Abstract
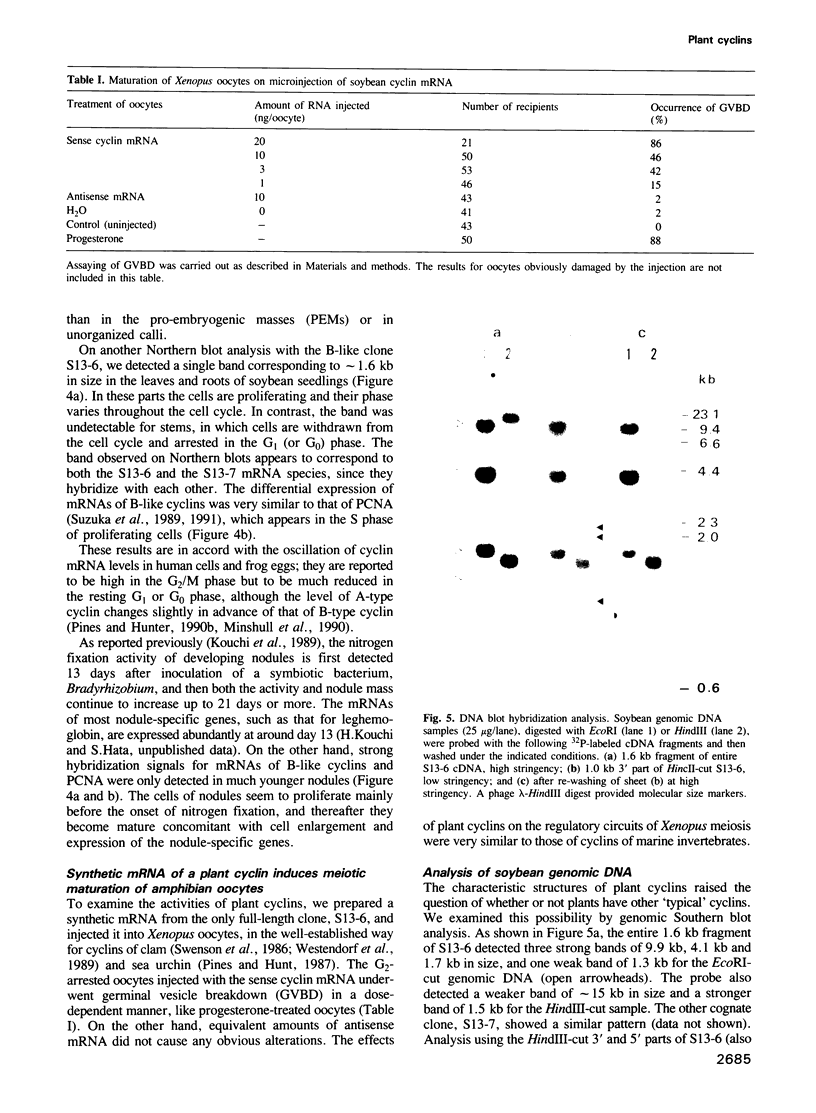
We have isolated and sequenced a carrot cDNA and two soybean cDNAs encoding mitotic cyclin homologs. The soybean clones were derived from nearly identical cognate genes. The carrot cyclin and soybean cyclins were slightly more similar to A-type and B-type cyclins thus far defined, respectively. However, they had divergent amino acid sequences in the portion that is most highly conserved in known cyclins and we could not easily include them in either of the phylogenetic types. Since the homology between carrot and soybean cyclins was low, each of them might define a novel and distinct type. The mRNA of carrot cyclin, 1.5 kb in length, was expressed concomitant with somatic embryogenesis of cultured cells. Expression of soybean cyclin mRNAs, 1.6 kb in length, was localized in proliferating parts of seedlings. As in the case of cyclin genes of marine invertebrates, microinjection of a synthetic mRNA for the soybean cyclin induced the maturation of Xenopus oocytes. Other cyclin genes may be present because, on Southern blot analysis of soybean genomic DNA, the isolated soybean cDNA probe hybridized with additional genes under low stringency.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borkird C., Choi J. H., Jin Z. H., Franz G., Hatzopoulos P., Chorneau R., Bonas U., Pelegri F., Sung Z. R. Developmental regulation of embryonic genes in plants. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6399–6403. doi: 10.1073/pnas.85.17.6399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J. H., Liu L. S., Borkird C., Sung Z. R. Cloning of genes developmentally regulated during plant embryogenesis. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1906–1910. doi: 10.1073/pnas.84.7.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyclin in fission yeast. Cell. 1988 Sep 9;54(6):738–740. doi: 10.1016/s0092-8674(88)90933-6. [DOI] [PubMed] [Google Scholar]
- Edelman A. M., Blumenthal D. K., Krebs E. G. Protein serine/threonine kinases. Annu Rev Biochem. 1987;56:567–613. doi: 10.1146/annurev.bi.56.070187.003031. [DOI] [PubMed] [Google Scholar]
- Evans T., Rosenthal E. T., Youngblom J., Distel D., Hunt T. Cyclin: a protein specified by maternal mRNA in sea urchin eggs that is destroyed at each cleavage division. Cell. 1983 Jun;33(2):389–396. doi: 10.1016/0092-8674(83)90420-8. [DOI] [PubMed] [Google Scholar]
- Feiler H. S., Jacobs T. W. Cell division in higher plants: a cdc2 gene, its 34-kDa product, and histone H1 kinase activity in pea. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5397–5401. doi: 10.1073/pnas.87.14.5397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin M. G., Purohit S. K., Verma D. P. The primary structure of soybean (Glycine max) ubiquitin is identical to other plant ubiquitins. Nucleic Acids Res. 1988 Dec 9;16(23):11377–11377. doi: 10.1093/nar/16.23.11377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura T., Komamine A. Synchronization of somatic embryogenesis in a carrot cell suspension culture. Plant Physiol. 1979 Jul;64(1):162–164. doi: 10.1104/pp.64.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glotzer M., Murray A. W., Kirschner M. W. Cyclin is degraded by the ubiquitin pathway. Nature. 1991 Jan 10;349(6305):132–138. doi: 10.1038/349132a0. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B., Lane C. D., Woodland H. R., Marbaix G. Use of frog eggs and oocytes for the study of messenger RNA and its translation in living cells. Nature. 1971 Sep 17;233(5316):177–182. doi: 10.1038/233177a0. [DOI] [PubMed] [Google Scholar]
- Hata S., Clabby M., Devlin P., Spits H., De Vries J. E., Krangel M. S. Diversity and organization of human T cell receptor delta variable gene segments. J Exp Med. 1989 Jan 1;169(1):41–57. doi: 10.1084/jem.169.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata S. cDNA cloning of a novel cdc2+/CDC28-related protein kinase from rice. FEBS Lett. 1991 Feb 11;279(1):149–152. doi: 10.1016/0014-5793(91)80271-4. [DOI] [PubMed] [Google Scholar]
- John P. C., Sek F. J., Lee M. G. A homolog of the cell cycle control protein p34cdc2 participates in the division cycle of Chlamydomonas, and a similar protein is detectable in higher plants and remote taxa. Plant Cell. 1989 Dec;1(12):1185–1193. doi: 10.1105/tpc.1.12.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. Functional messenger RNAs are produced by SP6 in vitro transcription of cloned cDNAs. Nucleic Acids Res. 1984 Sep 25;12(18):7057–7070. doi: 10.1093/nar/12.18.7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner C. F., O'Farrell P. H. Drosophila cdc2 homologs: a functional homolog is coexpressed with a cognate variant. EMBO J. 1990 Nov;9(11):3573–3581. doi: 10.1002/j.1460-2075.1990.tb07568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner C. F., O'Farrell P. H. Expression and function of Drosophila cyclin A during embryonic cell cycle progression. Cell. 1989 Mar 24;56(6):957–968. doi: 10.1016/0092-8674(89)90629-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner C. F., O'Farrell P. H. The roles of Drosophila cyclins A and B in mitotic control. Cell. 1990 May 4;61(3):535–547. doi: 10.1016/0092-8674(90)90535-m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin B. Driving the cell cycle: M phase kinase, its partners, and substrates. Cell. 1990 Jun 1;61(5):743–752. doi: 10.1016/0092-8674(90)90181-d. [DOI] [PubMed] [Google Scholar]
- Minshull J., Blow J. J., Hunt T. Translation of cyclin mRNA is necessary for extracts of activated xenopus eggs to enter mitosis. Cell. 1989 Mar 24;56(6):947–956. doi: 10.1016/0092-8674(89)90628-4. [DOI] [PubMed] [Google Scholar]
- Minshull J., Golsteyn R., Hill C. S., Hunt T. The A- and B-type cyclin associated cdc2 kinases in Xenopus turn on and off at different times in the cell cycle. EMBO J. 1990 Sep;9(9):2865–2875. doi: 10.1002/j.1460-2075.1990.tb07476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurse P. Universal control mechanism regulating onset of M-phase. Nature. 1990 Apr 5;344(6266):503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- Ohtsuka E., Matsuki S., Ikehara M., Takahashi Y., Matsubara K. An alternative approach to deoxyoligonucleotides as hybridization probes by insertion of deoxyinosine at ambiguous codon positions. J Biol Chem. 1985 Mar 10;260(5):2605–2608. [PubMed] [Google Scholar]
- Pines J., Hunt T. Molecular cloning and characterization of the mRNA for cyclin from sea urchin eggs. EMBO J. 1987 Oct;6(10):2987–2995. doi: 10.1002/j.1460-2075.1987.tb02604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pines J., Hunter T. Human cyclin A is adenovirus E1A-associated protein p60 and behaves differently from cyclin B. Nature. 1990 Aug 23;346(6286):760–763. doi: 10.1038/346760a0. [DOI] [PubMed] [Google Scholar]
- Pines J., Hunter T. Isolation of a human cyclin cDNA: evidence for cyclin mRNA and protein regulation in the cell cycle and for interaction with p34cdc2. Cell. 1989 Sep 8;58(5):833–846. doi: 10.1016/0092-8674(89)90936-7. [DOI] [PubMed] [Google Scholar]
- Pines J., Hunter T. p34cdc2: the S and M kinase? New Biol. 1990 May;2(5):389–401. [PubMed] [Google Scholar]
- Roy L. M., Singh B., Gautier J., Arlinghaus R. B., Nordeen S. K., Maller J. L. The cyclin B2 component of MPF is a substrate for the c-mos(xe) proto-oncogene product. Cell. 1990 Jun 1;61(5):825–831. doi: 10.1016/0092-8674(90)90192-h. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuttleworth J., Godfrey R., Colman A. p40MO15, a cdc2-related protein kinase involved in negative regulation of meiotic maturation of Xenopus oocytes. EMBO J. 1990 Oct;9(10):3233–3240. doi: 10.1002/j.1460-2075.1990.tb07522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standart N., Minshull J., Pines J., Hunt T. Cyclin synthesis, modification and destruction during meiotic maturation of the starfish oocyte. Dev Biol. 1987 Nov;124(1):248–258. doi: 10.1016/0012-1606(87)90476-3. [DOI] [PubMed] [Google Scholar]
- Suzuka I., Daidoji H., Matsuoka M., Kadowaki K., Takasaki Y., Nakane P. K., Moriuchi T. Gene for proliferating-cell nuclear antigen (DNA polymerase delta auxiliary protein) is present in both mammalian and higher plant genomes. Proc Natl Acad Sci U S A. 1989 May;86(9):3189–3193. doi: 10.1073/pnas.86.9.3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuka I., Hata S., Matsuoka M., Kosugi S., Hashimoto J. Highly conserved structure of proliferating cell nuclear antigen (DNA polymerase delta auxiliary protein) gene in plants. Eur J Biochem. 1991 Jan 30;195(2):571–575. doi: 10.1111/j.1432-1033.1991.tb15739.x. [DOI] [PubMed] [Google Scholar]
- Swenson K. I., Farrell K. M., Ruderman J. V. The clam embryo protein cyclin A induces entry into M phase and the resumption of meiosis in Xenopus oocytes. Cell. 1986 Dec 26;47(6):861–870. doi: 10.1016/0092-8674(86)90801-9. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Chenivesse X., Henglein B., Bréchot C. Hepatitis B virus integration in a cyclin A gene in a hepatocellular carcinoma. Nature. 1990 Feb 8;343(6258):555–557. doi: 10.1038/343555a0. [DOI] [PubMed] [Google Scholar]
- Westendorf J. M., Swenson K. I., Ruderman J. V. The role of cyclin B in meiosis I. J Cell Biol. 1989 Apr;108(4):1431–1444. doi: 10.1083/jcb.108.4.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield W. G., González C., Sánchez-Herrero E., Glover D. M. Transcripts of one of two Drosophila cyclin genes become localized in pole cells during embryogenesis. Nature. 1989 Mar 23;338(6213):337–340. doi: 10.1038/338337a0. [DOI] [PubMed] [Google Scholar]






