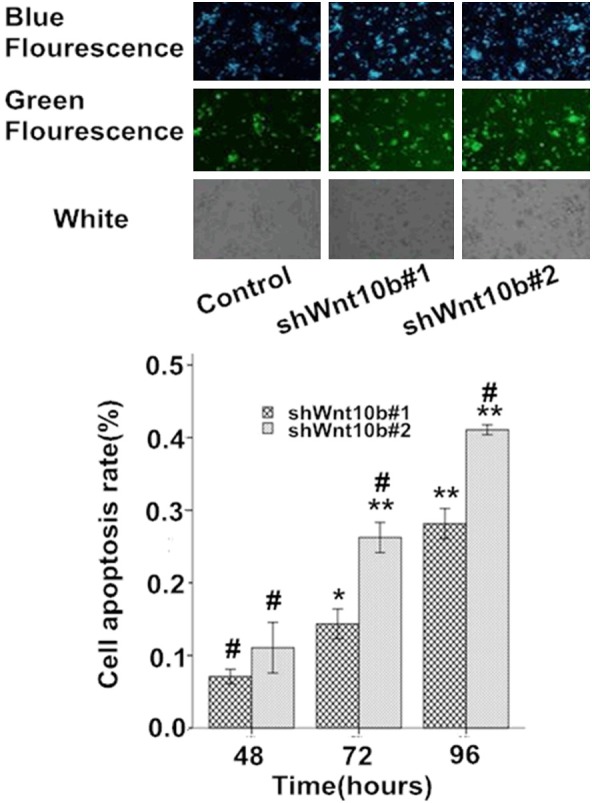
Silencing of Wnt10B promotes apoptosis in HepG2 cells in culture. HepG2 cells transfected with pLVTHM (control), pLV-shwnt10b1 (shWnt10b#1), or pLV-shwnt10b2 (shWnt10b#2) were cultured under serum-free DMEM for 48-96 hours, stained with Hoechst 33342, and analyzed by fluorescence microscopy. Photos: representative images of cells cultured for 72 hours from three independent experiments. Bar graph: data are showed as mean ± SD (n=3), *P<0.05, **P<0.01, vs. control; #P<0.05, vs. shWnt10b#1.
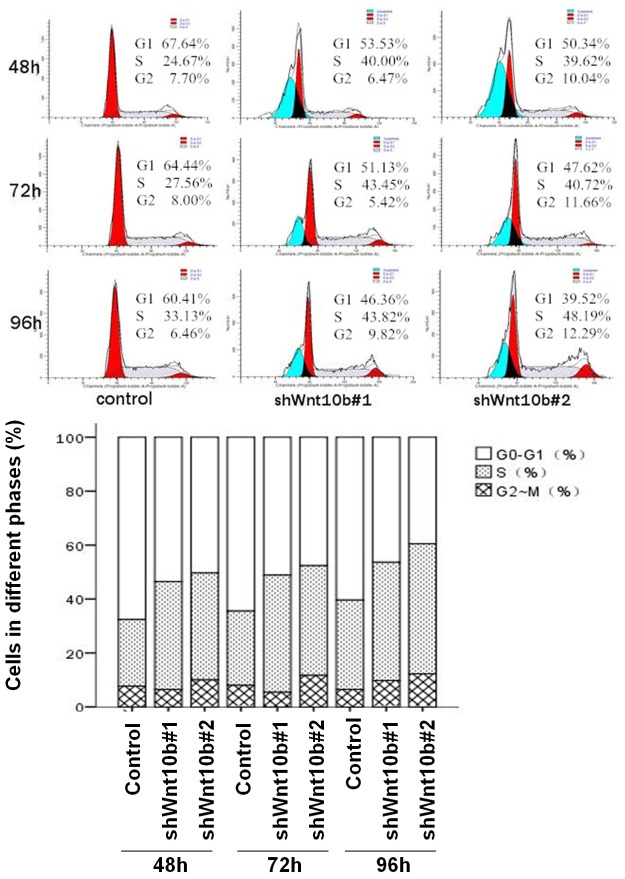
Increased arrest of cell cycle in Wnt10B-silencing HepG2 cells
In the FACScan analysis (Figure 8), the number of cells at G0-G1 were significant decreased and that at S phase increased, with no significant change in G2-M phase cells, in the Wnt10B-silencing cells, in comparison with the control, indicating the cell cycle retarded in the silencing cells. During the periods of cell cultures for 48-96 hours, there were ~62-67% cells at G0-G1 phases and ~20-25% at S phase in the control group (cells without Wnt10B silencing), and in the Wnt10B-silencing cells ~47-55% at G0-G1 and ~32-41% at S in the shWnt10b#1, and ~40-50% at G0-G1 and ~33-45% at S in the shWnt10b#2.
Figure 8.
Silencing of Wnt10B in cultured HepG2 cells decreases cells at G0-G1 phase, increases S phase cells, and does not changed the number of G2-M cells. HepG2 cells transfected with pLVTHM (control), pLV-shwnt10b1 (shWnt10b#1), or pLV-shwnt10b2 (shWnt10b#2) were analyzed with FACS as described in Materials and Methods. A representative result from three independent experiments presented here indicates the percentage of cells in the different phases.
Discussion
Previous studies [10-12] showed an aberrant overexpression of Wnt10B gene in tissues and cultured cells from HCC. Here, we demonstrated that silencing of this gene results in reduction of cell proliferation, colony formation, migration and invasion, and increasing in serum deprivation-induced cell apoptosis in HCC HepG2 cells in culture. Consistently, FACS can analysis indicates a retardation of the cell cycle, with increasing G0-G1 cells and decreasing cells at S phase in the stably Wnt10B-silencing cells. To the best of our knowledge, the present study demonstrates, for the first time, that silencing of Wnt10B directly leads a reduction of the viability of HCC cells.
Wnt/β-catenin signaling plays a crucial role in carcinogenesis and progression of cancers. Different Wnt ligands, through activating the β-catenin-dependent pathway, play important roles in deferent kinds of cancers. The major function of Wnt10B is, cooperated with other Wnt members, to promote the canonical Wnt (β-catenin) signaling pathway, in which accumulated β-catenin complexes with T cell factor (Tcf)/Lymphatic enhance transcription factors (Lef) in the nuclear and regulates the target genes, resulting in biological activities [4]. Over activation of this signaling pathway may contribute to maintain many of the abnormal biological activities of cancer cells, including vigorous proliferation and invasion [3] as well as high mitochondrial production of energy for these activities [9]. Recent studies make efforts to understand the role and possible mechanisms of Wnt10B in the development of human cancers. Studies of patients with osteosarcoma found that an aberrant high expression of Wnt10B and β-catenin was present in metastatic osteosarcoma tissue, associated to the invasion and metastasis of the tumor, and correlated with the reduced survival [5,14]. Overexpression of Wnt10B might play a vital role in the development of breast carcinoma, in which it could induce high-mobility group protein A2 via activating Wnt/β-catenin pathways and accelerate the proliferation of high invasive breast cancer cells [6]. The expression of Wnt10B was involved in the activation of the Wnt/β-catenin pathway and in the carcinogenesis of endometrial cancer, particularly in endometrioid carcinomas, while the clinical significance of Wnt10B overexpression remain unclear [7].
Although at this time data concerning the role of Wnt10B in HCC remain limited, studies have provided the evidence of a close association between an aberrant high activity of Wnt/β-catenin signaling and the liver cancer. For example, in the HCC-transplanted mice [15], WIF-1 and SFRP1, inhibitors of Wnt signaling pathways, could slow cell growth, induce cell apoptosis, and inhibit the tumor angiogenesis, resulting in suppression of the tumor growth. Liver stem cells marker epithelial cell adhesion molecules (EpCAM) was a target gene of Wnt/β-catenin signal transduction, activation of Wnt/β-catenin promoted the expression of EpCAM, and HCC with EpCAM-positive HCC cells could be highly invasive tumor [16,17]. Up to date, the role of Wnt10B in HCC remains largely unclear. A recent study showed that Wnt10B might express at high or low levels in HCC cells, thus it could be deregulated by either overexpression or silencing in HCC [12]. In addition, study of HCC HuH-7 (not HepG2) cells showed that, although Wnt10B was able to act on the oncogenic β-catenin pathway synergistically with the fibroblast growth factor (FGF), the up-regulated Wnt10B might suppress the cell growth via non-canonical Wnt pathways, the mechanisms independent of the β-catenin signaling [12]. HepG2 cells, the HCC cell line used in the present study, expressed Wnt10B (more significantly than HuH-7 cells) [11] as well as FGF [18], and consistently in this study, stable silencing of Wnt10B significantly reduced the cell proliferation and invasion possibly via weakness of the Wnt/β-catenin signaling. The present study generated some novel information on the Wnt signaling in HCC. Further studies are needed to examine the impact of Wnt10B silencing in HCC cells under in vivo conditions.
In summary, this in vitro study provides the evidence that stable silencing of Wnt10B directly reduces the viability, including reduction of cell proliferation and invasion as well as increased intendancy of apoptosis in HCC cells, implicating that Wnt10B plays an oncogenic role in HCC and is a potential therapeutic target. The role played by Wnt10B in HCC, however, still requires further investigation.
Acknowledgements
This work was supported by the Science and Technology Department of Guangxi Zhuang Autonomous Region in 2012 (Grant No. 12300017) and the Graduate Student Research Innovation Project in 2013(Grant No. JG2013117) in China. We thank Dr Wu (Guilin Medical University) in assistance of establishment of stable Wnt10B silencing HepG2 cells and Dr Ling (Guest Professor of GMU) for his assistance in the manuscript preparation.
Disclosure of conflict of interest
None.
References
- 1.Venook AP, Papandreou C, Furuse J, Guevara LL. The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncologist. 2010;15:5–13. doi: 10.1634/theoncologist.2010-S4-05. [DOI] [PubMed] [Google Scholar]
- 2.Zhang CY, Huang TR, Yu JH, Zhang ZQ, Li JL, Deng W, Ye SY, Zhou DH, He ZF. Epidemiological analysis of primary liver cancer in the early 21st century in Guangxi province of China. Chin J Cancer. 2010;29:545–550. doi: 10.5732/cjc.009.10510. [DOI] [PubMed] [Google Scholar]
- 3.Wend P, Wend K, Krum SA, Miranda-Carboni GA. The role of WNT10B in physiology and disease. Acta Physiol (Oxf) 2012;204:34–51. doi: 10.1111/j.1748-1716.2011.02296.x. [DOI] [PubMed] [Google Scholar]
- 4.Lee FS, Lane TF, Kuo A, Shackleford GM, Leder P. Insertional mutagenesis identifies a member of the Wnt gene family as a candidate oncogene in the mammary epithelium of int-2/Fgf-3 transgenic mice. Proc Natl Acad Sci U S A. 1995;92:2268–72. doi: 10.1073/pnas.92.6.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brun J, Dieudonné FX, Marty C, Müller J, Schüle R, Patiño-García A, Lecanda F, Fromigué O, Marie PJ. FHL2 silencing reduces Wnt signaling and osteosarcoma tumorigenesis in vitro and in vivo. PLoS One. 2013;8:e55034. doi: 10.1371/journal.pone.0055034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wend P, Runke S, Wend K, Anchondo B, Yesayan M, Jardon M, Hardie N, Loddenkemper C, Ulasov I, Lesniak MS, Wolsky R, Bentolila LA, Grant SG, Elashoff D, Lehr S, Latimer JJ, Bose S, Sattar H, Krum SA, Miranda-Carboni GA. WNT10B/β-catenin signaling induces HMGA2 and proliferation in metastatic triple-negative breast cancer. EMBO Mol Med. 2013;5:264–279. doi: 10.1002/emmm.201201320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen H, Wang Y, Xue F. Expression and the clinical significance of Wnt10a and Wnt10b in endometrial cancer are associated with the Wnt/β-catenin pathway. Oncol Rep. 2013;29:507–514. doi: 10.3892/or.2012.2126. [DOI] [PubMed] [Google Scholar]
- 8.Wang L, Xu XQ, Wang JF. The expression and significance of Wnt10b in stomach cancer. Jining Med Univ. 2014;37:30–36. [Google Scholar]
- 9.Lehwald N, Tao GZ, Jang KY, Papandreou I, Liu B, Liu B, Pysz MA, Willmann JK, Knoefel WT, Denko NC, Sylvester KG. β-Catenin regulates hepatic mitochondrial function and energy balance in mice. Gastroenterology. 2012;143:754–764. doi: 10.1053/j.gastro.2012.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fu F, Fan X, Sun L. The expression of Wnt10b in hepatocellular carcinoma. Chongqing Med J. 2014;43:3753–6. [Google Scholar]
- 11.Fan X, Sun L. Expression and significance of Wnt10b in hepatocellular carcinoma HepG2, Huh7, and Bel7402 cells. Health World. 2014:252–3. [Google Scholar]
- 12.Yoshikawa H, Matsubara K, Zhou X, Okamura S, Kubo T, Murase Y, Shikauchi Y, Esteller M, Herman JG, Wei Wang X, Harris CC. WNT10B functional dualism: beta-catenin/Tcf-dependent growth promotion or independent suppression with deregulated expression in cancer. Mol Biol Cell. 2007;18:4292–4303. doi: 10.1091/mbc.E06-10-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zufferey R, Nagy D, Mandel RJ, Naldini L, Trono D. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat Biotechnol. 1997;15:871–5. doi: 10.1038/nbt0997-871. [DOI] [PubMed] [Google Scholar]
- 14.Chen K, Fallen S, Abaan HO, Hayran M, Gonzalez C, Wodajo F, MacDonald T, Toretsky JA, Uren A. Wnt10b induces chemotaxis of osteosarcoma and correlates with reduced survival. Pediatr Blood Cancer. 2008;51:349–55. doi: 10.1002/pbc.21595. [DOI] [PubMed] [Google Scholar]
- 15.Hu J, Dong A, Fernandez-Ruiz V, Shan J, Kawa M, Martínez-Ansó E, Prieto J, Qian C. Blockade of Wnt Signaling Inhibits Angiogenesis and Tumor Growth in Hepatocellular Carcinoma. Cancer Res. 2009;69:6951–9. doi: 10.1158/0008-5472.CAN-09-0541. [DOI] [PubMed] [Google Scholar]
- 16.Yamashita T, Budhu A, Forgues M, Wang XW. Activation of hepatic stem cell marker EpCAM by Wnt-beta-catenin signalling in hepatocellular carcinoma. Cancer Res. 2007;67:10831–9. doi: 10.1158/0008-5472.CAN-07-0908. [DOI] [PubMed] [Google Scholar]
- 17.Yamashita T, Ji J, Budhu A, Forgues M, Yang W, Wang HY, Jia H, Ye Q, Qin LX, Wauthier E, Reid LM, Minato H, Honda M, Kaneko S, Tang ZY, Wang XW. EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology. 2009;136:1012–24. doi: 10.1053/j.gastro.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miura S, Mitsuhashi N, Shimizu H, Kimura F, Yoshidome H, Otsuka M, Kato A, Shida T, Okamura D, Miyazaki M. Fibroblast growth factor 19 expression correlates with tumor progression and poorer prognosis of hepatocellular carcinoma. BMC Cancer. 2012;12:56–71. doi: 10.1186/1471-2407-12-56. [DOI] [PMC free article] [PubMed] [Google Scholar]