Abstract
Norepinephrine released within primary sensory circuits from locus coeruleus afferent fibers can produce a spectrum of modulatory actions on spontaneous or sensory-evoked activity of individual neurons. Within the ventral posterior medial thalamus, membrane currents modulated by norepinephrine have been identified. However, the relationship between the cellular effects of norepinephrine and the impact of norepinephrine release on populations of neurons encoding sensory signals is still open to question. To address this lacuna in understanding the net impact of the noradrenergic system on sensory signal processing, a computational model of the rat trigeminal somatosensory thalamus was generated. The effects of independent manipulation of different cellular actions of norepinephrine on simulated afferent input to the computational model were then examined. The results of these simulations aided in the design of in vivo neural ensemble recording experiments where sensory-driven responses of thalamic neurons were measured before and during locus coeruleus activation in waking animals. Together the simulated and experimental results reveal several key insights regarding the regulation of neural network operation by norepinephrine including: 1) cell specific modulatory actions of norepinephrine, 2) mechanisms of norepinephrine action that can improve the tuning of the network and increase the signal-to-noise ratio of cellular responses in order to enhance network representation of salient stimulus features and 3) identification of the dynamic range of thalamic neuron function through which norepinphrine operates.
Keywords: whisker, trigeminal, computer model
1. Introduction
The regulation of membrane response properties by norepinephrine (NE) during the waking state could have profound effects on sensory signal processing. For example, neurophysiological data showing NE-induced enhancement of synaptic efficacy (Waterhouse et al., 1990; Waterhouse & Woodward, 1980) provides the basis for postulating a means through which synaptically-released NE may regulate sensory signal transmission during periods of arousal, vigilance, or sustained attention. However, it is difficult to explain how a small modification in cellular response properties manifests itself in a neuronal network and leads to changes in whole animal behavior.
A key advance in determining the role of NE in brain function has been elucidation of the cellular mechanisms underlying actions of NE in vitro. Previous intracellular studies have identified the membrane currents that are modulated by NE in thalamic relay cells and thus regulate the electrophysiological properties of these neurons. Direct application of NE decreases the leak current, ILeak, that contributes to the resting membrane potential of individual cells (McCormick & Pape, 1990a, b). Additional in vitro studies have shown that locally applied NE can: 1) augment the membrane actions of GABA in the cerebellum (Cheun & Yeh, 1992) and cerebral cortex (Sessler et al., 1995), 2) modulate potassium currents in the cerebral cortex (Foehring et al., 1989) and hippocampus (Madison & Nicoll, 1986a,b, 1988), and 3) increase the probability of evoked spiking activity in the cerebral cortex (Waterhouse et al., 2000). Furthermore, Lee and McCormick (1996) showed that NE also decreases the potassium leak current of cells in the thalamic reticular nucleus (nRT)
An important efferent target of NE-containing projections from the brainstem nucleus locus coeruleus (LC) is the somatosensory thalamus. Previous computational models of the thalamus focused on its oscillatory mode of operation during sleep and regulation of this functional state by brainstem modulatory systems (Bazhenov et al., 2000; Destexhe et al., 1993;1996a,b; Huguenard & McCormick, 1992; Lytton et al., 1996; 1997; McCormick & Huguenard, 1992; Wang et al., 1991; Destexhe et al., 1993). NE input increases as animals emerge from sleep or are performing tasks requiring attention. While there is general agreement that NE can modulate electrical properties of thalamic neurons and alter responses of these neurons to non-monoaminergic synaptic inputs (Berridge & Waterhouse, 2003;Foote et al., 1983; Woodward et al., 1979) and that these changes are important for how these networks respond to sensory stimuli, the relationship between the cellular effects of NE and the impact of NE on populations of neurons encoding sensory signals is still open to question. To address this gap in our understanding of the dynamics of NE modulatory influences on thalamic circuits of mammalian brain, we used a computational model of the rat trigeminal somatosensory system. Our model simulated the anatomical and physiological relationships of the thalamo-reticular network and was used to evaluate the net influence of a variety of previously demonstrated membrane and cellular effects of NE on this network. We used the model to address three specific questions: 1) do the cellular actions of NE produce a uniform effect on network transmission of sensory signals?, 2) can changes in the magnitude of excitatory or inhibitory post-synaptic potentials, both postulated NE actions, modulate the responses of thalamic cells to whisker stimulation in a manner similar to that seen in vivo? and, 3) which mechanisms of NE action account for changes in magnitude and/or latency of cellular responses to synaptic input? To validate the predictions of the model, and demonstrate that these predictions were potentially physiologic, we implanted arrays of microelectrodes within the rat VPM thalamus and recorded whisker-evoked somatosensory responses from ensembles of neurons in freely moving animals before, during and after periods of tonic electrical activation of the LC-NE system.
2. Results
To determine which of the known membrane actions of NE could account for increases or decreases in responsiveness of VPM cells to afferent input, we evaluated the effects of NE-mediated changes in membrane function on simulated afferent volleys of EPSP’s such as those that are known to occur in response to whisker stimulation. In addition, we tested our hypothesis that NE could produce the changes in sensory responsiveness seen in vivo by modulating the magnitude of EPSP’s and IPSP’s within modeled neurons.
2.1 Effect of reducing the leak current on neuronal activity in the model
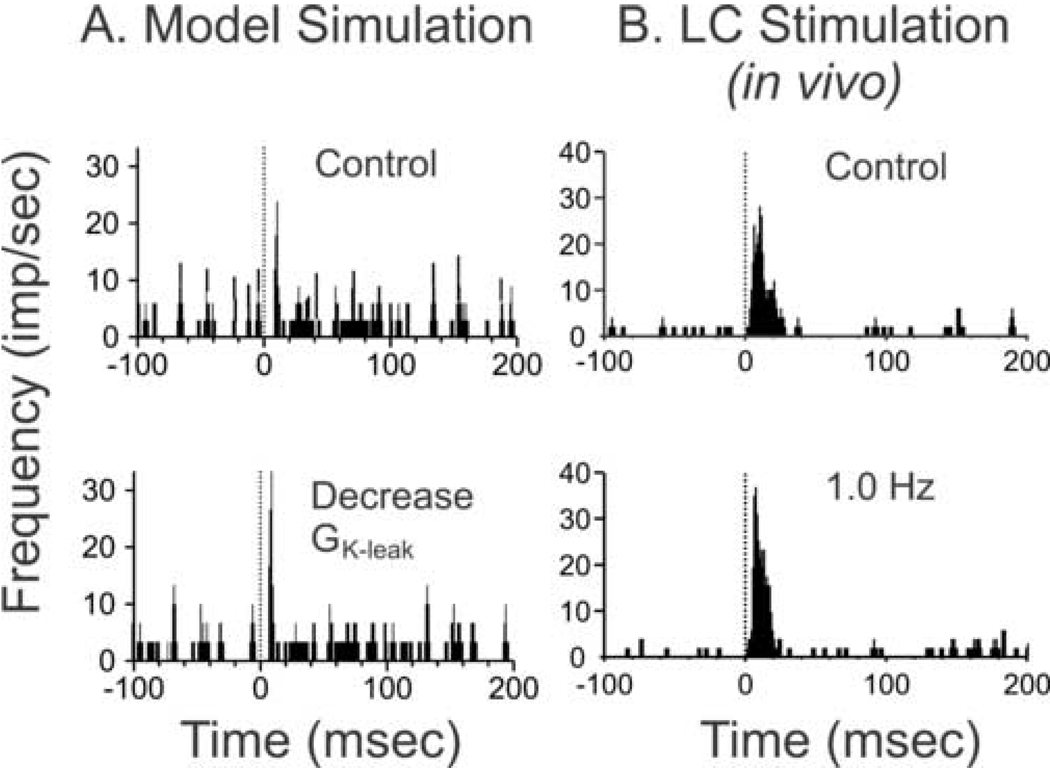
To model the effects of NE on the membrane potential, the value of the potassium-leak current was modified for all cells. GK-leak was decreased to simulate the effect of NE on the leaky membrane current. When GK-leak was decreased from 2.5 nS (nano-siemens), a level that simulated minimal NE receptor activation in the waking state, to 0.0 nS, a level that simulated maximum NE receptor activation, the resting membrane potential increased. These increases in the resting membrane potential were expected based on a previous report (McCormick & Pape, 1990a). To evaluate the effects of modifying the GK-leak on the response of VPM cells to synaptic inputs, afferent volleys to the VPM were generated by simulation of single whisker activation. Individual modeled VPM cells were more likely to fire action potentials in response to synaptic input and modal latencies were decreased in most cells (Table 1) when GK-leak was decreased to half the baseline level in both populations of cells. This effect is represented in the sample PSTH (Fig 2A) as an increase in the probability of the response and a decrease in the modal latency. Under initial conditions of the model the peak response was 243 Hz with a latency of 7 ms. The peak response increased 133 % (324 Hz) with a decrease in modal latency of 29 % (5 ms) when NE-activation was simulated by decreasing GK-leak to 0.5 X baseline conditions. This increase in responsiveness is comparable to the enhanced stimulus-driven discharge and reduced response latency observed in VPM thalamic cells during tonic (1.0 Hz) electrical activation of the LC in awake but quietly resting rats (see Fig 2B). In this case the response probability to whisker pad stimulation was increased approximately 31% from control conditions and the modal latency was reduced 22%, from 11.2 to 8.8 ms.
Table 1. Effects of Simulated NE Modulation of GK Leak on VPM Thalamic Network Dynamics.
Distribution of changes in cell responsiveness and latency to simulated synaptic input after modulation of GK Leak current. The first column on the left identifies the magnitude of leak current changes and the cell population where such changes were implemented. Numbers in the four columns to the right indicate the percentage of cells in the model that underwent a change in responsiveness to synaptic input following leak current changes.
| Increased Responsiveness |
Decreased Responsiveness |
Both increased Responsiveness and decreased Latency |
Unchanged | |
|---|---|---|---|---|
| 50% decrease in both populations |
20% | 10% | 5% | 0 |
| 25% decrease in VPM only |
0 | 95% | 0 | 5% |
| 75% decrease in VPM only |
75% | 20% | 0 | 5% |
| Decrease in nRT only |
0 | 0 | 0 | 100% |
| 75% decrease in both populations |
20% | 75% | 0 | 5% |
Figure 2.
Effects of simulated NE actions and LC activation on sensory thalamic neuronal responsiveness to synaptic input. Post-stimulus time histograms (PSTHs) were used to measure the responses of single cells to simulation or physiological activation of afferent inputs. A.) In the model the magnitude and modal latency of the response to simulated single whisker stimulation were measured at the peak of the largest bin post-stimulus. Under initial (control) model conditions, the network approximated the state of an awake but quietly resting animal (i.e. absence of sleep spindles and low probability of activation of the low threshold calcium current). When single whisker stimulation was simulated under this initial condition, the peak response was 243 Hz at a modal latency of 7 ms post-stimulus (above). As “simulated NE” was added to the model, the leaky potassium current was reduced and the resting membrane potential increased to −45.3 mV. When single whisker stimulation was simulated under this ‘NE-activated’ condition, the peak response was 324 Hz (133% increase) at a modal latency of 5 ms post stimulus (below). B.) In the intact, quietly resting, awake rat VPM thalamic neuron responses to whisker pad electrical stimulation were recorded before (control - above) and during tonic (1.0 Hz - below) activation of the ipsilateral LC nucleus. In the case shown each histogram represents the neuronal discharge pattern generated during n=30 whisker stimulus presentations (stimulus onset = 0). For this cell whisker-evoked discharge was enhanced 31% above control levels (28.1 to 36.8 Hz) during LC stimulation and demonstrated a 22% decrease in modal latency (11.3 ms to 8.8 ms).
2.2 Effects of differential leak current modulation: VPM vs nRT
The most frequent outcome of modulating the leak current on model cells was an increase in response probability often accompanied by a decrease in modal latency (Table 2). However, not every cell responded this way. For example, when GK-Leak was reduced by 50% (from a minimal NE effect, GK-leak=2.5 nS to GK-leak =1.25 nS), 5% of the VPM cells increased their response probability and decreased the modal latency of response while 20% of VPM cells only increased the probability of their responses. At the same time, response probability was reduced in nearly 10% of modeled VPM cells (n = 96). The remaining VPM cells showed no change in response probability or modal latency.
Table 2. Effects of Simulated NE Modulation of PSP Amplitude on VPM Thalamic Network Dynamics.
Distribution of changes in cell responsiveness and latency to sensory driven synaptic input after modulation of EPSP or IPSP amplitude. The first column on the left indicates how PSP magnitude was modified. The next column indicates the number of initially unresponsive cells that became responsive to simulated synaptic input following PSP change(s), i.e. ‘gating’ effect. The remaining five columns indicate the number of initially responsive cells (percentages in parentheses) that expressed a change or no change in responsiveness to simulated synaptic input following PSP modulation. The percentages are based upon the total number of cells displaying responses under baseline conditions (n=213).
| Cells Gated |
Increased Responsiveness |
Decreased Responsiveness |
Both Increased Responsiveness and Decreased Latency |
Decreased Latency |
Unchanged | |
|---|---|---|---|---|---|---|
| Double EPSP |
223 | 0 | 30 (14%) # |
0 | 11 (5.3%) |
162 (76%) |
| Double IPSP |
0 | 0 | 60 (28%) |
0 | 0 | 153 72% |
| Double Both |
336 | 10 (4.7%)## |
34 (16%) |
71 (33.3%) |
15 (7%) |
72 (34%) |
| Triple EPSP* |
223 | 3 (1.5%) |
14 (6.6%) |
0 | 156 (73%) |
15 (7%) |
| Triple IPSP |
0 | 0 | 90 (42%) |
0 | 0 | 123 (58%) |
| Triple Both** |
90 | 0 | 113 (53%) |
60 (28%) |
23 (10.8%) |
2 (1%) |
| Triple EPSP, Double IPSP |
223 | 3 (1.5%) ### |
14 (6.5%) |
149 (70%) |
0 | 48 17% |
| Triple IPSP Double EPSP |
214 | 9 (4.1%) |
109 (51%) |
38 (18%) |
0 | 57 (26.9%) |
25 cells (11.8%) increased latency not included in table or phase plot
15 cells (7%) increased latency not included in table or phase plot
10 cells (4.7%) decreased responsiveness and latency, not included in table or phase plot
11 cells (5.2%) increased responsiveness and latency, not included in table or phase plot
12 cells (5.9%) increased responsiveness and latency, not included in table or phase plot
As illustrated by the phase diagram of neuronal function (Fig. 3), response properties of VPM neurons were dependent on the magnitude of reduced leak current in VPM versus nRT circuitry. First, as GK-leak current was decreased in the VPM (holding the leak current constant in the nRT) the probability of a VPM cell firing in response to synaptic input was modulated in a non-linear manner. Initially, the response probability decreased in most VPM cells (95% of 213 cells; the remaining 5% were unchanged), however as GK-leak current was decreased further the response probability of these cells was then increased. Second, when GK-leak was decreased in the nRT but held constant in the VPM there was no change in the response properties of the VPM cells. Third, moderate decreases in GK-leak in both VPM and nRT resulted in an increase of the response probability and, sometimes, a decrease in the modal latency of VPM cells. However, large decreases in GK-leak in both VPM and nRT produced a decrease in the probability of a response from VPM neurons (see Fig 3). Collectively, these findings emphasize the variable nature of the relationship between NE’s net impact on neuronal function in noradrenergic terminal fields and the sensitivity of individual postsynaptic mechanisms to NE action.
Figure 3.
NE modulation of GK Leak. To better understand the complex nature of the response of the VPM/nRT network to simulated activation of NE postsynaptic mechanisms, phase diagrams were used to explore the working space that defines the response domains of individual cells to simulated activation of NE. X-axis equals the percent decrease in GK-leak conductance in the nRT and the y-axis equals the percent decrease in GK-leak conductance in the VPM. The effect of activating NE receptors in the VPM and the nRT were independently modulated by varying the model parameter GKleak (refer to Figure 1 for an explanation). The effect of increasing NE activation was to decrease Ileak and thereby depolarize the cell’s resting membrane potential. Simulating an increase in NE activation in VPM but not nRT (x-axis at 0) initially caused no change in the response to simulated single whisker stimulation. However, as the Ileak current was halved (y-axis approximately 50%) the responsiveness of VPM cells to simulated sensory input, as measured by the PSTH, decreased. When NE activation was further increased the responsiveness of VPM cells increased compared to baseline conditions. As NE action was moderately increased in both populations (i.e. both x and y axes at about 50%), cells responded with an increased probability of discharge and sometimes a decreased modal latency. However, for large increases in NE action in both populations (x and y axes greater than 75%) the probability of cell responses decreased. Changes stated within the diagram are for the majority of cells. Refer to Table 1 for details on the distribution of responses throughout the network. B) Illustrative PSTHs demonstrate the representative cases from each area of the phase space. PSTH notations are the same as Figure 2A.
2.3 Effect of increasing the amplitude of EPSP’s and IPSP’s on network activity
To test whether NE could produce changes in VPM response probability and modal latency by enhancing the amplitude of EPSP’s and IPSP’s, we modulated EPSP and IPSP effects independently in the model and assessed their impact on neuronal and network response properties (Fig. 4). The simulations were performed similarly as above, however instead of decreasing the GK-leak, the amplitudes of the EPSP’s or IPSP’s generated in VPM cells or nRT cells were increased (i.e. either doubled or tripled in amplitude).
Figure 4.
NE Modulation of PSP Amplitude. The diagram shown here illustrates the effects of changing excitatory and inhibitory synaptic efficacies in VPM and nRT cells. The X-axis indicates increasing amplitude of the IPSP and the y-axis indicates increasing amplitude of the EPSP. The net effect of changing PSP amplitudes was similar to the effects observed by altering the leak current. However, a much larger operating region, resulting in increased responsiveness and decreased modal latency of VPM cells, was observed with alteration of PSP amplitudes. Although increasing the amplitude of the EPSPs alone did not change the responsiveness of VPM cells to simulated sensory input (x-axis = 1.0), concomitant moderate increases in IPSP amplitude yielded increases in the amplitude of the response and decreases in modal latency (x-axis = 2.0). If both the EPSP amplitude and the IPSP amplitude were tripled, the responsiveness of VPM cells decreased. Changes stated within the diagram are for the majority of cells. Refer to Table 2 for details on the distribution of responses throughout the network. B) Example PSTHs illustrate representative modeled effects within PSP amplitude phase space. PSTH notations are the same as Figure 2A.
A complex set of effects was observed by altering the amplitude of the EPSP’s and IPSP’s (Fig. 4). In general, two types of changes were observed most frequently, occupying the most space on the neuronal function phase diagram: an increase in response probability and/or a decrease in the response modal latency. However, other changes were observed within this parameter space. For example, the response probability of VPM cells was decreased from baseline responsiveness when Gmax for IPSP’s alone was increased or when Gmax for both IPSP’s and EPSP’s was tripled (i.e. maximal NE effect).
Furthermore, under certain conditions, cells that were initially unresponsive to simulated input became responsive. This phenomenon of ‘gating’ cells into the pool of responsive neurons following simulated increases in NE action has been observed in vivo during iontophoretic application of NE (Waterhouse et al., 1988; 1990) or activation of the LC efferent path (Devilbiss & Waterhouse, 2004). For example, the number of cells responding to afferent input more than doubled, from n=213 to n=436 cells, following increases in EPSP amplitude alone. When the magnitude of both EPSP’s and IPSP’s were doubled, the number of cells responding to simulated input peaked at 549 or more than half of the units in the network.
The effects of increasing EPSP amplitude on the 213 cells that were initially responsive to simulated inputs were also quantified (Table 2). When the amplitude of the EPSP’s were doubled (holding Gmax for IPSP’s constant), most cells in this group (162/213, 76%) did not change their response properties from baseline conditions. A small number of cells showed a decrease in responsiveness (n=30) or both a decrease in responsiveness and a decrease in modal latency (n=10). An additional 11 cells showed a decrease in modal latency alone. As the amplitude of the EPSP’s was further increased, most of the initially responsive cells (155/213, 73%) showed a decrease in modal latency, while only 3 cells showed an increase in responsiveness. Overall, these results suggest that modulating the EPSP amplitude alone can greatly increase the number of cells in a network responding to afferent input (i.e. ‘gating-in’) but this action is not sufficient to increase the probability of response from cells that were already responsive under baseline conditions.
By contrast, when the amplitude of both the EPSP’s and the IPSP’s were doubled, the majority of initially responding cells showed an increased response probability and decreased modal latency (71/ 213 cells) or a decrease in responsiveness alone (34/213 cells) compared to baseline conditions. Additionally, a small number of cells showed an increase in responsiveness with (n=11; not listed in table) or without (n=10) a concomitant increase in modal latency. As the amplitude of EPSP’s was further increased, most cells continued to show an increase in responsiveness followed by a decrease in modal latency. Combined with the predominate decrease in modal latency observed when EPSP’s alone are increased, these data suggest that increasing both EPSP amplitude and IPSP amplitude has the additive effect of increasing the probability of a response from a cell as well as decreasing the latency of that response. However, when the amplitude of both the EPSP’s and the IPSP’s were tripled, a majority of cells (113 / 213 cells) showed a decrease in responsiveness from baseline conditions. Thus, although a large increase in the amplitude of EPSP’s alone (3 times baseline) results in a generalized increase in response probability, this increase in neuronal responsiveness can be reversed with concurrent increases in the amplitude of IPSP’s (3 times baseline). Nevertheless, it was observed that moderate increases in both IPSP’s and EPSP’s (twice baseline) generally increase the probability of a response. Overall, these data suggest that increases in the probability of a response by moderate (2 fold) increases changes in EPSP and IPSP magnitude can be negated by larger increases in PSP amplitude.
Modifying the amplitude of the IPSP’s alone reduced the probability of a response to simulated input. Initially there was no change in the response of neurons to simulated input as the IPSP amplitude increased. However, when the amplitude of the IPSP was double its initial value, the probability of a response began to decrease (28% of cells). Further increases in IPSP amplitude resulted in more cells (42%) exhibiting reduced response probability, although the total number of cells responding to simulated input did not change.
2.4 Comparison of Simulated NE Effects on GK-Leak vs PSP’s
While the effects of PSP changes were similar to the effects seen when modeling NE’s effect on the potassium leak current, there were some notable differences. First, by increasing EPSP amplitude without affecting IPSP, the modal latency of the response decreased with a rare change in response probability. In addition, within the operational domain of the network there was a much larger working region (i.e. range of parameters) where response probability was increased and/or response latency was decreased. This endpoint occurred over a broader range of PSP amplitudes than either increased amplitude and/or increased modal latency alone. If one hypothesizes that increased response probability and a decrease in modal latency results in optimal signal processing, then the effect of NE on PSP amplitudes does not have to be tightly controlled to achieve the desired signal processing outcome. Additionally, decreases in response probability were much less likely when NE’s effect on PSP size was modeled than when NE’s effect on GK-leak was modeled across the range of parameters studied. Nevertheless, decreased response probability was principally observed when EPSP and IPSP amplitudes were increased substantially (3 times baseline values) or when resting membrane potential was increased as observed when GK-leak is modulated by NE. Finally, a large number of cells were ‘gated’ into the response pool when the amplitude of the PSPs was modulated, but not when GK-leak was altered in VPM or nRT cells.
2.5 In Vivo Studies
The in vivo effects of endogenous NE release were measured by simultaneously recording from multiple single VPM neurons before and during tonic (0.5, 1.0 and 5.0 Hz) electrical stimulation of the ipsilateral LC nucleus. Previous work from our laboratory and elsewhere has shown that increased output from the LC results in increased NE levels in noradrenergic terminal fields (Berridge & Abercrombie, 1999; Florin-Lechner et al., 1996; Page & Abercrombie, 1999; Waterhouse & Devilbiss, 2002). Tonic activation of the LC-noradrenergic efferent pathway in the awake but quietly resting rat, produced heterogeneous effects on response probability and modal latency that were similar to those seen in the model. For example, as predicted by the model, NE-mediated reduction of the potassium-leak current or alteration of the balance between EPSP and IPSP amplitudes should result in an increase in response magnitude of VPM neurons to sensory stimuli. Figure 2B illustrates a representative neuron from in vivo recordings that demonstrated an increase in response probability during periods of LC stimulation. In this example the response was increased 31% above control. Moreover the model predicted a decrease in modal latency, an effect achieved by by reducing the potassium-leak current or altering the balance of EPSP and IPSP amplitudes. This predicted outcome was also evident in the response of the representative neuron shown in Fig. 2B, i.e. in addition to the increase in response probability, this cell exhibited a 22% decrease in modal latency (11.3 ms to 8.8 ms). Furthermore, as predicted by the model, we simultaneously recorded neighboring VPM neurons whose responses to whisker pathway stimulation were suppressed during LC stimulation. Cell-specific, LC-induced modulation of VPM neuronal responsiveness to whisker pad stimulation was observed in all six animals studied. The distribution of these effects as observed in vivo are listed in Table 3.
Table 3. Effect of LC stimulation on VPM Thalamic Neuron Responsiveness to Sensory Driven Synaptic Input.
Distribution of changes in cell responsiveness and latency during electrical stimulation of the LC. The first column on the left identifies the type of neuromodulatory action observed. The adjacent columns to the right indicate the number and percentage of cells that underwent changes from the baseline conditions, respectively. These data are based on a total of 74 cells recorded from 6 animals. “Gating” effects were observed in 34/74 cells that were initially unresponsive to whisker pad stimulation.
| Thalamus | ||
|---|---|---|
| Facilitation | 29/40 | 72.5% |
| Suppression | 8/40 | 20.0% |
| No effect “Gating” |
3/40 22/34 |
7.5% 64.7% |
Based upon the assumption that NE has uniform effects on GK-leak conductance in nRT and VPM cells, respectively, or produces equivalent effects on the amplitude of EPSP’s and IPSP’s in these thalamic regions, the diagonal through either neuronal function phase diagram (Fig. 3 or 4) indicates the prediction that VPM neuronal responses to afferent stimuli will be increased and subsequently decreased as NE’s effects are increased. During increasing frequencies of LC stimulation in the intact animal, a substantial number of neurons demonstrated an increased response probability at low levels of LC stimulation followed by suppression from this peak responsiveness at subsequently higher LC stimulation frequencies (Fig. 5). However, not all VPM cells demonstrated this LC stimulation-response relationship. Essentially, a spectrum of modulatory effects was observed with increasing frequencies of LC stimulation that were cell specific and frequency dependent.
Figure 5.
Modulation of thalamic neuron responsiveness to excitatory synaptic input across a range of tonic LC output. PSTH’s illustrate the bi-phasic (inverted-U) response relationship observed for LC-mediated modulation of the response of a single VPM thalamic neuron to whisker pad electrical stimulation. Each histogram sums unit activity during an equivalent number of whisker pad stimulus presentations. Data were collected during control and tonic ipsilateral LC stimulation periods (0.5, 1.0, 5.0 Hz; 10 uA). For this cell, peak enhancement (above Control) of whisker-evoked discharge was observed at 0.5 Hz LC stimulation. Higher frequencies (1.0, 5.0 Hz) of LC tonic stimulation also enhanced, but to a lesser degree, the whisker evoked excitatory response of the cell.
For a given cell, increasing LC stimulation either monotonically suppressed single neuron response probability or initially produced an increase in response probability at low levels of LC output followed by suppression at higher LC activation frequencies. For example, Figure 6 illustrates one (cell A) of three simultaneously recorded, neighboring neurons whose response to whisker-related synaptic input was monotonically suppressed across increasing LC stimulation frequencies. During the same experimental period, cell B demonstrated an increased probability of response at all frequencies of LC stimulation tested. By contrast, the response probability of neighboring cell C was increased during 0.5 Hz LC stimulation, but was suppressed from peak responsiveness at subsequently higher LC stimulation frequencies. Of the 29 neurons that demonstrated an initial facilitation of the sensory-evoked discharge during LC stimulation, 18 showed further increases in response probability with increasing LC activation, whereas the remaining 11 exhibited the parabolic or inverted-U LC stimulus-response relationship (n=6 animals). These data suggest that increasing the output of the LC-noradrenergic system and, therefore, raising local extracellular concentrations of NE can produce a number of trajectories through the potassium-leak current or EPSP-IPSP neuronal function phase diagrams.
Figure 6.
LC-noradrenergic pathway modulation of sensory thalamic neuron responsiveness. Poststimulus time histograms (PSTH’s) histograms illustrate the effects of increasing tonic frequencies of locus coeruleus (LC) electrical stimulation on whisker-evoked responses of simultaneously recorded VPM thalamic neurons. Spike train activity for individual cells was recorded from an awake rat before (control) and during tonic activation (0.5, 1.0, or 5.0 Hz at 10 uA) of the ipsilateral LC nucleus. Each histogram represents the neuronal discharge pattern generated during n=30 whisker stimulus presentations (stimulus onset = 0). A. In some cells (top row) an increasing range of tonic activation of LC produced a monotonic suppression of whisker-evoked discharge. B. In other cells (middle row) whisker-evoked discharge was progressively enhanced across the range of stimulus frequencies tested. C. However, in many cells (bottom row) stimulus-evoked responses were enhanced at low LC stimulation frequencies (0.5–1.0 Hz) and suppressed at higher stimulus frequencies (5.0 Hz), yielding an inverted-U response function for LC-induced modulatory actions in these neurons. Thus, both facilitation and suppression of responsiveness to synaptic input can be observed simultaneously in neighboring thalamic neurons with increased tonic output from the LC. Inset numbers represent the summed probability that the neuron will discharge in response to the whisker pad stimulation.
3 Discussion
In the present study we used a computational model of the rat thalamic circuitry to study the effects of known post-synaptic actions of NE on the responses of single cells and local circuits to activation of afferent synaptic pathways. The goal was to obtain a better understanding of the impact of the LC-NE system on local circuit dynamics in noradrenergic terminal fields. Simulation of NE activity as modeled in this study had two major outcomes. First, changing the efficacy of NE in our model produced a complex and varied array of neuromodulatory effects on cells within a simulated thalamic network. These disparate effects had previously been shown to manifest themselves at the single cell level in vivo, but it was not clear how or if they would be expressed simultaneously in neighboring neurons across a noradrenergically-innervated network. Overall, the results obtained from our model indicate that the predominant outcome of NE actions on intrinsic membrane properties or synaptic efficacy was to increase the probability and/or decrease the modal latency of individual VPM thalamic neuron responses to afferent input. However, NE did not produce a uniform effect on the response properties of cells in the thalamic relay circuitry. For example, simulation of NE action enhanced the response to afferent synaptic volleys for some cells in the modeled circuit while the response of other cells under the same conditions was suppressed. This intriguing result was confirmed in subsequent neural ensemble recordings from intact, awake animals.
Second, both of the synaptic effects of NE that were modeled, e.g. either decreasing leaky membrane current, or increasing the strength of post-synaptic potentials, resulted in the same array of changes in network dynamics. In all cases these effects depended upon the magnitude of simulated NE action. Moreover, many individual cells within the model followed an inverted-U sensory response function for magnitude and latency depending on the strength of simulated NE action. This was true regardless of the NE action simulated (i.e. decreasing leaky membrane current, or increasing the strength of post-synaptic potentials). Thus, the model predicts that across a range of increasing NE influences, a neuron in the thalamic network can transition between different levels of responsiveness to synaptic inputs depending on the output of the LC-noradrenergic system. This result was also confirmed in subsequent neural ensemble recordings in vivo.
3.1 Modeling Constraints
The computational model used in this study was constructed so as to reflect the rat trigeminal thalamic circuitry including several known anatomical and electrophysiological properties of the biological network. First unlike many other networks and most generic excitatory-inhibitory networks studied, there are no excitatory collaterals between neurons of the VPM thalamus. In addition, neurons in the VPM (glutamatergic) thalamus and nRT (GABAergic) are neurochemically and morphologically homogeneous. In contrast, networks of sensory cortical neurons are quite heterogeneous with regard to transmitter expression, morphology, hodology, and physiology. The homogeneity of the thalamic circuitry makes it a desirable network to study but also raises new questions about the diversity of NE-mediated responses that were observed.
However, since the effects of NE are similar across many different brain regions, the model parameters and outcomes derived here can be generalized to other central circuits that receive noradrenergic innervation. The parameters used in the model were based on realistic anatomical constraints of most excitatory-inhibitory networks and biologically-based measurements of membrane currents and NE actions across many neuronal populations. Indeed, these types of responses to NE on populations of cells in the hippocampus (Madison & Nicoll, 1986a,b, 1988), cerebellum (Cheun & Yeh, 1992) and primary somatosensory cortex (Sessler et al., 1995; Waterhouse et al., 2000) have been previously demonstrated in-vivo.
Despite this generality, single cells in the model exhibited response properties like those observed in tissue slice preparations, intact animals and other computer simulations of the thalamic circuitry. Upon activation by simulated afferent volleys the cells in our thalamic network responded with evoked discharge patterns like those observed in intact animals (Moxon & Chapin, 1999). The effects of NE were modeled using three known cellular mechanisms: 1) reduction of GK-leak (Foerhing et al., 1989; McCormick & Pape, 1990a), 2) enhancement of GABAA mediated chloride current (IPSP) (Cheun & Yeh, 1992; 1996; Sessler et al., 1995) and 3) enhancement of glutamate-mediated or synaptically evoked excitatory responses (Mouradian et al., 1991; Waterhouse et al., 2000). Despite the fact that the precise mechanism responsible for NE-modulation of glutamate action is unknown, our effect of generating an increase in the amplitude of the EPSP is a reasonable approximation of this noradrenergic action. Furthermore, the results of the modeling study were verified with simultaneous recordings from ensembles of single VPM thalamic neurons in vivo. In either the model or the waking animal, increasing effects of NE resulted in cell specific modulation of excitatory discharge patterns.
Direct application of NE also enhances a hyperpolarizing activated cation current, the H-current, IH. Subsequent studies showed that persistent activation of IH results in abolition of the low threshold calcium current, IT, and cessation of spindle oscillations. This mechanism shifts the cells from a burst discharge mode associated with sleep to single spiking relay mode associated with the waking state (Bal & McCormick, 1996). Previous models of thalamic circuitry (Bazhenov et al., 2000; Destexhe et al., 1993;1996a,b; Huguenard & McCormick, 1992; Lytton et al., 1996; 1997; McCormick & Huguenard, 1992; Wang et al., 1991; Destexhe et al., 1993) explored the relationship between these membrane currents and network oscillations. Since only a few neurons were required to examine this effect, these models used the Hodgkin-Huxley formalism (Hodgkin & Huxley, 1952), which is computationally intensive. It has been hypothesized that these membrane currents regulate the physiological properties of thalamic neurons across the sleep-wake continuum and during different states of arousal (McCormick & Pape, 1990b; Bal & McCormick 1996) as a function of their response to release of different endogenous neuromodulatory substances, including NE. During sleep, NE output is reduced and there is a tendency for enhanced hyperpolarization of postsynaptic target neurons, such as those in thalamus, followed by activation of the T-current (Bal & McCormick, 1996; McCormick & Pape, 1990b;) and subsequent generation of thalamic network oscillations (Jahnsen & Llinas, 1984a,b; von Krosigk et al., 1993). As the animal transitions from sleep to waking, NE output is increased, thalamic cells become depolarized and the oscillations are abolished. Because we were concerned primarily with NE effects under waking conditions, we did not include the T-current or the H-current in our model. However, since the H-current may play a role in thalamic network dynamics under waking conditions, an important next step in the development of this model would be to study the influence of the H-current on cell responsiveness in the context of simulated NE actions.
Because it was necessary to simulate the activity of a large number of cells in order to examine the frequency of each type of ‘cellular response’ (i.e. combinations of changes in firing probability and latency), we simplified our model in two important ways to reduce the time to compute a solution. First, we did not include the fast sodium channels responsible for the action potential. Instead, if a cell reached threshold for firing an action potential, synaptic activation was transmitted to downstream cells. In addition, the potassium delayed rectifier, the high threshold calcium current and the calcium dependent potassium current were computationally simplified to eliminate the time constant of activation (refer to Appendix). Accordingly, the amount of activation per integration step is held constant and the current was increased by an amount equal to the activation occurring during one time step. The limitation of this method is that the voltage dependence of the activation is constant instead of integrated as a function of membrane potential. Since these equations are always numerically integrated and the activation time constants for these parameters are fast, the numerical results are similar within the range of membrane potentials under investigation. Previous modeling studies using these simplified formulations showed that the responses of model cells accurately reflect data recorded from networks of cells in vivo (Moxon et al., 1999a, b).
3.2 Implications for Network Actions of NE
In the rat, LC neurons discharge tonically in a relatively slow and highly-regular state-dependent manner within the range of 0.1–5.0 Hz (Hobson et al., 1975; Aston-Jones & Bloom, 1981a; Foote et al., 1980). As these tonic discharge rates increase, extracellular NE increases linearly in LC efferent target regions (Berridge & Abercrombie, 1999). Moreover, changes in tonic LC impulse activity are correlated with changes in arousal level (Foote et al., 1980) and state dependent information processing. As such, the highest output from LC is observed during waking with very slow rates of discharge occurring during slow-wave sleep, and virtually no activity during REM sleep (Aston-Jones & Bloom, 1981a,b; Foote et al., 1980; Hobson et al., 1975). Within waking, LC neurons discharge most rapidly in anticipation of EEG and behavioral changes that signal enhanced arousal or attentiveness (Aston-Jones & Bloom, 1981a,b; Foote et al., 1980). LC neurons also exhibit burst firing associated with presentation of novel or salient environmental stimuli (Foote et al., 1980). However, model parameters of Gk-leak, IPSP, and EPSP were held constant during simulations, a constraint that most closely resembles tonic levels of NE release. Combined, these and other observations suggest that LC output might contribute to the modulation of sensory information acquisition (e.g. alert waking) and information processing.
Since NE is capable of exerting a number of different influences on the responsiveness of individual neurons to synaptic input through several different mechanisms including its actions on intrinsic membrane properties and post-synaptic second messenger systems, its impact on network dynamics was not obvious. Our model demonstrates three modes of operation whereby NE can influence network dynamics and have an important impact on the functional output of the thalamus during waking conditions. First, since every cell in our model population of VPM neurons was the same yet cells responded differently to singular changes in NE effect, we conclude that each of these noradrenergic actions can produce a multitude of modulatory outcomes at the cellular level. This is a likely consequence of the fact that not all neurons maintain the same “response state” at the same time owing in part, to differences in the synaptic inputs to each cell, as was demonstrated in the simulation presented here. In biological networks therefore, the effect of synaptically-released NE is likely due to the dynamic state of individual neurons and the neural networks within which they are embedded.
Second, increases in cell responsiveness coupled with decreases in response latency as observed with simulated NE input in the model have been postulated to enhance the tuning of a local circuit to sensory stimulation in vivo (Berridge & Waterhouse, 2003; Lorden et al., 1980; Selden et al., 1990). Our simulations show that modulating both EPSP and IPSP amplitude at the same time is more likely to decrease the latency and increase the responsiveness of thalamic cells than modulating either alone or modulating the leak current. Therefore, enhancing the tuning of a local circuit to synaptic input in vivo is most likely accomplished with a combination of both EPSP and IPSP amplitude modulation. Furthermore, the inverted-U response curve observed in vivo is most likely to be dependent on continuous changes in both EPSP and IPSP amplitude. Our model demonstrates that modulating both postulated mechanisms of NE action, PSP amplitude and the leak current, can produce this effect as long as it occurs in both populations of thalamic cells (i.e. increasing EPSPs and IPSPs at the same time or decreasing the leak current in both VPM and nRT cells simultaneously). However, changing PSP amplitude generates a greater likelihood of enhancing the tuning of cells in the network than modulating the leak current.
Finally, previous studies have shown that NE can produce changes in response threshold such that previously unresponsive or weakly responsive cells are gated into the response pool (Ciombor et al., 1999; Devilbiss & Waterhouse, 2004; Jiang et al., 1996; Waterhouse et al., 1988). Our combined results from the model and ensemble recording in vivo demonstrate that some cells can be gated-into a response pool while other cells have their response probability suppressed. This leads to an intriguing possibility for a mechanism to enhance the signal-to-noise in a neuronal network – cells representing an important dimension of the signal are recruited while cells discharging to a less salient feature of the stimulus are suppressed. Our model shows that, these gating-in conditions are likely to exist when the PSP amplitude is modulated but not when the leak current is modulated. There are likely to be many transmitter-mediated mechanisms through which the system could regulate effects on leak current vs. PSP amplitude and, thus, bias the response of the network according to different physiological conditions and behavioral contingencies. Further modeling and in vivo work is necessary to explore this possibility.
3.3 Potential Mechanisms Underlying NE Actions
To summarize, the mechanisms underlying NE-modulation of neuronal discharge are most likely explained by Gk-leak and PSP amplitude parameters. For example, decreasing the Gk-leak parameter in the model depolarizes VPM and nRT cells, making them more excitatory. Additionally, an increase in the amplitude of EPSP’s resulting from afferent input will additionally raise the probability of a neuron’s response to synaptic input, representing a purely bottom-up phenomenon. Increases in IPSP amplitude, due to recurrent inhibition of nRT cells, decrease the probability of a response before the afferent input can induce the cell to fire an action potential. Together, the balance of excitatory and inhibitory influences on VPM neurons, either by modulating Gk-leak for both VPM and nRT cell populations or PSP amplitude produces the observed spectrum of modulatory effects on VPM neuronal discharge. In other words, the cell specific modulation of excitatory discharge patterns as observed in the model are due to the interaction of overall increases in excitability of inhibitory and excitatory neuronal populations.
Comparisons between modeling data and in vivo data suggest that when either EPSP amplitude is high concordant with moderate increases in IPSP amplitude or there is a moderate Gk-leak for both VPM and nRT cell populations best matches in vivo conditions when the LC is stimulated at a low frequency of 0.5 Hz. This observation is supported by evidence indicating that the modulatory actions demonstrated by the model under these conditions was not significantly different from the modulatory actions of increased LC output (chi-square = 8.3, df =2, p>0.05 with Bonferroni correction). Nonetheless, the model is also capable of demonstrating a wider dynamic range of possible effects of NE on thalamic neurons than observed in the awake behaving animal. The detail of modulatory NE actions that this model provides begins to offer a better mechanistic understanding beneath the spectrum of putative modulatory actions of NE under normal conditions and possibly abnormal or disease states.
Importantly, the results of this modeling study can now provide a framework to pursue further in vivo studies. For example, we are particularly interested in the role of alpha vs. beta noradrenergic receptors in shaping thalamic responsiveness to afferent input since because, pharmacologically, these two receptors subtypes mediate different cellular and network actions and are linked to are related to different neurologic and psychiatric disorders. While some information about the effect of activation of different receptor subtypes is known, much work is still needed. For example, it is known that activation of the beta receptor, increases the H-current, reducing oscillations during the sleep cycle and essentially shifting these cells from an oscillatory state to a single-spike mode. The simulations in our model confirm this well established observation. Furthermore, it is well established that activation of the alpha 1 receptor subtype decreases the potassium leak current. As our simulations show, this can have interesting effects depending on whether the alpha 1 receptor subtype is preferentially activated in the thalamus, the reticular nucleus or some combination of the two. We are currently using these simulations to guide new in vivo experiments.
Future studies will address the effects of combined modulation of Gk-leak, IPSPs, and EPSPs on neuronal response probability. However, testing interactions of all possible combinations of cell types (nRT vs. VPM), mechanisms (Gk-leak, IPSP, EPSP), and a full range of current for PSP magnitudes in a computational model is very complex and requires additional in vivo studies to narrow the possible interactions tested. For example, the current modeling data demonstrates the effects of modulating EPSP or IPSP separately or in combination (Figure 4) on neuronal responsiveness. Additional pharmacological studies are necessary to determine whether specific NE receptor subtypes are responsible for modification of EPSPs or IPSPs and the extent to which these PSP's can be modulated under physiological conditions. These data are necessary to provide dose response information that can be used to provide a realistic limit to the number of Gk-leak current and PSP interactions modeled. This line of study will provide a formalized relationship between activating alpha and beta receptors and the resultant effects on neural discharge properties.
Conclusions
In conclusion, our model provides a strong rationale for designing experiments to identify the specific cell populations that are capable of expressing different NE modulatory actions and the threshold for activating these noradrenergically-mediated mechanisms. For example, various cellular and membrane neuromodulatory actions of NE have been linked to specific adrenoceptors and second-messenger cascades (Cheun & Yeh, 1992; 1996; Foehring et al., 1989; Madison & Nicoll, 1986b; Mouradian et al., 1991; Sessler et al., 1989; 1995; Waterhouse et al., 1981; 1982). Differential expression of adrenergic receptors among different neuronal populations would significantly alter the potential response of a network of cells to NE release. Likewise, if the thresholds for activation of these noradrenergic mechanisms were different, the net effect of NE on an ensemble of cells would depend upon local tissue concentrations of this agent. Impulse activity from the locus coeruleus and the efficacy of local noradrenergic reuptake mechanisms would thus be important factors in assessing the impact of synaptically-released NE on signal processing functions of local arrays of neurons.
The findings of the present study begin to establish a framework for evaluating the ability of NE to differentially modulate cellular response properties at different levels within complex neural networks (e.g. brainstem, thalamic and cortical levels of ascending sensory pathways) under waking conditions. In this regard these simulated responses and physiological data revealed at least three important considerations for understanding NE’s moment to moment regulation of neural network operation: 1) expression of cell specific noradrenergic modulatory action(s), 2) description of the relative proportion of cells that are modulated by NE release, and 3) identification of the NE mediated physiological parameter space in which VPM cells may operate.
4. Experimental Procedures
4.1 Model Network Structure
We modeled the rat VPM thalamus and nRT network within the trigeminal somatosensory system. This system is advantageous to model because the anatomical connections and the physiology of this sensory circuit are well documented (Belford & Killackey, 1979a,b; Chiaia et al., 1991a;b; Diamond et al., 1992a;b; Harris, 1986; 1987; Killackey et al., 1989; van der Loos, 1976; Waite 1973a,b). Within the VPM, anatomical specializations commonly called barreloids, are comprised of small populations of neurons (approximately 200) that project to a corresponding cortical barrel and are most likely to respond to a single whisker (Nicolelis & Chapin, 1994, Chapin & Nicolelis, 1999). There are no recurrent connections, nor collaterals between cells within the rat VPM (Harris, 1986), however axonal divergence from corresponding brainstem sensory nuclei permit VPM neurons to respond to several adjacent whiskers on the contralateral face. All VPM neurons are morphologically similar and make excitatory synaptic connections with cortical neurons and the adjacent nRT. Cells of the nRT are all inhibitory (de Biasi et al., 1986; Lee et al., 1994; Cox et al., 1997). They receive excitatory input from the ipsilateral somatosensory cortex and ipsilateral VPM (Carmen et al., 1964; Lozsadi et al., 1996; Liu & Jones, 1999), and project onto neighboring nRT cells and back to cells in the VPM (Minderhoud, 1971; Jones 1975; Ohara et al., 1980; Montero & Scott, 1981; Ohara & Lieberman, 1981,1985; Harris 1987; Ohara, 1988; Spreafico et al., 1991; Chen et al., 1992; Gonzalo-Ruiz & Lieberman, 1995; Crabtree, 1996).
Our model of the rat somatosensory VPM-nRT network simulates five thalamic barreloids and consists of two types of neurons: a) excitatory VPM neurons (n=1000) and b) inhibitory nRT cells (n=1000). As such, 200 VPM cells are modeled for each thalamic barreloid and associated region within the nRT. The large number of modeled neurons allowed us to simulate the response of individual cells to a broad range of stimuli, mimicking the input that would be expected from principal and surround whiskers. Moreover, we wanted to maximize the sample size of responding neurons in order to detect the full range of possible behaviors that have been observed in vivo. In accordance with existing knowledge of rat VPM anatomy, we did not model connections between individual VPM cells (Harris et al., 1986). Although connections exist between VPM and nRT cells, the extent of such connectivity is unknown. In lieu of this, previous modeling studies have assumed sparse connectivity between VPM cells and nRT (Wang et al., 1991). I In our model each VPM cell contacts on average 2% of nRT cells via excitatory synapses. Additionally, each nRT cell contacts, on average, 2% of VPM cells and 1% of nRT cells via inhibitory GABAA type synapses and 0.5% of VPM cells and 0.5% of nRT cells via inhibitory GABAB type synapses.
4.2 Membrane Currents
In our model, cells were constructed using a two-compartment equivalent circuit representation that contained 5 membrane currents, based on an integrate-and-fire model (Macgregor, 1987; Fig. 1). The membrane conductances in the soma compartment included a leaky membrane conductance mainly due to potassium and sodium ions (Gleak), and two conductances to potassium that acted as delayed rectifiers GK1, GK2. The membrane conductances in the dendritic compartment included a high threshold, voltage dependent conductance to calcium (GCa) and a calcium dependent conductance to potassium (GKCa) described by Jahnsen and Llinas (1984b) for thalamic cells. With the exception of the leakage membrane current, the activation and inactivation of each current was described using differential equation and exponential integration methods previously described (MacGregor, 1977) and verified for the currents described here (Rybak et al., 1997) using a 0.1 msec time step.
Figure 1.
Membrane current models for each cell. The soma had a leak current, Gleak, that consists of a sodium and potassium current. This current was responsible for maintaining the resting membrane potential. There was a voltage dependent potassium current, GK1 which acted as a delayed rectifier. The low threshold calcium current, GT, was responsible for the internal oscillations and GH was a hyperpolarizing activated cation current. Neither GT nor GH (shaded grey) were explicitly modeled (refer to text). GK2 was a second voltage dependent potassium current with a longer time constant and was responsible for the between burst after-hyperpolarization. The dendritic currents include a calcum current GCa and a calcium dependent potassium current GK[Ca].
As the goal of these simulations was to provide guidance for subsequent multi-neuron recording studies, simplifications of neuronal activity were introduced to minimize the number of parameters and focus attention of the study on the effect of NE on network dynamics. This approach allowed the results of this model to be extended to other brain nuclei identified (see Introduction) that exhibit similar dynamic behavior. First, since the shape of the action potential was not critical for interpreting the results of this model, the fast sodium current was not explicitly modeled. Therefore, when the cell reached a threshold for a sodium spike, the model presumed an action potential and transmitted synaptic activation to downstream neurons. Second, when the potential of the dendritic compartment exceeded the dendritic threshold, the calcium conductance was increased by a fixed amount and decayed with constant time constant (MacGregor, 1987). Third, while these changes in calcium concentration change the equilibrium potential for calcium according to the Nernst equation (Rybak et al., 1997), the equilibrium potential for calcium was held constant for the simulations in this study. This formalism represents a simplification that reduces computational overhead while maintaining a sufficiently accurate representation of the network dynamics.
VPM and nRT cells also contain other membrane currents, including a low threshold calcium current or T-current (Jahnsen & Llinas, 1984a; b) and a hyperpolarizing cation current or H-current (McCormick & Pape, 1990a), which were not modeled. These currents are primarily involved in the generation of 7–12 Hz oscillations associated with sleep spindles (McCormick & Pape, 1990b; von Krosigk et al., 1993). The H-current is enhanced by NE (Bal & McCormick, 1996), which depolarizes the cell limiting the occurrence of low threshold calcium spikes (Jahnsen & Llinas, 1984b). Reduction of low threshold calcium spikes and 7–12 Hz oscillations by NE’s enhancement of the H-current suggests that the H-current participates in the transition from sleep to wakefulness. Initially, the model was validated by demonstrating that oscillations were generated when the H-current and T-current were included (data not shown). However to simulate the waking state, the leak current was adjusted to include the activated H-current which effectively depolarized the cell (resting membrane potential approx. −57.3 mV) and allowed us to remove the H- and T-currents from the model. This approach represents the currents of VPM and nRT neurons in a reasonable manner that minimizes computational overhead and addresses circuit operations under the waking conditions modeled in these simulations.
4.3 Synaptic Currents
Three synaptic currents were simulated in the model. Excitatory post-synaptic potentials (EPSP’s) were considered to result from a release of glutamate at excitatory synapses, and inhibitory post-synaptic potentials (IPSP’s) resulted from activation of GABAA and GABAB-type synapses. The EPSP that is generated in situ when glutatmate binds to post-synaptic receptors, opens Na+ channels which in turn increases Na+ conductance, GNa. The other two synaptic potentials were inhibitory post-synaptic potentials (IPSP’s) that are generated in situ when GABA binds to GABAA or GABAB postsynaptic receptors. Activation of both types of GABA receptors results in an increase in chloride conductance although the time courses are substantially different. GABAB receptors operate through an intracellular second messenger cascade that is not explicitly modeled but nevertheless results in an increase in potassium conductance that is slower than the GABAA conductance.
These three postsynaptic potentials (PSPs) were each simulated using a dual-alpha function equation (see Appendix for equations). The parameters that distinguish the different PSP types (i.e. resulting from the activation of a glutamate receptor as opposed to GABAA or GABAB receptors) are: 1) the amplitude of the PSP, gmax, 2) the time course, described by τ1 and τ2, and 3) the reversal potential, Eeq. The amplitudes of the PSPs were modulated in the model to simulate changes brought about by fluctuations in LC output and resultant alterations in tissue concentrations of NE (described in detail below – ‘Modeling the Effects of NE’). The time courses of the PSPs were set based on experimental data. The glutamate receptor and the GABAA receptor (GABAA-R) are ligand gated ion-channels and therefore each has a relatively fast time course. In the model, the EPSP reaches a peak in 1–2 ms and decays in about 15 ms. Activation of the GABAA receptor generates an IPSP that peaks within 5 msec and lasts for less than 100 ms (Pearce, 1993; Thompson & Gahwiler, 1992). The GABAB receptors that activate postsynaptic second messenger systems take longer to peak (approximately 180 msec) (see Peet & McLennan, 1986) and have a duration of more than 300 msec (Dutar & Nicoll, 1988). The reversal potentials were also established on the basis of experimental data. Activation of ionotropic glutamate channels increases the permeability to sodium and calcium that moves the membrane potential towards a reversal potential of about 0 millivolts (mV) whereas chloride ion influx, via the GABAA receptors, has a reversal potential of −65 mV (Dingledine & Langmoen, 1980).
4.4 Simulated Afferent Stimulation
Afferent activity simulating sensory input from the periphery (mystacial vibrissae) via the brainstem was modeled using data from single whisker stimulation studies (Nicolelis & Chapin, 1994; Foffani & Moxon, 2004) and was represented as an EPSP incident on VPM cells in the model (Moxon & Chapin, 1999). Afferent volleys were simulated by activating model fiber populations that made synaptic contact with VPM thalamic cells. Each fiber made 20 synaptic contacts with cells of the VPM. For each trial, the probability of an input fiber firing an action potential was set so that an average of 175 fibers were activated for 5 milliseconds. The result of an input fiber simulating an action potential was to generate an EPSP at all of its 20 synapses. Each trial was repeated 50 times. For each trial a different, randomly chosen, subset of afferent fibers was activated to simulate the stochastic nature of brainstem input. These 50 trials simulated afferent input (i.e. whisker deflection) similar to repetitive deflection of a single whisker as occurs during receptive field mapping studies in vivo (Nicolelis & Chapin, 1994). Each trial consisted of simulating whisker-related afferent volleys to the thalamus. The objective of these simulations was to model standard experimental practice as employed in single whisker mapping studies (Foffani & Moxon, 2004). The responses for each neuron were then presented in the form of a post-stimulus time histogram (PSTH). The probability of a response (i.e. action potential generation) and the latency of that response to stimulus presentation (n=50) were quantified using the PSTH.
4.5 Modeling the Effects of NE
In vitro studies showed that direct application of NE decreased the leak current, ILeak that contributes to the resting membrane potential of individual cells (McCormick & Pape, 1990a). In intact preparations, local application of NE by iontophoresis or activation of the LC-NE efferent pathway enhanced neuronal responses to putative transmitters, glutamate or GABA, (Moises et al., 1979; Moises & Woodward, 1980; Waterhouse et al.,, 1980; Waterhouse et al.,, 1982; Mouradian et al.,, 1991). Receptor-linked second messenger mechanisms have been implicated in mediating noradrenergic regulation of GABA-induced membrane hyperpolarizations (Cheun & Yeh, 1992; 1996; Sessler et al., 1995) and glutamate-evoked discharges (Mouradian et al., 1990). In the simulations presented here, we postulate second messenger-mediated effects of NE on the amplitude of the EPSP’s and IPSP’s.
In the model, we simulated NE modulation of the leak current in both the VPM thalamus and the nRT. In addition we separately simulated modulation of excitatory and inhibitory post-synaptic potentials (EPSP and IPSP, respectively) to mimic previously observed changes in synaptic efficacy (Waterhouse et al., 2000) and GABA-induced whole cell currents (Cheun & Yeh, 1992), respectively. To model effects of NE, the conductances of individual membrane currents were modulated by modifying the Gmax parameter for that current (refer to Appendix for equations of individual currents). To simulate a reduction in the leaky membrane current, the value of GK-leak was decreased, thereby depolarizing the cells. To model the effects of NE on EPSP’s and IPSP’s the amplitudes of GNa-Glu and GCl-GABA were modulated respectively. In its final form the model was fixed in the ‘waking’ state to determine if NE-like influences on membrane currents could result in cellular and network effects like those seen in in vivo experiments. As such noted above, the low threshold calcium current and the H-current were not modeled.
4.6 Intact Animal Studies
Spike train activity was recorded extracellularly from multiple single VPM thalamic neurons in six adult male Long-Evans hooded rats (weight = 250–450 g - Charles River Laboratories, Wilmington, MA) during the waking state (Devilbiss & Waterhouse, 2002). Animals were housed in a light (12on-12off), temperature and humidity controlled environment with food and water available ad libitum. Neuronal recordings were obtained during daylight hours (i.e. when the lights were normally on). Animals were tethered to the recording equipment but allowed to freely move within the recording chamber and recordings were made while the animal remained in a state of quiet rest, verified by video analysis. Animals used in this study were treated in accordance to NIH guidelines on research animal care. Furthermore, these experiments were approved by the Institutional Animal Care and Use Committee of Drexel University.
4.6.1 Surgery
All animals received microwire implants in the VPM thalamus and the ipsilateral nucleus LC. Additionally, a stimulation electrode was passed under the skin and anchored in the whisker pad. These methods have been described previously (Devilbiss & Waterhouse, 2002). In brief, under anesthesia the rat was placed in a stereotaxic apparatus and the skull exposed. The head was placed at a 15° angle (nose down) to the horizontal plane and a small craniotomy was performed at 1.2 mm lateral and 3.6 mm caudal to the intersection of the midline and lambda to allow the vertical placement of the microwire bundle into the LC. Five stainless steel screws (MX-080-2; Small Parts Inc. Miami, FL) were placed into the skull for electrical grounding and to ensure proper anchoring of the probes. A recording/stimulation probe, containing a bundle of eight 50 µm Teflon-coated, stainless steel microwires (SB103, NB Labs, Dennison, TX), tied together with a silk suture was lowered to an approximate depth of 6.0 mm. During placement of the electrode bundle, neuronal electrical activity was monitored to guide accurate placement within the LC. The characteristic spontaneous discharge rate (~ 0.1 – 5 Hz) and the biphasic response to tail pinch (Akaike 1982) were two criteria used to identify putative LC neurons. The skull opening was then sealed with Gelfoam and the probe was attached to the skull with dental acrylic.
The rat’s head was then re-positioned parallel to the horizontal plane and an additional skull opening was made at −3.3 A/P and −2.8 M/L (with respect to bregma) for placement of a VPM microwire probe (coordinates from the atlas of Paxinos & Watson, 1986 and Waite, 1973a,b). Neuronal electrical activity was monitored to guide placement of the electrode into the VPM at an approximate depth of 5.5 mm (from dura). The VPM thalamic region that represented sensory information for the C3 vibrissae was targeted. After electrode placement, the skull opening overlying the cortex was filled with Gelfoam and the probe was secured to the skull with dental acrylic. Before all of the skull screws were encapsulated with dental acrylic the grounding wires from each electrode assembly were wrapped around the exposed screws for electrical grounding.
A whisker pad stimulating electrode was prepared by placing the twisted pair of 7-stranded stainless steel wire (793200, A-M Systems Inc. Carlsborg, WA) inside a 20 gauge needle cut blunt and polished. The needle was used to tunnel under the skin from the initial skull incision into the whisker pad. By carefully removing the needle, the twisted pair was left hooked around a whisker follicle. The free ends of the twisted pair were inserted through a 15 mm section of Silastic tubing (602-231, Dow Corning), protecting the wire from rubbing against the dental acrylic. The free ends of the wire were crimped into a connector and attached to the skull with dental acrylic. The skin was loosely sealed around the dental cement and the animal was allowed to recover for 5 – 10 days.
4.6.2 Electrophysiological Recording
Prior to experimentation, the animals were habituated to the testing chamber, the experimenter, and the experimental procedure (whisker pad stimulation). During this habituation period multiple single units were discriminated (see Devilbiss & Waterhouse, 2002) from periods of spontaneous discharge and again from periods of repeated whisker pad stimulation. The experimental session was initiated after confirmation that the units were responsive to whisker stimulation. The whisker pad was stimulated with biphasic pulses (range 1–3 mA) that were threshold for producing a rostral twitch of a single vibrissae. The stimulus current chosen produced robust excitatory discharge in a majority of the simultaneously recorded units, and produced evoked-responses in VPM neurons comparable to single vibrissa whisker deflection (Devilbiss & Waterhouse, 2002). During an initial control period and in all subsequent periods, single stimulus pulses to the whisker pad (n=90) were presented randomly (pulse duration 1 msec; mean inter-trial interval 2 sec; range 1.5 – 2.5 sec) to prevent stimulus habituation. Following the control period, the order of three additional control periods and six LC stimulation conditions were randomly assigned. For each of the LC stimulation conditions a train of single electrical shocks ranging from 3 µA to 300 µA were delivered at a frequency of 0.5 Hz to 5.0 Hz. The experimental session was videotaped with a video counter timer providing time stamps (resolution = 0.1 sec) synchronized to the multiunit recording and behavioral control systems so that we could confirm that the animal remained in a state of quiet rest throughout the experiment.
4.6.3 Histology
Following each experiment, the animal was deeply anesthetized and 60 µA of current was passed across pairs of bundle microwires for 45 seconds. The animal was then perfused with 0.9% saline followed by a 10% formalin solution containing 5% potassium ferrocyanide, which produces a Prussian blue reaction product. The brains were then removed and stored in phosphate buffer containing 15% sucrose. Coronal 80 µm sections were cut on a freezing microtome and collected through the VPM recording and LC stimulation sites and Nissl-Stained.
4.6.4 Data Analysis
Post-stimulus time histograms (PSTH’s) were examined to identify and quantify changes in the probability of a response (i.e. action potential generation) and the temporal coding of stimulus-evoked discharge. Probability of a response was measured as spikes/stimulus or frequency of discharge during a fixed response epoch in control and LC stimulation histograms. Increases or decreases in response latency were quantified by determining changes in the latency to the statistical mode of the stimulus-evoked response, or the latency to the response peak discharge was calculated and referred to here as the modal latency. Decreases in this measure indicate a concentrated burst of activity near the onset of the stimulus-evoked response or a more focused discharge per unit of time. The statistical significance of LC-mediated changes in the magnitude or latency of stimulus-evoked discharge were assessed by comparing values obtained during LC simulation conditions to control conditions with students T-tests for each neuron (with bonferroni corrections).
Acknowledgements
This work was supported by NIH grants NS32461, NS34808, and DA05117 to BDW.
Abbreviations
- A/P
Anterior-Posterior
- CNS
Central Nervous System
- Eeq
Reversal Potential
- EPSP
Excitatory Postsynaptic Potential
- GCa
Conductance of Calcium
- GCl-GABA
Conductance of Chloride mediated by Gamma-Aminobutyric Acid
- GK1
Conductance of Potassium (fast)
- GK2
Conductance of Potassium (slow)
- GKca
Conductance of Potassium (calcium dependent)
- Gleak
Conductance of a Leaky Membrane
- gmax
Amplitude of a Postsynaptic Potential
- GNa
Conductance of Sodium
- GNa-Glu
Conductance of Sodium mediated by Glutamate
- GABA
Gamma-Aminobutyric Acid
- GABAA; GABAB
Gamma-Aminobutyric Acid Receptor Subtypes
- Hz
Hertz (frequency)
- IPSP
Inhibitory Postsynaptic Potential
- LC
Locus Coeruleus
- LC-NE
Locus Coeruleus - Norepinephrine
- M/L
Medial-Lateral
- mA
Milliamp
- Msec
Millisecond
- mV
Millivolts
- NE
Norepinephrine
- nRT
Thalamic Reticular Nucleus
- nS
Nano-Siemens
- PSP
Postsynaptic Potential
- PSTH
Post-Stimulus Time Histogram
- τ1; τ2
Decay Time(s)
- THD
dendritic threshold
- VPM
Ventral Posteromedial Thalamus
Appendix
The model of each VPM cell was constructed using a two-compartment equivalent circuit representation that contained 5 membrane currents and 3 synaptic currents (Equation 1), which was a simplification of previous models (Destexhe et al., 1993, 1996a, b; Lytton et al., 1996, 1997; Rush & Rinzel, 1994; Wang et al., 1991;).
| Equation 1 |
The magnitude of the currents was determined by evaluating the conductance of the current at each time step in the model, which is generally a function of the cell’s membrane potential. The membrane conductances in the soma compartment included a leaky membrane conductance mainly due to sodium and potassium ions (Gleak), a voltage dependent conductance to potassium that acted as a delayed rectifier (GK1) and a second, slower potassium conductance (GK2), which was mainly responsible for the inter-burst interval. When the potential of the soma reached threshold, the conductance to potassium was increased by an amount Bi and decayed with time constant τGKi (Equation 2).
| Equation 2 |
Dendritic Membrane Conductances
The membrane conductances in the dendritic compartment included a high threshold conductance to calcium (GCa) and a calcium dependent conductance to potassium (GKCa) (Jahnsen & Llinas, 1984b). The conductance to calcium, GCa, was voltage-dependent (Equation 3). When the potential of the dendritic compartment exceeded the dendritic threshold, THD, the calcium conductance was increased by an amount D and decayed with time constant τGCa.
| Equation 3 |
The potassium current, GKCa, was dependent on the concentration of calcium. The calcium concentration, [Ca2+], was proportional to the conductance to calcium (Equation 4). A represented the amplitude of calcium concentration rise in proportion to calcium conductance and τCa was the time constant for changes in calcium concentration.
| Equation 4 |
When calcium conductance in the dendritic compartment increased the concentration of calcium above a threshold, Cao there was an increase in dendritic potassium conductance, GKCa (Equation 5). BD, the amplitude of potassium conductance increase, corresponded to the given level of [Ca2+]. In the model, GKCa increased incrementally (in proportion to BD) as long as [Ca2+] was above Cao. When the membrane potential of the dendritic compartment dropped below its threshold, THD, the calcium conductance was no longer driven upward and tended to decay with time constant τGCa toward zero. As GCa approached zero, the calcium concentration also decreased. When it fell below Cao, then GKCa was no longer being augmented and it in turn began to decay exponentially back to zero.
| Equation 5 |
Parameter values are given in Table 4. This formalism represents a simplification that reduces computational overhead while maintaining a sufficiently accurate representation of the network dynamics. Since we are not addressing questions of precise mechanisms underlying network oscillations, this model is sufficient. With the exception of the leakage membrane current, the activation and inactivation of each current was described using these differential equation and integrated using methods previously described (MacGregor, 1977).
Table 4. Parameters for each conductance described in the model.
Table of constant values and time constants used for each equation in the appendix to model synaptic currents and membrane conductance’s. The first column on the left identifies the type of cellular mechanism being modeled. The adjacent columns to the right indicate the parameters, values, and equilibrium potential used in modeling equations. Parameters of leak current (Gmax) are used for ohm’s law (the integrative form is Eg. 1); parameters of the potassium conductance’s are used in Eq. 2; calcium conductance parameters are used in Eg. 3; calcium concentration parameters are used in Eq. 4; calcium activated potassium parameters are used in Eq. 5
| Conductance | Parameter | Value | Equilibrium Potential (mV) |
|---|---|---|---|
| Leak Current | Gmax | 4.4 | −70.0 |
| Potassium – rectifying, GK1 | B1 τGK1 |
20.00 5 |
−85.0 |
| Potassium – slow GK2 | B2 τGK2 |
5.00 100 |
−85.0 |
| Calcium Conductance, GCa | D τGCa |
0.2 5.0 |
120 |
| Calcium Concentration, [Ca2+] | A τCA |
0.1 20.0 |
|
| Calcium activated Potassium, GKD | BD τGKD |
200 100 |
−85.0 |
Synaptic Currents
The postsynaptic potentials (PSPs) were simulated using a dual-alpha function equation (Equation 6). The parameters that distinguish the different PSPs (i.e. resulting from the activation of a glutamate receptor as opposed to a GABA receptor) are the amplitude of the PSP, gmax, and the time course, described by τ1 and τ2 (Table 5 and refer to Methods).
| Equation 6 |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CLASSIFICATION TERMS: Computational and Theoretical Neuroscience
References
- Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J. Neurosci. 1981a;1:876–886. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom FE. Norepinephrine-containing locus coeruleus neurons in behaving rats exhibit pronounced responses to non-noxious environmental stimuli. J. Neurosci. 1981b;1:887–900. doi: 10.1523/JNEUROSCI.01-08-00887.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike T. Periodic bursting activities of locus coerulleus neurons in the rat. Brain Res. 1982;239:629–633. doi: 10.1016/0006-8993(82)90540-6. [DOI] [PubMed] [Google Scholar]
- Bal T, McCormick DA. What stops synchronized thalamocortical oscillations? Neuron. 1996;17:297–308. doi: 10.1016/s0896-6273(00)80161-0. [DOI] [PubMed] [Google Scholar]
- Bazhenov M, Timofeev I, Steriade M, Sejnowski T. Spiking-bursting activity in the thalamic reticular nucleus initiates sequences of spindle oscillations in thalamic networks. J Neurophysiol. 2000;84:1076–1087. doi: 10.1152/jn.2000.84.2.1076. [DOI] [PubMed] [Google Scholar]
- Belford GR, Killackey HP. The development of vibrissae representation in subcortical trigeminal centers of the neonatal rat. J Comp Neurol. 1979a;188:63–74. doi: 10.1002/cne.901880106. [DOI] [PubMed] [Google Scholar]
- Belford GR, Killackey HP. Vibrissae representation in subcortical trigeminal centers of the neonatal rat. J Comp Neurol. 1979b;183:305–321. doi: 10.1002/cne.901830207. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Abercrombie ED. Relationship between locus coeruleus discharge rates and rates of norepinephrine release within neocortex as assessed by in vivo microdialysis. Neuroscience. 1999;93:1263–1270. doi: 10.1016/s0306-4522(99)00276-6. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Carman JB, Cowan WM, Powell TPS. Cortical connections of the thalamic reticular nucleus. J Anat. 1964;98:587–598. [PMC free article] [PubMed] [Google Scholar]
- Chapin JK, Nicolelis MAL. Principal component analysis of neuronal ensemble activity reveals multidimensional somatosensory representations. J Neurosci Methods. 1999;94:121–140. doi: 10.1016/s0165-0270(99)00130-2. [DOI] [PubMed] [Google Scholar]
- Chen S, Raos V, Bentivoglio M. Connections of the thalamic reticular nucleus with the contralateral thalamus in the rat. Neurosci Lett. 1992;147(1):85–88. doi: 10.1016/0304-3940(92)90780-b. [DOI] [PubMed] [Google Scholar]
- Cheun JE, Yeh HH. Modulation of GABAA receptor-activated current by norepinephrine in cerebellar Purkinje cells. Neuroscience. 1992;51:951–960. doi: 10.1016/0306-4522(92)90532-7. [DOI] [PubMed] [Google Scholar]
- Cheun JE, Yeh HH. Noradrenergic potentiation of cerebellar Purkinje cell responses to GABA: cyclic AMP as intracellular intermediary. Neuroscience. 1996;74:835–844. doi: 10.1016/0306-4522(96)00130-3. [DOI] [PubMed] [Google Scholar]
- Chiaia NL, Rhoades RW, Fish SE, Killackey HP. Thalamic processing of vibrissal information in the rat. I. Afferent input to the medial ventral posterior and posterior nuclei. J Comp Neurol. 1991a;314:201–216. doi: 10.1002/cne.903140202. [DOI] [PubMed] [Google Scholar]
- Chiaia NL, Rhoades RW, Fish SE, Killackey HP. Thalamic processing of vibrissal information in the rat: II. Morphological and functional properties of medial ventral posterior nucleus and posterior nucleus neurons. J. Comp. Neurol. 1991b;314:217–236. doi: 10.1002/cne.903140203. [DOI] [PubMed] [Google Scholar]
- Ciombor KJ, Ennis M, Shipley MT. Norepinephrine increases rat mitral cell excitatory responses to weak olfactory nerve input via alpha-1 receptors in vitro. Neuroscience. 1999;90:595–606. doi: 10.1016/s0306-4522(98)00437-0. [DOI] [PubMed] [Google Scholar]
- Cox CL, Huguenard JR, Prince DA. Nucleus reticularis neurons mediate diverse inhibitory effects in thalamus. Proc Natl Acad Sci USA. 1997;94(16):8854–8859. doi: 10.1073/pnas.94.16.8854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree JW. Organization in the somatosensory sector of the cat's thalamic reticular nucleus. J comp Neurol. 1996;366(2):207–222. doi: 10.1002/(SICI)1096-9861(19960304)366:2<207::AID-CNE2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- de Biasi S, Frassoni C, Spreafico R. GABA immunoreactivity in the thalamic reticular nucleus of the rat. A light and electron microscopical study. Brain Res. 1986;399(1):143–147. doi: 10.1016/0006-8993(86)90608-6. [DOI] [PubMed] [Google Scholar]
- Destexhe A, Bal T, McCormick DA, Sejnowski TJ. Ionic mechanisms underlying synchronized oscillations and propagating waves in a model of ferret thalamic slices. J Neurophysiol. 1996a;76:2049–2070. doi: 10.1152/jn.1996.76.3.2049. [DOI] [PubMed] [Google Scholar]
- Destexhe A, Bal T, McCormick DA, Sejnowski TJ. In vivo, in vitro and computational analysis of dendritic calcium currents in thalamic reticular neurons. J Neurosci. 1996b;16:169–185. doi: 10.1523/JNEUROSCI.16-01-00169.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destexhe A, McCormick DA, Sejnowski TJ. A model for 8–10 Hz spindling in interconnected thalamic relay and reticularis neurons. Biophys J. 1993;65:2473–2477. doi: 10.1016/S0006-3495(93)81297-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devilbiss DM, Waterhouse BD. Determination and quantification of pharmacological, physiological, or behavioral manipulations on ensembles of simultaneously recorded neurons in functionally related neural circuits. J Neurosci Methods. 2002;121:181–198. doi: 10.1016/s0165-0270(02)00263-7. [DOI] [PubMed] [Google Scholar]
- Devilbiss DM, Waterhouse BD. The effects of tonic locus ceruleus output on sensory-evoked responses of ventral posterior medial thalamic and barrel field cortical neurons in the awake rat. J Neurosci. 2004;24:10773–10785. doi: 10.1523/JNEUROSCI.1573-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond M, Armstrong-James M, Ebner F. Somatic sensory responses in the rostral sector of the posterior group (POm) and in the ventral posterior medial nucleus (VPM) of the rat thalamus. J. Comp. Neurol. 1992a;318:462–476. doi: 10.1002/cne.903180410. [DOI] [PubMed] [Google Scholar]
- Diamond M, Armstrong-James M, Budway M, Ebner F. Somatic sensory responses in the rostral sector of the posterior group (POm) and in the ventral posterior medial nucleus (VPM) of the rat thalamus: dependence on the barrel field cortex. J. Comp. Neurol. 1992b;319:66–79. doi: 10.1002/cne.903190108. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Langmoen IA. Conductance changes and inhibitory actions of hippocampal recurrent IPSPs. Brain Res. 1980;185:277–287. doi: 10.1016/0006-8993(80)91068-9. [DOI] [PubMed] [Google Scholar]
- Dutar P, Nicoll RA. Pre- and postsynaptic GABAB receptors in the hippocampus have different pharmacological properties. Neuron. 1988;1:585–591. doi: 10.1016/0896-6273(88)90108-0. [DOI] [PubMed] [Google Scholar]
- Florin-Lechner SM, Druhan JP, Aston-Jones G, Valentino RJ. Enhanced norepinephrine release in prefrontal cortex with burst stimulation of the locus coeruleus. Brain Res. 1996;742:89–97. doi: 10.1016/s0006-8993(96)00967-5. [DOI] [PubMed] [Google Scholar]
- Foehring RC, Schwindt PC, Crill WE. Norepinephrine selectively reduces slow Ca2+- and Na+-mediated K+ currents in cat neocortical neurons. J.Neurophysiol. 1989;61:245–256. doi: 10.1152/jn.1989.61.2.245. [DOI] [PubMed] [Google Scholar]
- Foffani G, Moxon KA. PSTH-based classification of sensory stimuli using ensembles of single neurons. J.Neurosci.Methods. 2004;135:107–120. doi: 10.1016/j.jneumeth.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Foote SL, Aston-Jones G, Bloom FE. Impulse activity of locus coeruleus neurons in awake rats and monkeys is a function of sensory stimulation and arousal. Proc Natl Acad Sci U S A. 1980;77:3033–3037. doi: 10.1073/pnas.77.5.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote SL, Bloom FE, Aston-Jones G. Nucleus locus ceruleus: new evidence of anatomical and physiological specificity. Physiol Rev. 1983;63:844–914. doi: 10.1152/physrev.1983.63.3.844. [DOI] [PubMed] [Google Scholar]
- George MJ. Modification of receptive fields of posteriomedial barrel subfield neocortical single units by known concentrations of iontophoresed noradrenaline in the rat. Int J Neurosci. 1992;65:69–81. doi: 10.3109/00207459209003279. [DOI] [PubMed] [Google Scholar]
- Gonzalo-Ruiz A, Lieberman AR. GABAergic projections from the thalamic reticular nucleus to the anteroventral and anterodorsal thalamic nuclei of the rat. J Chem Neuroanat. 1995;9(3):165–174. doi: 10.1016/0891-0618(95)00078-x. [DOI] [PubMed] [Google Scholar]
- Harris RM. Morphology of physiologically identified thalamocortical relay neurons in the rat ventrobasal thalamus. J Comp Neurol. 1986;251:491–505. doi: 10.1002/cne.902510405. [DOI] [PubMed] [Google Scholar]
- Harris RM. Axon collaterals in the thalamic reticular nucleus from thalamocortical neurons of the rat ventrobasal thalamus. J.Comp Neurol. 1987;258:397–406. doi: 10.1002/cne.902580308. [DOI] [PubMed] [Google Scholar]
- Hobson JA, McCarley RW, Wyzinski PW. Sleep cycle oscillation: reciprocal discharge by two brainstem neuronal groups. Science. 1975;189:55–58. doi: 10.1126/science.1094539. [DOI] [PubMed] [Google Scholar]
- Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952;117:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huguenard JR, McCormick DA. Simulation of the currents involved in rhythmic oscillations in thalamic relay neurons. J.Neurophysiol. 1992;68:1373–1383. doi: 10.1152/jn.1992.68.4.1373. [DOI] [PubMed] [Google Scholar]
- Jahnsen H, Llinas R. Electrophysiological properties of guinea-pig thalamic neurones: an in vitro study. J.Physiol. 1984a;349:205–226. doi: 10.1113/jphysiol.1984.sp015153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnsen H, Llinas R. Ionic basis for the electro-responsiveness and oscillatory properties of guinea-pig thalamic neurones in vitro. J Physiol. 1984b;349:227–247. doi: 10.1113/jphysiol.1984.sp015154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Griff ER, Ennis M, Zimmer LA, Shipley MT. Activation of locus coeruleus enhances the responses of olfactory bulb mitral cells to weak olfactory nerve input. J.Neurosci. 1996;16:6319–6329. doi: 10.1523/JNEUROSCI.16-19-06319.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killackey HP, Koralek KA, Chiaia NL, Rhodes RW. Laminar and areal differences in the origin of the subcortical projection neurons of the rat somatosensory cortex. J.Comp Neurol. 1989;282:428–445. doi: 10.1002/cne.902820309. [DOI] [PubMed] [Google Scholar]
- Kossl M, Vater M. Noradrenaline enhances temporal auditory contrast and neuronal timing precision in the cochlear nucleus of the mustached bat. J Neurosci. 1989;9:4169–4178. doi: 10.1523/JNEUROSCI.09-12-04169.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecas JC. Locus coeruleus activation shortens synaptic drive while decreasing spike latency and jitter in sensorimotor cortex. Implications for neuronal integration. Eur.J.Neurosci. 2004;19:2519–2530. doi: 10.1111/j.0953-816X.2004.03341.x. [DOI] [PubMed] [Google Scholar]
- Lee KH, McCormick DA. Abolition of spindle oscillations by serotonin and norepinephrine in the ferret lateral geniculate and perigeniculate nuclei in vitro. Neuron. 1996;17:309–321. doi: 10.1016/s0896-6273(00)80162-2. [DOI] [PubMed] [Google Scholar]
- Lee SM, Friedberg MH, Ebner FF. The role of GABA-mediated inhibition in the rat ventral posterior medial thalamus. I. Assessment of receptive field changes following thalamic reticular nucleus lesions. J.Neurophysiol. 1994;71:1702–1715. doi: 10.1152/jn.1994.71.5.1702. [DOI] [PubMed] [Google Scholar]
- Liu XB, Jones EG. Predominance of corticothalamic synaptic inputs to thalamic reticular nucleus neurons in the rat. J.Comp Neurol. 1999;414:67–79. [PubMed] [Google Scholar]
- Lorden JF, Callahan M, Dawson R., Jr. Depletion of central catecholamines alters amphetamine- and fenfluramine-induced taste aversions in the rat. J.Comp Physiol Psychol. 1980;94:99–114. doi: 10.1037/h0077650. [DOI] [PubMed] [Google Scholar]
- Lozsadi DA, Gonzalez-Soriano J, Guillery RW. The course and termination of corticothalamic fibres arising in the visual cortex of the rat. Eur.J.Neurosci. 1996;8:2416–2427. doi: 10.1111/j.1460-9568.1996.tb01205.x. [DOI] [PubMed] [Google Scholar]
- Lytton WW, Contreras D, Destexhe A, Steriade M. Dynamic interactions determine partial thalamic quiescence in a computer network model of spike-and-wave seizures. J.Neurophysiol. 1997;77:1679–1696. doi: 10.1152/jn.1997.77.4.1679. [DOI] [PubMed] [Google Scholar]
- Lytton WW, Destexhe A, Sejnowski TJ. Control of slow oscillations in the thalamocortical neuron: a computer model. Neuroscience. 1996;70:673–684. doi: 10.1016/s0306-4522(96)83006-5. [DOI] [PubMed] [Google Scholar]
- MacGregor RJ. Neural and brain modeling. San Diego: Academic Press; 1987. [Google Scholar]
- MacGregor RJ, Lewis ER. Neural Modelling: Electric Signal Processing in the Nervous System. New York: Plenum Press; 1977. [Google Scholar]
- Madison DV, Nicoll RA. Actions of noradrenaline recorded intracellularly in rat hippocampal CA1 pyramidal neurones, in vitro. Journal of Physiology. 1986a;372:221–244. doi: 10.1113/jphysiol.1986.sp016006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison DV, Nicoll RA. Cyclic adenosine 3',5'-monophosphate mediates beta-receptor actions of noradrenaline in rat hippocampal pyramidal cells. Journal of Physiology. 1986b;372:245–259. doi: 10.1113/jphysiol.1986.sp016007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison DV, Nicoll RA. Norepinephrine decreases synaptic inhibition in the rat hippocampus. Brain Res. 1988;442:131–138. doi: 10.1016/0006-8993(88)91440-0. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Huguenard JR. A model of the electrophysiological properties of thalamocortical relay neurons. J.Neurophysiol. 1992;68:1384–1400. doi: 10.1152/jn.1992.68.4.1384. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Pape HC. Noradrenergic and serotonergic modulation of a hyperpolarization-activated cation current in thalamic relay neurones. J.Physiol. 1990a;431:319–342. doi: 10.1113/jphysiol.1990.sp018332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Pape HC. Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurones. J.Physiol. 1990b;431:291–318. doi: 10.1113/jphysiol.1990.sp018331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minderhoud JM. An anatomical study of the efferent connections of the thalamic reticular nucleus. Exp.Brain Res. 1971;112:435–446. doi: 10.1007/BF00234497. [DOI] [PubMed] [Google Scholar]
- Moises HC, Waterhouse BD, Woodward DJ. Locus coeruleus stimulation potentiates Purkinje cell responses to afferent input: the climbing fiber system. Brain Res. 1981;222:43–64. doi: 10.1016/0006-8993(81)90939-2. [DOI] [PubMed] [Google Scholar]
- Moises HC, Waterhouse BD, Woodward DJ. Locus coeruleus stimulation potentiates local inhibitory processes in rat cerebellum. Brain Res.Bull. 1983;10:795–804. doi: 10.1016/0361-9230(83)90211-3. [DOI] [PubMed] [Google Scholar]
- Moises HC, Woodward DJ. Potentiation of GABA inhibitory action in cerebrllum by locus coeruleus stimulation. Brain Res. 1980;182:327–344. doi: 10.1016/0006-8993(80)91192-0. [DOI] [PubMed] [Google Scholar]
- Moises HC, Woodward DJ, Hoffer BJ, Freedman R. Interactions of norepinephrine with Purkinje cell responses to putative amino acid neurotransmitters applied by microiontophoresis. Exp.Neurol. 1979;64:493–515. doi: 10.1016/0014-4886(79)90227-9. [DOI] [PubMed] [Google Scholar]
- Montero VM, Scott GL. Synaptic terminals in the dorsal lateral geniculate nucleus from neurons of the thalamic reticular nucleus: a light and electron microscope autoradiographic study. Neuroscience. 1981;6:2561–2577. doi: 10.1016/0306-4522(81)90102-0. [DOI] [PubMed] [Google Scholar]
- Mouradian RD, Sessler FM, Waterhouse BD. Noradrenergic potentiation of excitatory transmitter action in cerebrocortical slices: evidence for mediation by an alpha 1 receptor-linked second messenger pathway. Brain Res. 1991;546:83–95. doi: 10.1016/0006-8993(91)91162-t. [DOI] [PubMed] [Google Scholar]
- Moxon KA, Chapin JK. Cortico-thalamic interactions in response to whisker stimulation in a computer model of the rat barrel system. Neurocomputing. 1999;26–27:809–822. [Google Scholar]
- Moxon KA, Gerhardt GA, Adler LE. Memory and Emotion. Proceedings of the International School of Biocybertnetics. Casamicciola, Napoli, Italy: World Scientific; 1999a. Sensory gating in a computer model of the hippocampus; pp. 138–162. [Google Scholar]
- Moxon KA, Leiser S, Markowitz R, Chapin JK. Memory and Emotion. Proceedings of the International School of Biocybertnetics. Casamicciola, Napoli, Italy: World Scientific; 1999b. Differential gating of somatosensory input during active and passive stimulation; pp. 163–180. [Google Scholar]
- Nicolelis MAL, Chapin JK. Spatiotemporal structure of somatosensory responses of many-neuron ensembles in the rat ventral posterior medial nucleus of the thalamus. J Neurosci. 1994;14:3511–3532. doi: 10.1523/JNEUROSCI.14-06-03511.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara PT. Synaptic organization of the thalamic reticular nucleus. J.Electron Microsc.Tech. 1988;10:283–292. doi: 10.1002/jemt.1060100306. [DOI] [PubMed] [Google Scholar]
- Ohara PT, Lieberman AR. Thalamic reticular nucleus: anatomical evidence that cortico-reticular axons establish monosynaptic contact with reticulo-geniculate projection cells. Brain Res. 1981;207:153–156. doi: 10.1016/0006-8993(81)90685-5. [DOI] [PubMed] [Google Scholar]
- Ohara PT, Lieberman AR. The thalamic reticular nucleus of the adult rat: experimental anatomical studies. J.Neurocytol. 1985;14:365–411. doi: 10.1007/BF01217752. [DOI] [PubMed] [Google Scholar]
- Ohara PT, Sefton AJ, Lieberman AR. Mode of termination of afferents from the thalamic reticular nucleus in the dorsal lateral geniculate nucleus of the rat. Brain Res. 1980;197:503–506. doi: 10.1016/0006-8993(80)91136-1. [DOI] [PubMed] [Google Scholar]
- Page ME, Abercrombie ED. Discrete local application of corticotropin-releasing factor increases locus coeruleus discharge and extracellular norepinephrine in rat hippocampus. Synapse. 1999;33:304–313. doi: 10.1002/(SICI)1098-2396(19990915)33:4<304::AID-SYN7>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1986. [Google Scholar]
- Pearce RA. Physiological evidence for two distinct GABAA responses in rat hippocampus. Neuron. 1993;10:189–200. doi: 10.1016/0896-6273(93)90310-n. [DOI] [PubMed] [Google Scholar]
- Peet MJ, McLennan H. Pre-and postsynaptic actions of baclofen: blockade of the late synaptically-evoked hyperpolarization of CA1 hippocampal neurones. Exp Brain Res. 1986;61:567–574. doi: 10.1007/BF00237582. [DOI] [PubMed] [Google Scholar]
- Rybak IA, Paton JF, Schwaber JS. Modeling neural mechanisms for genesis of respiratory rhythm and pattern. II. Network models of the central respiratory pattern generator. J Neurophysiol. 1997;77:2007–2026. doi: 10.1152/jn.1997.77.4.2007. [DOI] [PubMed] [Google Scholar]
- Sato H, Fox K, Daw NW. Effect of electrical stimulation of locus coeruleus on the activity of neurons in the cat visual cortex. J Neurophysiol. 1989;62:946–958. doi: 10.1152/jn.1989.62.4.946. [DOI] [PubMed] [Google Scholar]
- Selden NR, Robbins TW, Everitt BJ. Enhanced behavioral conditioning to context and impaired behavioral and neuroendocrine responses to conditioned stimuli following ceruleocortical noradrenergic lesions: support for an attentional hypothesis of central noradrenergic function. J.Neurosci. 1990;10:531–539. doi: 10.1523/JNEUROSCI.10-02-00531.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessler FM, Liu W, Kirifides ML, Mouradian RD, Lin RCS, Waterhouse BD. Noradrenergic enhancement of GABA-induced input resistance changes in layer V regular spiking pyramidal neurons of rat somatosensory cortex. Brain Res. 1995;675:171–182. doi: 10.1016/0006-8993(95)00060-4. [DOI] [PubMed] [Google Scholar]
- Sessler FM, Mouradian RD, Cheng JT, Yeh HH, Liu WM, Waterhouse BD. Noradrenergic potentiation of cerebellar Purkinje cell responses to GABA: evidence for mediation through the beta-adrenoceptor-coupled cyclic AMP system. Brain Res. 1989;499:27–38. doi: 10.1016/0006-8993(89)91132-3. [DOI] [PubMed] [Google Scholar]
- Snow PJ, Andre P, Pompeiano O. Effects of locus coeruleus stimulation on the responses of SI neurons of the rat to controlled natural and electrical stimulation of the skin. Arch Ital Biol. 1999;137:1–28. [PubMed] [Google Scholar]
- Spreafico R, Battaglia G, Frassoni C. The reticular thalamic nucleus (RTN) of the rat: cytoarchitectural, Golgi, immunocytochemical, and horseradish peroxidase study. J.Comp Neurol. 1991;304:478–490. doi: 10.1002/cne.903040311. [DOI] [PubMed] [Google Scholar]
- Thompson SM, Gahwiler BH. Comparison of the actions of baclofen at pre-and postsynaptic receptors in the rat hippocampus in vitro. J Physiol. 1992;451:329–345. doi: 10.1113/jphysiol.1992.sp019167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Loos H. Barreloids in mouse somatosensory thalamus. Neuroscience Letters. 1976:1–6. doi: 10.1016/0304-3940(76)90036-7. [DOI] [PubMed] [Google Scholar]
- Von Krosigk M, Bal T, McCormick DA. Cellular mechanisms of a synchronized oscillation in the thalamus. Science. 1993;261:361–364. doi: 10.1126/science.8392750. [DOI] [PubMed] [Google Scholar]
- Waite PM. Somatotopic organization of vibrissal responses in the ventro-basal complex of the rat thalamus. J Physiol. 1973a;228:527–540. doi: 10.1113/jphysiol.1973.sp010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waite PM. The responses of cells in the rat thalamus to mechanical movements of the whiskers. J Physiol. 1973b;228:541–561. doi: 10.1113/jphysiol.1973.sp010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse BD, Azizi SA, Burne RA, Woodward DJ. Modulation of rat cortical area 17 neuronal responses to moving visual stimuli during norepinephrine and serotonin microiontophoresis. Brain Res. 1990;514:276–292. doi: 10.1016/0006-8993(90)91422-d. [DOI] [PubMed] [Google Scholar]
- Waterhouse BD, Devilbiss DM. Effects of tonic locus coeruleus stimulation on sensory - evoked responses of thalamic and cortical neurons in the awake rat. 682.20. Society for Neuroscience; 11-6-2002; Washington, DC. 2002. Ref Type: Conference Proceeding. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse BD, Moises HC, Woodward DJ. Noradrenergic modulation of somatosensory cortical neuronal responses to iontophoretically applied putative neurotransmitters. Exp.Neurol. 1980;69:30–49. doi: 10.1016/0014-4886(80)90141-7. [DOI] [PubMed] [Google Scholar]
- Waterhouse BD, Moises HC, Woodward DJ. Alpha-receptor-mediated facilitation of somatosensory cortical neuronal responses to excitatory synaptic inputs and iontophoretically applied acetylcholine. Neuropharmacology. 1981;20:907–920. doi: 10.1016/0028-3908(81)90020-4. [DOI] [PubMed] [Google Scholar]
- Waterhouse BD, Moises HC, Woodward DJ. Phasic activation of the locus coeruleus enhances responses of primary sensory cortical neurons to peripheral receptive field stimulation. Brain Res. 1998;790:33–44. doi: 10.1016/s0006-8993(98)00117-6. [DOI] [PubMed] [Google Scholar]
- Waterhouse BD, Moises HC, Yeh HH, Woodward DJ. Norepinephrine enhancement of inhibitory synaptic mechanisms in cerebellum and cerebral cortex: mediation by beta adrenergic receptors. J.Pharmacol.Exp.Ther. 1982;221:495–506. [PubMed] [Google Scholar]
- Waterhouse BD, Mouradian R, Sessler FM, Lin RCS. Differential modulatory effects of norepinephrine on synaptically driven responses of layer V barrel field cortical neurons. Brain Res. 2000;868:39–47. doi: 10.1016/s0006-8993(00)02261-7. [DOI] [PubMed] [Google Scholar]
- Waterhouse BD, Sessler FM, Cheng JT, Woodward DJ, Azizi SA, Moises HC. New evidence for a gating action of norepinephrine in central neuronal circuits of mammalian brain. Brain Res Bull. 1988;21:425–432. doi: 10.1016/0361-9230(88)90154-2. [DOI] [PubMed] [Google Scholar]
- Waterhouse BD, Woodward DJ. Interaction of norepinephrine with cerebrocortical activity evoked by stimulation of somatosensory afferent pathways in the rat. Exp Neurol. 1980;67:11–34. doi: 10.1016/0014-4886(80)90159-4. [DOI] [PubMed] [Google Scholar]
- Woodward DJ, Moises HC, Waterhouse BD, Hoffer BJ, Freedman R. Modulatory actions of norepinephrine in the central nervous system. Fed.Proc. 1979;38:2109–2116. [PubMed] [Google Scholar]
- Woolsey TA, Van der Loos H. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. The description of a cortical field composed of discrete cytoarchitectonic units. Brain Res. 1970;17:205–242. doi: 10.1016/0006-8993(70)90079-x. [DOI] [PubMed] [Google Scholar]