Abstract
Metastatic soft tissue sarcomas (STS) represent enormous challenges to improve the low survival rate, which is almost the same as past 2 decades ago, although surgery, radiotherapy and radiofrequency ablation has been accepted in the treatment of metastatic STS. Moreover, STS varies between elderly and younger victims in the aspect of diagnoses, prognosis, and treatment strategies. In order to evaluate the role of local treatment in improving prognosis for patients with metastatic STS and select the proper candidates who will benefit from local therapy, a single-institution nearly 50-year experience were collected and reviewed. Finally, we found that local treatments could improve treatment response and survival, but overall survival advantage could not be seen in elderly patients. This conclusion from a single institution could serve as a basis for future prospective multi-institutional large-scale studies.
Keywords: Soft tissue sarcoma, metastasis, local treatment, prognosis
Introduction
Soft tissue sarcomas (STS), arising from almost any embryonic mesodermal tissue, account for nearly 1% of newly diagnosed malignancies annually [1]. Under multimodality treatment, patients with localized disease have estimated 5-year survival rates of about 70% [2-4]. However, metastatic STS still represents enormous challenges to improve the low survival rate [5]. Despite advances in chemotherapy, radiotherapy and surgery, the 3-year survival of patients with metastatic STS is 20-45%, which is almost the same as past 2 decades ago [6-9].
Surgery, based on existing data shown in numerous studies in prolonging survival, is one of the most common therapy option for advanced-stage STS [7,10-14]. However, not all metastatic individuals are fit for surgical treatment. Therefore, it is necessary to select the proper candidates who will benefit from surgical procedures and carefully evaluate for possible resection. Radiotherapy, aiming to adequate local control, remains controversial in ideal treatment sequence with surgery and improvement in survival [15,16]. Nevertheless, no data were available in comparing outcomes of surgery and radiotherapy in treatment of metastatic STS. Although most centers employed combinational regiments of neo-adjuvant or adjuvant treatment for aggressive STS, supporting evidence remains rare [15]. Based on literatures, we would expect that radiotherapy might reduce local recurrence [17]. Nowadays, radiofrequency ablation has also been accepted in the treatment of unresectable metastatic STS [18-21]. Additionally, STS varies between elderly and younger victims in the aspect of diagnoses, histologic subtypes and prognosis [22], which leads to distinct treatment strategies for these 2 group patients suffered from STS.
Our aim of this study is to determine whether local treatment (including surgery, radiotherapy and radiofrequency ablation) is critical in improving prognosis for patients with metastatic STS and select the proper candidates who will benefit from local therapy.
Method
This study was approved by the institutional review board of Sun Yat-sen University Cancer Center (SYSUCC) and informed consent was obtained from each participant. Chart review was performed on 154 consecutive patients who suffered from metastatic STS with metastases between July 1965 and May 2013. Only patients with STS were included in current study, whereas those with osteosarcoma were not. Under these criteria, 142 of the 154 patients were enrolled in the final analysis, which meant 12 patients with STS were excluded from analysis because of incomplete records. Characteristics of patients and tumors at initial diagnosis of STS and development of metastases were collected and tested for relationships with progress free survival (PFS) and overall survival (OS), including the following factors: patient age, gender (male vs. female), primary tumor size, and tumor depth (superficial vs. deep) at diagnosis. In current study, WHO classification [23] was used for determination of pathological diagnosis and tumor grade. In addition, elderly, and younger patients were defined as age at diagnosis > 60 years, or < 18 years, respectively [24]. All data were reviewed and confirmed by two independent consultant pathologists and radiologists.
Local Treatment defined as underwent one or more procedure of surgery, radiotherapy or radiofrequency ablation. In detail, treatment regiments varied, including bilateral metastasis sternotomy, thoracotomy, and thorascopic surgery in surgery treatment; conventional fractionated radiotherapy and SBRT with different dose in radiotherapy; and different procedure of power and time in radiofrequency ablation. Furthermore, response to treatment was classified according to RECIST criteria (version 1.1) [25].
Statistical analysis
PFS and OS curves were estimated using the Kaplan-Meier method. PFS was calculated from the date of metastasis treatment to the time of metastasis progression or end of follow-up, and similarly OS from the date of initial diagnosis to the time of death reported or end of follow-up. Risk factors of PFS and OS were then assessed by univariate analysis with log rank test and multivariate analysis with Cox proportional hazards regression. Next, multivariate analysis was performed using Cox proportional hazard model. Cut-off value of PFS was established by the receiver operating characteristic (ROC) curve statistical analyses. Additionally, all models for survival analyses were adjusted for age at diagnosis. P < 0.05 was considered to be significant in all statistical analyses. Data analysis was performed using SPSS 18.0 (PASW Statistics 18) for Windows (SPSS Inc, Chicago, IL).
Result
142 of 154 patients with metastatic STS were eligible for the final analysis. In this group of 142 patients, the mean age was 44.35 years (range: 5-71 years, median 47.5 years); 28 patients (19.7%) belong to elderly group, 114 patients (80.3%) to younger group. 60 patients were male (42.3%) and 82 female (57.7%). Explicitly, the tumors pathological subtypes included so-called fibrohistiocytic tumors in 22 patients (15.5%), undifferentiated sarcomas in 96 (67.6%), smooth muscle tumors in 22 (15.5%), and fibroblastic/myofibroblastic tumors in 2 (1.4%). The mean follow-up for survivors as of December 2014 was 71.05 months (range: 2.97-476.17 months, median 49.38 months). Besides, the mean tumor size at diagnosis was 6.68 cm (range 0.5-20 cm, median 5.5 cm). 79 patients (55.6%) underwent local treatment, whereas 63 patients (44.4%) not (Table 1).
Table 1.
Clinicopathologic characteristics of patients with metastatic STS
| Characteristic | Patients with metastatic STS (n = 142) | |
|---|---|---|
| Age, yrs | 47.5† (range: 5-71) | |
| Gender (%) | ||
| Male | 60 | 42.3% |
| Female | 82 | 57.7% |
| Primary tumor size (cm) | 5.5† (range: 0.5-20) | |
| Primary tumor depth (%) | ||
| Superficial | 44 | 31% |
| Deep | 98 | 69% |
| Pathological subtypes (%) | ||
| So-called fibrohistiocytic tumors | 22 | 15.5% |
| Undifferentiated sarcomas | 96 | 67.6% |
| Smooth muscle tumors | 22 | 15.5% |
| Fibroblastic/Myofibroblastic tumors | 2 | 1.4% |
| Pathological grade (%) | ||
| 1 | 9 | 6.3% |
| 2 | 13 | 9.2% |
| 3 | 120 | 84.5% |
| Follow-up (months) | ||
| Median | 49.38 | |
| Range | 2.97-476.17 | |
| Mean | 71.05 | |
| Local Treatment (%) | ||
| With | 79 | 55.6% |
| Without | 63 | 44.4% |
Median values are listed.
For treatment with metastatic tumor, of the 79 patients underwent local therapy in this study, 48 (60.8%) underwent surgery, 11 (13.9%) radiotherapy, 7 (8.9%) patients underwent radiofrequency ablation and 13 (16.4%) both surgery and radiotherapy.
After metastases treatment, 81 patients (57.1%) responded with CR (including radical resection), 29 (20.4%) with PR, 6 (4.2%) with SD, and 26 (18.3%) with PD. Response varies, but significant difference could be observed between patients underwent local treatment or not, although no statistical differences were seen in different sarcoma types (Table 2).
Table 2.
Response to metastases treatment of patients with/without local treatment
| Response | With local treatment (N = 79) | Without local treatment (N = 63) |
|---|---|---|
| CR | 54 (68.4%) | 27 (42.9%) |
| PR | 13 (16.4%) | 16 (25.4%) |
| SD | 1 (1.3%) | 5 (7.9%) |
| PD | 11 (13.9%) | 15 (23.8%) |
†: Higher proportion of patients responded with CR and lower proportion with PR, SD and PD in with-local-treatment group than without-local-treatment group (P = 0.012).
Univariate analysis showed that age (P = 0.238), gender (P = 0.783), size of primary tumor (P = 0.425), tumor depth (P = 0.484), pathological subtypes (P = 0.861) and pathological grade (P = 0.965) did not have any significant impact on OS. Median OS was 2411 days and 28.2% of the patients were alive without disease, 25.4% were alive with disease, 45.8% dies of disease, while 0.7% (1 patients) died from other causes (heart disease). The overall 1-, 3- and 5-year OS rates were 88%, 61% and 44% each, respectively (Figure 1).
Figure 1.
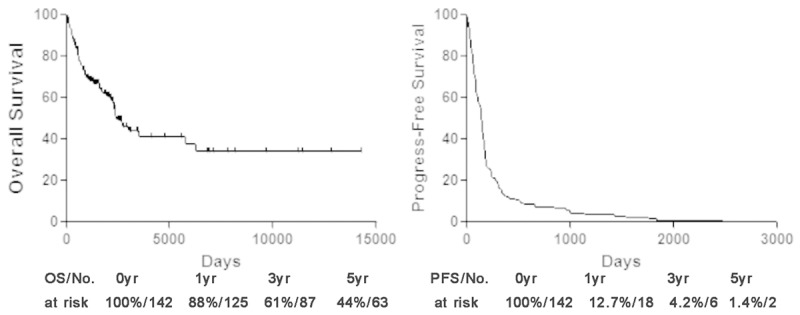
OS and PFS of patients with metastatic STS. PFS progress-free survival, OS overall survival.
Similarly, no significant impact on PFS when analyzing with age (P = 0.801), gender (P = 0.309), size of primary tumor (P = 0.427), tumor depth (P = 0.404), pathological subtypes (P = 0.796) or pathological grade (P = 0.900). Median PFS was 147 days and the overall 1-, 3- and 5-year PFS rates were 12.7%, 4.2% and 1.4% each, respectively (Figure 1).
OS was significantly worse in the without local treatment group (median OS 1638 days) than the local treatment group (median OS 6262 days) (P < 0.001). Likewise, patients without local treatment had a significantly worse PFS (median PFS 74 days) than those with local treatment (median PFS 195 days) (P < 0.001). The OS and PFS curves for the two groups are shown in Figure 2.
Figure 2.
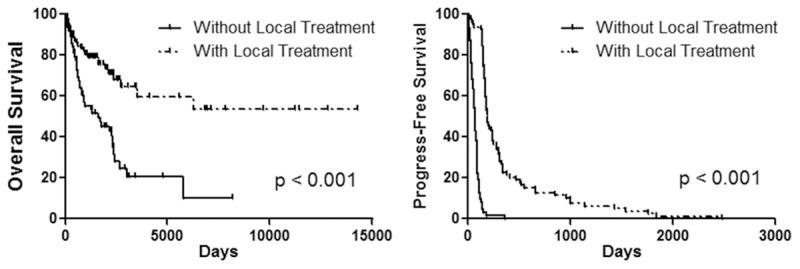
OS and PFS of patients with/without local treatment. PFS progress-free survival, OS overall survival.
Importantly, although the benefit for PFS of local treatment could be observed in both elder and younger group, the benefit for OS of local treatment was not present in elder patients group (P = 0.7066, Figure 3).
Figure 3.
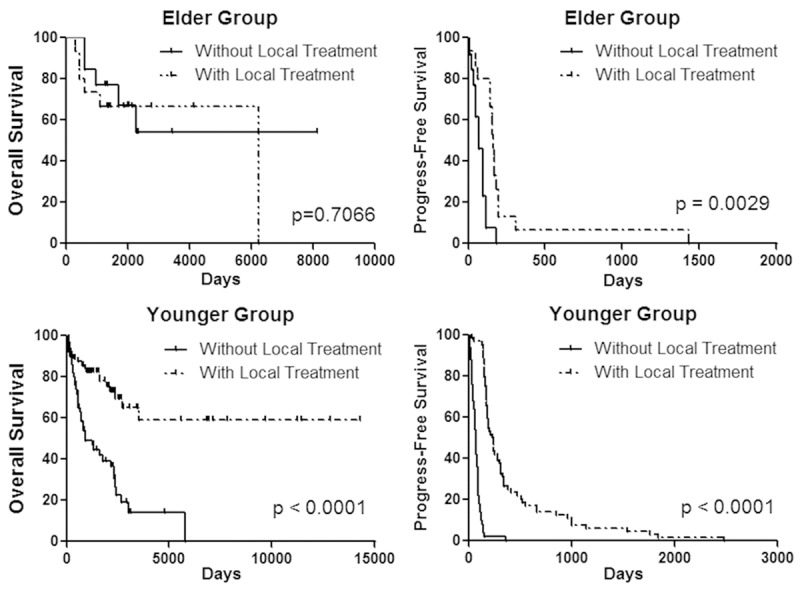
OS and PFS of patients with/without local treatment in different age group. PFS progress-free survival, OS overall survival.
Discussion
Here we presented series of patients representing a single-institution nearly 50-year experience in the management of metastatic STS. As an important subgroup of STS [26,27], metastatic STS still have an unsatisfying prognosis, despite of continuous treatment development [28-30]. Previous studies reported little prognosis improvement of metastatic STS in last decades [28,30]. This is why we set to determine whether local treatment (including surgery, radiotherapy and radiofrequency ablation) is useful in improving prognosis for patients with metastatic STS, and select the proper candidates who will benefit from local therapy.
Although it is generally considered to be incurable of metastatic diseases, patients underwent surgical resection with pulmonary metastatic STS has been reported a relatively remarkable proportion of long-term survivors, which leads surgical approaches becoming a cornerstone of management of pulmonary metastatic STS [31]. Previous studies in lung metastases of sarcoma indicated the utility and a statistically better OS in those underwent aggressive surgical approaches [7,14,32]. Several studies [10,12] even showed a curable subset of patients if a complete response of metastatic disease could be achieved by surgery. Although phase III studies comparing surgical procedures to other options in metastatic sarcoma are still lacking, an advantage survival for aggressive resection in these patients was supported by substantial retrospective data.
However, both physiologically and medically preoperative assessments are key to identifying patients might benefit most from surgery of metastatic STS. RFA or radiotherapy would also provide acceptable local control, thus representing reasonable alternative to surgery for oncological inoperable patients.
Radiotherapy has been proved to serve a consistent role in reducing local recurrence rate and a trend in survival advantage, thus providing an additional option in effective local disease control [33]. Although conformal treatment techniques have been in use for many decades with affordable toxicities, continuous technologic advances, including intensity-modulated radiotherapy and proton beam radiotherapy, could minimize normal tissue exposure and decrease late effects [34]. Additionally, due to the reason that most patients could not be suitable for repeating thoracotomies, it would be reasonable to choose radiotherapy as a more safe and effective method for achieving a similar benefit, especially for patients with restricted cardiopulmonary reserve or unsatisfying performance status when disease recurred again [35-37].
RF ablation, another relatively safe and effective therapeutic options, has also been accepted in patients with unresectable primary and metastatic diseases, even in selected elderly patients and advantaging trends in survival have been observed in some literatures [18-21,38,39].
Both systemic and local treatment have important contribution to survival improvement [29,40]. The efficacy would be better when local treatment of metastatic STS companied with effective systemic treatment. Moreover, multidisciplinary treatment combining local and systemic treatment should be highly recommended [41]. Nonetheless, local treatment remains a remarkable and challenging therapeutic issue in metastatic STS [26,42].
Although evidence of local treatment proved the efficacy in metastases therapy, the role of aggressive local treatment remains controversial in elderly patients [43,44]. Only few literatures concerning the management of metastatic STS in elderly patients, but reports indicated different therapy strategy should be adopted because of widely differences in the aspect of life expectancy and tolerance for aggressive therapeutic regimens [45,46].
Several limitations remain in this study. First, all the data were retrospectively collected, thus clinical and survival comparisons might be influenced by selection bias due to its retrospective nature. Second, a relatively small number of elder group were examined in this study, due to the reason that metastatic STS are extremely rare. It is substantial that the result of local treatment not improving OS in elder group might be caused by a Type II error, although it has been showed to be sufficient number in elder group to identify the significance of PFS improvement. Third, local treatment regimens varied among the retrospectively reviewed patients, which weakens the strength of our conclusions.
In current study, local treatments were found to be effective and significant procedures in achieving better treatment response and improving both OS and PFS for patients with metastatic STS. Remarkably, elder metastatic STS patients should be carefully assessed before local treatment. Although PFS was extended under local treatment, the improvement of OS could not be observed. This conclusion from a single institution could serve as a basis for future prospective multi-institutional large-scale studies.
Acknowledgements
We wish to thank Prof. Shan Yan from Department of English, School of Foreign Languages (SYSU) for language editing.
Disclosure of conflict of interest
The authors declare no competing interests.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Ludwig JA. Ewing sarcoma: historical perspectives, current state-of-the-art, and opportunities for targeted therapy in the future. Curr Opin Oncol. 2008;20:412–418. doi: 10.1097/CCO.0b013e328303ba1d. [DOI] [PubMed] [Google Scholar]
- 3.Cotterill SJ, Ahrens S, Paulussen M, Jurgens HF, Voute PA, Gadner H, Craft AW. Prognostic factors in Ewing’s tumor of bone: analysis of 975 patients from the European Intergroup Cooperative Ewing’s Sarcoma Study Group. J. Clin. Oncol. 2000;18:3108–3114. doi: 10.1200/JCO.2000.18.17.3108. [DOI] [PubMed] [Google Scholar]
- 4.Subbiah V, Anderson P, Lazar AJ, Burdett E, Raymond K, Ludwig JA. Ewing’s sarcoma: standard and experimental treatment options. Curr Treat Options Oncol. 2009;10:126–140. doi: 10.1007/s11864-009-0104-6. [DOI] [PubMed] [Google Scholar]
- 5.Corey RM, Swett K, Ward WG. Epidemiology and survivorship of soft tissue sarcomas in adults: a national cancer database report. Cancer Med. 2014;3:1404–1415. doi: 10.1002/cam4.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsuchiya H, Kanazawa Y, Abdel-Wanis ME, Asada N, Abe S, Isu K, Sugita T, Tomita K. Effect of timing of pulmonary metastases identification on prognosis of patients with osteosarcoma: the Japanese Musculoskeletal Oncology Group study. J. Clin. Oncol. 2002;20:3470–3477. doi: 10.1200/JCO.2002.11.028. [DOI] [PubMed] [Google Scholar]
- 7.Briccoli A, Rocca M, Salone M, Bacci G, Ferrari S, Balladelli A, Mercuri M. Resection of recurrent pulmonary metastases in patients with osteosarcoma. Cancer. 2005;104:1721–1725. doi: 10.1002/cncr.21369. [DOI] [PubMed] [Google Scholar]
- 8.Kempf-Bielack B, Bielack SS, Jurgens H, Branscheid D, Berdel WE, Exner GU, Gobel U, Helmke K, Jundt G, Kabisch H, Kevric M, Klingebiel T, Kotz R, Maas R, Schwarz R, Semik M, Treuner J, Zoubek A, Winkler K. Osteosarcoma relapse after combined modality therapy: an analysis of unselected patients in the Cooperative Osteosarcoma Study Group (COSS) J. Clin. Oncol. 2005;23:559–568. doi: 10.1200/JCO.2005.04.063. [DOI] [PubMed] [Google Scholar]
- 9.Carter SR, Grimer RJ, Sneath RS, Matthews HR. Results of thoracotomy in osteogenic sarcoma with pulmonary metastases. Thorax. 1991;46:727–731. doi: 10.1136/thx.46.10.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeMatteo RP, Shah A, Fong Y, Jarnagin WR, Blumgart LH, Brennan MF. Results of hepatic resection for sarcoma metastatic to liver. Ann Surg. 2001;234:540–548. doi: 10.1097/00000658-200110000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bauer S, Hartmann JT. Locally advanced and metastatic sarcoma (adult type) including gastrointestinal stromal tumors. Crit Rev Oncol Hematol. 2006;60:112–130. doi: 10.1016/j.critrevonc.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 12.Abdalla EK, Pisters PW. Metastasectomy for limited metastases from soft tissue sarcoma. Curr Treat Options Oncol. 2002;3:497–505. doi: 10.1007/s11864-002-0069-1. [DOI] [PubMed] [Google Scholar]
- 13.Bacci G, Mercuri M, Briccoli A, Ferrari S, Bertoni F, Donati D, Monti C, Zanoni A, Forni C, Manfrini M. Osteogenic sarcoma of the extremity with detectable lung metastases at presentation. Results of treatment of 23 patients with chemotherapy followed by simultaneous resection of primary and metastatic lesions. Cancer. 1997;79:245–254. [PubMed] [Google Scholar]
- 14.Snyder CL, Saltzman DA, Ferrell KL, Thompson RC, Leonard AS. A new approach to the resection of pulmonary osteosarcoma metastases. Results of aggressive metastasectomy. Clin Orthop Relat Res. 1991:247–253. [PubMed] [Google Scholar]
- 15.Pisters PW, O’Sullivan B, Maki RG. Evidence-based recommendations for local therapy for soft tissue sarcomas. J. Clin. Oncol. 2007;25:1003–1008. doi: 10.1200/JCO.2006.09.8525. [DOI] [PubMed] [Google Scholar]
- 16.Prosnitz LR, Maguire P, Anderson JM, Scully SP, Harrelson JM, Jones EL, Dewhirst M, Samulski TV, Powers BE, Rosner GL, Dodge RK, Layfield L, Clough R, Brizel DM. The treatment of high-grade soft tissue sarcomas with preoperative thermoradiotherapy. Int J Radiat Oncol Biol Phys. 1999;45:941–949. doi: 10.1016/s0360-3016(99)00272-2. [DOI] [PubMed] [Google Scholar]
- 17.Blakely ML, Spurbeck WW, Pappo AS, Pratt CB, Rodriguez-Galindo C, Santana VM, Merchant TE, Prichard M, Rao BN. The impact of margin of resection on outcome in pediatric nonrhabdomyosarcoma soft tissue sarcoma. J Pediatr Surg. 1999;34:672–675. doi: 10.1016/s0022-3468(99)90353-6. [DOI] [PubMed] [Google Scholar]
- 18.Dupuy DE, Zagoria RJ, Akerley W, Mayo-Smith WW, Kavanagh PV, Safran H. Percutaneous radiofrequency ablation of malignancies in the lung. AJR Am J Roentgenol. 2000;174:57–59. doi: 10.2214/ajr.174.1.1740057. [DOI] [PubMed] [Google Scholar]
- 19.Suh RD, Wallace AB, Sheehan RE, Heinze SB, Goldin JG. Unresectable pulmonary malignancies: CT-guided percutaneous radiofrequency ablation--preliminary results. Radiology. 2003;229:821–829. doi: 10.1148/radiol.2293021756. [DOI] [PubMed] [Google Scholar]
- 20.Akeboshi M, Yamakado K, Nakatsuka A, Hataji O, Taguchi O, Takao M, Takeda K. Percutaneous radiofrequency ablation of lung neoplasms: initial therapeutic response. J Vasc Interv Radiol. 2004;15:463–470. doi: 10.1097/01.rvi.0000126812.12853.77. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura T, Matsumine A, Yamakado K, Matsubara T, Takaki H, Nakatsuka A, Takeda K, Abo D, Shimizu T, Uchida A. Lung radiofrequency ablation in patients with pulmonary metastases from musculoskeletal sarcomas [corrected] . Cancer. 2009;115:3774–3781. doi: 10.1002/cncr.24420. [DOI] [PubMed] [Google Scholar]
- 22.Ferrari A, Dileo P, Casanova M, Bertulli R, Meazza C, Gandola L, Navarria P, Collini P, Gronchi A, Olmi P, Fossati-Bellani F, Casali PG. Rhabdomyosarcoma in adults. A retrospective analysis of 171 patients treated at a single institution. Cancer. 2003;8:71–580. doi: 10.1002/cncr.11550. [DOI] [PubMed] [Google Scholar]
- 23.Rosenberg AE. WHO Classification of Soft Tissue and Bone, fourth edition: summary and commentary. Curr Opin Oncol. 2013;25:571–573. doi: 10.1097/01.cco.0000432522.16734.2d. [DOI] [PubMed] [Google Scholar]
- 24.Martin-Ponce E, Hernandez-Betancor I, Gonzalez-Reimers E, Hernandez-Luis R, Martinez-Riera A, Santolaria F. Prognostic value of physical function tests: hand grip strength and six-minute walking test in elderly hospitalized patients. Sci Rep. 2014;4:7530. doi: 10.1038/srep07530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 26.Breneman JC, Lyden E, Pappo AS, Link MP, Anderson JR, Parham DM, Qualman SJ, Wharam MD, Donaldson SS, Maurer HM, Meyer WH, Baker KS, Paidas CN, Crist WM. Prognostic factors and clinical outcomes in children and adolescents with metastatic rhabdomyosarcoma--a report from the Intergroup Rhabdomyosarcoma Study IV. J. Clin. Oncol. 2003;21:78–84. doi: 10.1200/JCO.2003.06.129. [DOI] [PubMed] [Google Scholar]
- 27.Rodeberg D, Arndt C, Breneman J, Lyden E, Donaldson S, Paidas C, Andrassy R, Meyer W, Wiener E. Characteristics and outcomes of rhabdomyosarcoma patients with isolated lung metastases from IRS-IV. J Pediatr Surg. 2005;40:256–262. doi: 10.1016/j.jpedsurg.2004.09.045. [DOI] [PubMed] [Google Scholar]
- 28.Carli M, Colombatti R, Oberlin O, Bisogno G, Treuner J, Koscielniak E, Tridello G, Garaventa A, Pinkerton R, Stevens M. European intergroup studies (MMT4-89 and MMT4-91) on childhood metastatic rhabdomyosarcoma: final results and analysis of prognostic factors. J. Clin. Oncol. 2004;22:4787–4794. doi: 10.1200/JCO.2004.04.083. [DOI] [PubMed] [Google Scholar]
- 29.Crist W, Gehan EA, Ragab AH, Dickman PS, Donaldson SS, Fryer C, Hammond D, Hays DM, Herrmann J, Heyn R, Et A. The Third Intergroup Rhabdomyosarcoma Study. J. Clin. Oncol. 1995;13:610–630. doi: 10.1200/JCO.1995.13.3.610. [DOI] [PubMed] [Google Scholar]
- 30.Koscielniak E, Harms D, Henze G, Jurgens H, Gadner H, Herbst M, Klingebiel T, Schmidt BF, Morgan M, Knietig R, Treuner J. Results of treatment for soft tissue sarcoma in childhood and adolescence: a final report of the German Cooperative Soft Tissue Sarcoma Study CWS-86. J. Clin. Oncol. 1999;17:3706–3719. doi: 10.1200/JCO.1999.17.12.3706. [DOI] [PubMed] [Google Scholar]
- 31.Karnak I, Emin SM, Kutluk T, Tanyel FC, Buyukpamukcu N. Pulmonary metastases in children: an analysis of surgical spectrum. Eur J Pediatr Surg. 2002;12:151–158. doi: 10.1055/s-2002-32728. [DOI] [PubMed] [Google Scholar]
- 32.Kandioler D, Kromer E, Tuchler H, End A, Muller MR, Wolner E, Eckersberger F. Long-term results after repeated surgical removal of pulmonary metastases. Ann Thorac Surg. 1998;65:909–912. doi: 10.1016/s0003-4975(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 33.Gadd MA, Casper ES, Woodruff JM, McCormack PM, Brennan MF. Development and treatment of pulmonary metastases in adult patients with extremity soft tissue sarcoma. Ann Surg. 1993;218:705–712. doi: 10.1097/00000658-199312000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rehders A, Hosch SB, Scheunemann P, Stoecklein NH, Knoefel WT, Peiper M. Benefit of surgical treatment of lung metastasis in soft tissue sarcoma. Arch Surg. 2007;142:70–76. doi: 10.1001/archsurg.142.1.70. [DOI] [PubMed] [Google Scholar]
- 35.Weiser MR, Downey RJ, Leung DH, Brennan MF. Repeat resection of pulmonary metastases in patients with soft-tissue sarcoma. J Am Coll Surg. 2000;191:184–191. doi: 10.1016/s1072-7515(00)00306-9. [DOI] [PubMed] [Google Scholar]
- 36.Blackmon SH, Shah N, Roth JA, Correa AM, Vaporciyan AA, Rice DC, Hofstetter W, Walsh GL, Benjamin R, Pollock R, Swisher SG, Mehran R. Resection of pulmonary and extrapulmonary sarcomatous metastases is associated with long-term survival. Ann Thorac Surg. 2009;88:877–885. doi: 10.1016/j.athoracsur.2009.04.144. [DOI] [PubMed] [Google Scholar]
- 37.Stragliotto CL, Karlsson K, Lax I, Rutkowska E, Bergh J, Strander H, Blomgren H, Friesland S. A retrospective study of SBRT of metastases in patients with primary sarcoma. Med Oncol. 2012;29:3431–3439. doi: 10.1007/s12032-012-0256-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ding JH, Chua TC, Glenn D, Morris DL. Feasibility of ablation as an alternative to surgical metastasectomy in patients with unresectable sarcoma pulmonary metastases. Interact Cardiovasc Thorac Surg. 2009;9:1051–1053. doi: 10.1510/icvts.2009.218743. [DOI] [PubMed] [Google Scholar]
- 39.von Meyenfeldt EM, Prevoo W, Peyrot D, Lai AFN, Burgers SJ, Wouters MW, Klomp HM. Local progression after radiofrequency ablation for pulmonary metastases. Cancer. 2011;117:3781–3787. doi: 10.1002/cncr.25958. [DOI] [PubMed] [Google Scholar]
- 40.Maurer HM, Beltangady M, Gehan EA, Crist W, Hammond D, Hays DM, Heyn R, Lawrence W, Newton W, Ortega J, Et A. The Intergroup Rhabdomyosarcoma Study-I. A final report. Cancer. 1988;61:209–220. doi: 10.1002/1097-0142(19880115)61:2<209::aid-cncr2820610202>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 41.Dantonello TM, Winkler P, Boelling T, Friedel G, Schmid I, Mattke AC, Ljungman G, Bielack SS, Klingebiel T, Koscielniak E. Embryonal rhabdomyosarcoma with metastases confined to the lungs: report from the CWS Study Group. Pediatr Blood Cancer. 2011;56:725–732. doi: 10.1002/pbc.22862. [DOI] [PubMed] [Google Scholar]
- 42.Raney B, Anderson J, Breneman J, Donaldson SS, Huh W, Maurer H, Michalski J, Qualman S, Ullrich F, Wharam M, Meyer W. Results in patients with cranial parameningeal sarcoma and metastases (Stage 4) treated on Intergroup Rhabdomyosarcoma Study Group (IRSG) Protocols II-IV, 1978-1997: report from the Children’s Oncology Group. Pediatr Blood Cancer. 2008;51:17–22. doi: 10.1002/pbc.21492. [DOI] [PubMed] [Google Scholar]
- 43.Ginsberg RJ, Hill LD, Eagan RT, Thomas P, Mountain CF, Deslauriers J, Fry WA, Butz RO, Goldberg M, Waters PF, Et A. Modern thirtyday operative mortality for surgical resections in lung cancer. J Thorac Cardiovasc Surg. 1983;86:654–658. [PubMed] [Google Scholar]
- 44.Deslauriers J, Ginsberg RJ, Piantadosi S, Fournier B. Prospective assessment of 30-day operative morbidity for surgical resections in lung cancer. Chest. 1994;106:329S–330S. doi: 10.1378/chest.106.6_supplement.329s. [DOI] [PubMed] [Google Scholar]
- 45.Osaka S, Sugita H, Osaka E, Yoshida Y, Ryu J. Surgical management of malignant soft tissue tumours in patients aged 65 years or older. J Orthop Surg (Hong Kong) 2003;11:28–33. doi: 10.1177/230949900301100107. [DOI] [PubMed] [Google Scholar]
- 46.Torigoe T, Terakado A, Suehara Y, Kurosawa H, Yazawa Y, Takagi T. Bone versus soft-tissue sarcomas in the elderly. J Orthop Surg (Hong Kong) 2010;18:58–62. doi: 10.1177/230949901001800113. [DOI] [PubMed] [Google Scholar]


