Abstract
It has been reported that Retinoic acid receptor responder 3 (RARRES3) could suppress the metastasis of colorectal cancer (CRC). However, the underlying mechanism by which RARRES3 suppresses metastasis remains unknown. To investigate the functional involvement of RARRES3 in CRC, we first analyzed the expression of this protein between human CRC clinical samples and their corresponding normal controls and tested its correlation with clinicopathology as well as prognosis of CRC. We also examined the endogenous expression of RARRES3 by western-blot in a panel of CRC cell lines with different metastatic capacity. Cell proliferation, migration and invation of the CRC cell lines with either knockdown or reexpression of RARRES3 were examined by MTT, transwell and wound healing assays, respectively. The intrecellular signaling pathways affected by manipulations of RARRES3 in CRC cells were determined by western blot. Immunoprecipitation (IP) was employed to assess the interactionbetween proteins. To investigate the metastatic ability in vivo, CRC cell lines with manipulations of RARRES3 expression were inoculated in nude mice through tail vein injection. We confirmed that RARRES3 was significantly down-regulated in CRC tissues compared with normal controls. RARRES3 expression was not correlated with prognosis but significantly associated with CRC differentiation and lymphnodes metastases. We also found that RARRES3 was able to significantly suppress the metastasis of CRC cells both in vitro and in vivo through the regulation of epithelial-mesenchymal transition (EMT) process during which RARRES3 interactedwith MTDH in an opposite way. Taken together, we for the first time found that RARRES3 was able to suppress the metastasis of CRC both in vitro and in vivo via suppression of MTDH so as to regulate EMT.
Keywords: Colorectal cancer, RARRES3, β-catenin, EMT, metastasis
Introduction
Colorectal cancer (CRC) is the third leading cause of death from cancer worldwide [1], and ranks the fifth among cancer related death in China, with its incidence continually increasing [2]. Despite of the advances in the treatment modalities including new surgical techniques, novel radiotherapy and chemotherapy over the last decades, the overall survival rate of patients with CRC has been dismal, with a 5-year survival rate being less than 10% for stage IV patients whose have CRC cells metastasized to distant organs [3]. Distant metastases to lung and liver are fairly common in CRC [4,5]. A recent research provided clinical and molecular evidence showing that different MAPK signalling pathways are implicated in the mechanisms that allow colon cancer cells to form liver and lung metastases [6].
Retinoic acid receptor responder 3 (RARRES3), also named TIG3 [7] or RIG1 [8], has been identified and characterized as a tumor suppressor being able to repress lung metastasis in breast cancer via the regulation of adhesion and differentiation [9,10]. It is a consensus that RARRES3 is significantly down-regulated in CRC tissues compared with normal controls, and that its expression is significantly associated with CRC differentiation but not with prognosis [11,12]. Additionally, it has been shown that RARRES3 is able to regulate keratinocyte differentiation and loss of RARRES3 in transformed cells contributes to the malignant phenotype [13], probably due to a direct involvement of TP53 [14]. Nevertheless, there has been little molecular mechanism linking RARRES3 and metastasis suppression in colorectal cancer.
MTDH has been reported to significantly promote cancer metastasis by inducing epithelial-mesenchymal transition [15,16] in cooperation with SND1 [17,18], via regulating the PI3K-AKT [19], ERK [20], p38 and NF-kB [21] signal pathways. There has been, however, no report regarding the mechanism underlying MTDH mediates metastasis in the setting of RARRES3 changes in CRC.
To investigate the possible mechanism by which RARRES3 suppresses metastasis in colorectal cancer, in the present study, we analyzed the clinicopathological as well as prognostic significance of RARRES3 in CRC tissues. We also determined the malignant behaviors of CRC cell lines after genetic manipulations of RARRES3 expression in vitro via both knock-down and over-expression strategies. We found that RARRES3 was significantly down-regulated in human CRC tissues compared to the corresponding normal controls and that the level of RARRES3 significantly associated CRC differentiation, ableit no prognostic association was identified. Mechanistically, we found that RARRES3 was able to significantly suppress CRC metastasis through modulating the epithelial-mesenchymal transition (EMT) process by interacting with MTDH in an opposite way. Our study, for the first time, identified RARRES3 mediates suppression of CRC metastasis through MTDH and the regulation of EMT.
Materials and methods
CRC clinical tissues
The present study was approved by the Medical Ethics Committee of Ruijin Hospital, School of Medicine, Shanghai Jiaotong University and signed informed consent was obtained before obtaining clinical tissues. 78 cases of normal colorectal tissues and 86 cases of colorectal cancer tissues in formalin-fixed paraffin-embedded (FFPE) blocks were retrieved from the department of pathology from 2006 to 2013 in our hospital. None of the recruited patients received treatment before surgery, and the clinical-pathological information for all the patients was available. Representative hematoxylin and eosin (H&E)-stained slides from each patient were retrospectively reviewed blindly and separately by two pathologists. Both samples were separately excised by experienced pathologists and were frozen in liquid nitrogen within 30 min after surgery and stored at -80°C until analysis.
CRC cell culture and transfection
The human CRC cell lines HT29, DLD-1, HCT-15, CoL0320, SW480 and SW620 were all purchased from American Type Culture Collection (Rockville, MD). Cells were cultured in DMEM supplemented with 10% fetal bovine serum and penicillin/streptomycin in a 5% CO2 humidified incubator at 37°C. For knockdown experiments, lentiviral short hairpin RNA (shRNA) vector pGFP-C-shLenti- RARRES3 (TL 320510) and shRNA scramble control were all purchased from Origene (Origene Technologies, Inc, USA). shRNA target sequences were selected using the on-line tool (http://rnaidesigner.lifetechnologies.com/rnaiexpress/). Additionally, eukaryotic over-expression vector harboring full length cDNA of RARRES3 (NM_004585.2) pCMV-XL5-RARRES3 (SC 117294) was also purchased from Origene (Origene Technologies, Inc, USA).
Immunohistochemistry (IHC)
Hematoxylin and eosin-stained slides and unstained slides for immunohistochemical analysis were prepared from formalin-fixed, paraffin-embedded blocks of CRC tissues. Immunohistochemical stains were performed using heat-induced epitope retrieval, an avidin-biotin complex method. The rabbit anti-RARRES3 antibody (TA 308191, Origene Technologies, Inc, USA) was diluted with 1:100. The sections were evaluated by light microscopic examination, and cellular localization of the protein and immunostaining level in each section were assessed by two pathologists. The staining patterns were scored as follows: negative, weak (less than 30% of cells with positive staining), moderate (less than 60% but more than 30% of cells with positive staining) and strong positive (more than 60% of cells with positive staining) according to the immunostaining intensity.
Western blotting
Seventy-two hours after transfection, HT-29 and SW-620 cells were harvested in RIPA lysis buffer (Bioteke, Beijing, China) and 50 μg of cellular protein were subjected to 8% SDS-PAGE separation. Proteins were transferred to PVDF microporous membrane (Millipore, Boston, MA, USA) and blots were probed with rabbit polyclonal antibodies against RARRES3 (TA 308191, Origene Technologies, Inc, USA), MTDH (#14065), Fibronectin (#9699), Snail (#4719), E-cadherin (#3195), vimentin (#3932), β-catenin (#9582) (Cell Signaling Technology, USA). β-tubulin (sc-9104) and GAPDH (sc-25778, Santa Cruz Biotechnology, CA, USA). β-tubulin was chosen as an internal control and the blots were visualized with Western Breeze Kit (WB7105, Invitrogen Life Technologies, CA, USA).
Co-immunoprecipitation
Cells transfected with a control vector or pCMV-XL5-RARRES3 were used in this study. The samples were pre-absorbed with 25 μl of protein A/G-Sepharose (50%) for 10 min and immunoprecipitation was performed using 4 μg/ml anti-FLAG at 4°C for 1 hr and then incubated with 30 μl of A/G-Sepharose for an additional hour or overnight. After three washes, the pellets were resuspended in 40 μl of SDS sample buffer and boiled for 5 min. The entire supernatant was subjected to Western blotting.
Cell proliferation assay
Methylthiazolyl blue tetrazolium (MTT; Sigma-Aldrich, St Louis, MO) spectrophotometric dye assay was used to observe and compare cell proliferation ability. HT-29 and SW-620 cells were plated in 96-well plates at a density of 5 × 103 cells per well. After transfection experiments, cell proliferation was assessed. Cells were incubated for 4 h in 20 μL MTT at 37°C. The color was developed by incubating the cells in 150 μL dimethyl sulfoxide (DMSO), the absorbance was detected at 490 nm wave length. The data were obtained from three independent experiments.
Cell migration and invasion assays in vitro
Cell migration ability was calculated by the wound healing assay. HT-29 cells were plated in 6-well plate at a concentration of 4 × 105 cells/well and allowed to form a confluent monolayer for 24 h. After the transfection experiment, the monolayer was scratched with a sterile pipette tip (10 μL), washed with serum free medium to remove floated and detached cells and photographed (time 0 h and 48 h) by inversion fluorescence microscope (Olympus, Japan). Cell culture inserts (24-well, pore size 8 μm; BD Biosciences) were seeded with 5 × 103 cells in 100 μL of medium with 0.1% FBS. Inserts pre-coated with Matrigel (40 μL, 1 mg/mL; BD Biosciences) were used for invasion assays. Medium with 10% FBS (400 μL) was added to the lower chamber and served as a chemotactic agent. Noninvasive cells were wiped from the upper side of the membrane and cells on the lower side were fixed in cold methanol (-20°C) and air dried. Cell were stained with 0.1% crystal violet (dissolved in methanol) and counted using the inverted microscope. Each individual experiment had triplicate inserts, and 4 microscopic fields were counted per insert.
Metastasis assay in nude mice
Briefly, 1 × 105 cells (four groups including HT-29-shRNA-scramble, HT-29-shRNA-RARRES3, SW-620-pCMV-XL5-vector, and SW-620-pCMV-XL5-RARRES3) were injected intravenously through the tail vein into 4-5-week-old severe combined immunodeficient nude mice (BALB/c) (five mice per group). After 12 weeks, the number of tumour nodules formed on the liver surfaces was counted. Livers were excised and embedded in paraffin for further study.
Statistics
Data were expressed as mean ± SD and were analyzed by Student’s t test, one-way ANOVA and χ2 test as appropriate using SPSS for Windows version 16.0 (SPSS, Chicago, USA). Kaplan-Meier survival curves were plotted and log rank test was done. The significance of various variables for survival was analyzed by Cox proportional hazards model in a multivariate analysis. P<0.05 in all cases was considered statistically significant.
Results
The expression of RARRES3 was significantly down-regulated in CRC tissues and positively associated with differentiation
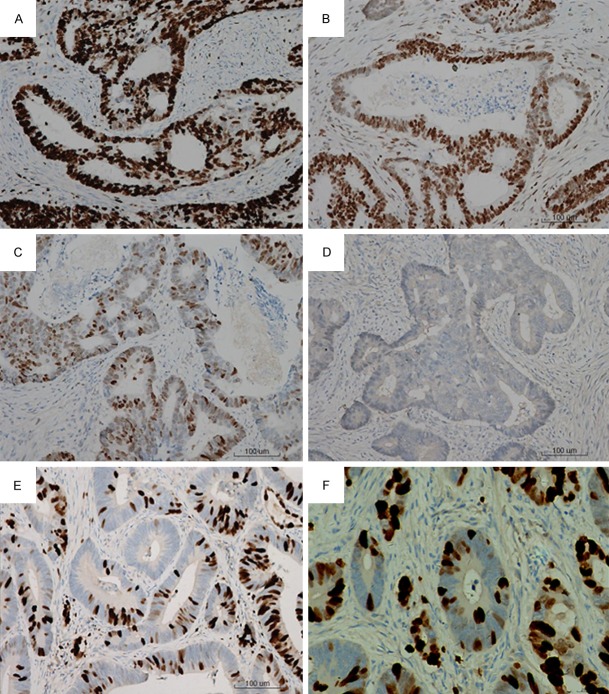
To understand the clinicopathological significance of RARRES3, we analyzed the expression of RARRES3 in a total of 86 CRC tissues and 78 distal normal tissues by immunohistochemistry (IHC). The expression of RARRES3 was heterogeneous in CRC tissues, ranging from weak to moderate to strong positive staining. Compared with CRC tissues, all paired normal control tissues had strong positive staining of RARRES3. RARRES3 was found to localize in the supranuclear regions of tumor and normal mucosal cells (Figure 1). Some random granular patterns of RARRES3 staining were observed in tumors cells. Representative results of RARRES3 expression were shown in Figure 1. Clinicopathological analyses of RARRES3 expression were seen in Table 1. There was significant difference between RARRES3 expression and tumor differentiation (P=0.005), lymph node metastases (P=0.016) as well as CRC and normal tissues among all the clinicopathological parameters available. No significant association was found between RARRES3 expression and other parameters.
Figure 1.
RARRES3 was significantly down-regulated in CRC tissues. Shown were immunohistochemical analysis of the expression of RARRES3 in CRC tissues. A. Strong expression of RARRES3. B. Moderate expression of RARRES3. C. Weak expression of RARRES3. D. Negative expression of RARRES3 in CRC tissues. E and F. Were immunohistochemical analysis of the expression of RARRES3 in paired normal tissues (× 200).
Table 1.
Correlation between RARRES3 expression and clinicopathologic information of CRC
| Parameters | n | RARRES3 expression | x2 | P value | ||
|---|---|---|---|---|---|---|
|
| ||||||
| Low (+, -) | High (++, +++) | |||||
| Adjacent normal tissues | 78 | 11 | 67 | 47.748 | - | |
| CRC | 86 | 47 | 39 | |||
| Gender | Male | 64 | 37 | 27 | 1.009 | 0.315 |
| Female | 22 | 10 | 12 | |||
| Age (years) | >60 | 57 | 30 | 27 | 0.278 | 0.598 |
| ≤60 | 29 | 17 | 12 | |||
| Clinical stage | I | 6 | 3 | 3 | 4.169 | 0.124 |
| II | 43 | 21 | 22 | |||
| III | 32 | 23 | 9 | |||
| T classification | T1-T2 | 17 | 9 | 8 | 0.087 | 0.558 |
| T3-T4 | 65 | 37 | 28 | |||
| N classification | N0 | 50 | 22 | 28 | 5.775 | 0.016 |
| N1-N3 | 34 | 24 | 10 | |||
| Differentiation degree | Good | 47 | 24 | 23 | 10.670 | 0.005 |
| Moderate | 11 | 6 | 5 | |||
| Poor | 18 | 17 | 1 | |||
| Tumor volume (cm3) | <10 | 23 | 17 | 6 | 0.760 | 0.684 |
| 10-20 | 27 | 17 | 10 | |||
| >20 | 34 | 24 | 10 | |||
| Gross classification | Mucinous type | 46 | 29 | 17 | 0.743 | 0.690 |
| Signet-ring type | 6 | 3 | 3 | |||
| Glandular type | 24 | 13 | 11 | |||
| Undifferentiated type | 3 | 1 | 2 | |||
To confirm whether there was association between RARRES3 expression and prognosis, Kaplan-Meier survival curve for different expression status of RARRES3 was performed. Based on 86 cases of patients with CRC, no significant difference in survival was found between patients with weak, moderate or strong RARRES3 staining in tumors (P=0.924) (Figure 2). Similarly, multivariate analysis showed there was no significant survival difference between patients with negative and weak (P=0.929, data not shown) or between patients with negative and strong RARRES3 staining in tumors (P=0.458, data not shown).
Figure 2.
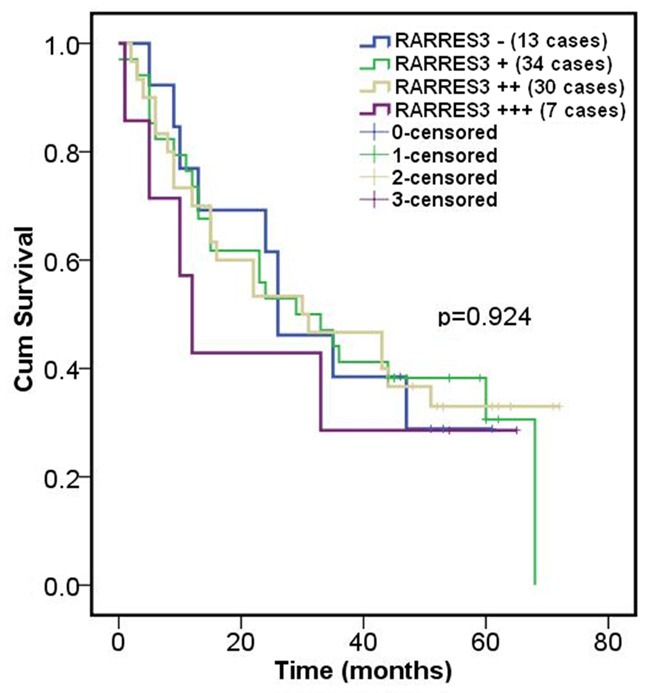
RARRES3 expression wasn’t significantly associated with prognosis in CRC. Immunostaining of RARRES3 and overall survival in CRC was calculated by the Kaplan-Meier survival curves. There wasn’t significant difference between RARRES3 expression and prognosis (P>0.05, using log rank test) after statistical analysis.
RARRES3 could suppress EMT process
To investigate the role of RARRES3 in CRC, we used shRNA as well as over-expression vectors to manipulate RARRES3 expression in culture. We selected 3 candidate interference target sequences (Supplementary Table 1) and therefore constructed 3 shRNA vectors. The knockdown efficiency of the 3 shRNA vectors was determined by western-blot (Figure 3A). It can be seen that among the 3 candidate target sequences, the knock-down efficiency of shRNA-RARRES3 was most significant of all and thus was chosen in the following functional analysis.
Figure 3.
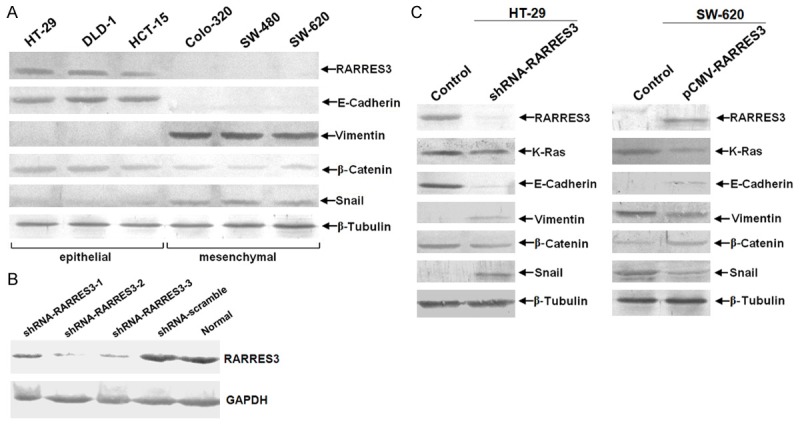
RARRES3 could suppress EMT process while involving the up-regulation of β-catenin. A. Knock-down efficiency of candidate shRNA target sequences was determed by western-blotting. Both the 3 different shRNA vectors as well as shRNA control (scramble) vetor were transfected in CRC cell line HT-29. shRNA-RARRES3-2 was chosen as the best which was abbreviated for shRNA-RARRES3 in the following funcational exmperiment. B. The endogenous expression of RARRES3 in a panel of CRC cell lines was detected using immunoblotting. β-Tubulin was loading control. 80μg total protein was loaded per lane, which was separated by 10% SDS-PAGE followed by visualization with Western Breeze kit (Invitrogen, USA). C. Immunoblotting assay for HT-29 and SW-620 after knock-down and over-expression of RARRES3 respectively using retroviral shRNA and over-expression vector transfection for 96 hours.
To determine the basal expression of RARRES3 in CRC cell lines, western-blot was performed on a panel of CRC cell lines including both epithelial and mesenchymal phenotypes. We found that RARRES3 expression was positively associated with E-cadherin expression but negatively correlated with Snail and vimentin, typical markers of mesenchymal. Meanwhile, we also found that expression of β-Catenin was also reduced in parallel to RARRES3 (Figure 3B). As a further confirmation, we employed the gain- and loss-of-function strategies. We knocked-down RARRES3 in HT-29 cells which had a highest basal level of RARRES3 and over-expressed RARRES3 in SW-62 cells that were negative for RARRES3 (Figure 3C). Thus, based on the results from CRC cell lines, we concluded that RARRES3 was able to inhibit the EMT process in CRC.
RARRES3 could suppress both invasion and migration but not proliferation in CRC cell lines in vitro
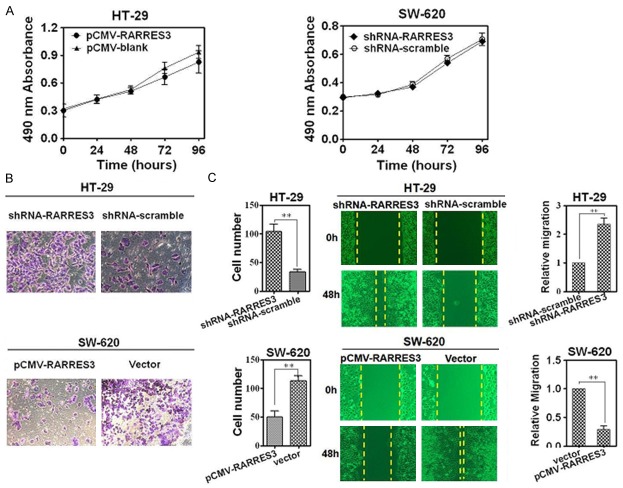
To understand the role of RARRES3 in the metastasis of CRC, we over-expressed and knocked-down RARRES3 in CRC cell lines HT-29 and SW-620, respectively, using transfection. We found that loss of RARRES3 protein failed to inhibit proliferation (Figure 4A) but significantly promoted invasive (Figure 4B) and migratory (Figure 4C) abilities of HT-29 cell line. On the contrary, over-expression of RARRES3 in SW-620 CRC cell line, which expressed little endogenous RARRES3, significantly compromised both the invasion (Figure 4B) and migration (Figure 4C), despite leaving the proliferation unaffected (Figure 4A).
Figure 4.
RARRES3 could suppress both the invasion and migration ability but not proliferation in CRC cell lines. A. MTT assay for HT-29 and SW-620 after knock-down of RARRES3 using over-expression vector and retroviral shRNA vector transfection for 0 h, 24 h, 48 h, 72 h and 96 h; B. Transwell assays for HT-29 and SW-620 after knock-down and over-expression of RARRES3 using retroviral shRNA vector and over-expression vector transfection for 48 hours. The left is qualification assay; the right is quantitation assay of Transwell; C. Wound-healing assay for HT-29 and SW-620 after knock-down and over-expression of RARRES3 using retroviral shRNA vector and over-expression vector transfection for 96 hours. (**P<0.01, *P<0.05 compared with control group using independent Student’s t test or one-way ANOVA analysis). Images of migratory/Invasion cells from the scratched boundary/transwell chamber were observed and acquired with light microscope (10 × 10). Similar results were obtained in three independent experiments, and shown were representative figures.
RARRES3 interacted with MTDH in an opposite way
Based on the finding that knock-down of RARRES3 up-regulated β-catenin expression so as to regulate EMT process and given that MTDH, another pivotal metastasis-associated factor known to regulate EMT process through β-catenin pathway, we tried to detect whether or not there was any change of MTDH after manipulations of RARRES3. We found that MTDH expression was increased in response to RARRES3 suppression (Figure 5A). To confirm the change of β-catenin expression, we detected the distribution of β-catenin in cytoplasm and nucleus fractions. We found that β-catenin expression was increased in nuclear compartment and decreased in cytoplasm compartment after knock-down of RARRES3, suggesting that β-catenin might translocate from cytoplasm to nuclear after ectopic expression of RARRES3 (Figure 5B). To investigate whether or not there was any physical interaction between RARRES3 and MTDH, we performed IP experiment. The result showed that RARRES3 and MTDH physically interacted with each other in an opposite way (Figure 5C).
Figure 5.
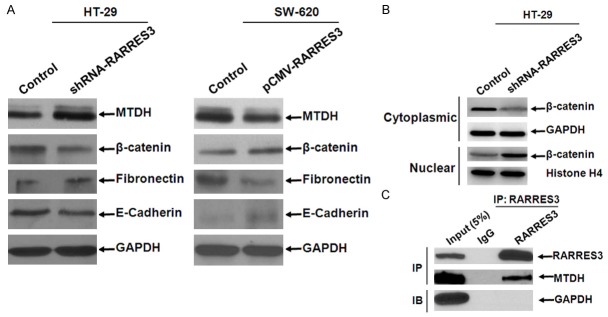
RARRES3 interacted with MTDH in an opposite way. A. Expression variation of MTDH, β-catenin, Fibronectin, E-cadherin after ectopic expression of RARRES3 in HT-29 and SW-620 where basal expression of RARRES3 was highest and lowest respectively; B. Expression of β-catenin both in cytoplasmic and nuclear compartment was detected using western-blotting; GAPDH and Histone H4 were as loading control. 100 μg total protein was loaded per lane, which was separated by 10% SDS-PAGE followed by visualization with Western Breeze kit (Invitrogen, USA). C. Immunoprecipitation analysis of RARRES3 and MTDH. 293T cells transfected with the eukaryotic expression vector of RARRES3 that contains Flag tag on N-terminus were harvested for immunoprecipitation (IP) using anti-FLAG M2-agarose beads. FLAG precipitates were subjected for immunoblot using anti-RARRES3, anti-MTDH, or anti-GAPDH antibody.
RARRES3 could suppress metastasis in vivo
To further investigate the suppressive effect of RARRES3 on tumor metastasis, we conducted an experimental metastasis in nude mice by injecting CRC cells with manipulations of RAARES3 by shRNA or overexpression vectors through tail vein, with five mice per group. shRNA-scramble and pCMV-XL5-blank vectors were used as controls. Mice were euthanized and the metastatic nodules were counted on the surface of liver after 12 weeks. The number of visible nodules formed in the liver induced by HT-29-shRNA-RARRES3 were significantly increased when compared to those from the mice injected with HT-29-shRNA-scramble (Figure 6A); meanwhile, the number of visible nodules formed in the liver induced by SW-620-pCMV-XL5-RARRES3 were significantly less than those induced by SW-620-pCMV-XL5-vector (Figure 6B). These results suggested that RARRES3 could effectively suppress tumor metastasis in vivo.
Figure 6.
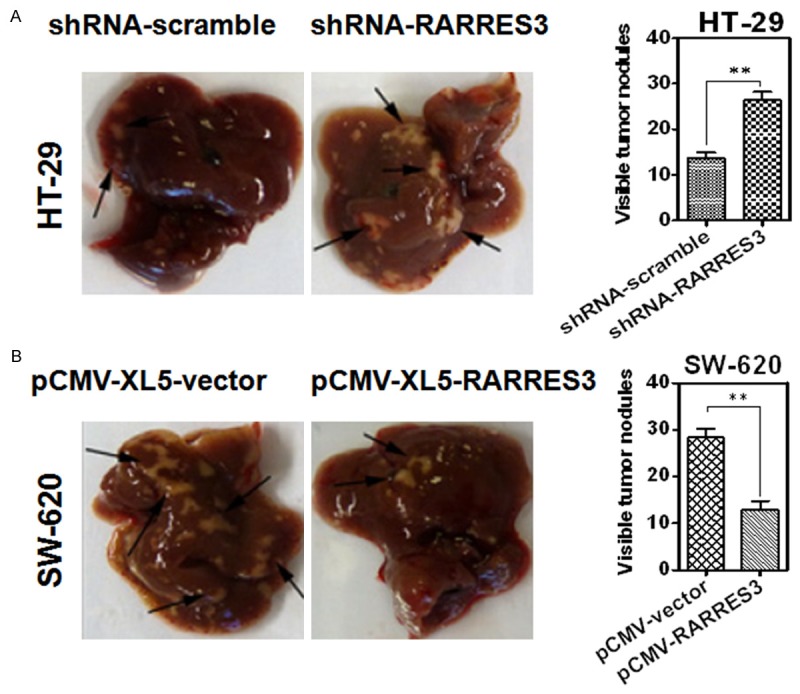
RARRES3 could suppress colorectal cancer (CRC) metastasis in vivo. RARRES3 could suppress colorectal cancer (CRC) metastasis in vivo. Representative livers derived from severe combined immunodeficient (BALB/c) mice in HT-29 (A) and SW-620 (B) were shown. The formation of metastatic nodules at the liver surface could be significantly promoted by HT-29-shRNA-RARRES3 and significantly suppressed by SW-620-pCMV-RARRES3.
Discussion
Despite RARRES3 has been firstly reported and found to be significantly associated with tumor differentiation but not prognosis in CRC by the same group [11,12], little evidence was available in regard to the role of RARRES3 in the suppressinon of CRC metastasis. given the role of RARRES3 as a metastasis suppressor identified in breast cancer [9,10]. In the present study, we confirmed that RARRES3 was significantly reduced in CRC tissues in comparison with normal tissue controls using IHC, and that there was significant difference between RARRES3 expression and CRC differentiation. In addition, we showed that RARRES3 was able to suppress the EMT process via interaction with MTDH in an opposite way. To our knowledge, this is the first report that identified RARRES3 and MTDH were interacted in an opposite way in the suppression of CRC metastasis.
The present study revealed a new aspect of the molecular mechanism by which RARRES3 suppressed metastasis involving the interaction between RARRES3 and MTDH in an opposite way so as to convert the CRC cells from the mesenchymal to the epithelial state. As a matter of fact, there were limited studies available regarding the mechanism of RARRES3 in malignant tumors, if any, those were mainly focused on breast cancer [9,10,22,23] and keratinocyte of the epidermis [13,24] as well as other epithelial cancers [25] including cervical cancer [26], head and neck squamous cell carcinoma [27] and ovarian cancer [28]. Based on the reports mentioned above, the roles of RARRES3 include the regulation of cell differentiation and inhibition of malignant transformation [13,27]. Some studies found that the N-termial hydrophilic region of RARRES3 is required for RARRES3 to exert its tumor suppressive function [24,26,29,30]. Others reported that expression of RARRES3 reduced proliferation, promoted cellular apoptosis through redistribution of microfilament [25], and Golgi apparatus [31]. As for the variation of signal pathway, Huang et al observed that RARRES3 negatively regulated extracellular signal-regulated kinase (EKR), c-Jun N-terminal kinase (JNK) and p38 mitogen-activated kinase both in cervical and gastric cancer cell lines [32], which was partly consistent with and supported by Tsai et al [31] that RARRES3 suppressed Ras activation. On the other hand, Ou et al reported that down-regulation of HER2 by RARRES3 was mediated by the PI3K/AKT pathway in ovarian cancer cells [28]. Wu et al discovered that RARRES3-prostaglandin D2 (PGD2) signaling pathway might play an important role in cancer suppression in testis [33]. In hepatocellular carcinoma cells, RARRES3 was found to be directly regulated by p53 [14]. In our present study, we found that expression of RARRES3 negatively regulated K-Ras expression in CRC cell lines, which was consistent with published study [31]. In addition, RARRES3 can also increase the expression of E-cadherin and β-catenin, the former of which was a typical epithelial biomarker, whereas decreased the expression of vimentin, N-cadherin and Snail, typical mesenchymal biomarkers. The results obtained demonstrated that RARRES3 could suppress the EMT process involving the up-regulation of β-catenin signaling pathway. Because that MTDH could promote metastasis through induction of EMT [16,34] and nuclear translocation of β-catenin [15,16], and that RARRES3 can suppress the MTDH expression, there is a remarkable meaning that RARRES3 suppresses the EMT process through modulating nuclear translocation of β-catenin.
With respect to the subcellular localization of RARRES3, there has been inconsistent reports depending on the different cancer cell lines examed or different methodologies employed. Tsai et al reported that using confocal microscopy RARRES3 was found to localize in the endoplasmic reticulum (ER) and Golgi apparatus in HtTA cervical cancer cells [31], whereas Scharadin et al [25] observed in epidermal squamous cell carcinoma SCC-13 cells that RARRES3 localized near the centrosome without mentioning what approach was taken. Shyu RY and colleagues found that RARRES3 was localized at the supranuclear region in CRC tissues using IHC on the premise that the antibody against RARRES3 was higher specific [12], which supports our findings at the tissue level.
Taken together, our results demonstrated that RARRES3 was significantly reduced in CRC tissues compared to normal controls, and that RARRES3 was significantly associated with cell differentiation. Mechanistically, we found, for the first time, that RARRES3 interacted with MTDH in an opposite way to suppress metastasis of CRC through the regulation of EMT process.
Acknowledgements
The work was supported by the Department of Gastroenterology and Department of Nuclear Medicine, Ruijin Hospital, School of Medicine, and Shanghai Jiaotong University. We thank Mr. Jiangwei Liu for his excellent technical assistance, and Dr Jiayi Gu for his critical review of this manuscript.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Haraldsdottir S, Einarsdottir HM, Smaradottir A, Gunnlaugsson A, Halfdanarson TR. [Colorectal cancer - review] . Laeknabladid. 2014;100:75–82. doi: 10.17992/lbl.2014.02.531. [DOI] [PubMed] [Google Scholar]
- 2.Chen WQ, Zheng RS, Zhang SW, Zeng HM, Zou XN. The incidences and mortalities of major cancers in China, 2010. Chin J Cancer. 2014;33:402–405. doi: 10.5732/cjc.014.10084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao L, Ding YQ. [Progression in metastasis of colorectal carcinoma] . Zhejiang Da Xue Xue Bao Yi Xue Ban. 2014;43:486–493. doi: 10.3785/j.issn.1008-9292.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 4.Jegatheeswaran S, Satyadas T, Sheen AJ, Treasure T, Siriwardena AK. Thoracic surgical management of colorectal lung metastases: a questionnaire survey of members of the Society for Cardiothoracic Surgery in Great Britain and Ireland. Ann R Coll Surg Engl. 2013;95:140–143. doi: 10.1308/003588413X13511609956336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berry MF. Role of segmentectomy for pulmonary metastases. Ann Cardiothorac Surg. 2014;3:176–182. doi: 10.3978/j.issn.2225-319X.2014.02.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Urosevic J, Garcia-Albeniz X, Planet E, Real S, Cespedes MV, Guiu M, Fernandez E, Bellmunt A, Gawrzak S, Pavlovic M, Mangues R, Dolado I, Barriga FM, Nadal C, Kemeny N, Batlle E, Nebreda AR, Gomis RR. Colon cancer cells colonize the lung from established liver metastases through p38 MAPK signalling and PTHLH. Nat Cell Biol. 2014;16:685–694. doi: 10.1038/ncb2977. [DOI] [PubMed] [Google Scholar]
- 7.DiSepio D, Ghosn C, Eckert RL, Deucher A, Robinson N, Duvic M, Chandraratna RA, Nagpal S. Identification and characterization of a retinoid-induced class II tumor suppressor/growth regulatory gene. Proc Natl Acad Sci U S A. 1998;95:14811–14815. doi: 10.1073/pnas.95.25.14811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang SL, Shyu RY, Yeh MY, Jiang SY. Cloning and characterization of a novel retinoid-inducible gene 1(RIG1) deriving from human gastric cancer cells. Mol Cell Endocrinol. 2000;159:15–24. doi: 10.1016/s0303-7207(99)00207-5. [DOI] [PubMed] [Google Scholar]
- 9.Morales M, Arenas EJ, Urosevic J, Guiu M, Fernandez E, Planet E, Fenwick RB, Fernandez-Ruiz S, Salvatella X, Reverter D, Carracedo A, Massague J, Gomis RR. RARRES3 suppresses breast cancer lung metastasis by regulating adhesion and differentiation. EMBO Mol Med. 2014;6:865–881. doi: 10.15252/emmm.201303675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Errico A. Breast cancer: RARRES3-suppressing metastases to the lung in breast cancer. Nat Rev Clin Oncol. 2014;11:378. doi: 10.1038/nrclinonc.2014.103. [DOI] [PubMed] [Google Scholar]
- 11.Wu CC, Shyu RY, Chou JM, Jao SW, Chao PC, Kang JC, Wu ST, Huang SL, Jiang SY. RARRES1 expression is significantly related to tumour differentiation and staging in colorectal adenocarcinoma. Eur J Cancer. 2006;42:557–565. doi: 10.1016/j.ejca.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 12.Shyu RY, Jiang SY, Chou JM, Shih YL, Lee MS, Yu JC, Chao PC, Hsu YJ, Jao SW. RARRES3 expression positively correlated to tumour differentiation in tissues of colorectal adenocarcinoma. Br J Cancer. 2003;89:146–151. doi: 10.1038/sj.bjc.6601049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scharadin TM, Eckert RL. TIG3: an important regulator of keratinocyte proliferation and survival. J Invest Dermatol. 2014;134:1811–1816. doi: 10.1038/jid.2014.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu TH, Chu CC, Jiang SY, Hung MW, Ni WC, Lin HE, Chang TC. Expression of the class II tumor suppressor gene RIG1 is directly regulated by p53 tumor suppressor in cancer cell lines. FEBS Lett. 2012;586:1287–1293. doi: 10.1016/j.febslet.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 15.Li X, Kong X, Huo Q, Guo H, Yan S, Yuan C, Moran MS, Shao C, Yang Q. Metadherin enhances the invasiveness of breast cancer cells by inducing epithelial to mesenchymal transition. Cancer Sci. 2011;102:1151–1157. doi: 10.1111/j.1349-7006.2011.01919.x. [DOI] [PubMed] [Google Scholar]
- 16.Zhu K, Dai Z, Pan Q, Wang Z, Yang GH, Yu L, Ding ZB, Shi GM, Ke AW, Yang XR, Tao ZH, Zhao YM, Qin Y, Zeng HY, Tang ZY, Fan J, Zhou J. Metadherin promotes hepatocellular carcinoma metastasis through induction of epithelialmesenchymal transition. Clin Cancer Res. 2011;17:7294–7302. doi: 10.1158/1078-0432.CCR-11-1327. [DOI] [PubMed] [Google Scholar]
- 17.Blanco MA, Aleckovic M, Hua Y, Li T, Wei Y, Xu Z, Cristea IM, Kang Y. Identification of staphylococcal nuclease domain-containing 1 (SND1) as a Metadherin-interacting protein with metastasis-promoting functions. J Biol Chem. 2011;286:19982–19992. doi: 10.1074/jbc.M111.240077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo F, Wan L, Zheng A, Stanevich V, Wei Y, Satyshur KA, Shen M, Lee W, Kang Y, Xing Y. Structural insights into the tumor-promoting function of the MTDH-SND1 complex. Cell Rep. 2014;8:1704–1713. doi: 10.1016/j.celrep.2014.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee SG, Su ZZ, Emdad L, Sarkar D, Franke TF, Fisher PB. Astrocyte elevated gene-1 activates cell survival pathways through PI3K-Akt signaling. Oncogene. 2008;27:1114–1121. doi: 10.1038/sj.onc.1210713. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J, Zhang Y, Liu S, Zhang Q, Wang Y, Tong L, Chen X, Ji Y, Shang Q, Xu B, Chu M, Wei L. Metadherin confers chemoresistance of cervical cancer cells by inducing autophagy and activating ERK/NF-kappaB pathway. Tumour Biol. 2013;34:2433–2440. doi: 10.1007/s13277-013-0794-z. [DOI] [PubMed] [Google Scholar]
- 21.Sarkar D, Park ES, Emdad L, Lee SG, Su ZZ, Fisher PB. Molecular basis of nuclear factorkappaB activation by astrocyte elevated gene-1. Cancer Res. 2008;68:1478–1484. doi: 10.1158/0008-5472.CAN-07-6164. [DOI] [PubMed] [Google Scholar]
- 22.Paroni G, Fratelli M, Gardini G, Bassano C, Flora M, Zanetti A, Guarnaccia V, Ubezio P, Centritto F, Terao M, Garattini E. Synergistic antitumor activity of lapatinib and retinoids on a novel subtype of breast cancer with coamplification of ERBB2 and RARA. Oncogene. 2012;31:3431–3443. doi: 10.1038/onc.2011.506. [DOI] [PubMed] [Google Scholar]
- 23.Shyu RY, Chang SC, Yu JC, Hsu SJ, Chou JM, Lee MS, Jiang SY. Expression and regulation of retinoid-inducible gene 1 (RIG1) in breast cancer. Anticancer Res. 2005;25:2453–2460. [PubMed] [Google Scholar]
- 24.Scharadin TM, Adhikary G, Shaw K, Grun DJ, Xu W, Eckert RL. Pericentrosomal localization of the TIG3 tumor suppressor requires an N-terminal hydrophilic region motif. J Invest Dermatol. 2014;134:1220–1229. doi: 10.1038/jid.2013.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scharadin TM, Jiang H, Jans R, Rorke EA, Eckert RL. TIG3 tumor suppressor-dependent organelle redistribution and apoptosis in skin cancer cells. PLoS One. 2011;6:e23230. doi: 10.1371/journal.pone.0023230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsai FM, Shyu RY, Lin SC, Wu CC, Jiang SY. Induction of apoptosis by the retinoid inducible growth regulator RIG1 depends on the NC motif in HtTA cervical cancer cells. BMC Cell Biol. 2009;10:15. doi: 10.1186/1471-2121-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higuchi E, Chandraratna RA, Hong WK, Lotan R. Induction of TIG3, a putative class II tumor suppressor gene, by retinoic acid in head and neck and lung carcinoma cells and its association with suppression of the transformed phenotype. Oncogene. 2003;22:4627–4635. doi: 10.1038/sj.onc.1206235. [DOI] [PubMed] [Google Scholar]
- 28.Ou CC, Hsu SC, Hsieh YH, Tsou WL, Chuang TC, Liu JY, Kao MC. Downregulation of HER2 by RIG1 involves the PI3K/Akt pathway in ovarian cancer cells. Carcinogenesis. 2008;29:299–306. doi: 10.1093/carcin/bgm263. [DOI] [PubMed] [Google Scholar]
- 29.Zhang LY, Liu LY, Qie LL, Ling KN, Xu LH, Wang F, Fang SH, Lu YB, Hu H, Wei EQ, Zhang WP. Anti-proliferation effect of APO866 on C6 glioblastoma cells by inhibiting nicotinamide phosphoribosyltransferase. Eur J Pharmacol. 2012;674:163–170. doi: 10.1016/j.ejphar.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 30.Han BG, Cho JW, Cho YD, Kim SY, Yoon HJ, Song HK, Cheong HK, Jeon YH, Lee DK, Lee S, Lee BI. Expression, purification and biochemical characterization of the N-terminal regions of human TIG3 and HRASLS3 proteins. Protein Expr Purif. 2010;71:103–107. doi: 10.1016/j.pep.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 31.Tsai FM, Shyu RY, Jiang SY. RIG1 suppresses Ras activation and induces cellular apoptosis at the Golgi apparatus. Cell Signal. 2007;19:989–999. doi: 10.1016/j.cellsig.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 32.Huang SL, Shyu RY, Yeh MY, Jiang SY. The retinoid-inducible gene I: effect on apoptosis and mitogen-activated kinase signal pathways. Anticancer Res. 2002;22:799–804. [PubMed] [Google Scholar]
- 33.Wu CC, Shyu RY, Wang CH, Tsai TC, Wang LK, Chen ML, Jiang SY, Tsai FM. Involvement of the prostaglandin D2 signal pathway in retinoid-inducible gene 1 (RIG1)-mediated suppression of cell invasion in testis cancer cells. Biochim Biophys Acta. 2012;1823:2227–2236. doi: 10.1016/j.bbamcr.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 34.Tang J, Shen L, Yang Q, Zhang C. Overexpression of metadherin mediates metastasis of osteosarcoma by regulating epithelial-mesenchymal transition. Cell Prolif. 2014;47:427–434. doi: 10.1111/cpr.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.