Abstract
Backgrounds: Radiotherapy (RT) and chemotherapy (CT) can potentiate systemic antitumor immune effect. However, immunomodulation during RT or CT and their clinical implications in rectal cancer have not been thoroughly investigated. Methods: We investigated alterations in the densities of tumor infiltrating lymphocytes (TILs) during chemoradiation and their clinical utilities in patients with rectal cancer. We analyzed 136 rectal cancer patients who underwent neoadjuvant RT, CT or chemoradiotherapy (CRT), followed by radical resection retrospectively. Pretreatment biopsy specimens and posttreatment resected specimens of all patients were immunostained for CD3 and CD8. The predictive value of TILs to neoadjuvant treatment and prognosis were examined. Results: Densities of CD3+ and CD8+TILs in posttreatment specimens after RT, CT or CRT were all significantly higher than those in pretreatment specimens. There were no significant differences between each two of these three groups. High pretreatment CD3+ and CD8+TILs were associated with good response (TRG ≥ 3) after neoadjuvant treatments (P = 0.033 and 0.021). High CD3+TILs and CD8+TILs in pretreatment biopsy specimens were significantly associated with favorable disease free survival (DFS) (P = 0.010 and P = 0.022) and overall survival (OS) (P = 0.019 and P = 0.003). Conclusions: We may, thus, conclude that chemoradiation can enhance local immune response by increased TILs. High TILs densities before treatment are associated with good response to neoadjuvant chemoradiotherapy and a favorable prognosis.
Keywords: Rectal cancer, lymphocytes, inflammation, chemoradiotherapy, prognosis
Introduction
Rectal cancer is one of the most common causes of cancer death in the world [1]. Radiation therapy (RT) and fluoropyrimidine based chemotherapy (CT), before or after surgery, reduce local recurrence risk and increase sphincter preservation rate of rectal cancer [2]. Nowadays, neoadjuvant chemoradiotherapy (nCRT) combined with surgery has been a standard treatment strategy for locally advanced rectal cancer (LARC) [3]. Pathological complete response (pCR) after nCRT has been considered a strong indicator for chemoradiosensitivity and survival. However, response to nCRT ranges from none to complete [4]. Given the wide spectrum of tumor response, it is critical to find a new tool to predict the response of rectal cancer to nCRT, as well as a better surrogate to select nonresponder for an early surgery [5].
Tumor infiltrating lymphocytes (TILs) are frequently found in tumors, which may suggest that tumors trigger an immune response [6]. Many studies have reported that high abundance of TILs is associated with a favorable clinical outcome [7-9]. CD8+ cytotoxic T-lymphocytes, especially, have been found to indicate better survival in many kinds of cancer, including non-small cell lung cancer (NSCLC), colonic, esophageal, as well as urothelial cancers and melanoma [10-13]. Galon et al. [14] have established an immunoscore system that is a more valuable prognostic tool than the histopathological methods currently being used in the treatment of colorectal cancer. Chemotherapy and radiotherapy have previously been considered immunosuppressants, because lymphocytes are very sensitive to the effects of radiation and cytotoxic agents targeting tumors [15]. However, recently, more and more data suggest that RT and CT can potentiate systemic antitumor immune effects by causing immunogenic tumor cell death and inducing T cell response [16]. The particular mechanisms have not yet been elucidated. A number of studies have indicated an association between the presence of extensive lymphocytic infiltration and high response rates to RT or CT [17]. For rectal cancer, it has been found that density of TILs in biopsy samples before treatment can be a predictor of tumor response to CRT [18]. However, differences of immunomodulation during RT or CT and their clinical implications in rectal cancer have not been thoroughly investigated.
In this study, we examined the densities of two TIL subtypes, CD3+, a general T-lymphocyte marker, and CD8+, so far the most popular lymphocyte marker for prognosis [6] before and after CRT in rectal cancer patients to determine their clinical relevance. To clarify the respective effect of RT and CT to TILs, we investigated alterations in the densities of these TIL subsets during RT, CT or CRT and their predictive values on tumor regressions and prognosis by using pretreatment biopsy samples and posttreatment resected tissues for 136 patients with neoadjuvant treatments.
Materials and methods
Patients
Since January 2000, 136 patients in Shandong cancer hospital and institute who received neoadjuvant RT, CT or CRT for locally advanced (radiological T3-T4 or N+) rectal cancer were included in this study. All patients had no evidence of distant metastases and had not received any CRT previously. The study was approved by the scientific review and ethics committee of Shandong Cancer Hospital and Institute. All patients signed informed consents.
All patients received neoadjuvant RT, CT or CRT followed by surgery. Some patients didn’t receive standard neoadjuvant concomitant CRT for local advanced rectal cancer. In our study, to clarify the respect effects of radiation and chemotherapy on TILs, we analyzed some patients with neoadjuvant RT or CT retrospectively. These patients were not able to tolerate standard nCRT because of poor performance or refusal to receive combinatory treatment. Briefly, most patients received 40-45 Gy in 25-28 fractions to the pelvis with/or without a boost of 5.4 Gy to the primary gross tumor using three-dimensional conformal irradiation or a 4-field box technique over 5 weeks. Chemotherapy was administered intravenously alone or concomitant with RT, which was single-agent or two-agents based on fluoropyrimidine. The mean interval between nCRT and surgery was 45.1 ± 5.4 days. The median follow-up period was 57 months. Two survival end-points were evaluated: disease free survival (DFS), calculated from the initiation date of treatment to the date of any evidence of local or systemic cancer recurrence; and overall survival (OS), calculated from the initiation date of treatment to the date of death or censored date of last contact.
Histopathology and tumor regression grading
Histopathological examination was performed by two independent pathologists. Tumor response was evaluated using the tumor regression grade (TRG) system established by Dworak et al. [19] as follows: Grade 0: no regression; Grade 1: minor regression, residual tumor outgrowing fibrosis; Grade 2: moderate regression, few tumor cell fibrosis in 26% to 50% of the tumor mass; Grade 3: major regression, very few tumor cells in fibrotic tissue with or without mucous substance; Grade 4: total regression, no tumor cells, only fibrotic mass. In our present study, TRG 3-4 were defined as “good response” and TRG 0-2 were defined as “poor response” [5].
Immunohistochemistry
Pretreatment biopsy specimens and posttreatment surgically resected specimens of all patients were retrieved to perform the immunohistochemistry. All 4-μm thin sections were deparaffinized, rehydrated and then exposed to antigen retrieval system, which was performed under high pressure for 2 minutes. The sections were stained with primary specific CD3 antibody (clone ZM-0417, ready-to-use; Beijing Zhongshan Golden Bridge Biotechnology Company, Beijing, China) and specific CD8 antibody (clone ZA-0508, ready-to-use; Beijing Zhongshan Golden Bridge Biotechnology Company, Beijing, China) in humidified chamber at 37°C for 60 minutes. Reactive sites were identified by exposure to a secondary goat anti-rabbit antibody (Beijing Zhongshan Golden Bridge Biotechnology Company, Beijing, China) incubated at 37°C for 15 minutes. CD3 and CD8 staining was then visualized by using diaminobenzidine (DAB) and then counterstained in hematoxylin. Finally, sections were dehydrated and mounted.
Evaluations of CD3+TILs and CD8+TILs were carried out by two independent pathologists who were blinded to clinicopathologic information. The densities of CD3+TILs or CD8+TILs were defined as the percentage of tumor stroma containing positive CD3+T lymphocytes or CD8+ T lymphocytes in a manner similar to that of a previous publication [20]. To sub-divide the patients and analyze the predictive effects of TILs, densities of CD3+TILs or CD8+TILs were graded as either high or low according to the mean values for the analyses presented [20].
Statistical analysis
The associations of TILs with clinicopathologic parameters (age, sex, cT, cN and differentiation), including the TRG, were assessed using the chi-squared test. Comparisons of TILs between pretreatment biopsy specimens and posttreatment resected specimens were performed by unpaired t-tests. Patient survival curves were generated by the Kaplan-Meier method and compared by log-rank tests. All the statistical tests were two sided, and considered significant when P ≤ 0.05. Statistical analyses were performed using SPSS_ version 19.0 (IBM SPSS, Chicago, Illinois, USA).
Results
Patient characteristics
A total of 136 patients who underwent neoadjuvant RT (30%), CT (24%) or CRT (46%) and potentially curative resection of rectal cancer were included. There were 75 male and 61 female with a median age of 62 years old. Among the 136 patients, 75 were assessed as clinical T3 (cT3) and 61 as clinical T4 (cT4). Clinical lymph node negative (cLN-) and positive (cLN+) were identified in 44 and 92 patients, respectively. Additionally, 78 patients had differentiated histology and 58 patients had undifferentiated histology. According to the TRG system, there were 64 (47%) patients with good response and 72 (53%) patients with poor response (Table 1).
Table 1.
Clinicopathological characteristics of 136 patients with rectal cancer
| Clinicopathological parameters | Cases (%) |
|---|---|
| Age | |
| < 65 | 64 (47) |
| ≥ 65 | 72 (53) |
| Sex | |
| Male | 75 (55) |
| Female | 61 (45) |
| Histology | |
| Differentiated | 78 (57) |
| Undifferentiated | 58 (43) |
| White cell count | |
| < 4 (×109/L) | 41 (30) |
| 4-8 (×109/L) | 58 (42) |
| > 8 (×109/L) | 37 (28) |
| cT | |
| 3 | 75 (55) |
| 4 | 61 (45) |
| cN | |
| Negative | 44 (32) |
| Positive | 92 (68) |
| Neoadjuvant treatment | |
| Radiotherapy | 40 (30) |
| Chemotherapy | 33 (24) |
| Chemoradiotherapy | 63 (46) |
| TGR | |
| Good response | 64 (47) |
| Poor response | 72 (53) |
Relationships between TILs in pretreatment biopsy specimens and clinical-pathological characteristics
As shown in Figure 1, CD3 and CD8 were clearly stained in the cell membrane of interstitial infiltrates. The average value of CD3+TILs and CD8+TILs in 136 pretreatment biopsy specimens was 30% and 15%, respectively. The CD8+TILs in specimens with cN-tumors were significantly more than those in specimens with cN+ patients (68.2% vs. 48.9%, P = 0.035). Patients with high CD3+TILs seemed to have better N stage; however, the associations were not statistically significant. CD3+ and CD8+TILs expressions were not correlated with age, sex, histology, white blood cells (WBCs) or T stage (Table 2).
Figure 1.
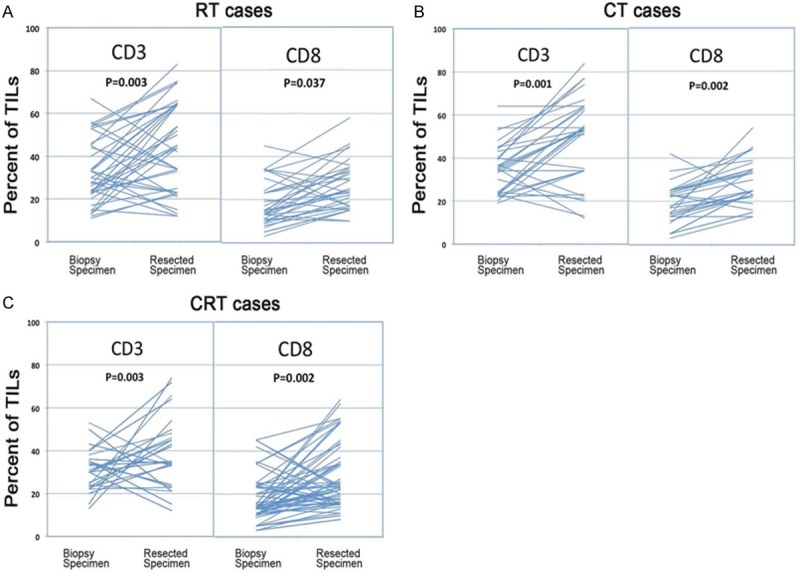
Corresponding CD3+ and CD8+TIL densities in biopsy and surgically resected specimens for all cases. A. Alterations in the CD3+ and CD8+TILs during radiotherapy (RT); B. Alterations in the CD3+ and CD8+TILs during chemotherapy (CT); C. Alterations in the CD3+ and CD8+TILs during chemoradiotherapy (CRT). CD3+ and CD8+ were significantly enhanced during neoadjuvant treatments in all cases. Unpaired t-tests revealed that there were no significant differences between each two of these three groups.
Table 2.
Relationships of TILs in pretreatment biopsy specimens and clinicopathological characteristics in patients with rectal cancer
| Clinicopathological parameters | CD3 | P value | CD8 | P value | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| High | Low | High | Low | |||
| Age | ||||||
| <65 | 37 | 27 | 0.453 | 36 | 28 | 0.807 |
| ≥ 65 | 37 | 35 | 39 | 33 | ||
| Sex | ||||||
| Male | 42 | 33 | 0.680 | 46 | 29 | 0.108 |
| Female | 32 | 29 | 29 | 32 | ||
| Histology | ||||||
| Differentiated | 43 | 35 | 0.846 | 38 | 40 | 0.966 |
| Undifferentiated | 31 | 27 | 30 | 28 | ||
| White cell count | ||||||
| <4 (×109/L) | 25 | 16 | 0.541 | 24 | 17 | 0.419 |
| 4-8 (×109/L) | 31 | 27 | 34 | 24 | ||
| > 8 (×109/L) | 18 | 19 | 17 | 20 | ||
| cT | ||||||
| 3 | 44 | 31 | 0.269 | 46 | 29 | 0.108 |
| 4 | 30 | 31 | 29 | 32 | ||
| cN | ||||||
| Negative | 29 | 15 | 0.063 | 30 | 14 | 0.035* |
| Positive | 45 | 47 | 45 | 47 | ||
Statistically significant.
Associations of TILs between biopsy and resected specimens
After neoadjuvant RT, CT or CRT, densities of CD3+ and CD8+ T lymphocytes were all significantly higher than those before treatment (RT cases: (average CD3+: 33% vs. 45% P = 0.003; CD8+: average 21% vs. 27% P = 0.037); CT cases: (CD3+: average 34% vs. 48% P = 0.001; CD8+: average 19% vs. 28% P = 0.002); CRT cases: (CD3+: average 31% vs. 39% P = 0.003; CD8+: average 18% vs. 25% P = 0.002). In Figure 2A-C, most of the lines showed an upslope. To show the differences among RT, CT and CRT cases, the increases of CD3+ and CD8+TILs in biopsy and resected specimens were compared. Unpaired t-tests revealed that there were no significant differences between each two of these three groups (RT vs. CT vs. CRT: 6.6% vs. 8.6% vs. 8.4% for CD3; 11.0% vs. 6.7% vs. 9.2% for CD8; all P > 0.05), which suggested that there were no differences in the abilities to enhance TILs by RT, CT or CRT in rectal cancer between the groups. Representative microscopic appearances are shown in Figure 1.
Figure 2.
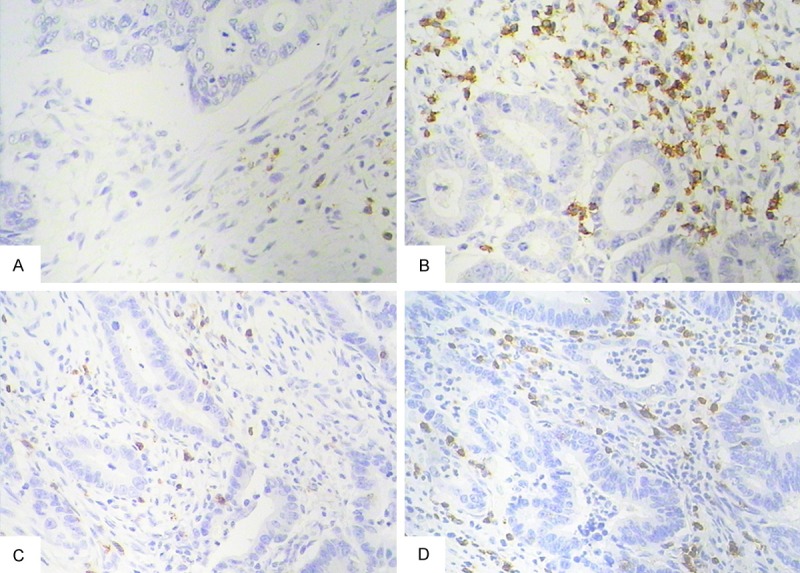
Chemoradiotherapy (CRT)-induced alterations in the microscopic appearance of rectal cancer tissues stained for CD3+ and CD8+ from the same patient. A. CD3+TILs in a pretreatment biopsy specimen; B. Enhanced CD3+TILs in a post-CRT resected specimen; C. CD8+TILs in a pretreatment biopsy specimen; D. Enhanced CD8+TILs in a post-CRT resected specimen (magnification for panels a and b: ×200).
Predictive values to neoadjuvant treatment of TILs
As shown in Table 3, patients who had tumors with cT3 and cN- were more likely to achieve good tumor regression (P = 0.049 and 0.002, respectively). Statistical analyses revealed that pretreatment with CD3+TILs and CD8+TILs were associated with good response (TRG ≥ 3) after neoadjuvant treatment. There were 55.4% patients with high pretreatment CD3+TILs and 37.1% patients with low pretreatment CD3+TILs who achieved good responses (P = 0.033). Patients with high CD8+TILs had better responses to neoadjuvant treatment than patients with low CD8+TILs (56% vs. 36.1%; P = 0.021).
Table 3.
Correlations between clinicopathological parameters and tumor response to neoadjuvant treatment
| Clinicopathological Parameters | Good response |
|---|---|
| High vs. Low CD3+TILs | 55.4% vs. 37.1%; P = 0.033* |
| High vs. Low CD8+TILs | 56% vs. 36.1%; P = 0.021* |
| Age < 65 vs. age ≥ 65 | 45.3% vs. 48.6%; P = 0.700 |
| Male vs. Female gender | 46.6% vs. 47.5%; P = 0.919 |
| Differentiated vs. Undifferentiated history | 53.4% vs. 42.3%; P = 0.198 |
| cT3 vs. cT4 stage | 54.6% vs. 37.7%; P = 0.049* |
| Node negative vs. Node positive | 65.9% vs. 38.0%; P = 0.002* |
Statistically significant.
Prognostic values of TILs
The median follow-up period for the survivors in the study was 57 months (range 23-111). The prognostic values of host characteristics, including TILs, are shown in Table 4. High CD3+TILs and CD8+TILs in pretreatment biopsy specimens were significantly associated with better prognosis. The 5y-DFS of patients with high pretreatment CD3+TILs was significantly higher than that of patients with low CD3+TILs (59% vs. 40%, P = 0.010). Patients with high pretreatment CD3+TILs had significant better 5y-OS than patients with low CD3+TILs (75% vs. 56%, P = 0.019) (Figure 3A). The 5y-DFS and 5y-OS of patients with high pretreatment CD8+TILs were significantly longer than those of patients with low CD8+TILs (P = 0.002, P = 0.003) (Figure 3B). As shown in Table 4, T stage, N stage, neoadjuvant regimens and TRG were associated with DFS (P = 0.033, 0.012, 0.023, and 0.001, respectively). T stage, N stage and TRG were sensitive predictors for OS (P = 0.008, 0.014, and 0.001, respectively). Compared with neoadjuvant RT or CT, patients in neoadjuvant CRT group had longer OS, but the difference was not statistically significant (P = 0.128).
Table 4.
Correlations between clinicopathological parameters and survival in patients with rectal caner
| Parameters | 5-year DFS (%) | P value | 5-year OS (%) | P value |
|---|---|---|---|---|
| Age | ||||
| <65 | 59% | 0.149 | 71% | 0.238 |
| ≥ 65 | 43% | 62% | ||
| Sex | ||||
| Male | 56% | 0.160 | 70% | 0.302 |
| Female | 44% | 62% | ||
| Histology | ||||
| Differentiated | 51% | 0.711 | 69% | 0.622 |
| Undifferentiated | 50% | 65% | ||
| White cell count | ||||
| <4 (×109/L) | 43% | 0.390 | 58% | 0.323 |
| 4-8 (×109/L) | 51% | 72% | ||
| > 8 (×109/L) | 56% | 67% | ||
| cT | ||||
| 3 | 56% | 0.033* | 76% | 0.008* |
| 4 | 44% | 55% | ||
| cN | ||||
| Negative | 65% | 0.012* | 81% | 0.014* |
| Positive | 43% | 59% | ||
| Neo-adjuvant therapy | ||||
| Chemotherapy alone | 36% | 0.023* | 70% | 0.128 |
| Radiotherapy alone | 45% | 48% | ||
| Chemo-radiotherapy | 61% | 74% | ||
| TRG | ||||
| Good response | 64% | 0.001* | 81% | 0.001* |
| Poor response | 38% | 54% | ||
| CD3+TILs | ||||
| High | 59% | 0.010* | 75% | 0.019* |
| Low | 40% | 56% | ||
| CD8+ | ||||
| High | 61% | 0.002* | 77% | 0.003* |
| Low | 37% | 54% |
Statistically significant.
Figure 3.
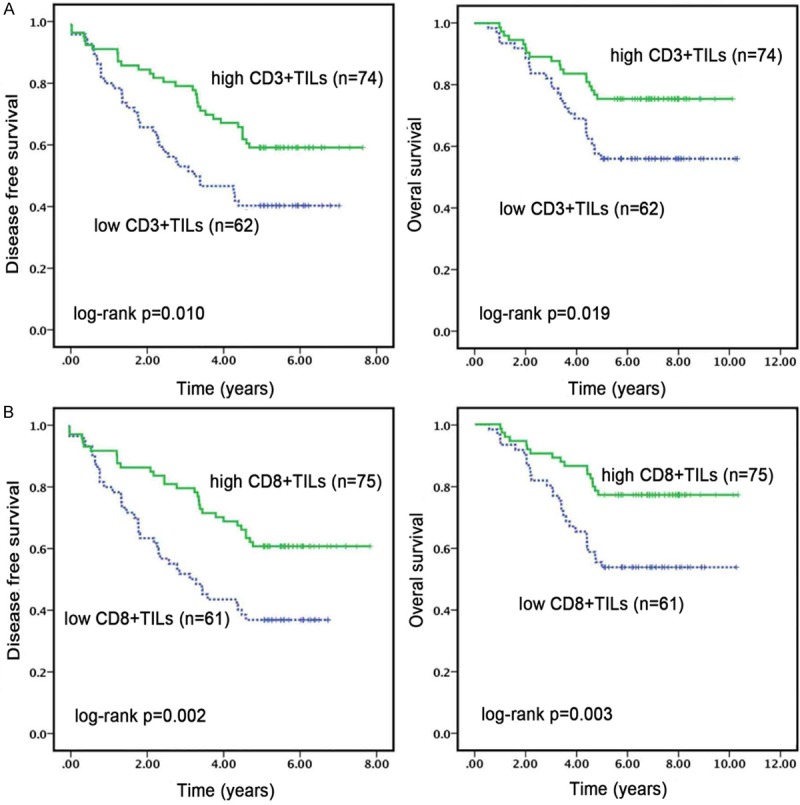
Kaplan-Meier analyses of DFS and OS according to CD3+TILs and CD8+TILs in pretreatment biopsy specimens. Patients with high CD3+TILs (A) and CD8+TILs (B) achieved better DFS and OS than patients with low CD3+ and CD8+TILs.
Discussion
The results of this study demonstrated that local antitumor immunity was enhanced by increased CD3+ and CD8+TILs after neoadjuvant RT, CT or CRT in rectal cancer. These findings were in accordance with the results of previous study, which suggested that TILs were increased after CRT [21]. To the best of our knowledge, this is the first study to evaluate the respective effect of RT, CT and CRT on the numbers and compositions of TIL subsets. The data indicated that CD3+ and CD8+TILs in resected specimens after treatment were significantly higher than those in biopsy specimens in all cases (RT, CT and CRT). The evolution of a local immune response was possibly triggered by the RT- or CT-mediated release of tumor antigens and by chemotaxis toward certain mediators such as proinflammatory cytokine interferon-γ. Benjamin et al. have summarized that the necrotic tumor cell death is induced by multimodal treatments and finally leads to antitumor immunity [22]. According to the data obtained from multiple cancer models, some of the effects of ionizing radiation and chemotherapy contribute to systemic antitumor immunity [23]. A recent example of the abscopal effect in a patient with melanoma treated with ipilimumab and radiotherapy supports this view [24]. Our results of increased TILs during treatment may provide evidences supporting the role of radiotherapy and chemotherapy as immune adjuvants.
Certainly, each tumor treatment modality has its advantages and disadvantages when viewed separately. Improved RT techniques allow a maximum local tumor control by concomitantly minimizing the normal tissue side actions, but do not primarily result in systemic antitumor responses. Low dose CT is beneficial for shifting the tumor microenvironment into an activating one, but may not have the sufficient cytotoxicity to kill the tumor cells. These considerations are highly relevant to decisions on the best approaches to deliver chemoradiation and to integrate it with other more systemic therapies, such as immunotherapy. In this study, there were no differences in the ability to enhance TILs in rectal cancer between RT, CT or CRT. It is known that patients with LARC have better local control after nCRT than those with RT or CT monotherapy [3]. Besides, according to our results, local antitumor immunity was also enhanced after CRT. It demonstrated that nCRT, as the current standard treatment of LARC, was still immune-active and higher doses may be required to suppress the antitumor immunity.
We also found that CD3+ and CD8+TILs in pretreatment specimens were associated with good responses after neoadjuvant RT, CT and CRT, which indicated that tumors attracting T cells were more sensitive to chemoradiation. Our results were consistent with the results of other reports in the literature [18,21]. Basic research studies have demonstrated that RT or CT is more efficient in immunocompetent mice, and that immunosuppression compromised CRT efficacy [25,26]. Growing evidence has indicated that radiation exerts its antitumor activity not only by inducing an irreversible DNA damage, but also by promoting tumor-specific immune response [27]. In addition, preclinical studies have demonstrated that cytotoxic agents induce immune response against tumor cells, which significantly contributes to their antitumor effects [28]. Chemoradiation may promote antitumor effects by further inducing the expression of tumor associated antigens in tumors with a higher number of TILs, because these tumors are originally immunogenic. Our results provided further evidence for a mechanical linkage between tumor response to chemoradiation and host immunity. Given the predictive effect of pretreatment local immunity for chemoradiotherapy, it may indicate chemoradiosensitivity and help to determine whether to undergo additional CRT or have surgery early. In addition, in our results, CD8+ seemed to be a more efficient predictor for nCRT than CD3+. The potential reason may be that CD8+cytotoxic T lymphocytes are directly capable of killing tumor cells; while CD3+ represents the total general T lymphocytes, but not directly target tumor cells. The capability of chemoradiation to kill tumor cells is more related to the CD8+ than the CD3+ subtype. Further studies are required to elucidate the detailed mechanisms.
A large number of studies have assessed the prognostic value of TILs in colorectal cancer [8,14,18,21,29]. Our results demonstrated that CD3+TILs and CD8+TIL subtypes in pretreatment biopsy specimens were significantly associated with DFS and OS. The results were consistent with those of previous studies. Richards et al. have demonstrated that CD3+TIL at the invasive margin and CD8+TIL in the cancer cell nests are strong predictor of survival in colorectal cancer [29]. Shinto et al. have found that post-CRT CD8+ lymphocytes in tumor stroma are independent prognostic factors for rectal cancer [21]. It is speculated that intratumoral T cells can attenuate the metastatic potential of tumor cells by modifying tumor stroma or tumor cells. This suggests that the state of the local adaptive immune response partly determines the time of recurrence and overall survival time. However, T-cell activation is the result of a balance between positive and negative signals. The modulation of signaling via costimulatory or coinhibitory receptors on T cells has proven to be a potent way to affect antitumor immune responses [30]. Ipilimumab, an antibody that blocks the coinhibitory receptor cytotoxic T lymphocyte-associated antigen-4 (CTLA-4), has been approved by FDA for the treatment of unresectable or metastatic melanoma. Other agents that target a coinhibitory receptor, programmed death 1 (PD-1), and its ligand, PD-L1, are in clinical development [30]. With these inhibitory checkpoints, the effect may be more significant when TILs and these checkpoints are used in combination as predictors of prognosis.
There were some limitations of this study. First, single RT or CT before surgery was not standard treatment for local advanced rectal cancer. In our study, to distinguish the respect effect of radiotherapy and chemotherapy on TILs, we analyzed some patients with neoadjuvant RT or CT retrospectively. These patients were not able to tolerate standard neoadjuvant CRT because of poor performance or their decision to refuse to receive combinatory treatment. Secondly, we chose to assess the density of T-cell infiltration by observers rather than utilizing automated imaging software, which may result in variability. However, automated imaging software distinguishes cells by different depths of color without considering the shapes, features and positions. In contrast, in the present study, evaluation of T-cell density was carried out by two independent investigators blinded to clinicopathologic information. They could give a broader examination of full sections, avoiding ecrotic areas and without missing tumor compartments. In view of the heterogeneity of T-cell density within different single sections, we believe that this technique shows more advantage than analysis performed with automated imaging software. Finally, we did not analyze TILs in different areas of the tumor microenvironment, such as invasive margin and cancer cell nests. However, previous studies have suggested that inflammatory cells in all areas of the tumor microenvironment may be important indicators of prognosis, and the density of infiltration is prior to the type and location of inflammatory cells [29,31,32].
To the best of our knowledge, this is the first study demonstrating that RT, CT and CRT can all enhance local immune response by increased TILs. Moreover, there was no significant difference among these three cases. Our results provided further evidences for a mechanical linkage between tumor response to chemoradiotherapy and host immunity. It may be a new potential tool to predict the response of rectal cancer to nCRT, as well as a better surrogate to select nonresponders for an early surgery. In addition, recent preclinical studies have suggested that the combination of chemoradiaion and immune therapies shows significantly better control of tumor than single treatment, but some subpopulations fail to respond. The local tumor immunity may be an effective predictor for these combinations, but the correlation or alteration in the number of tumor infiltrating and circulating T lymphocytes remains to be clarified. These information may provide a more convenient and efficient tool to monitor treatment effects in the future.
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.van Gijn W, Marijnen CA, Nagtegaal ID, Kranenbarg EM, Putter H, Wiggers T, Rutten HJ, Pahlman L, Glimelius B, van de Velde CJ. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011;12:575–582. doi: 10.1016/S1470-2045(11)70097-3. [DOI] [PubMed] [Google Scholar]
- 3.Rödel C, Liersch T, Becker H, Fietkau R, Hohenberger W, Hothorn T, Graeven U, Arnold D, Lang-Welzenbach M, Raab HR, Sülberg H, Wittekind C, Potapov S, Staib L, Hess C, Weigang-Köhler K, Grabenbauer GG, Hoffmanns H, Lindemann F, Schlenska-Lange A, Folprecht G, Sauer R German Rectal Cancer Study Group. Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: initial results of the German CAO/ARO/AIO-04 randomised phase 3 trial. Lancet Oncol. 2012;13:679–687. doi: 10.1016/S1470-2045(12)70187-0. [DOI] [PubMed] [Google Scholar]
- 4.Kuerer HM, Newman LA, Smith TL, Ames FC, Hunt KK, Dhingra K, Theriault RL, Singh G, Binkley SM, Sneige N, Buchholz TA, Ross MI, McNeese MD, Buzdar AU, Hortobagyi GN, Singletary SE. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J. Clin. Oncol. 1999;17:460–469. doi: 10.1200/JCO.1999.17.2.460. [DOI] [PubMed] [Google Scholar]
- 5.Meng X, Wang R, Huang Z, Zhang J, Feng R, Xu X, Zhu K, Dou X, Chen D, Yu J. Human epidermal growth factor receptor-2 expression in locally advanced rectal cancer: association with response to neoadjuvant therapy and prognosis. Cancer Sci. 2014;105:818–824. doi: 10.1111/cas.12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer. 2011;105:93–103. doi: 10.1038/bjc.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fridman WH, Galon J, Pages F, Tartour E, Sautes-Fridman C, Kroemer G. Prognostic and predictive impact of intra- and peritumoral immune infiltrates. Cancer Res. 2011;71:5601–5605. doi: 10.1158/0008-5472.CAN-11-1316. [DOI] [PubMed] [Google Scholar]
- 8.Laghi L, Bianchi P, Miranda E, Balladore E, Pacetti V, Grizzi F, Allavena P, Torri V, Repici A, Santoro A, Mantovani A, Roncalli M, Malesci A. CD3+ cells at the invasive margin of deeply invading (pT3-T4) colorectal cancer and risk of post-surgical metastasis: a longitudinal study. Lancet Oncol. 2009;10:877–884. doi: 10.1016/S1470-2045(09)70186-X. [DOI] [PubMed] [Google Scholar]
- 9.Dieci MV, Criscitiello C, Goubar A, Viale G, Conte P, Guarneri V, Ficarra G, Mathieu MC, Delaloge S, Curigliano G, Andre F. Prognostic value of tumor-infiltrating lymphocytes on residual disease after primary chemotherapy for triple-negative breast cancer: a retrospective multicenter study. Ann Oncol. 2014;25:611–618. doi: 10.1093/annonc/mdt556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horne ZD, Jack R, Gray ZT, Siegfried JM, Wilson DO, Yousem SA, Nason KS, Landreneau RJ, Luketich JD, Schuchert MJ. Increased levels of tumor-infiltrating lymphocytes are associated with improved recurrence-free survival in stage 1A non-small-cell lung cancer. J Surg Res. 2011;171:1–5. doi: 10.1016/j.jss.2011.03.068. [DOI] [PubMed] [Google Scholar]
- 11.Schumacher K, Haensch W, Roefzaad C, Schlag PM. Prognostic significance of activated CD8 (+) T cell infiltrations within esophageal carcinomas. Cancer Res. 2001;61:3932–3936. [PubMed] [Google Scholar]
- 12.Wahlin BE, Sander B, Christensson B, Kimby E. CD8+ T-cell content in diagnostic lymph nodes measured by flow cytometry is a predictor of survival in follicular lymphoma. Clin Cancer Res. 2007;13:388–397. doi: 10.1158/1078-0432.CCR-06-1734. [DOI] [PubMed] [Google Scholar]
- 13.Pelletier MP, Edwardes MD, Michel RP, Halwani F, Morin JE. Prognostic markers in resectable non-small cell lung cancer: a multivariate analysis. Can J Surg. 2001;44:180–188. [PMC free article] [PubMed] [Google Scholar]
- 14.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 15.Order SE. The effects of therapeutic irradiation on lymphocytes and immunity. Cancer. 1977;39:737–743. doi: 10.1002/1097-0142(197702)39:2+<737::aid-cncr2820390708>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 16.Kalbasi A, June CH, Haas N, Vapiwala N. Radiation and immunotherapy: a synergistic combination. J Clin Invest. 2013;123:2756–2763. doi: 10.1172/JCI69219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balermpas P, Rodel F, Weiss C, Rodel C, Fokas E. Tumor-infiltrating lymphocytes favor the response to chemoradiotherapy of head and neck cancer. Oncoimmunology. 2014;3:e27403. doi: 10.4161/onci.27403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yasuda K, Nirei T, Sunami E, Nagawa H, Kitayama J. Density of CD4 (+) and CD8 (+) T lymphocytes in biopsy samples can be a predictor of pathological response to chemoradiotherapy (CRT) for rectal cancer. Radiat Oncol. 2011;6:49. doi: 10.1186/1748-717X-6-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dworak O, Keilholz L, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis. 1997;12:19–23. doi: 10.1007/s003840050072. [DOI] [PubMed] [Google Scholar]
- 20.Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, Rouas G, Francis P, Crown JP, Hitre E, de Azambuja E, Quinaux E, Di Leo A, Michiels S, Piccart MJ, Sotiriou C. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J. Clin. Oncol. 2013;31:860–867. doi: 10.1200/JCO.2011.41.0902. [DOI] [PubMed] [Google Scholar]
- 21.Shinto E, Hase K, Hashiguchi Y, Sekizawa A, Ueno H, Shikina A, Kajiwara Y, Kobayashi H, Ishiguro M, Yamamoto J. CD8+ and FOXP3+ tumor-infiltrating T cells before and after chemoradiotherapy for rectal cancer. Ann Surg Oncol. 2014;21(Suppl 3):S414–421. doi: 10.1245/s10434-014-3584-y. [DOI] [PubMed] [Google Scholar]
- 22.Frey B, Rubner Y, Kulzer L, Werthmoller N, Weiss EM, Fietkau R, Gaipl US. Antitumor immune responses induced by ionizing irradiation and further immune stimulation. Cancer Immunol Immunother. 2014;63:29–36. doi: 10.1007/s00262-013-1474-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst. 2013;105:256–265. doi: 10.1093/jnci/djs629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, Mu Z, Rasalan T, Adamow M, Ritter E, Sedrak C, Jungbluth AA, Chua R, Yang AS, Roman RA, Rosner S, Benson B, Allison JP, Lesokhin AM, Gnjatic S, Wolchok JD. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366:925–931. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plotnikov A, Niego B, Ophir R, Korenstein R, Keisari Y. Effective treatment of mouse metastatic prostate cancer by low electric field enhanced chemotherapy. Prostate. 2006;66:1620–1630. doi: 10.1002/pros.20435. [DOI] [PubMed] [Google Scholar]
- 26.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, Yang H, Amigorena S, Ryffel B, Barrat FJ, Saftig P, Levi F, Lidereau R, Nogues C, Mira JP, Chompret A, Joulin V, Clavel-Chapelon F, Bourhis J, Andre F, Delaloge S, Tursz T, Kroemer G, Zitvogel L. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 27.Gupta A, Sharma A, von Boehmer L, Surace L, Knuth A, van den Broek M. Radiotherapy supports protective tumor-specific immunity. Oncoimmunology. 2012;1:1610–1611. doi: 10.4161/onci.21478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andre F, Dieci MV, Dubsky P, Sotiriou C, Curigliano G, Denkert C, Loi S. Molecular pathways: involvement of immune pathways in the therapeutic response and outcome in breast cancer. Clin Cancer Res. 2013;19:28–33. doi: 10.1158/1078-0432.CCR-11-2701. [DOI] [PubMed] [Google Scholar]
- 29.Richards CH, Roxburgh CS, Powell AG, Foulis AK, Horgan PG, McMillan DC. The clinical utility of the local inflammatory response in colorectal cancer. Eur J Cancer. 2014;50:309–319. doi: 10.1016/j.ejca.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 30.Callahan MK, Wolchok JD. At the bedside: CTLA-4- and PD-1-blocking antibodies in cancer immunotherapy. J Leukoc Biol. 2013;94:41–53. doi: 10.1189/jlb.1212631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nielsen HJ, Hansen U, Christensen IJ, Reimert CM, Brunner N, Moesgaard F. Independent prognostic value of eosinophil and mast cell infiltration in colorectal cancer tissue. J Pathol. 1999;189:487–495. doi: 10.1002/(SICI)1096-9896(199912)189:4<487::AID-PATH484>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 32.Roxburgh CS, McMillan DC. The role of the in situ local inflammatory response in predicting recurrence and survival in patients with primary operable colorectal cancer. Cancer Treat Rev. 2012;38:451–466. doi: 10.1016/j.ctrv.2011.09.001. [DOI] [PubMed] [Google Scholar]


