Highlights
-
•
Duodenal metastasis.
-
•
Melena and microcytic anemia.
-
•
Lung carcinoma.
Keywords: Melena, Duodenal metastasis, Lung adenocarcinoma, Endoscopic resection
Abstract
Introduction
We report a rare case of duodenal metastasis from primary lung adenocarcinoma presented with history of melena and weight loss.
Presentation of case
A 52-year-old smoker man presented with six months history of epigastric pain, melena and weight loss. Esophago-gastroduodenoscopy revealed a 10 mm ulcerative lesion in the fourth part of duodenum. Histopathology of resected lesion showed poorly differentiated adenocarcinoma. Tumor cells showed immunopositivity for cytokeratin-7 (CK7), thyroid transcription factor 1 (TTF-1), and immunonegativity for CK20, Villin, CDX2 and thyroglobulin, supporting the diagnosis of metastatic adenocarcinoma of the lung origin. Computed tomography (CT) of chest revealed left hilar mass encasing the main pulmonary artery associated with ipsilateral hilar and contralateral mediastinal lymphadenopathy. Bronchoscopy assisted biopsy of lung mass confirmed the diagnosis of primary adenocarcinoma. Patient was staged as T4N3M1. After the resection of duodenal metastasis followed by three cycles of cisplatinum based chemotherapy with Bevacizumab, melena resolved completely.
Discussion
Duodenal metastases from lung adenocarcinoma are extremely uncommon, and rarely produce symptoms. Most of cases require duodenectomy or pancreatico-duodenectomy for symptomatic relief. For smaller duodenal metastatic lesions (≤1 cm) endoscopic resection is a feasible therapeutic option.
Conclusion
Although rare, duodenal metastasis from lung adenocarcinoma should also be included in the differential diagnosis of melena. Smaller lesions (≤1 cm) can safely be managed with endoscopic resection.
1. Introduction
Small bowel as initial site of distant metastasis is relatively rare clinical entity, and mostly has been reported with colon, uterus, cervix, ovaries, and breast malignancies [1]. Distant metastases from lung cancer are usually found in the adrenal glands, bone, liver, and brain; however, metastasis in the small bowel is extremely uncommon [2]. Among small bowel metastasis, the jejunum is the most frequent site of involvement (50.9%), followed by the ileum (33.3%), and the duodenum (15.8%) [3]. Duodenal metastases rarely show any symptoms; however, duodenal involvement of lung cancer can elicit melena, hypochromic microcytic anemia, upper gastrointestinal (GI) bleeding, malabsorption, intussusception and obstruction [4,5].
Herein we report our experience with a case of melena and weight loss secondary to metastatic lung adenocarcinoma at the time of presentation.
2. Presentation of case
A 52-year-old Syrian male patient presented with the six months history of epigastric pain and melena. He also complained of anorexia, lethargy, and weight loss of 4 kilograms over past four months. Epigastric pain was dull in nature, aggravated by food intake, and it had increased in intensity over two weeks, for which he was taking oxycodone/acetaminophen, but minimal pain relief. His previous medical and surgical history was unremarkable. He was active smoker with one pack a day for 15 years; however, he denied any alcohol consumption.
On physical examination, he was found in good general condition, and his vitals were stable. On abdominal examination, there was mild epigastric tenderness without any rigidity, guarding, or rebound tenderness. The rest of systemic examination was unremarkable.
Complete blood count (CBC) showed hemoglobin (Hb) 8.4 gm/dl ↓; mean corpuscular volume (MCV) 75 femtoliters (fL) ↓; mean corpuscular hemoglobin (MCH) 24 picograms (pg)↓, white blood cells (WBC) 7400/μl; red blood cells (RBCs) 4 × 106/μl; and platelets 356.000/μl. Liver and renal function tests were within normal limits. Fecal occult blood (FOB) test was found positive. Two units of packed RBCs were transfused to the patient before elective esophago-gastroduodenoscopy and colonoscopy. Esophago-gastroduodenoscopy revealed a 10 mm ulcerative lesion in the fourth part of duodenum with no bleeding, which was resected with cold forceps (Fig. 1). The examination of esophagus, stomach and gastroesophageal junction appeared normal, and colonoscopy was also unremarkable. Histopathology of resected duodenal lesion showed duodenal mucosal ulceration beneath of which there was subepithelial tumor infiltration, and necrosis. The neoplasm had nests, cords and single cell growth pattern in addition to glandular formations. Tumor cells were polygonal shaped with high nuclear/cytoplasmic ratio. There was also marked nuclear pleomorphism and frequent mitoses (Fig. 2). The overall picture was that of poorly differentiated adenocarcinoma. Immunohistochemical analysis showed that the tumour was positive for cytokeratin-7 (CK7), thyroid transcription factor 1 (TTF-1), and negative for CK20, Villin, CDX2 and thyroglobulin (Fig. 3). These findings strongly supported the diagnosis of metastatic adenocarcinoma of the lung origin.
Fig. 1.
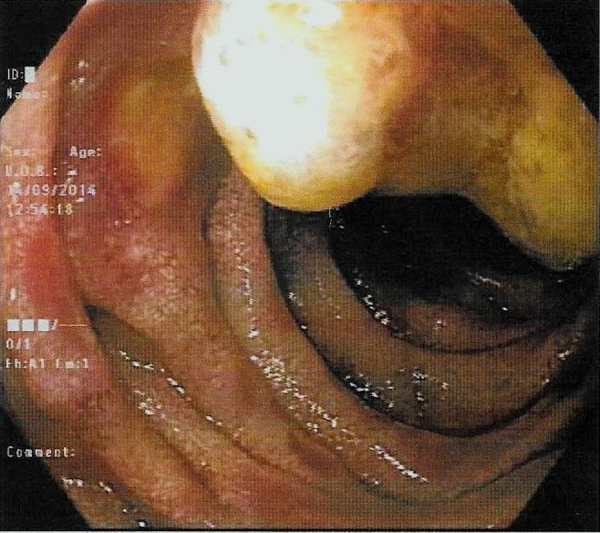
Esophagastroduodenoscopy showing an ulcerative lesion in the fourth part of duodenum with no active bleeding.
Fig. 2.
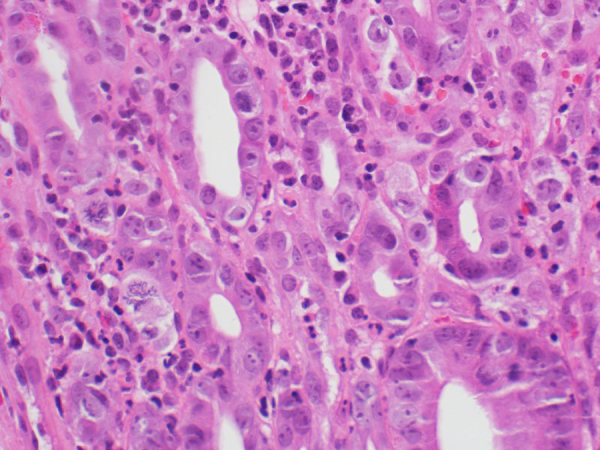
Biopsy of duodenal lesion showing neoplasm forming glandular and cords pattern with frequent mitoses (H & E stain, 400 × magnifications).
Fig. 3.
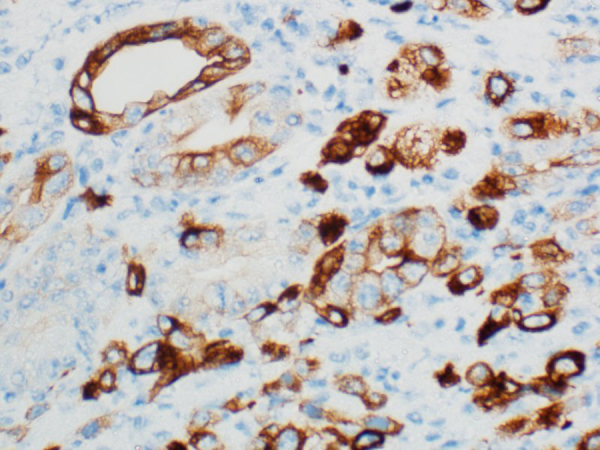
Biopsied duodenal lesion showing CK7 immunopositive tumor cells (CK7 immunostain, 400 × magnifications) suggesting metastatic adenocarcinoma of lung origin.
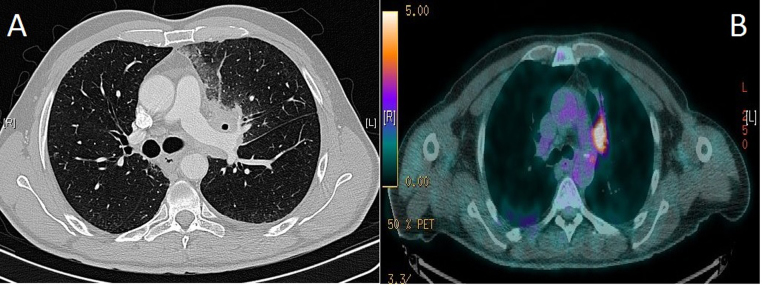
Computed tomography (CT) of chest showed ill-defined necrotic mass measuring 4 × 3.2 cm in left hilar region involving and extending to the anterior segment of left upper lobe. The mass was encasing the left main pulmonary artery, alongwith ipsilateral hilar and contra-lateral para-tracheal, peri-carinal, sub-carinal and hilar lymph nodes (Fig. 4A). CT abdomen was unremarkable. CT-positron emission tomography (CT-PET) showed 18flouro-deoxyglucose (FDG) avid left upper lobe elongated lung mass [standardized uptake volume (SUVmax) 9.3] extending from the left hilum to the pleural surface in the left apical region. There were also FDG avid right upper paratracheal (SUVmax 4.9), left hilar (SUVmax 4.7), and subcarinal (SUVmax 6.6) lymph nodes (Fig. 4B). The rest of staging work up was negative. Bronchoscopy assisted biopsy of lung lesion confirmed the diagnosis of primary adenocarcinoma with negative epidermal growth factor receptor mutation (EGFR-). Patient was staged as T4N3M1.
Fig. 4.
(A) axial view of computed tomography chest showing left hilar mass encasing the left main pulmonary artery and causing narrowing of left upper lobe bronchus, and (B) CT-PET imaging showing 18FDG avid left upper lobe elongated lung mass (standardized uptake volume 9.3) extending from the left hilum to the pleural surface in the left apical region.
After discussing the case in a multidisciplinary tumor board meeting, patient was started on cisplatinum based chemotherapy with Bevacizumab. After three cycles of chemotherapy, melena resolved completely; however, he developed multiple brain metastases. After the completion of whole brain radiation therapy, he was started on oral continuous daily dose of 150 mg Erlotinib.
3. Discussion
Duodenal involvement as delayed site of distant metastasis or as initial manifestation of primary lung carcinoma is extremely rare. Signs and symptoms depend on the anatomic site of duodenal involvement Table 1 [6–16].
Table 1.
Previously published case reports of duodenal metastasis from primary lung carcinoma.
| Reference | Age (years)/sex | Symptoms | Time of diagnosis | Location | Histopathology | Treatment | Follow-up | Status |
|---|---|---|---|---|---|---|---|---|
| [16] | 63/M | Melena, microcytic anemia | 24 months after treatment for primary lung cancer | 3rd part of duodenum | Squamous cell carcinoma | Duodenectomy | 5 months | Dead with progressive disease |
| [12] | 66/M | Perforation | During chemoradiation for primary lung cancer | 4th part of duodenum | Squamous cell carcinoma | Duodenectomy followed by chemotherapy | – | – |
| [14] | 75/M | Melena, microcytic anemia, intussusception | At time of diagnosis of primary lung cancer | 2nd and 3rd parts of duodenum | Small cell carcinoma | Pancreaticoduodenectomy | – | – |
| [6] | 58/M | Obstruction | 2 years after treatment for primary lung cancer | 2nd part of duodenum | Large cell carcinoma | Pancreatico-duodenectomy followed by chemotherapy | 46 months | Alive disease free |
| [7] | 46/F | Melena, microcytic anemia | 20 days after treatment for primary lung cancer | 4th part of duodenum | Large cell carcinoma | Duodenectomy followed by chemotherapy | 12 months | Dead Brain metastasis |
| [8] | 61/M | Melena, weight loss, hemoptysis | At time of diagnosis of primary lung cancer | 4th part of duodenum | Adenocarcinoma | Endoscopic resection, Chemotherapy Blood transfusion, Erythropoietin |
7 months | Dead Progressive lung disease |
| [9] | 55/M | Upper GI bleeding | – | 3rd part of duodenum invading SMA | Adenocarcinoma | – | Few days | Dead Massive GI bleeding |
| [10] | 66/M | Upper GI bleeding | 8 months after treatment for primary lung cancer | – | Adenocarcinoma | – | Few weeks | Dead with massive GI bleeding |
| [11] | 65/M | Jaundice, obstruction | – | 2nd part of duodenum | Squamous cell carcinoma | Endoscopic resection | – | – |
| [13] | 54/M | Dysphagia | During chemoradiation for primary lung cancer | 1st and 2nd parts of duodenum | Squamous cell carcinoma | Endoscopic resection | 2 months | Dead |
| [15] | 69/M | Incidental on imaging | 36 months after treatment for primary lung cancer | 2nd part of duodenum | Small cell carcinoma | Palliative RT 30Gy in 10 fractions | – | – |
M = male, F = female, SMA = superior mesenteric artery, GI = gastrointestinal, RT = radiation therapy
Duodenal metastasis poses a diagnostic dilemma, as radiologic imaging is often unremarkable as seen our patient. Endoscopic evaluation and biopsy should be performed in such cases to establish a definitive diagnosis especially if the cause of melena or microcytic anemia cannot be ascertained [6]. Endoscopic ultrasonography (EUS) may be helpful for localization of submuscoal duodenal metastasis in some cases [5–7].
The treatment of duodenal metastasis is also challenging, and it depends on the site of duodenal involvement and size of these lesions. However, most of cases require duodenectomy or pancreatico-duodenectomy for symptomatic relief [6–10]. Endoscopic resection of smaller duodenal metastatic lesions (≤1 cm) appears to be safe and effective, especially in cases in which these metastases may be removed completely by endoscopic methods, as seen in our patient [11–15]. The role of radiation therapy needs to be investigated, as only case report utilizing radiation therapy for duodenal metastasis has been reported [16].
Duodenal metastasis is associated with dismal prognosis. Only a few cases have survived more than 12 months after surgical resection of duodenal metastases, with the exception of one patient who survived 46 months [9].
In conclusion, duodenal metastasis from lung adenocarcinoma is extremely rare entity and should also be included in the differential diagnosis of melena. Smaller lesions (≤1 cm) can safely be managed with endoscopic resection.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Author contributions
All authors have made substantial contributions to all of the following: (1) data collection, analysis and interpretation of data, (2) manuscript writing and editing it critically for important intellectual content, and (3) final approval of the version to be submitted.
Conflict of interest
No potential conflict of interest among authors, and no grants or funds received for this case report.
Contributor Information
Eyad Fawzi AlSaeed, Email: ealsaeed@yahoo.ca.
Mutahir A. Tunio, Email: mkhairuddin@kfmc.med.sa.
Khalid AlSayari, Email: kalsayari@kfmc.med.sa.
Sadiq AlDandan, Email: saldandan@kfmc.med.sa.
Khalid Riaz, Email: kalsayari@kfmc.med.sa.
References
- 1.Song Y., Li M., Shan J., Ye X., Tang S., Fang X. Acute small bowel obstruction: a rare initial presentation for the metastasis of the large-cell carcinoma of the lung. World J. Surg. Oncol. 2012;10 doi: 10.1186/1477-7819-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rivera N.T., Katz H., Weisbaum G., Guarneri R., Bray N., Constanza-Guaqueta D. Solitary metastasis to the small bowel from primary adenocarcinoma of the lung. J. Gastrointest. Cancer. 2014;45(Suppl. 1):91–95. doi: 10.1007/s12029-013-9567-6. [DOI] [PubMed] [Google Scholar]
- 3.Liu W., Zhou W., Qi W.L., Ma Y.D., Xu Y.Y. Gastrointestinal hemorrhage due to ileal metastasis from primary lung cancer. World J. Gastroenterol. 2015;21:3435–3440. doi: 10.3748/wjg.v21.i11.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goh B.K., Teo M.C., Chng S.P., Tan H.W., Koong H.N. Upper gastrointestinal bleed secondary to duodenal metastasis: a rare complication of primary lung cancer. J. Gastroenterol. Hepatol. 2006;21:486–487. doi: 10.1111/j.1440-1746.2006.04111.x. [DOI] [PubMed] [Google Scholar]
- 5.Lee K.A., Lee S.K., Seo D.W., Kim M.H. Duodenal metastasis from lung cancer presenting as obstructive jaundice. Gastrointest. Endosc. 2001;54:228. doi: 10.1067/mge.2001.116896. [DOI] [PubMed] [Google Scholar]
- 6.Hirai S., Hamanaka Y., Mitsui N., Sato K., Chatani N. Solitary metachnonous jejunum and duodenum metastasis after surgical resection of lung. Cancer, Kyobu Geka. 2010;63:129–132. [PubMed] [Google Scholar]
- 7.Hinoshita E., Nakahashi H., Wakasugi K., Kaneko S., Hamatake M., Sugimachi K. Duodenal metastasis from large cell carcinoma of the lung: report of a case. Surg. Today. 1999;29:799–802. doi: 10.1007/BF02482332. [DOI] [PubMed] [Google Scholar]
- 8.Kostakou C., Khaldi L., Flossos A., Kapsoritakis A.N., Potamianos S.P. Melena a rare complication of duodenal metastases from primary carcinoma of the lung. World J. Gastroenterol. 2007;13:1282–1285. doi: 10.3748/wjg.v13.i8.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steinhart A.H., Cohen L.B., Hegele R., Saibil F.G. Upper gastrointestinal bleeding due to superior mesenteric artery to duodenum fistula: rare complication of metastatic lung carcinoma. Am. J. Gastroenterol. 1991;86:771–774. [PubMed] [Google Scholar]
- 10.Cremon C., Barbara G., De Giorgio R., Salvioli B., Epifanio G., Gizzi G. Upper gastrointestinal bleeding due to duodenal metastasis from primary lung carcinoma. Dig. Liver Dis. 2002:141–143. doi: 10.1016/s1590-8658(02)80245-6. [DOI] [PubMed] [Google Scholar]
- 11.Misra S.P., Dwivedi M., Misra V., Dharmani S., Gupta M. Duodenal metastases from squamous cell carcinoma of the lung: endoscopic management of bleeding and biliary and duodenal obstruction. Indian J. Gastroenterol. 2004;23:185–186. [PubMed] [Google Scholar]
- 12.Yamada H., Akahane T., Horiuchi A., Shimada R., Shibuya H., Hayama T. A case of lung squamous cell carcinoma with metastases to the duodenum and small intestine. Int. Surg. 2011;96:176–181. doi: 10.9738/1380.1. [DOI] [PubMed] [Google Scholar]
- 13.Hu J.B., Zhu Y.H., Jin M., Sun X.N. Gastric and duodenal squamous cell carcinoma: metastatic or primary? World J. Surg. Oncol. 2013;11:204. doi: 10.1186/1477-7819-11-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jarmin R., Azman A., Rahim R., Kosai N.R., Das S. A rare case of intussusception associated with metastasized small cell carcinoma of lung. Acta Med. Iran. 2012;50:782–784. [PubMed] [Google Scholar]
- 15.Ito Y., Suzuki M., Oyamada Y., Kou H., Takeshita K., Asano K., Yamaguchi K. A case of relapsed small cell lung cancer recognized by simple metastasis to the duodenum. Nihon Kokyuki Gakkai Zasshi. 2001;39:30–34. [PubMed] [Google Scholar]
- 16.Kamiyoshihara M., Otaki A., Nameki T., Kawashima O., Otani Y., Morishita Y. Duodenal metastasis from squamous cell carcinoma of the lung; report of a case. Kyobu Geka. 2004;57:151–153. [PubMed] [Google Scholar]