Abstract
Twist1 is a highly conserved basic helix-loophelix transcription factor, and has been shown to play an important role in carcinogenesis of many tumors including colorectal cancer (CRC). Here we aimed to investigate the role of Twist1 in the clinical significance and chemoresistance in CRC. In this study, we examined the correlation between Twist1 expression and clinicopathological characteristics using immunohistochemistry in patients with CRC. The molecular mechanisms of Twist1 expression and its effects on chemosensitivity to 5-Fluorouracil and oxaliplatin were also explored by MTT assay, colony forming assay, flow cytometry assay. The results indicate that Twist1 is overexpressed in cancer tissue, and its positive expression are related to histological grade (P=0.004), T-stage (P=0.033), N-stage (P=0.000), M-stage (P=0.040), TNM stage (P=0.002) and recurrence (P=0.023). Moreover, positive Twist1 expression is correlated with poor overall survival in CRC patients (P<0.0001), and is a significant independent prognostic indicator. In addition, we show that knockdown of Twist1 inhibits proliferation, and increased the percentage of apoptotic cells of CRC cell lines. Our findings suggest that Twist1 promotes proliferation and chemoresistance of CRC cells. Twist1 may be a potential prognostic marker and a molecular target for therapies.
Keywords: Twist1, colorectal cancer, prognosis, epithelial-mesenchymal transition, chemoresistance
Introduction
Colorectal cancer (CRC) is the third most common human malignancies worldwide. Despite recent advances in treatment with chemotherapy, CRC remains the third leading cause of cancer death, and patients may have a poor prognosis [1]. Thus, there is a crucial need to explore novel cancer-related genes that may serve as diagnostic markers and molecular targets in CRC therapy.
The introduction of platinum derivative drugs, like oxaliplatin (OXA), shows good therapeutic results by increasing survival rates [2,3]. The molecular mechanism of OXA in tumor cells is not yet clear but the drug shows powerful cytotoxic properties and its function is based on the ability to covalently bind DNA, forming platinum-DNA adducts which lead to apoptosis [4]. 5-Fluorouracil (5-FU) exerts its anticancer effects by repressing the thymidylate synthase and incorporating its metabolites into RNA and DNA, and has been extensively used in the treatment of solid tumors [5].
Recently, development of multidrug resistance (MDR) is an important problem in chemotherapeutic treatment of metastatic cancers. A number of researches also displayed that epithelial-mesenchymal transition (EMT) is involved in drug resistance in cancer cells [6,7]. Although cancer cells undergoing EMT develop resistance to anticancer agents, EMT can also be induced by anticancer agents. In breast and ovarian cancer, cancer cells resistant to cisplatin and paclitaxel undergo EMT with increased expression of Twist1.
Although many studies have been undertaken to disclose the mechanisms of chemoresistance, the mechanisms remain really unclear. In this study, the aim was to examine the prognostic effect and the biological significance of Twist1 in CRC. Here, we found that Twist1 was an independent prognostic factor, and expression of Twist1 promoted the potential of cancer cells against the chemoresistance. Our data demonstrated that the Twist1 may play an important role in the development of chemoresistance.
Material and methods
Patients and samples
This study was conducted using the formalin-fixed, paraffin-embedded tissues obtained from 95 patients with primary CRC who underwent curative surgery at the First People’s Hospital of Shunde Affiliated to Southern Medical University (Foshan, China) from January 2005 to December 2005. Cancer tissues were cut in wedge shapes and normal tissues were cut at least 10 cm away from tumor margin. Inclusion criteria were primary resection, no preoperative treatment with chemotherapy or radiotherapy, complete medical records, and the availability of all histopathologic slides of the resected specimens, because of the difficulty of finding a suitable tumor for determining gene expression in these patients’ surgical samples. The diagnosis and histological differentiation were evaluated according to the World Health Organization classification, and the tumor staging was based on the American Joint Committee on Cancer TNM staging system. The research protocol was approved by the Ethics Committee of the First People’s Hospital of Shunde and consents were acquired from all patients for the study. The clinicopathological features of patients are summarized in Table 1.
Table 1.
Correlations between Twist expression and clinicopathological characteristics in colorectal cancer patients
| Characteristics | Total (n=95) | Twist expression | P-value | |
|---|---|---|---|---|
|
| ||||
| n (%) | Negative | Positive | ||
| Sex | 0.099* | |||
| Male | 58 (61.1) | 17 (29.3) | 41 (70.7) | |
| Female | 37 (38.9) | 17 (45.9) | 20 (54.1) | |
| Age (y) | 0.515* | |||
| ≤60 | 49 (51.6) | 14 (28.6) | 35 (71.4) | |
| >60 | 46 (48.4) | 16 (34.8) | 30 (65.2) | |
| Histological type | 0.076* | |||
| Tubular | 65 (68.4) | 19 (29.2) | 46 (70.8) | |
| Mucinous | 15 (15.8) | 9 (60.0) | 6 (40.0) | |
| Papillary | 15 (15.8) | 6 (40.0) | 9 (60.0) | |
| Histological grade | 0.004* | |||
| G1 | 17 (17.9) | 12 (70.6) | 5 (29.4) | |
| G2 | 64 (67.4) | 18 (28.1) | 46 (71.9) | |
| G3 | 14 (14.7) | 4 (28.6) | 10 (71.4) | |
| T-stage | 0.033* | |||
| T1-2 | 15 (15.8) | 9 (60.0) | 6 (40.0) | |
| T3-4 | 80 (84.2) | 25 (31.3) | 55 (68.8) | |
| N-stage | 0.000* | |||
| N0 | 51 (53.7) | 28 (54.9) | 23 (45.1) | |
| N1-2 | 44 (46.3) | 6 (13.6) | 38 (86.4) | |
| M-stage | 0.040* | |||
| M0 | 83 (87.4) | 40 (48.2) | 43 (51.8) | |
| M1 | 12 (12.6) | 2 (16.7) | 10 (83.3) | |
| Tumor differentiation | 1.000** | |||
| Moderate/Well | 86 (90.5) | 31 (36.0) | 55 (64.0) | |
| Poor/other | 9 (9.5) | 3 (33.3) | 6 (66.7) | |
| Colorectal cancer type | 0.940* | |||
| Colon cancer | 61 (64.2) | 22 (36.1) | 39 (63.9) | |
| Rectal cancer | 34 (35.8) | 12 (35.3) | 22 (64.7) | |
| TNM stage | 0.002* | |||
| I-II | 41 (43.2) | 22(53.7) | 19 (46.3) | |
| III-IV | 54 (56.8) | 12 (22.2) | 42 (77.8) | |
| Recurrence | 0.023* | |||
| Yes | 17 (17.9) | 2 (11.8) | 15 (88.2) | |
| No | 78 (82.1) | 32 (41.0) | 46 (59.0) | |
Pearson Chi-square test.
Continuity Correction test.
Immunohistochemical analysis
Immunohistochemical staining of sections for Twist1 protein expression was performed in CRC tissues. Briefly, the sections were deparaffinized in xylene and rehydrated with a series of graded ethanol solutions. Antigen retrieval was carried out by heating in a microwave oven for 10 min in 0.05% Tween-20 in 0.1 M sodium citrate buffer, pH 6.0. The sections were incubated with 0.3% hydrogen peroxide in phosphate buffer saline (PBS) to block the endogenous peroxidase activity. After blocking non-specific binding with 10% skim milk, the sections were then incubated with rabbit anti-Twist1 (H-81; Santa Cruz Biotechnology, Santa Cruz, CA) at 4°C overnight. After washing, sections were incubated with peroxidase-conjugated Envision secondary antibodies (Dako, Glostrup, Denmark). The peroxidase activity was visualized with a DAB substrate kit (Vector Laboratories, Burlingame, CA, USA). All tissues were examined by at least two experienced pathologists and checked for the presence of tumor cells. For evaluation of Twist expression, staining intensity was scored as 0 (negative), 1 (weak), 2 (medium), and 3 (strong). Extent of staining was scored as 0 (0%), 1 (1%-25%), 2 (26%-50%), 3 (51%-75%), and 4 (76%-100%) according to the percentage of the positive staining areas in relation to the whole cancer areas. The sum of the intensity and extent score was used as the final staining score (0-7) for Twist [14]. Negative Twist1 expression was defined as a final score of <3 and ≥3 as positive Twist1 expression.
Patients’ treatment and follow-up
After surgery, adjuvant treatment based on OXA and 5-FU was administered to patients with stage-III/IV tumors without medical contra-indications who gave their written informed consent.
Patients were followed up until death or until June 1, 2014. All patients were monitored by physical examination, routine blood tests [including serum carcinoembryonic antigen (CEA) concentration], chest X-ray and abdominal ultrasonography every 2 mo in the first year after surgery, and every 3-6 mo thereafter. A computed tomography scan (CT) or magnetic resonance imaging was performed every 6 mo or immediately when a recurrence/metastasis was suspected. If needed, a whole-body fludeoxyglucose positron emission tomography/CT was performed. The follow-up data were recorded during the postoperative examination in our hospital, while patients who were examined in another hospital were followed up by telephone or letter. Recurrence was determined by at least two imaging examination results. The overall survival (OS) was the interval between the surgery and death or the last follow-up examination.
Cell culture and culture conditions
Human CRC cancer cell lines, SW480, HCT116 and LoVo, were obtained from American Type Culture Collection (Manassas, VA, USA) and routinely maintained in our lab. These cell lines were cultured in RPMI 1640 (Gibco, CA, USA) with 100 IU/ml penicillin, 100 mg/ml streptomycin and 10% fetal bovine serum in a humidified 5% CO2 atmosphere at 37°C.
Immunofluorescent analysis
Cells were grown on glass chamber slides fixed with 4% paraformaldehyde in PBS for 30 min. Then cells were permeabilized in 0.1% Triton X-100 for 30 min and blocked with 0.5% bovine serum albumin in PBS for 30 min at room temperature. After washing with PBS, the cells were incubated at 4°C overnight with specific primary antibodies for Twist1. After being washed with PBS, the cells were incubated with appropriate fluorescein isothiocyanate-conjugated secondary antibodies for 1 h and then stained with 4’, 6-diamidino-2-phenylindole (DAPI). The images were visualized with an Olympus microscope.
Chemotherapeutic agents in vitro
5-Fluorouracil (Sigma-Aldrich, MO, USA) was reconstructed in normal saline. Oxaliplatin (Sigma-Aldrich, MO, USA) was reconstructed in glucose saline. For the MTT test, we treated cells with different concentrations of OXA and 5-FU, ranging between 0-200 µg/ml, and determined the IC50. For the apoptosis and colony forming assay evaluations, we used a single dose of IC50 for OXA and IC50 for 5-FU in tandem. Evaluations were made 48 hours after treatment for cell proliferation. Each drug was dissolved at a concentration 100 times of its IC50 and stored at 4°C under the protection from light and was diluted at appropriate IC50 in culture medium immediately before use.
Twist1 SiRNA synthesis and cell transfection
This study included two human siRNAs (GenePharma, Shanghai, China) designed against Twist1. One negative random siRNA (GenePharma, Shanghai, China) exhibiting no significant sequence similarity to human gene sequence, served as a negative control. The sequences for Twist1 siRNAs and control siRNA were: siRNA: Twist1-Homo-864 (sense: 5’-GAUGGCAAGCUGCAGCUAUTT-3’; antisense: 5’-AUAGCUGCAGCUUGCCAUCTT-3’); and the negative control siRNA (sense: 5’-UUCUCCGAACGUGUCACGUTT-3’; anti-sense: 5-ACGUGACACGUUCGGAGAATT-3’). The cells were split into 3 groups, including the control group supplemented with only the transfection reagent, the negative control group added with a nontargeting control siRNA and the transfection reagent, and the last groups supplemented with different Twist1 siRNA and the transfection reagents. For cell transfection, cells were plated on 96-well (5×103 cells) and 6-well plates (5×105 cells) in RPMI 1640 with 10% FBS and were allowed to attach for 24 h, and then treated with 20 pmol siRNA per well. Equimolar amounts of siRNAs were incubated with Lipofectamine 3000 Transfection Reagent (Invitrogen, Madison, WI, United States) according to the manufacturer’s instructions. Transfected cells were grown at 37°C for 6 h, followed by incubation with complete medium. Cells were maintained for 48 h before experiments, unless otherwise described.
Twist1 overexpression transfection
For transfection, cells were seeded in culture plates, grown to 50-80% confluency and transfected with plasmids (Genechem, Shanghai, CHN) using Lipofectamine 3000 according to the manufacturer’s protocol. In DNA plasmid transfection, 2.5 µg DNA was used per well for 6-well culture plate. Following transfection, cells were incubated for another 48-72 h before being harvested for the gene expression testing or functional assays.
Real-time PCR analysis
The relative expression level of Twist1 was determined by real-time PCR as our previously described [8]. The Twist1 expression level was normalized with GAPDH. The primers for Twist1 were as follows: F: 5’-TTTCGGATGGGGTTGTTATC-3’; R: 5’-AAACGACCTAACCCGAACG-3’.
Western blotting analysis
Protein suspension from the whole-cell lysate (30 µg) was loaded onto a sodium dodecylsulfate-polyacrylamide gel (SDS-PAGE) for electrophoresis and then transferred to a PVDF membrane (Millipore, Biller ica, MA, USA). The membranes were first blocked with 5% (w/v) non-fat dry milk in TBST at room temperature for 1 h. The membranes were incubated overnight with the following primary antibodies at 4°C: Twist1 (1:1000), GAPDH (1:1000; Santa Cruz, CA, USA). After washing with TBST thrice, the membrane was incubated with secondary antibodies against rabbit IgG (1:1000) at room temperature for 2 h. Following three TBST washes, the membranes were developed using ECL Plus (Millipore, MA, USA) and the signals were visualized by ECL Western Blotting Detection System (KO). GAPDH served as an internal loading control. Results represent three independent experiments.
3-(4, 5-dimethyl thiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) assay
5,000 cells of each line were plated in 6 duplicate wells in 96-well plates and allowed to adhere to the plate for 24 h at 37°C. Cells were then treated for 48 h at 37°C with either 5-FU or OXA at various concentrations as mentioned above. Thereafter, 20 µl of 5 mg/ml MTT (Sigma-Aldrich, MO, USA) in PBS was added to each well, and the microtiter plates were incubated for additional 4 h at 37°C. The yellow tetrazolium salt MTT was reduced to dark-colored formasan by viable cells. After removing supernatants, formazan crystals were dissolved with 150 µl of DMSO, and optical densities (OD) were measured at 490 nm by an automated ELIZA reader (Beckman-Coulter Co., USA). The IC50 values were defined as the concentrations resulting in a 50% reduction in growth compared to control cell growth.
Colony forming assay
200 cells were seeded per well in 6-well plates and IC50 of OXA and 5-FU were added 24 h later for 14 days continuously. The cells were fixed in 70% ethanol and stained with 10% (v/v) Giemsa (MERCK, Damstadit, Germany). Colonies consisted of more than 50 cells were counted. Two wells were used for each dose and 2 wells treated with solvent were only used as controls. Colony forming ability after drug treatment was calculated as the ratio between the number of colonies in the treated wells and the untreated controls multiplied by 100. Results represented the average of 3 independent experiments.
Apoptosis-flow cytometry
Early and late cell apoptosis was measured by flowcytometry using Annexin V-FITC and PI provided in a commercial kit (KeyGEN Biotechnology Co., Nanjing, China) according to the manufacturer’s protocol. Briefly, LoVo, HCT116 and SW480 cells were seeded in a six-well plate at a density of 1×106 cells/well for 24 h at 37°C. Cells were then treated for 48 h at 37°C with IC50 of 5-FU and OXA until test. On the test day, 1×105 trypsinized cells were washed twice in PBS and resuspended in 100 µl of binding buffer. Cells were then exposed to Annexin V-FITC (5 µl) and PI (5 µl) for 15 min in dark. Stained cells were analyzed for apoptosis by collecting 10,000 counts per cell line using BD LSRFortessa flow cytometer equipped with FACSDIVA 6.2 software for cell acquisition and data analysis.
Statistical analysis
The SPSS 13.0 software (Chicago, I L, USA) and GraphPad Prism software (La Jolla, CA, USA) were used for statistical analysis. All the results were presented as mean ± SD. Paired t test was used to evaluate the difference of Twist1 expression between tumors and normal tissues. χ2 test was performed to determine the correlation between the expression of Twist1 and clinicopathologic parameters. Survival curves were estimated by the Kaplan-Meier method and compared with the log rank test. Multivariate analysis was performed using the Cox regression model to assess whether a factor was an independent predictor of OS. Student’s t-test and one-way ANOVA were used in either 2 or multiple groups for statistical significance. A two-tailed P-value of <0.05 was considered statistically significant.
Results
Relationship between Twist1 expression and clinicopathological factors
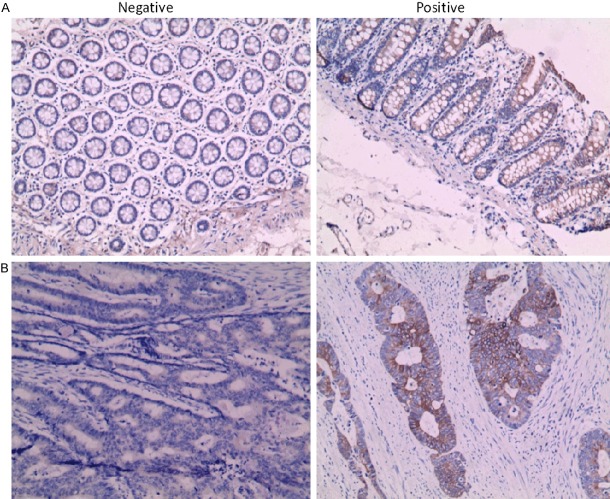
Patients were divided into two groups (negative or positive) according to whether the Twist1 expression level value. The expression of Twist1 in CRC tissues and matched adjacent non-tumor tissues were examined by immunohistochemistry. Positive expression of Twist1 was detected in 60/95 CRC samples and 19/95 normal tissues (P<0.01). Twist1 was located predominantly on cytoplasm, and showed different staining intensities in normal mucosa and cancers (Figure 1). The relationships between Twist1 and clinicopathological factors were examined (Table 1). On one side, positive Twist1 expression was found to be significantly correlated with histological grade, T-stage, N-stage, M-stage and recurrence. Moreover, Twist1 expression was more frequently observed in advanced TNM-stage tumors than in early TNM-stage tumors. On the other side, there were no significant differences between groups in age, Sex, histological type, T-stage, colorectal cancer type or tumor differentiation.
Figure 1.
The expression of Twist1 were performed by immunohistochemistry in clinical speimes. A. Twist expression was shown in norma mucos (×200). B. Twist expression was shown incancers (×200).
Impact of Twist1 expression on overall survival
The overall survival (OS) curves were stratified according to Twist1 expression levels. The OS was significantly longer in the negative Twist1 expression group compared to that in the positive Twist1 expression group (P<0.0001; Figure 2). In a univariate analysis, expression of Twist1 and various clinicopathological parameters were evaluated for their impact on OS. The OS was significantly associated with histological grade (P=0.017), N-stage (P=0.0000), M-stage (P=0.0023), TNM stage (P=0.0000) and the expression of Twist1 (P=0.0000) (Table 2). A multivariate Cox regression analysis demonstrated that the expression of Twist1 was a significant prognostic factor for OS (P=0.0000; Table 2). Among the other covariates, only N-stage was a significant prognostic factor (P=0.0000; Table 2).
Figure 2.
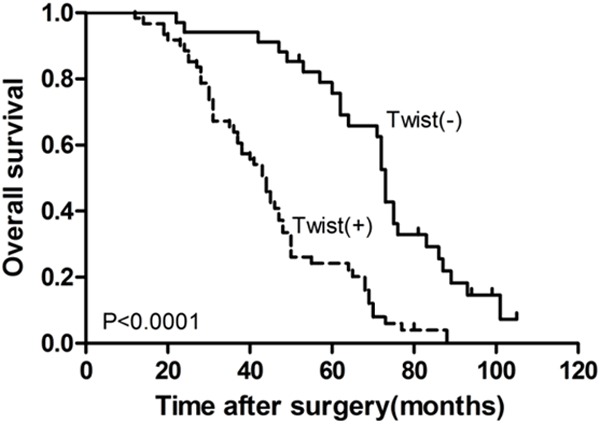
Kaplan-Meier method used for overall survival analysis to demonstrate that CRC patients with positive Twist1 expressions are significantly associated with a shorter survival time (P<0.0001).
Table 2.
Univariate and multivariate analysis of clinicopathologic parameters with OS by Cox proportional hazards regression
| Factors | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| OR | 95% CI | P | OR | 95% CI | P | |
| Sex | 0.853 | 0.538-1.355 | 0.502 | |||
| Age | 1.001 | 0.982-1.020 | 0.915 | |||
| Histological type | 1.262 | 0.872-1.827 | 0.218 | |||
| Histological grade | 0.625 | 0.424-0.920 | 0.017 | |||
| T-stage | 1.365 | 0.736-2.530 | 0.323 | |||
| N-stage | 6.340 | 3.300-12.180 | 0.000 | 4.981 | 2.514-9.867 | 0.000 |
| M-stage | 1.810 | 1.087-3.013 | 0.023 | |||
| Tumor differentiation | 0.787 | 0.361-1.715 | 0.547 | |||
| Tumor location | 0.992 | 0.617-1.593 | 0.972 | |||
| TNM stage | 3.058 | 1.873-4.993 | 0.000 | |||
| Recurrence | 1.629 | 0.939-2.827 | 0.083 | |||
| Twist1 expression | 3.723 | 2.237-6.196 | 0.000 | 2.970 | 1.734-5.089 | 0.000 |
The blank cells correspond to variables that showed no independent relationship with OS in the Multivariate Cox proportional hazards regression.
The expression of Twist1 in CRC cell lines with different treatments
The Twist1 expression in CRC cell lines was shown in Figure 3A. In addition, the expression level of Twist1 was examined by western blotting and Real-time PCR with different treatments in CRC cells (Figure 3B, 3C).
Figure 3.
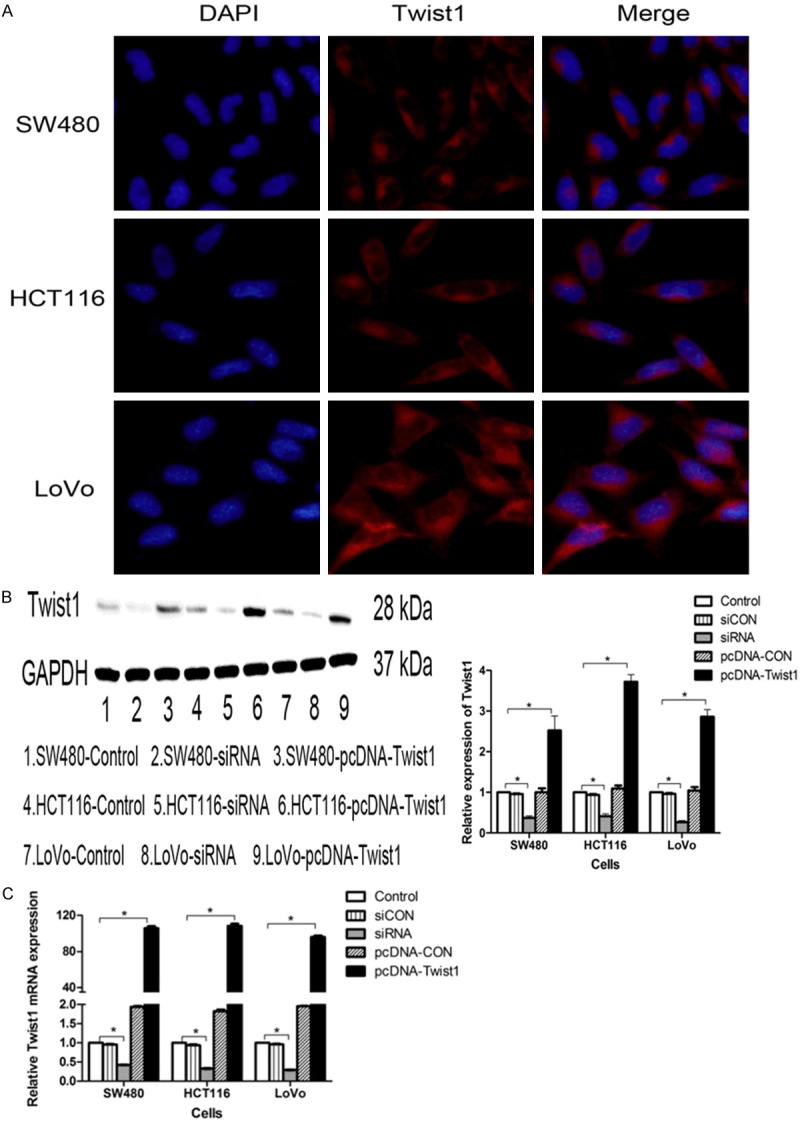
The expression of Twist1 were performed by immunofluorescence, western blotting and Real-time PCR with different treatent in CRC cells. A. The Twist1 expression was significant differences in LoVo or HCT116 compardto SW480 (P<0.05). B. Western blotting and the graph showed the expression of Twist1 with different transfections compared to control group (P<0.05), and there were no significant difference of the expression of Twist1 in the siCON group or the pcDNA-CON group compared to control group (P>0.05). C. The graph of RT-PCR showed that cells were transfected with siRNA or pcDNA-Twist1 compared to control group (P<0.05), the expression of Twist1 in the siCON group or the pcDNA-CON group was no differences compared to control group (P>0.05).
MTT in CRC cells with different treatments
Preliminary studies were done in order to evaluate the antiproliferative effect of OXA and 5-FU on the CRC cells (SW480, HCT116 and LoVo). The IC50 values for OXA and 5-FU are presented in Table 3. Treated with a dose of IC50 for 5-FU and OXA, CRC cells transfected with siRNA-Twist1 exhibited much lower resistance to the anticancer drugs than cells transfected with control at 48 h (P<0.05). Likewise, upregulation of Twist1 in CRC cells conferred stronger chemoresistance compared to cells transfected with control at 48 h (P<0.05). The MTT values 48 h post-treatment were represented as groups, plotted against OD 490 nm (Figure 4).
Table 3.
Overview of IC50 values for SW480, HCT116 and LoVo for OXA and 5-FU at 48 h (µg/ml)
| Compound | SW480 | HCT116 | LoVo |
|---|---|---|---|
| OXA | 1.87 | 11.86 | 94.83 |
| 5-FU | 0.66 | 5.75 | 27.66 |
Figure 4.
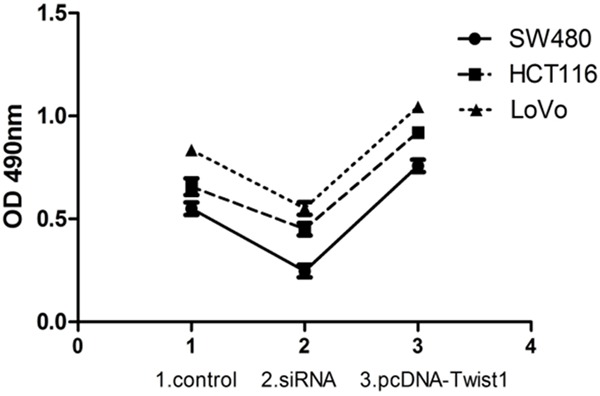
Chemoresistance shown as the number of viable colorectal cancer (CRC) cells transfected with siRNA or pcDNA-Twist1 compared to control group treated with a dose of IC50 for 5-FU and OXA at 48 h. CRC cells (SW480, HCT116 and LoVo) transfected with siRNA-Twist1 exhibited much lower resistance to the anticancer drugs than cells transfected with control at 48 h (P<0.05). Likewise, upregulation of Twist1 in SW480, HCT116 and LoVo cells conferred stronger chemoresistance compared to cells transfected with control at 48 h (P<0.05). Cell numbers were determined from the absorbance at 490 nm (OD 490).
Colony forming assay in CRC cells with different treatments
The upregulation or knockdown of Twist1 elicited significant cell chemoresistance and proliferation compared to with the control group (P<0.05). In addition, the number of colony formation were significantly differences in CRC cells with different treatments (P<0.05) (Figure 5).
Figure 5.
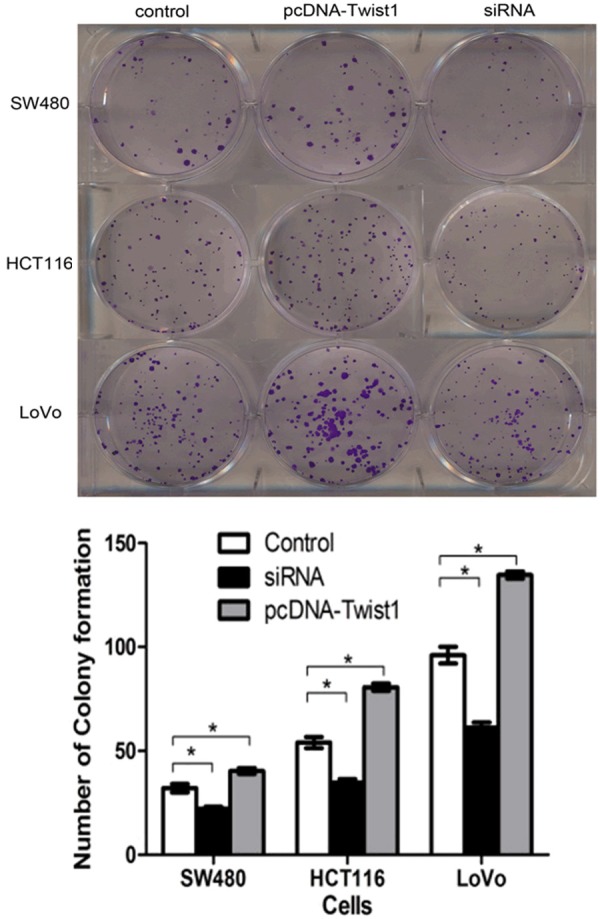
Chemoresistance and proliferation shown as the number of colony formation of colorectal cancer (CRC) cells transfected with siRNA or pcDNA-Twist1 compared to control group treated with a dose of IC50 for 5-FU and OXA. CRC cells with siRNA-Twist1 exhibited much lower chemoresistance and proliferation than the control group (P<0.05). Likewise, upregulation of Twist1 in SW480, HCT116 and LoVo cells conferred stronger chemoresistance and proliferation compared to the control group (P<0.05). In addition, the number of colony formation were significatl differences in CRC cells (P<0.05).
Apoptosis-Flow Cytometry in CRC cells with different treatments
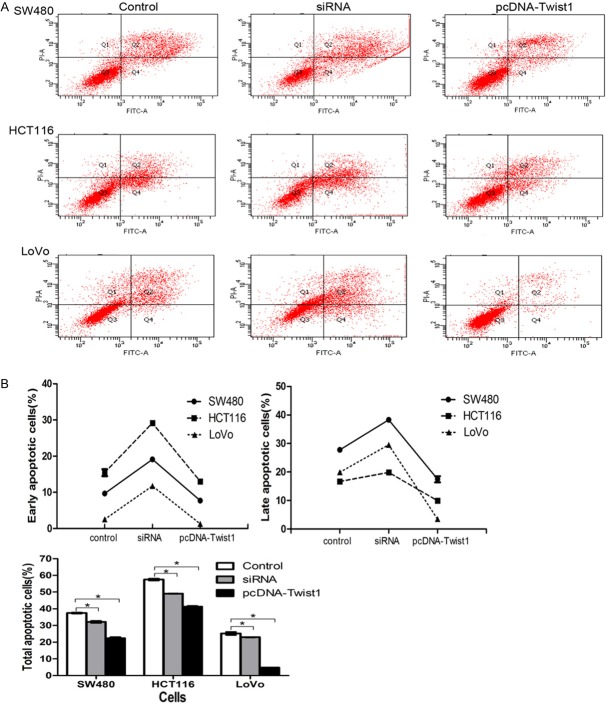
In the case of the combined treatment, upregulation of Twist1 expression inhibits CRC cells apoptosis in vitro. We investigated whether Twist1 could reduce CRC cells apoptosis. Cells were harvested and disposed by the Annexin V-FITC/PI assay with indicated treatment at 48 h after transfection in the cells. The rates of apoptosis were obviously decreased in SW480, HCT116 and LoVo (Figure 6). The Twist1 siRNA showed the highest apoptosis rate at 48 h in early, late or total apoptosis, suggesting that Twist1 expression can increase apoptosis of colorectal cancer cells in vitro.
Figure 6.
A. The effect of Twist1 on apoptosis with combined treatment of IC50 of XA and 5-FU in SW480, HCT116 and LoVo cells. B. As show in early, late and total apoptosis, the apoptotic rate of the Twist1 siRNA obviously increased in CRC cells compared with the control group (P<0.01). Besides, the apoptotic rate of the pcDNA-Twist1 obviously decreased in CRC cells compared with the control group (P<0.01). The experiment was done in triplicate. The figure represented one of the 3 experiments performed in duplicate.
Discussion
This study, which investigated the significance of Twist1 protein expression in CRC, identified some variable factors that had effects on the patients’ prognosis. It was showed that Twist1 expression was positively associated with histological grade, N-stage, M-stage, TNM stage and recurrence in patients with CRC. With regard to Twist1 in CRC, previous reports demonstrated its role in pathological activities, such as the induction of EMT in CRC cells in vitro [9]. In addition, there is a report showing that Twist expression is significantly associated with prognosis in patients with CRC [10,11]. Consistent with their results, the present study demonstrated that Twist1 expression is correlated with lymph node metastasis and TNM stage, suggesting that Twist may be involved in the invasion and metastasis of CRC. Histological grade, N-stage, M-stage, TNM stage, recurrence and the expression of Twist1 were valuable predictors for OS by univariate analysis. After adjustment by multivariate analysis, N-stage and Twist1 expression remained as independent risk factors for poor OS. Twist1 expression was identified to be an independent risk factor for poor OS. Kaplan-Meier analysis displayed that patients with Twist1-negative expression had significantly longer OS than the positive patients.
Invasion and metastasis are life-limiting aspects of malignant neoplasm. It has been shown in a series of researches that cancer cells use EMT to down-regulate their cell-cell adhesion and become motile and invasive [12]. A variety of studies showed that Twist1, a highly conserved basic helix-loop-helix transcription factor, is involved in EMT and suggested as an oncogene [13,14]. Besides, Twist1 is in relation to the regulation of cell movement and tissue reorganization during early embryogenesis [9]. And it has been shown to play an essential role in diverse ways, including tumor cell apoptosis, invasion and metastasis, which are involved in carcinogenesis and cancer progression [15]. Recently, some evidences also indicate that Twist1 can be a key factor responsible for metastasis of breast cancer by promoting EMT in an in vivo system, and suppression of Twist1 inhibits tumor metastasis and reduces the presence of tumor cells in the blood circulation and lymph node involvement [16]. For our results, it suggests that Twist1 could enhance the migration behavior of CRC cells, which is similar to their results and thus support these recent findings about Twist1.
Chemotherapy is a systemic treatment involving the use of chemical agents to stop cancer cells from growing. Although the chemotherapy based on OXA and 5-FU has been an effective method for eliminating CRC cells, chemoresistance has become a major therapeutic obstacle. Recently, a number of researches revealed that EMT is involved in multidrug-resistance in cancer cells. Many chemotherapeutic agents were shown to induce EMT in several types of cancers, including head and neck carcinoma [17], hepatocellular carcinoma [18], breast cancer [19], ovarian cancer [7], bladder cancer [20]. On the other side, EMT displayed that it could enhance the chemoresistance of the tumor cells in breast cancer, ovarian cancer, colorectal cancer and pancreatic cancer [7,19,21,22]. As a result, the above studies provide forceful evidence between MDR and EMT. In the present study, we found that CRC cells with chemoresistant undergo EMT process and the chemoresistant CRC cells were accompanied with enhancing metastatic potential, in which the Twist1 expression was up-regulated.
In view of the MDR concomitant EMT and Twist1, a question was raised: Did Twist1 play a role in the MDR after a certain period of treatment? Previously, numbers of surveys have discovered that Twist1 gene expression is associated with the development of acquired resistance to an anticancer drug, and overexpression of Twist1 leads to resistance to chemical agents [23-25]. Moreover, silencing of Twist1 sensitizes lung cancer cells to cisplatin via stimulating AMPK-induced mTOR inhibition [18,26]. These relationships of evidence made further efforts to suggest that Twist1 may be a positive factor in promoting resistance to anticancer drugs, which is one of the characteristics of advanced cancers. In order to answer the problem, our work revealed that Twist1 may assume a dual role because it has intrinsic potential effects on EMT as well as an antiapoptotic function. In addition, our results also implicate that Twist1 may be an important oncogene that induces tumorigenesis and promotes tumor progression in malignant cells.
In conclusion, the present study demonstrates that Twist1 plays a crucial role in the chemoresistance of colorectal cancer. Downregulation of Twist1 expression facilitated apoptosis and enhanced the sensitivity of chemotherapy. The results of the current study provide support for Twist1 to be a potential prognostic marker and a therapeutic strategy for chemoresistant colorectal cancer. However, further clarification of functional characterization must be required. Besides, the sample sizes are a limitation of our study, and the patients’ follow-up should be taken into consideration. Therefore, large sample sizes are still needed to confirm the molecular mechanism and effect of Twist1 on CRC in further study.
Acknowledgements
This work was supported, in part, by grants from the Guangdong Natural Science Foundation (S2011010005306), the Ministry of Science and Technology of Foshan (201108191), the Ministry of Science and Technology of Shunde (20110202085), the Ministry of Science and Technology of Foshan (201308231) and the Traditional Chinese Medicine Bureau of Guangdong Province (20141307).
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104–17. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 2.Board RE, Valle JW. Metastatic colorectal cancer: current systemic treatment options. Drugs. 2007;67:1851–67. doi: 10.2165/00003495-200767130-00004. [DOI] [PubMed] [Google Scholar]
- 3.Wang X, Li M, Wang J, Yeung CM, Zhang H, Kung HF, Jiang B, Lin MC. The BH3-only protein, PUMA, is involved in oxaliplatin-induced apoptosis in colon cancer cells. Biochem Pharmacol. 2006;71:1540–50. doi: 10.1016/j.bcp.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 4.Hato SV, de Vries IJ, Lesterhuis WJ. STATing the importance of immune modulation by platinum chemotherapeutics. Oncoimmunology. 2012;1:234–236. doi: 10.4161/onci.1.2.18126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kadoyama K, Miki I, Tamura T, Brown JB, Sakaeda T, Okuno Y. Adverse event profiles of 5-fluorouracil and capecitabine: data mining of the public version of the FDA Adverse Event Reporting System, AERS, and reproducibility of clinical observations. Int J Med Sci. 2012;9:33–9. doi: 10.7150/ijms.9.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pogribny IP, Filkowski JN, Tryndyak VP, Golubov A, Shpyleva SI, Kovalchuk O. Alterations of microRNAs and their targets are associated with acquired resistance of MCF-7 breast cancer cells to cisplatin. Int J Cancer. 2010;127:1785–94. doi: 10.1002/ijc.25191. [DOI] [PubMed] [Google Scholar]
- 7.Kajiyama H, Shibata K, Terauchi M, Yamashita M, Ino K, Nawa A, Kikkawa F. Chemoresistance to paclitaxel induces epithelial-mesenchymal transition and enhances metastatic potential for epithelial ovarian carcinoma cells. Int J Oncol. 2007;31:277–83. [PubMed] [Google Scholar]
- 8.Kyo S, Sakaguchi J, Ohno S, Mizumoto Y, Maida Y, Hashimoto M, Nakamura M, Takakura M, Nakajima M, Masutomi K, Inoue M. High Twist expression is involved in infiltrative endometrial cancer and affects patient survival. Hum Pathol. 2006;37:431–8. doi: 10.1016/j.humpath.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 9.Bell GP, Thompson BJ. Colorectal cancer progression: lessons from Drosophila. Semin Cell Dev Biol. 2014;28:70–7. doi: 10.1016/j.semcdb.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Valdés-Mora F, Gómez del Pulgar T, Bandrés E, Cejas P, Ramírez de Molina A, Pérez-Palacios R, Gallego-Ortega D, García-Cabezas MA, Casado E, Larrauri J, Nistal M, González-Barón M, García-Foncillas J, Lacal JC. TWIST1 overexpression is associated with nodal invasion and male sex in primary colorectal cancer. Ann Surg Oncol. 2009;16:78–87. doi: 10.1245/s10434-008-0166-x. [DOI] [PubMed] [Google Scholar]
- 11.Gomez I, Pena C, Herrera M, Munoz C, Larriba MJ, Garcia V, Dominguez G, Silva J, Rodriguez R, de Herreros AG, Bonilla F, Garcia JM. TWIST1 is expressed in colorectal carcinomas and predicts patient survival. PLoS One. 2011;6:e18023. doi: 10.1371/journal.pone.0018023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 13.Karreth F, Tuveson DA. Twist induces an epithelial-mesenchymal transition to facilitate tumor metastasis. Cancer Biol Ther. 2004;3:1058–9. doi: 10.4161/cbt.3.11.1302. [DOI] [PubMed] [Google Scholar]
- 14.Vernon AE, LaBonne C. Tumor metastasis: a new twist on epithelial-mesenchymal transitions. Curr Biol. 2004;14:R719–21. doi: 10.1016/j.cub.2004.08.048. [DOI] [PubMed] [Google Scholar]
- 15.Matsuo N, Shiraha H, Fujikawa T, Takaoka N, Ueda N, Tanaka S, Nishina S, Nakanishi Y, Uemura M, Takaki A, Nakamura S, Kobayashi Y, Nouso K, Yagi T, Yamamoto K. Twist expression promotes migration and invasion in hepatocellular carcinoma. BMC Cancer. 2009;9:240. doi: 10.1186/1471-2407-9-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–39. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 17.Maseki S, Ijichi K, Tanaka H, Fujii M, Hasegawa Y, Ogawa T, Murakami S, Kondo E, Nakanishi H. Acquisition of EMT phenotype in the gefitinib-resistant cells of a head and neck squamous cell carcinoma cell line through Akt/GSK-3beta/snail signalling pathway. Br J Cancer. 2012;106:1196–204. doi: 10.1038/bjc.2012.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uchibori K, Kasamatsu A, Sunaga M, Yokota S, Sakurada T, Kobayashi E, Yoshikawa M, Uzawa K, Ueda S, Tanzawa H, Sato N. Establishment and characterization of two 5-fluorouracil-resistant hepatocellular carcinoma cell lines. Int J Oncol. 2012;40:1005–10. doi: 10.3892/ijo.2011.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li QQ, Xu JD, Wang WJ, Cao XX, Chen Q, Tang F, Chen ZQ, Liu XP, Xu ZD. Twist1-mediated adriamycin-induced epithelial-mesenchymal transition relates to multidrug resistance and invasive potential in breast cancer cells. Clin Cancer Res. 2009;15:2657–65. doi: 10.1158/1078-0432.CCR-08-2372. [DOI] [PubMed] [Google Scholar]
- 20.McConkey DJ, Choi W, Marquis L, Martin F, Williams MB, Shah J, Svatek R, Das A, Adam L, Kamat A, Siefker-Radtke A, Dinney C. Role of epithelial-to-mesenchymal transition (EMT) in drug sensitivity and metastasis in bladder cancer. Cancer Metastasis Rev. 2009;28:335–44. doi: 10.1007/s10555-009-9194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gungor C, Zander H, Effenberger KE, Vashist YK, Kalinina T, Izbicki JR, Yekebas E, Bockhorn M. Notch signaling activated by replication stress-induced expression of midkine drives epithelial-mesenchymal transition and chemoresistance in pancreatic cancer. Cancer Res. 2011;71:5009–19. doi: 10.1158/0008-5472.CAN-11-0036. [DOI] [PubMed] [Google Scholar]
- 22.Hoshino H, Miyoshi N, Nagai K, Tomimaru Y, Nagano H, Sekimoto M, Doki Y, Mori M, Ishii H. Epithelial-mesenchymal transition with expression of SNAI1-induced chemoresistance in colorectal cancer. Biochem Biophys Res Commun. 2009;390:1061–5. doi: 10.1016/j.bbrc.2009.10.117. [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Ling MT, Guan XY, Tsao SW, Cheung HW, Lee DT, Wong YC. Identification of a novel function of TWIST, a bHLH protein, in the development of acquired taxol resistance in human cancer cells. Oncogene. 2004;23:474–82. doi: 10.1038/sj.onc.1207128. [DOI] [PubMed] [Google Scholar]
- 24.Zhou YT, Li K, Tian H. Effects of vinorelbine on cisplatin resistance reversal in human lung cancer A549/DDP cells. Asian Pac J Cancer Prev. 2013;14:4635–9. doi: 10.7314/apjcp.2013.14.8.4635. [DOI] [PubMed] [Google Scholar]
- 25.Shiota M, Kashiwagi E, Yokomizo A, Takeuchi A, Dejima T, Song Y, Tatsugami K, Inokuchi J, Uchiumi T, Naito S. Interaction between docetaxel resistance and castration resistance in prostate cancer: implications of Twist1, YB-1, and androgen receptor. Prostate. 2013;73:1336–44. doi: 10.1002/pros.22681. [DOI] [PubMed] [Google Scholar]
- 26.Jin HO, Hong SE, Woo SH, Lee JH, Choe TB, Kim EK, Noh WC, Lee JK, Hong SI, Kim JI, Park IC. Silencing of Twist1 sensitizes NSCLC cells to cisplatin via AMPK-activated mTOR inhibition. Cell Death Dis. 2012;3:e319. doi: 10.1038/cddis.2012.63. [DOI] [PMC free article] [PubMed] [Google Scholar]