Abstract
Forkhead box protein C2 (FOXC2) plays a vital role in carcinogenesis; however, its significance and prognostic value in colon cancer remain unclear. In this study, FOXC2 expression was analyzed in a tissue microarray (TMA) containing 185 samples of primary colon cancer tumor samples and in human colon cancer cell lines. The effect of FOXC2 on cell proliferation, tumorigenesis, and metastasis was examined in vitro and in vivo. FOXC2 was overexpressed in human colon cancer cells and tissues, and correlated with colon cancer progression and patient survival. Functional study demonstrated that FOXC2 promoted cell growth, cell migration, and tumor formation in nude mice, whereas knockdown of FOXC2 by short hairpin RNA (shRNAs) significantly suppressed cell growth, cell migration and tumor formation. Further study found that FOXC2 enhanced AKT activity with subsequent GSK-3β phosphorylation and Snail stabilization, and then induced epithelial-mesenchymal transition (EMT) and promoted tumor invasion and metastasis. Collectively, FOXC2 promotes colon cancer metastasis by facilitating EMT and acts as a potential prognostic factor and therapeutic target in colon cancer.
Keywords: FOXC2, colon cancer, metastasis, EMT, prognostic factor
Introduction
Colorectal cancer (CRC), one of the most common malignancies, is the third leading cause of cancer-related deaths in the United States [1]. The incidence of CRC in Asian countries is increasing rapidly and is likely similar to that in western countries [2,3]. In China, both the incidence and mortality rate of CRC are increasing [4]. Overall, about 50% of patients diagnosed with CRC succumbed to the disease, due to complications associated with distant metastasis [5]. The underlying molecular mechanisms in CRC metastasis are still unclear.
Epithelial mesenchymal transition (EMT) is the process by which epithelial cells are transformed into mesenchymal cells [6]. It may be physiological as part of embryological development, or pathological as part of cancer development. It is one of the key initiating events in the metastatic cascade. EMT has profound effects on tumor cell invasiveness, proliferation and motility.
Currently, the exact mechanism of EMT progress remains largely unexplored. However, the critical role of some transcriptional factors in the activation of EMT has been well documented, including Forkhead box (Fox) transcription factors family. For example, FoxM1 induces EMT by activating the uPA system/Slug pathway or upregulating Snail, leading to metastasis in pancreatic [7], breast cancer [8], and lung cancer [9]. Forkhead box Q1 promotes hepatocellular carcinoma EMT by transactivating ZEB2 and VersicanV1 expression [10]. FoxF1 promotes EMT and breast cancer metastasis through the inhibition of E-cadherin transcription [11]. FoxC1 promotes hepatocellular carcinoma metastasis through the induction of EMT and the up-regulation of NEDD9 expression [12]. In contrast, loss of FOXA1/2 is essential for the EMT in pancreatic cancer [13]. Downregulation of FOXO3a promotes tumor metastasis and is associated with metastasis-free survival of patients with clear cell renal cell carcinoma [14]. These studies indicate that Fox protein-mediated EMT is involved in tumor metastasis. The critical role of EMT in the induction of invasiveness and metastasis in CRC suggests that Fox proteins may be involved in CRC metastasis. Forkhead box protein C2 (FOXC2), also known as mesenchyme forkhead 1 is a gene encoding a transcription factor that controls the generation of mesodermal tissue such as vascular tissue and lymphatic tissue [15,16]. FOXC2 has been reported to be involved in the EMT process in various tumors. For example FOXC2 expression induces EMT in breast cancer [11,17,18] and extrahepatic cholangiocarcinoma [19], and high expression of FOXC2 is related to a poor prognosis and cancer progression in gastric [20], esophageal cancer [21] and extrahepatic cholangiocarcinoma [19]. However, the relationship between FOXC2 expression and CRC has not been fully investigated. Hence, we examined the expression of FoxC2 in a large number of colon cancer tissues and corresponding normal mucosa tissues to evaluate the clinical significance of FoxC2 expression. In additional, we examined the in vitro effects of FOXC2 expression on the proliferation, migration, and invasion of human colon cell lines and its relation with EMT markers.
Materials and methods
Patient information and tissue specimens
This study was approved by the Institutional Research Ethics Committee and written consents were obtained from all 185 patients with pathologically and clinically confirmed colon cancer, including 24 cases at stage I, 81 at stage II, and 80 at stage III. The matching adjacent noncancerous tissue, primary colon cancer tissue, and lymph node metastasis lesions from the 185 patients were fixed in formalin and embedded in paraffin for histological analysis and immunohistochemical studies. Fresh samples were dissected manually to remove connective tissues and were immediately stored in liquid nitrogen until Western blot analysis. None of the patients had received neoadjuvant therapy before surgery.
Construction of the tissue microarray (TMA) and immunohistochemistry (IHC) staining
Construction of the TMA and IHC staining were performed as described previously [22]. FOXC2 anti-human mouse polyclonal antibody was used at a dilution of 1:200 (WH0002303M2, Sigma-Aldrich); PBS was used as negative control. Immunoreactivity was evaluated independently by two researchers in a blinded fashion. A semiquantitative scoring system was used [23], which evaluated both staining intensity (0, no stain; 1+, weak stain; 2+, moderate stain; 3+,strong stain) and the percentage of stained cells (0, <5%; 1, 5-25%; 2, 26-50%; 3, 51-75%; and 4, >75%). Scores for staining intensity and percentage positivity of cells were then multiplied to generate the immunoreactivity score (IS) for each case. All cases were sorted into two groups according to the IS. High expression of FOXC2 was defined as detectable immunoreactions in the cytoplasm and nucleus with IS≥4 [20].
Cell culture
The human colon cancer cell lines (SW480, SW620, HT29, RKO, LoVo, COLO 205, LS174T and HCT116) were originally purchased from the American Type Culture Collection (Manassas, VA, USA). All cell lines were cultured in medium according to the Defense Technical Information Center recommendation supplemented with 10% FBS (Gibico, Life Technology, Austria).
Stable transfection of colon cancer cells
The coding sequence of human FOXC2 was amplified and cloned into a lentiviral expression vector, pCDH-CMV-MCS-EF1-Puro, to generate pCDH-FOXC2. Biologically active short hairpin RNAs (ShRNA) were generated using the lentiviral expression vector pLKO.1-puro. The shRNA target sequence for human FOXC2 was 5’-CCTGAGCGAGCAGAATTACTA-3’. PLKO.1-scramble shRNA with limited homology with any known sequences in the human was used as a negative control. SW480 cells were transfected with the pCDH-FOXC2 expression vector or the control vector. RKO cells were transfected with the pLKO.1-shFOXC2 expression vector or pLKO.1-scramble. The cells stably transfected were isolated using puromycin selection after the cells were transfected with expression vector or control plasmids.
CCK8 assays
96-well plates were seeded with each group of cells at a density of 2000 cells per well and cultured for 24, 48, 72 or 96 h, respectively. 10 μL of CCK8 solution was added to each well and incubated for 2 h at 37°C. Then, each solution was measured spectrophotometrically at 450 nm to determine cell viability. The cell viability of different groups at each measuring time point was compared.
Colony formation assay
6-well plates were seeded with each group of cells at a density of 200 cells per well and cultured for 14 days. The surviving colonies (>50 cells) were counted with crystal violet staining. Colony-forming efficiency (CFE %) was defined as the ratio of the number of colonies formed in culture to the number of cells inoculated.
In vitro migration and invasion assays
The migration and invasion assays were conducted in a 24-well Transwell chamber (Costar, Cambridge, MA) with uncoated membranes or membranes coated with Matrigel (BD Biosciences, San Jose, CA, USA). Briefly, 100 μL of cell suspensions (5×104 cells) prepared in serum-free medium was loaded in the upper well of the chamber, while medium containing 10% FBS was placed in the lower wells as a chemoattractant stimulus. The chamber was incubated for 48 h, and the nonmigratory and non-invasive cells in the upper chamber of the filter were removed with a cotton tip. The migrated cells on the bottom surface of the filter were fixed with 4% paraformaldehyde and stained with hematoxylin, then counted under a microscope in four randomly selected fields at ×100 magnification.
Western blot analysis
Total proteins from cells and tissues were extracted in lysis buffer (Pierce, Thermo Scientific, USA), and the concentration was determined using the BCA protein assay kit (Pierce, Thermo Scientific, USA). Cell lysates were electrophoresed on SDS-polyacrylamide gels and transferred onto polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). Membranes were blocked with 5% BSA in TBST buffer (0.1% Tween-20, 20 mM Tris-HCl pH 7.5, and 140 mM NaCl) and probed with primary antibodies (all from Abcam, UK) at 1:1000 dilution, followed by secondary antibody-horseradish peroxidase conjugate and bounded proteins were visualized using ECL (Pierce, Thermo Scientific, USA) and detected using a BioImaging System.
Confocal immunofluorescent analysis
Cells (5×104) were implanted onto a slide for 24 hours. Cells were fixed with paraformaldehyde for 30 minutes, then permeabilized with 0.1% Triton X-100 for 5 min at room temperature and thereafter the primary antibodies were added for incubation for 2 hours at room temperature, then cells were incubated with Alexa flours 594 TgG donkey anti-rabbit (1:500, Invitrogen, USA) for an hour at room temperature, nuclei were stained with propidium iodide for 5 minutes when necessary. Fluorescence images were photographed with a confocal microscopy.
Animal experiments
All procedures involving mice were conducted in accordance with Fudan University Shanghai Cancer Center Animal Care guidelines. All efforts were made to minimize animal suffering, to reduce the number of animals used, and to utilize possible alternatives to in vivo techniques. Tumor cells (5×106 per mouse for SW480/Vector and SW480/FOXC2 (n=5), 2×106 for RKO/Scramble and RKO/shFOXC2, n=5) on the forelimbs of each 4-6 week old Balb/C athymic nude mouse. Animals were sacrificed 6 weeks later or when they had become moribund. Tissue were fixed in 10% buffered formalin, immersed in an ascending series of alcohols, and paraffin embedded. 4 μm sections were cut and stained with hematoxylin and eosin (H & E).
Statistical analysis
Chi-square tests were used to test independence, and Student’s t-test to compare continuous data. The Kaplan-Meier method was used to analyze colon cancer patients’ cumulative survival rates. A Cox proportional hazards model was used to calculate univariate and multivariate hazard ratios for the study variables. All of statistical analyses were performed using the statistical software package SPSS for Windows, version 17 (IBM Corp, Armonk, NY, USA). Statistical significance was set at two-sided P<0.05.
Results
Aberrant overexpression of FOXC2 in colon cancer tissue and cell lines
Western blots (Figure 1A) and Real-time PCR (Figure 1B) analyses revealed that all eight colon cancer cells, including HT29, LS174T, SW480, SW620, RKO, HCT116, COLO205, and LoVo express varying levels of FOXC2. FOXC2 expression levels seems to be consistent with the invasive ability of the eight cancer cells.
Figure 1.
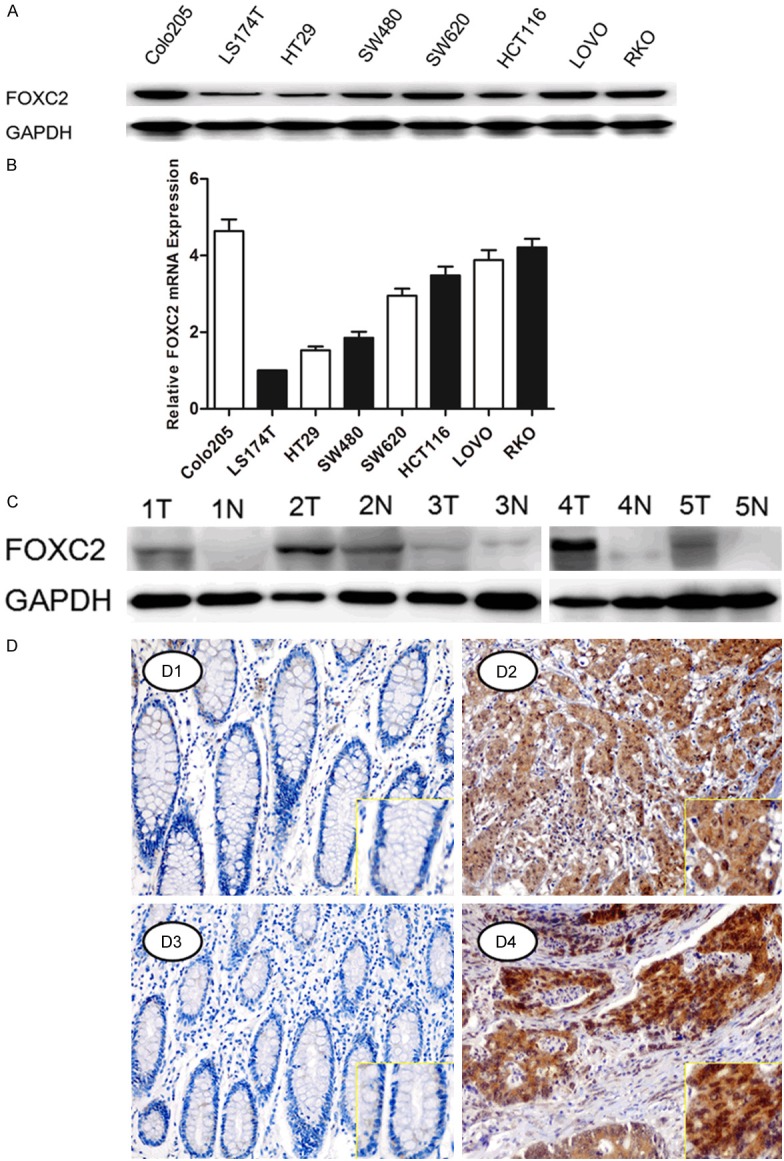
Analysis of FOXC2 expression in colon cancer cell lines and tissues. (A) Western blotting (A) and Real-time PCR (B) analysis were performed to examine FOXC2 expression in eight colon cancer cell lines. (C) Western blotting analysis was performed to examine FOXC2 expression in five cases of primary colon carcinomas (T) and paired noncancerous adjacent tissues (N). GAPDH ise loading control. (D) Immunohistochemical staining of FOXC2 in TMA.FOXC2 was localized within the cytoplasm/nucleus of cancer cells. Elevated FOXC2 expression in the tumor of colon cancer tissue (D2, D4) compared to adjacent normal mucosa with negative staining (D1, D3) (orignal magnification: 400X for the inserts, 100×X for all).
Then, we evaluated the expression levels of FOXC2 in colon cancer tissues by using western blots (Figure 1C) and IHC (Figure 1D) to analyze its protein expression in primary colon cancerous tissue and paired normal colonic mucosa. It showed that FOXC2 was highly expressed in cancerous tissue relative to non-cancerous mucosa in five cases of colon cancer patients’ specimens, suggesting that FOXC2 expression is upregulated in colon cancer.
Association between FOXC2 expression and the clinical features of colon cancer
To further determine the clinicopathologic significance of FOXC2 expression, IHC was performed to detect the expression of FOXC2 in a tissue array containing 185 cases of primary colon cancer paired with noncancerous tissue and the available 62 cases of lymph node metastases (LNM). As shown in Figure 1D, FOXC2 protein was localized in the cytoplasm and nucleus of the tumor cells, with weak or no staining in the normal mucosa. FOXC2 was significantly overexpressed in 55.68% (103/185) of primary cancer lesions and in 74.13% (46/62) of lymph node metastasis, when compared with adjacent normal colonic tissue. There was no high expression in normal mucosa (Table 1), suggesting FOXC2 may play a critical role in the metastasis of colon cancer.
Table 1.
Expression of FOXC2 in cancer tissues, lymph node metastasis and normal colonic mucosa
| Tissue sample | n | Expression of FOXC2 | χ2 Value | P Value | ||
|---|---|---|---|---|---|---|
|
|
||||||
| Negative | Low | High | ||||
| Cancerous tissue†, ‡ | 185 | 13 | 69 | 103 | 184.080 | <0.001 |
| Lymph node metastasis† | 62 | 3 | 13 | 46 | ||
| Normal mucosa | 185 | 82 | 103 | 0 | ||
P value is based on Fisher’s test.
Significant difference in the expression of FOXC2 between indicated group and the normal mucosa tissue sample. Cancerous tissue VS normal mucosa, χ2=158.911, P<0.001. Lymph node metastasis VS normal mucosa, χ2=169.422, P<0.001.
Significant difference in the expression of FOXC2 between cancerous tissue and the LNM tissue sample, χ2=6.713, P=0.035 (Fisher’s exact test).
Chi-square analysis showed that the expression levels of FOXC2 were closely correlated with the T classification (P=0.011), lymph node involvement (P<0.001), lymphovascular invasion (P=0.037), AJCC (American Joint Committee on Cancer) stage (P<0.001), and Ki67 expression level (P<0.001) in colon cancer patients (Table 2). Collectively, these data indicate that FOXC2 may be involved in colon cancer carcinogenesis and metastasis.
Table 2.
Association between FOXC2 expression and clinicopathological factors in colon cancers (n=185)
| Variable | n | FOXC2 Expression | χ2 Value | P value | |
|---|---|---|---|---|---|
|
|
|||||
| Negative/Low | High | ||||
| Gender | 0.001 | 0.974 | |||
| Male | 82 | 40 | 42 | ||
| Female | 103 | 50 | 53 | ||
| Age | 1.082 | 0.292 | |||
| ≤60 | 61 | 33 | 28 | ||
| >60 | 124 | 57 | 67 | ||
| T category | 11.096 | 0.011a | |||
| T1 | 7 | 7 | 0 | ||
| T2 | 23 | 14 | 9 | ||
| T3 | 73 | 36 | 37 | ||
| T4 | 82 | 33 | 49 | ||
| LNM | 12.622 | <0.001 | |||
| Negative | 107 | 64 | 43 | ||
| Positive | 78 | 26 | 52 | ||
| AJCC stage | 12.524 | <0.001 | |||
| I/II | 105 | 63 | 42 | ||
| III | 80 | 27 | 53 | ||
| Pathological grading | 1.182 | 0.277 | |||
| High/Moderate | 166 | 83 | 83 | ||
| Poor/undifferentiation | 19 | 7 | 12 | ||
| Lymphovascular invasion | 4.346 | 0.037 | |||
| Negative | 174 | 88 | 86 | ||
| Positive | 11 | 2 | 9 | ||
| Ki67 | 12.265 | <0.001 | |||
| Negative | 52 | 36 | 16 | ||
| Positive | 133 | 54 | 79 | ||
Fisher’s exact test.
Overexpression of FOXC2 is associated with poor clinical outcome in human colon cancer
After a median follow-up period of 62 months (range 12-89 months), 65 out of 185 (35.1%) patients relapsed and 57 out of 185 (30.8%) died from the disease. FOXC2 was overexpression in 43 out of 65 (66.15%) of patients who relapsed, and in 42 out of 57 (73.68%) of patients who died. The incidence of relapses and deaths was significantly higher in patients with FOXC2 overexpression than those of low/negative expression (P=0.003 and <0.001, respectively). Kaplan-Meier survival analysis and log-rank test also showed that the expression of FOXC2 was significantly correlated with overall survival OS (χ2=8.336, P=0.004) and DFS (χ2=5.071, P=0.024) of patients with colon cancer (Figure 2A, 2B).
Figure 2.
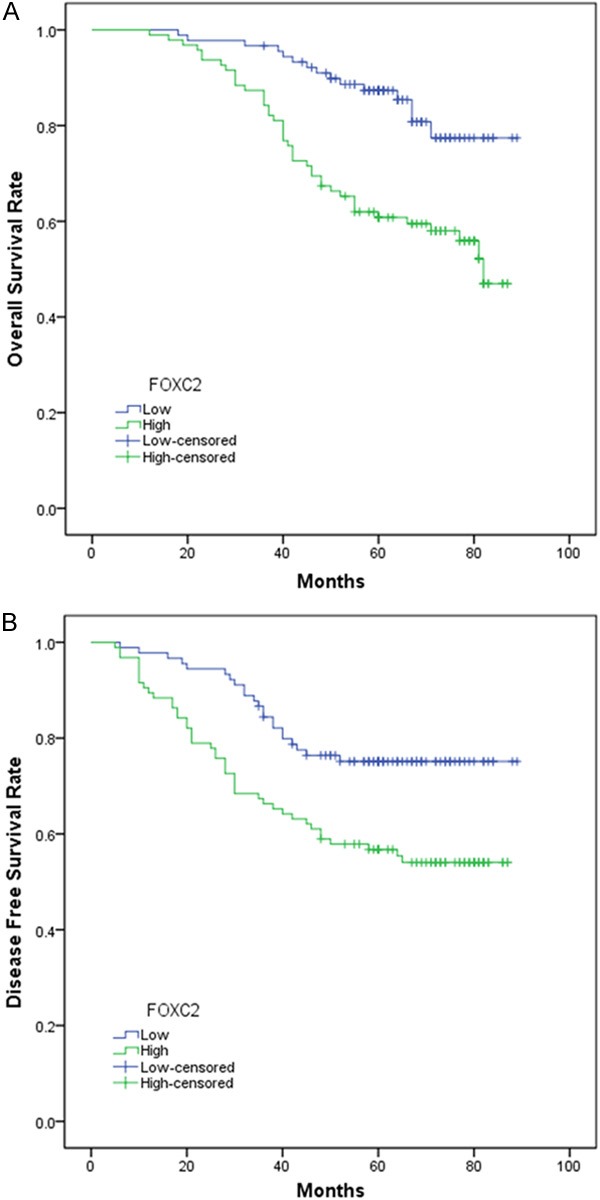
The disease-free survival (DFS) (A) and overall survival (OS) (B) rates were stimated by the Kaplan-Meier method. Both the DFS rate and OS rate of patients with FOXC2 positive primary tumor were significantly lower than that of patients with FOXC2 negative primarytmor (log-rank test, P<0.05).
FOXC2 shows strong oncogenicity function
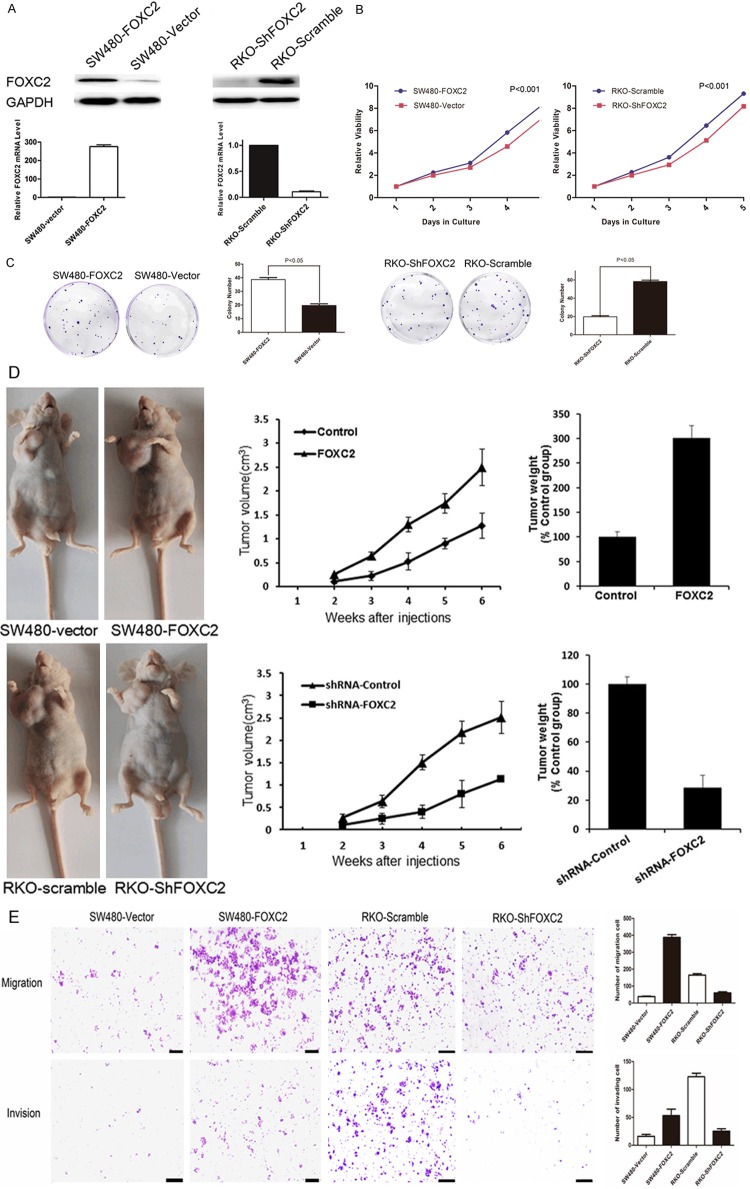
To determine whether FOXC2 has oncogenic function, we established stable FOXC2-expressing in SW480 cells, and ectopic expression of the FOXC2 in the cells was determined by RT-PCR and western blot (Figure 3A). Functional assays were used to characterize the tumorigenicity of FOXC2. Our CCK8 assay showed cell growth rates in FOXC2-transfected cells were significantly higher than those in the control cells at 24-, 48-, 72-, and 96-hour time points (P<0.001, Figure 3B). We also assessed the effect of FOXC2 overexpression on clone formation of cancer cells using clone formation assay. The results showed that the FOXC2-transfected cells yielded a higher number and larger colonies compared to the control cells (P<0.001, Figure 3C).
Figure 3.
FOXC2 Shows Strong Oncogenicity Function. Effect of overexpression of FOXC2 in SW480 cells and knockdown of FOXC2 in RKO cell analyzed by Western blot and RT-PCR (A). Ectopic expression of FOXC2 stimulates SW480 cell proliferation, conversely, knockdown of FOXC2 inhibits RKO cell proliferation as determined by CCK8 assays (B) and colon frmation assays (C). (D) SW480-vector/SW480-FOXC2 and RKO-scramb/RKO-ShFOXC2 cells (1×107) were injected in the forelimbs of nude mice. Gross tumors in the mice were showed in right. Tumor weight and tumor volumes were measuredonthe indicated days. (E) FOXC2 is associated with migration and invasive ability. Overexpression of FOXC2 promotes the migration and invasion of SW480 cells. Knockdown of FOXC2 represses migration and invasion of RKO cells. Scale bars = 100 μm. Data represent the mean ± SD and are representative of three independent experiments.
To confirm this effect in vivo, we performed tumorigenesis assays in nude mice by subcutaneous injection of control cells (SW480-vector) and SW480-FOXC2 cells. After 4 weeks the mice were sacrificed and the size and weight of the xenograft tumors were measured. The results showed that tumors developed from FOXC2-transfected cells were significantly larger and heavier than tumors from control cells (P<0.05) (Figure 3D).
Overexpression of FOXC2 promotes the migration and invasion of human CRC cells
As FOXC2 overexpression was significantly associated with colon cancer invasion, the role of FOXC2 in tumor cell migration and invasion was investigated. Transwell invasion assay further revealed significantly increased cell motility and invasion with FOXC2 overexpression, as compared with control cells (P<0.05, Figure 3E).
Knockdown of FOXC2 represses tumorigenicity and cell motility of human CRC cells
To further confirm the oncogenicity of FOXC2, the FOXC2 gene was silenced by shRNAs targeting the sequence CCTGAGCGAGCAGAATTACTA. The shRNAs were stably transfected into the colon cell line RKO. A scrambled shRNA was used asnegative control. Real-time PCR and western blot indicated that the FOXC2 mRNA and protein levels were efficiently silenced compared with scramble shRNA (Figure 3A). Knockdown of FOXC2 expression in the cells significantly inhibited the cell growth rate (P<0.05, Figure 3B) and frequency of clone formation (P<0.05, Figure 3C). To confirm this effect in vivo, we performed tumorigenesis assays in nude mice by subcutaneous injection of control cells (RKO-scramble) and RKO-ShFOXC2 cells. In contrast to control cells, the RKO-ShFOXC2 cells exhibited slower tumor growth and remarkably smaller tumor volumes (Figure 3D).
In transwell assay, the numbers RKO-ShFOXC2 cells, migrated and invaded into the lower compartment of the migration chamber, were significantly smaller than RKO-scramble (P<0.05, Figure 3E).
FOXC2 induces epithelial-mesenchymal transition in colon cancer
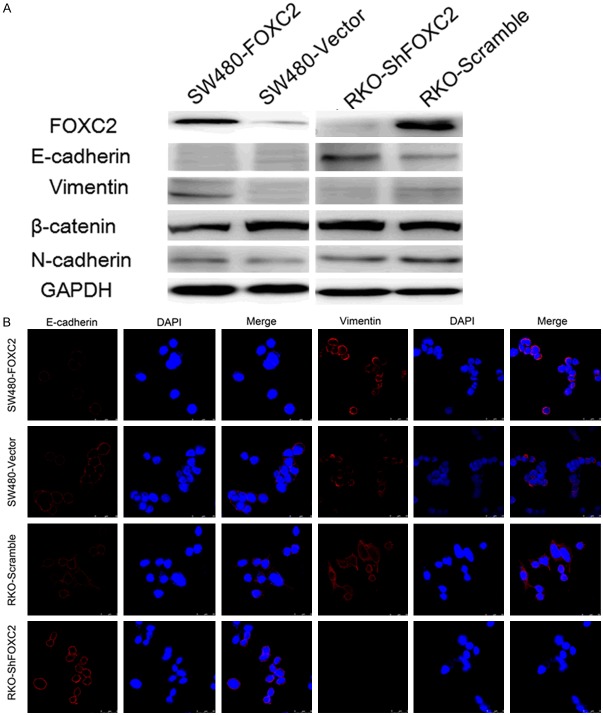
As EMT is one of the key events in tumor invasion and metastasis, the effect of FOXC2 on EMT was analyzed by investigating the expression levels of EMT markers and EMT-related transcription factors. We found that ectopic expression of the FOXC2 was correlated with overexpression of mesenchymal markers vimentin and N-cadherin, and repression of epithelial cell-specific protein β-catenin and E-cadherin, a central marker of epithelial cell phenotype. Besides, Snail, an EMT-related transcription factors, was also increased in FOXC2-transfected cells compared with the control cells. An opposite expression pattern of these genes was observed in FOXC2-silenced cells (Figure 4A). These results were confirmed by confocal microscopy examination in SW480 and RKO cells (Figure 4B).
Figure 4.
FOXC2 Induces Epithelial-Mesenchymal Tranitin in Colon Cancer. A. Western-blot analysis of phenotypic markers including E-cadherin, β-cadherin vimentin, N-cadherin in SW480-Vector/SW480-FOXC2 and RKO-ShFOXC2/RKO-Scramble. GAPDH expression was used s he loading control. B. Confocal microscopy analysis of phenotypic marker including E-cadherin, vimentin. The Red signal represents the staining of corresponding protein, and the green signal represents the nuclear DNA staining by DAPI.
FOXC2 activates the Akt/GSK-3β/Snail pathway to induce EMT
Activation of AKT plays an important role in inducing EMT by inhibiting GSK-3β, leading to the stabilization and nuclear localization of Snail, thereby triggering cell migration and EMT [24]. Therefore, we tested the expression of several proteins involved in the Akt/GSK-3β/Snail pathway by western blot analysis. We found that increased expression of Akt, phosphorylated Akt (p-AKT308 and 473), phosphorylated GSK3β (p-GSK-3β), and Snail could be detected in FOXC2-transfected cells. Conversely, expression levels of these proteins were decreased in FOXC2-silenced cells (Figure 5A).
Figure 5.
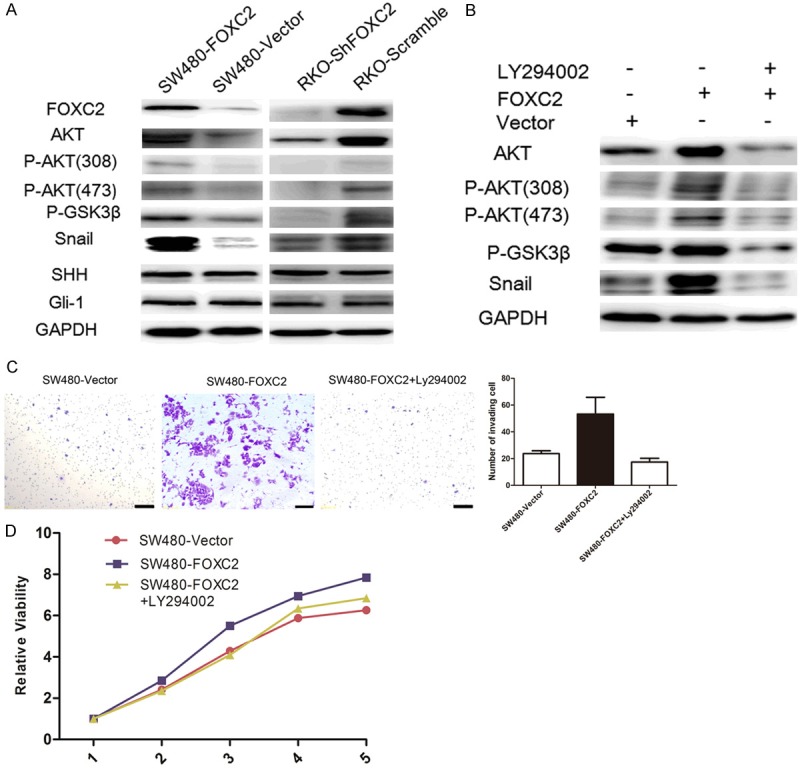
FOXC2 Activates the Akt/GSK-3β/Snail Pathway to Induce EMT. (A) Western-blot analysis of the expression of the indicated proteins in the indicated cells. (B) SW480-FOXC2 cells were treated with the AKT inhibitor LY294002 (20 μM) or DMSO for 24 h, then harvested to examine the expression levels of the indicated proteins by Western blotting. SW480-FOXC2 cell invasion ability was determined by Transwell analysis (C) and proliferation ability was determined by CCK8 assays (D) after treatment with LY294002. Scale bars = 100 μm. Data represent the mean ± SD and are representative of three independent experiments.
In additional, to confirm that the oncogenic effect of FOXC2 was by activating the Akt/GSK-3b/Snail pathway, the PI3K inhibitor LY294002 was used to explore the relationship between the inhibition of PI3K and the increased cell motility induced by FOXC2 in FOXC2 transfected SW480 cells. Our results showed that LY294002 could effectively decrease the levels of p-Akt, p-GSK3β, and Snail induced by FOXC2 (Figure 5B). Transwell assay and CCK8 analysis further confirmed that LY294002 could significantly decrease cell migration and proliferation ability in FOXC2-transfectants cells (P<0.05, Figure 5C, 5D).
Discussion
Aberrant expression of FOXC2 expression has been documented in several human cancers, including breast cancer, gastric cancer, ovarian cancer, oral squamous cancer, and glioblastoma [17,20,25-27]. However, this study is the first that reports the up-regulation of FOXC2 is associated with the process of EMT in colon cancer. The development of colon cancer is a multistep process involving the accumulation of multiple genetic and epigenetic alterations that lead to the activation of oncogenes and inactivation of tumor suppressor genes. In this study we explored the oncogenicity of FOXC2 in the pathogenesis and progression of colon cancer. Our results showed that high expression of FOXC2 protein was significantly correlated with the poor clinicopathological factors characteristics, such as tumor stage, LNM and eventually caused short CSS and DFS. Similar results were reported in breast cancer, and ovarian cancer [17,25]. These data strongly suggest FOXC2 as an oncogene that plays an important role in CRC progression. Our functional studies also demonstrated that FOXC2 had strong tumorigenicity. Overexpression of FOXC2 promoted cell growth, cell migration, clone formation, and tumor formation in nude mice. Conversely, knockdown of FOXC2 expression significantly inhibited these functions in colon cells.
Since accumulating evidence suggests EMT plays a critical role in cancer progression, through which tissue epithelial cancers invade and metastasis. We tested whether the function of FOXC2 on cancer cell was by way of induction of the EMT pathway. As anticipated, FOXC2 overexpression led to the decreased protein levels of epithelial marker (β-catenin, E-cadherin) and increased expression of mesenchymal marker (N-cadherin, vimentin), and suppressed FOXC2 expression led to a reversion of EMT progress,. In addition, the expression level of Snail, an important EMT transcription factor, was dramatically modulated by FOXC2 expression, increased with FOXC2 overexpression and decreased when FOXC2 was silenced.
Further study found that FOXC2 was able to induce EMT of colon cells by way of the activation of the AKT-GSK3β-Snail signaling pathway. We observed activation of Akt pathway in FOXC2-induced EMT, which correlates with hyper phosphorylated GSK-3β, Snail activation, and E-cadherin down-regulation. The transcriptional activity of Snail is regulated by GSK-3β, which is negatively regulated by Akt [24,28]. Treatment with Ly294002 abolished the FOXC2-induced cell function, accompanied by restoration of E-cadherin and decreased vimentin. Meanwhile, p-Akt was decreased and induction in Snail was reduced. Therefore, our experimental data demonstrated that the AKT-GSK3β-Snail signaling pathway might play a critical role in FOXC2-induced EMT. Previous studies also indicated that phosphorylation of AKT is associated with a loss of cell adhesion, decrease in cell-matrix adhesion, loss of apico-basolateral cell polarization and induction of cell motility[29]. PI3K/AKT pathway was frequently activated in various cancers and played an important role in promoting EMT [30].
In summary, this is the first report to describe that FOXC2 plays a critical role in colon cancer by regulating EMT pathway. This study provides new evidence supporting the role of FOXC2 in the progression of colon cancer. FOXC2 level appears to be an independent predictor of survival for patients with colon cancer. Inhibition of FOXC2 expression in colon cancer represents a promising option to prevent tumor local advance and distant metastases.
Acknowledgements
This study was partially supported by grants from the National Natural Science Foundation of China (No. 81372646, 81101586).
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Sung JJ, Lau JY, Young GP, Sano Y, Chiu HM, Byeon JS, Yeoh KG, Goh KL, Sollano J, Rerknimitr R, Matsuda T, Wu KC, Ng S, Leung SY, Makharia G, Chong VH, Ho KY, Brooks D, Lieberman DA, Chan FK. Asia Pacific consensus recommendations for colorectal cancer screening. Gut. 2008;57:1166–1176. doi: 10.1136/gut.2007.146316. [DOI] [PubMed] [Google Scholar]
- 3.Byeon JS, Yang SK, Kim TI, Kim WH, Lau JY, Leung WK, Fujita R, Makharia GK, Abdullah M, Hilmi I, Sollano J, Yeoh KG, Wu DC, Chen MH, Kongkam P, Sung JJ. Colorectal neoplasm in asymptomatic Asians: a prospective multinational multicenter colonoscopy survey. Gastrointest Endosc. 2007;65:1015–1022. doi: 10.1016/j.gie.2006.12.065. [DOI] [PubMed] [Google Scholar]
- 4.Lei T, Chen WQ, Zhang SW, Lei TH, Ying Q, He ZY, Wang XH. [Prevalence trend of colorectal cancer in 10 cities and counties in China from 1988 to 2002] . Zhonghua Zhong Liu Za Zhi. 2009;31:428–433. [PubMed] [Google Scholar]
- 5.de Krijger I, Mekenkamp LJ, Punt CJ, Nagtegaal ID. MicroRNAs in colorectal cancer metastasis. J Pathol. 2011;224:438–447. doi: 10.1002/path.2922. [DOI] [PubMed] [Google Scholar]
- 6.Lim SH, Becker TM, Chua W, Ng WL, de Souza P, Spring KJ. Circulating tumour cells and the epithelial mesenchymal transition in colorectal cancer. J Clin Pathol. 2014;67:848–53. doi: 10.1136/jclinpath-2014-202499. [DOI] [PubMed] [Google Scholar]
- 7.Huang C, Xie D, Cui J, Li Q, Gao Y, Xie K. FOXM1c promotes pancreatic cancer epithelial-to-mesenchymal transition and metastasis via upregulation of expression of the urokinase plasminogen activator system. Clin Cancer Res. 2014;20:1477–1488. doi: 10.1158/1078-0432.CCR-13-2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang C, Chen H, Tan G, Gao W, Cheng L, Jiang X, Yu L, Tan Y. FOXM1 promotes the epithelial to mesenchymal transition by stimulating the transcription of Slug in human breast cancer. Cancer Lett. 2013;340:104–112. doi: 10.1016/j.canlet.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Wei P, Zhang N, Wang Y, Li D, Wang L, Sun X, Shen C, Yang Y, Zhou X, Du X. FOXM1 promotes lung adenocarcinoma invasion and metastasis by upregulating SNAIL. Int J Biol Sci. 2015;11:186–198. doi: 10.7150/ijbs.10634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xia L, Huang W, Tian D, Zhang L, Qi X, Chen Z, Shang X, Nie Y, Wu K. Forkhead box Q1 promotes hepatocellular carcinoma metastasis by transactivating ZEB2 and VersicanV1 expression. Hepatology. 2014;59:958–973. doi: 10.1002/hep.26735. [DOI] [PubMed] [Google Scholar]
- 11.Zhang H, Meng F, Liu G, Zhang B, Zhu J, Wu F, Ethier SP, Miller F, Wu G. Forkhead transcription factor foxq1 promotes epithelial-mesenchymal transition and breast cancer metastasis. Cancer Res. 2011;71:1292–1301. doi: 10.1158/0008-5472.CAN-10-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xia L, Huang W, Tian D, Zhu H, Qi X, Chen Z, Zhang Y, Hu H, Fan D, Nie Y, Wu K. Overexpression of forkhead box C1 promotes tumor metastasis and indicates poor prognosis in hepatocellular carcinoma. Hepatology. 2013;57:610–624. doi: 10.1002/hep.26029. [DOI] [PubMed] [Google Scholar]
- 13.Song Y, Washington MK, Crawford HC. Loss of FOXA1/2 is essential for the epithelial-tomesenchymal transition in pancreatic cancer. Cancer Res. 2010;70:2115–2125. doi: 10.1158/0008-5472.CAN-09-2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ni D, Ma X, Li HZ, Gao Y, Li XT, Zhang Y, Ai Q, Zhang P, Song EL, Huang QB, Fan Y, Zhang X. Downregulation of FOXO3a promotes tumor metastasis and is associated with metastasisfree survival of patients with clear cell renal cell carcinoma. Clin Cancer Res. 2014;20:1779–1790. doi: 10.1158/1078-0432.CCR-13-1687. [DOI] [PubMed] [Google Scholar]
- 15.Kume T. Foxc2 transcription factor: a newly described regulator of angiogenesis. Trends Cardiovasc Med. 2008;18:224–228. doi: 10.1016/j.tcm.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kriederman BM, Myloyde TL, Witte MH, Dagenais SL, Witte CL, Rennels M, Bernas MJ, Lynch MT, Erickson RP, Caulder MS, Miura N, Jackson D, Brooks BP, Glover TW. FOXC2 haploinsufficient mice are a model for human autosomal dominant lymphedema-distichiasis syndrome. Hum Mol Genet. 2003;12:1179–1185. doi: 10.1093/hmg/ddg123. [DOI] [PubMed] [Google Scholar]
- 17.Hollier BG, Tinnirello AA, Werden SJ, Evans KW, Taube JH, Sarkar TR, Sphyris N, Shariati M, Kumar SV, Battula VL, Herschkowitz JI, Guerra R, Chang JT, Miura N, Rosen JM, Mani SA. FOXC2 expression links epithelial-mesenchymal transition and stem cell properties in breast cancer. Cancer Res. 2013;73:1981–1992. doi: 10.1158/0008-5472.CAN-12-2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mani SA, Yang J, Brooks M, Schwaninger G, Zhou A, Miura N, Kutok JL, Hartwell K, Richardson AL, Weinberg RA. Mesenchyme Forkhead 1 (FOXC2) plays a key role in metastasis and is associated with aggressive basallike breast cancers. Proc Natl Acad Sci U S A. 2007;104:10069–10074. doi: 10.1073/pnas.0703900104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watanabe A, Suzuki H, Yokobori T, Altan B, Kubo N, Araki K, Wada S, Mochida Y, Sasaki S, Kashiwabara K, Hosouchi Y, Kuwano H. Forkhead box protein C2 contributes to invasion and metastasis of extrahepatic cholangiocarcinoma, resulting in a poor prognosis. Cancer Sci. 2013;104:1427–1432. doi: 10.1111/cas.12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu JL, Song YX, Wang ZN, Gao P, Wang MX, Dong YL, Xing CZ, Xu HM. The clinical significance of mesenchyme forkhead 1 (FoxC2) in gastric carcinoma. Histopathology. 2013;62:1038–1048. doi: 10.1111/his.12132. [DOI] [PubMed] [Google Scholar]
- 21.Nishida N, Mimori K, Yokobori T, Sudo T, Tanaka F, Shibata K, Ishii H, Doki Y, Mori M. FOXC2 is a novel prognostic factor in human esophageal squamous cell carcinoma. Ann Surg Oncol. 2011;18:535–542. doi: 10.1245/s10434-010-1274-y. [DOI] [PubMed] [Google Scholar]
- 22.Li D, Peng Z, Tang H, Wei P, Kong X, Yan D, Huang F, Li Q, Le X, Xie K. KLF4-mediated negative regulation of IFITM3 expression plays a critical role in colon cancer pathogenesis. Clin Cancer Res. 2011;17:3558–3568. doi: 10.1158/1078-0432.CCR-10-2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sinicrope FA, Ruan SB, Cleary KR, Stephens LC, Lee JJ, Levin B. bcl-2 and p53 oncoprotein expression during colorectal tumorigenesis. Cancer Res. 1995;55:237–241. [PubMed] [Google Scholar]
- 24.Zhou BP, Deng J, Xia W, Xu J, Li YM, Gunduz M, Hung MC. Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat Cell Biol. 2004;6:931–940. doi: 10.1038/ncb1173. [DOI] [PubMed] [Google Scholar]
- 25.Liu B, Han SM, Tang XY, Han L, Li CZ. Overexpressed FOXC2 in ovarian cancer enhances the epithelial-to-mesenchymal transition and invasion of ovarian cancer cells. Oncol Rep. 2014;31:2545–2554. doi: 10.3892/or.2014.3119. [DOI] [PubMed] [Google Scholar]
- 26.Sasahira T, Ueda N, Yamamoto K, Kurihara M, Matsushima S, Bhawal UK, Kirita T, Kuniyasu H. Prox1 and FOXC2 act as regulators of lymphangiogenesis and angiogenesis in oral squamous cell carcinoma. PLoS One. 2014;9:e92534. doi: 10.1371/journal.pone.0092534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li W, Fu X, Liu R, Wu C, Bai J, Xu Y, Zhao Y, Xu Y. FOXC2 often overexpressed in glioblastoma enhances proliferation and invasion in glioblastoma cells. Oncol Res. 2013;21:111–120. doi: 10.3727/096504013X13814233062171. [DOI] [PubMed] [Google Scholar]
- 28.Bachelder RE, Yoon SO, Franci C, de Herreros AG, Mercurio AM. Glycogen synthase kinase-3 is an endogenous inhibitor of Snail transcription: implications for the epithelialmesenchymal transition. J Cell Biol. 2005;168:29–33. doi: 10.1083/jcb.200409067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bellacosa A, Kumar CC, Di Cristofano A, Testa JR. Activation of AKT kinases in cancer: implications for therapeutic targeting. Adv Cancer Res. 2005;94:29–86. doi: 10.1016/S0065-230X(05)94002-5. [DOI] [PubMed] [Google Scholar]
- 30.Larue L, Bellacosa A. Epithelial-mesenchymal transition in development and cancer: role of phosphatidylinositol 3’ kinase/AKT pathways. Oncogene. 2005;24:7443–7454. doi: 10.1038/sj.onc.1209091. [DOI] [PubMed] [Google Scholar]