Abstract
Background
Patient navigation (PN) may improve cancer care by identifying and removing patient-reported barriers to care. In 2012, the American College of Surgeons Commission on Cancer (CoC) announced that healthcare facilities seeking CoC-accreditation must have PN processes in place starting January 1, 2015. Given the unfunded mandates, hospitals are looking for cost-effective ways to implement PN. This study examined demographic and psychosocial predictors of barriers to diagnostic resolution among individuals with a cancer screening abnormality enrolled in the Ohio Patient Navigation Research Project (OPNRP).
Methods
Data were obtained from patients who received care at one of nine OPNRP intervention clinics. Descriptive statistics and logistic regression models were used.
Results
Of 424 participants, 151 (35.6%) reported a barrier to diagnostic resolution within 90 days of study consent. The most commonly reported barriers were misconception about a test or treatment (16.4%), difficulty communicating with their provider (15.0%), and scheduling problems (11.5%). Univariate analyses indicated that race, education, employment, income, insurance, clinic type, friend support, and physical and psychological functioning were significantly associated with reporting a barrier to diagnostic resolution. Multivariate analyses found having a comorbidity (OR=1.25, 95% CI=1.04, 2.61) and higher intrusive thoughts and feelings (OR=1.25, 95% CI=1.10,1.41) were significantly associated with reporting a barrier to diagnostic resolution.
Conclusion
Results suggest demographic and psychosocial factors are associated with barriers to diagnostic resolution. To assure CoC mandate compliance and provide timely care for all patients, CoC-accredited facilities can systematically identify patients most likely to have barriers to care, and assign them to PN.
Keywords: patient navigation, accreditation, barriers, diagnostic resolution, continuity of patient care
Introduction
Profound advances in cancer screening and treatment have contributed to increased longevity and quality of life among cancer survivors. Despite this improvement, cancer health disparities by race and socioeconomic status remain.1–3 One strategy to reduce cancer health disparities is through patient navigation (PN). PN is a patient-centered health care service delivery model that assists individuals, particularly the medically underserved, in overcoming barriers to care across the cancer care continuum. PN has been demonstrated to increase cancer screening rates, improve follow-up rates after an abnormal cancer screening test, reduce time from cancer diagnosis to treatment initiation, and decrease cancer treatment costs.4–8
Healthcare organizations have advocated PN as an important strategy towards more patient-centric oncology practices. In 2012, the American College of Surgeons Commission on Cancer (CoC) announced that facilities seeking CoC accreditation need to have PN processes in place by January 1, 2015. However, these mandates are currently unfunded. In March 2014, the Patient Navigation Assistance Act of 2014 (H.R. 4168) was introduced to provide payment for patient navigator services under title XIX (Medicaid) of the Social Security Act. To date, however, the Patient Navigation Assistance Act has not progressed further. As a result, cancer treatment centers are looking for cost-effective ways to implement PN processes (i.e., direct resources to those who are at risk for loss to follow-up). A vital step to implementing cost-effective PN processes is to identify patient populations who are in particular need of PN by identifying patients most likely to have barriers to care and thus, most likely to not receive proper, timely care.9,10 By identifying those most likely to need PN, scarce resources can be diverted to the patient population in most need and most likely to delay or not receive prompt appropriate care. The results of efficient deployment would be the reduction of health care costs as well as improvement in quality of care.
The primary objective of this study was to examine demographic and psychosocial predictors of barriers to diagnostic resolution of a cancer screening abnormality among 424 participants enrolled in a PN intervention study. These results may provide information to assist the design and targeting of future PN programs.
Methods
Study design and population
We used data collected as part of the Ohio Patient Navigation Research Project (OPNRP), a group-randomized trial with a nested cohort design. Details regarding the study design and population have been previously published.8 Briefly, the study initially recruited patients from eight primary care clinics from the Ohio State University (OSU) Primary Care Research Network and four Columbus-area federally qualified health centers (FQHCs). A total of 18 clinics were randomized to either the PN or comparison (usual care) conditions. The initial 12 clinics were matched according to clinic type and proportion of black patients, with 6 clinics randomized to each condition. The additional 6 clinics were matched according to specialization only (gynecology vs. gastroenterology vs. general medicine clinics).
Several different mechanisms were used to recruit participants. The first method was through medical charts and cytology and mammography reports to identify potential participants. If a potential participant was identified, a research staff member forwarded the name to the physician asking permission to contact the patient. The second method of recruitment was through a letter introducing the study sent to the patient prior to contact by the research staff. Potential participants were then called, the study was explained; and informed consent and the baseline questionnaire (which included demographic and psychosocial measures) were administered.8
To be eligible for participation, patients must have met the following requirements: 1) at least 18 years old; 2) a regular patient of the primary care clinic; 3) not cognitively impaired; 4) reside outside of a nursing home or institutional setting; 5) speak and understand English or Spanish; and 6) able to provide informed consent. In addition, participants must have been identified as having an abnormal breast, cervical, or colorectal cancer screening test. Participants with a positive history of previous medical navigation or cancer, with the exception of non-melanoma of the skin, were ineligible. The study was approved by The Ohio State University Institutional Review Board.
In the theoretically-based11–13 PN intervention, patients were contacted by an assigned patient navigator by telephone within 5 days of assignment to identify specific barriers to diagnostic resolution (i.e., pathologic diagnosis of an invasive cancer on biopsy). Navigators then tailored their assistance to eliminate the specific barriers of each patient through supportive listening, educational materials, referrals for psychological care, assistance with making appointments, and resolving transportation problems, etc.8 The PN intervention lasted until the navigated patient had a diagnostic resolution for the abnormal breast, cervical, or CRC screening tests (according to a medical record review (MRR)) or until the end of the study.
Measures
Survey measures obtained from patients at baseline have been described previously.8 We briefly list the measures examined in this analyses and include more information on the measures not previously described.
Demographic characteristics
Patients provided information about their age, gender, race, marital status, education, employment status, income, health insurance, clinic type, primary site of cancer, and comorbidities.
Anxiety
Anxiety was measured by the Beck Anxiety Inventory, a 21-item scale that is descriptive of subjective, somatic, or pain-related symptoms of anxiety.14
Depressive symptoms
Depressive symptoms were measured by the Center for Epidemiological Studies-Depression (CES-D) scale.15
Perceived social support
Perceived social support was measured with the Perceived Social Support-Friend Scale and Perceived Social Support-Family Scale.16
Trust in Physicians
Trust in physicians was measured by the Trust in Physician Scale.17
Quality of life
Overall health-related quality of life was assessed using eight dimensions on the Medical Outcomes Study 36-item short form (SF-36): physical functioning, role limitations, bodily pain, general health perceptions, vitality, social functioning, emotional well-being, and general perceptions of health status.18 In addition to eight specific subscales, the SF-36 measure has two summary scales, the Physical Component Summary (PCS) and the Mental Component Summary (MCS), which were used in the present analyses. Subscales were scored from 0 to 100, with higher scores indicating better health-related quality of life.18
Perceived Stress
Perceived stress was measured by the Perceived Stress Scale (PSS), a 14-item measure of overall stress as experienced in the past month. 19 Item responses range from 0=never to 4=very often, with higher scores indicating higher perceived stress.
Self-efficacy
The 12-item Communication and Attitudinal Self-Efficacy Scale (CASE) was used to assess participants’ self-efficacy to understand and participate in their care, seek and obtain information, and maintain a positive attitude.20 Responses are on a 4-point Likert scale (“strongly disagree” to “strongly agree”) with higher scores indicating higher self-efficacy.
Distress
The Impact of Events Scale (IES) was used to measure subjective distress caused by stressful events experienced during the past seven days.21 The IES scale consists of 15 items, seven of which measure intrusive symptoms (i.e., unintentional thoughts, images, or feelings) and eight avoidance symptoms (i.e., active attempts to suppress thinking) regarding a stressful event. Responses are a 4-point Likert scale (“not at all” to “often”) with higher scores indicating higher distress.
Barriers to care
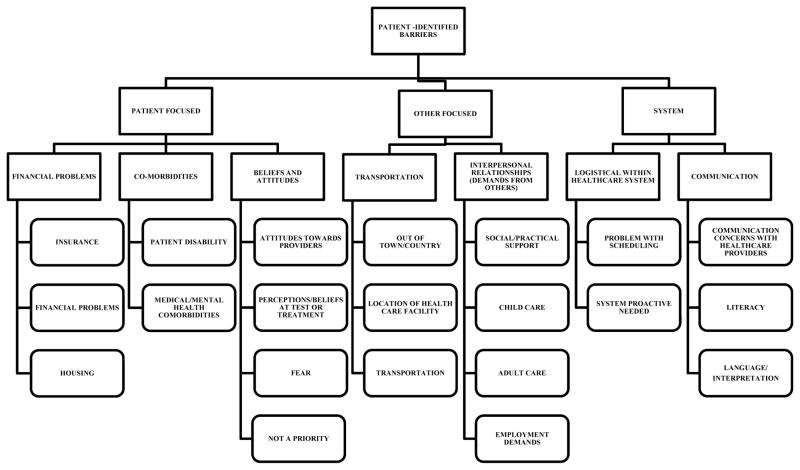
A 21-item measure enumerating the number and types of barriers to cancer care a participant experienced. Responses to each item were “yes” or “no”. Barriers to care were organized into patient-, system-, and other-focused barriers by the national PNRP research group.22 In Figure 1, the different barriers to care for each of the above categories are listed. The name given to each barrier was meant to describe the difficulty/problem that interfered with a participant’s progression to diagnostic resolution.
Figure 1.
Analyses
The primary outcome for this study was whether the patient reported a barrier to care in the first 90 days following consent. Univariate logistic regression was used to calculate the odds ratios of reporting a barrier in the first 90 days for each of the potential demographic and psychosocial predictors measured at baseline. A backwards selection process including all variables significant at the 0.05 level was used to obtain a multivariate model. Two-way interactions were explored in the final model and all analyses were conducted using SAS v9.3 (SAS Institute, Cary, NC).
Results
Sample characteristics
Baseline characteristics of the 424 participants in the PN clinics are shown in Table 1. The mean age of the participants was 46.7 years, and the majority of the participants were white (71.2%). Most participants reported being married or living as a couple (50.0%), having at least a high school education (94.3%), being employed full or part-time (61.8%), having household incomes ≥$50,000/year (53.3%), having private health insurance (67.1%), and receiving care at an academic medical center (90.8%). More than half of the participants (60.2%) had an abnormal breast screening test result, 35.1% of the participants had abnormal cervical screening results, and 4.7% had abnormal colorectal screening results.
Table 1.
Patient Characteristics of Participants in PN Arm, OPNRP (n=424)*
| Total (N = 424) N (%) |
|
|---|---|
| Age, Mean (SD) | 46.7 |
| Gender | |
| Female | 413 (97.4) |
| Male | 11 (2.6) |
| Race | |
| White | 302 (71.2) |
| African American | 87 (20.5) |
| Other | 35 (8.3) |
| Marital status | |
| Single | 114 (26.9) |
| Married | 212 (50.0) |
| Divorced/Widowed | 98 (23.1) |
| Education | |
| < High School | 24 (5.7) |
| High School | 70 (16.5) |
| Some College/Associate’s degree | 139 (32.8) |
| College graduate/Graduate degree | 191 (45.0) |
| Employment | |
| Full or part-time | 261 (61.8) |
| Retired/Disabled/Unemployed | 161 (38.2) |
| Household income/year | |
| < $50K | 183 (46.7) |
| ≥ $50K | 209 (53.3) |
| Insurance | |
| Private | 279 (67.1) |
| Public | 112 (26.9) |
| Uninsured | 25 (6.0) |
| Clinic Type | |
| Academic Medical Center | 254 (90.8) |
| Federally Qualified Health Center | 39 (9.2) |
| Cancer Site | |
| Breast | 255 (60.1) |
| Cervix | 149 (35.1) |
| Colorectal | 20 (4.7) |
Some variables do not total 424 due to missing data
Demographic predictors of reporting barriers
Of the 424 participants in the PN clinics, 151 (35.6%) reported a patient-, system-, or other-focused barrier to diagnostic resolution within the first 90 days from study consent. The most commonly reported barriers were misconception/beliefs about a test or treatment (16.4%), communication difficulty with their health care provider (15.0%), and problems with scheduling (11.5%).
Univariate analysis found that race, education, employment status, income, insurance, and clinic type were significant predictors of reporting a barrier in the first 90 days from consent. Specifically, participants who were non-white (P = 0.02), had lower education (P = 0.008), unemployed or retired (P = 0.04), earned less than $50,000/year (P = 0.006), and uninsured (P = 0.02) were significantly more likely to report a barrier to diagnostic resolution. Patients from FQHCs (P = 0.03) were significantly more likely to report at least one barrier to diagnostic resolution (Table 2).
Table 2.
Demographic Predictors of Reporting a Barrier to Diagnostic Resolution*
| Predictor | Level | No Barriers Reported (n = 273) n (%) |
Barriers reported (n = 151) n (%) |
OR for barrier (95% CI) | P |
|---|---|---|---|---|---|
| Age | Mean (SD) | 47.0 (14.4) | 46.1 (14.1) | 1.00 (0.98, 1.01) | 0.5569 |
| Gender | Female | 267 (64.6) | 146 (35.4) | 1.00 | 0.4927 |
| Male | 6 (54.5) | 5 (45.5) | 1.52 (0.46, 5.08) | ||
| White, non-Hispanic | No | 72 (55.8) | 57 (44.2) | 1.00 | 0.0152 |
| Yes | 201 (68.1) | 91 (31.9) | 0.59 (0.39, 0.90) | ||
| Education | < High School | 10 (41.7) | 14 (58.3) | 1.00 | 0.0080 |
| High School | 37 (52.9) | 33 (47.1) | 0.64 (0.25, 1.63) | ||
| Some College/Associate’s degree | 98 (70.5) | 41 (29.5) | 0.30 (0.21, 0.73) | ||
| College graduate/Graduate degree | 128 (67.0) | 63 (33.0) | 0.35 (0.15, 0.84) | ||
| Marital Status | Single | 75 (65.8) | 39 (34.2) | 1.00 | 0.3397 |
| Married | 141 (66.5) | 71 (33.5) | 0.97 (0.60, 1.57) | ||
| Divorced/Widowed | 57 (58.2) | 41 (41.8) | 1.38 (0.79, 2.42) | ||
| Working full-or part-time | No | 94 (58.4) | 67 (41.6) | 1.00 | 0.0413 |
| Yes | 178 (68.2) | 83 (31.8) | 0.65 (0.44, 0.98) | ||
| Income ≥ $50K | No | 106 (57.1) | 77 (42.1) | 1.00 | 0.0058 |
| Yes | 149 (71.3) | 60 (28.7) | 0.55 (0.30, 1.14) | ||
| Insurance | Uninsured | 10 (40.0) | 15 (60.0) | 1.00 | 0.0178 |
| Private | 191 (68.5) | 88 (31.5) | 0.31 (0.13, 0.71) | ||
| Public | 70 (62.5) | 42 (37.5) | 0.40 (0.16, 0.97) | ||
| Clinic Type | AMC | 254 (66.0) | 131 (34.0) | 1.00 | 0.0348 |
| FQHC | 19 (48.7) | 20 (51.3) | 2.04 (1.05, 3.96) | ||
| Primary site of cancer | Breast | 174 (68.2) | 81 (31.8) | 1.00 | 0.0908 |
| Cervix | 89 (59.7) | 60 (40.3) | 1.45 (0.95, 2.20) | ||
| Colorectal | 10 (50.0) | 10 (50.0) | 2.15 (0.86, 5.37) | ||
| Any comorbidity | No | 115 (71.0) | 47 (29.0) | 1.00 | 0.0531 |
| Yes | 158 (61.7) | 98 (38.3) | 1.52 (0.99, 2.32) |
Some variables do not total 424 due to missing data
Univariate psychosocial predictors of reporting barriers
Univariate analysis found that anxiety, depressive symptoms, friend support, physical and mental functioning, perceived stress, self-efficacy, and impact of life events were significant predictors of reporting a barrier in the first 90 days from consent. Specifically, patients that had higher anxiety (P = 0.005), higher depressive symptoms (P = 0.002), lower friend support (P = 0.02), lower physical (P = 0.002) and mental (P = 0.002) functioning, higher perceived stress (P = 0.05), lower self-efficacy (P = 0.001), and higher avoidance behaviors (P = 0.01) and intrusive thoughts and feelings (P = 0.001) were significantly more likely to report one or more barriers to diagnostic resolution (Table 3).
Table 3.
Psychosocial Predictors of Reporting a Barrier to Diagnostic Resolution
| Predictor | No Barriers Reported (n = 273) Mean (SD) |
Barriers reported (n = 151) Mean (SD) |
OR for barrier (95% CI) | P |
|---|---|---|---|---|
| Beck anxiety | 8.0 (8.9) | 11.6 (10.8) | 1.04 (1.02, 1.06) | 0.0005 |
| CES-D >15 (n(%)) | 57 (20.9) | 52 (34.9) | 2.03 (1.30, 3.17) | 0.0018 |
| Perceived Social Support-Friend | 16.9 (3.8) | 15.8 (4.5) | 0.94 (0.90, 0.99) | 0.0187 |
| Perceived Social Support-Family | 16.5 (5.2) | 15.7 (5.4) | 0.97 0.94, 1.01) | 0.1775 |
| Physician Trust | 45.2 (6.1) | 44.0 (6.7) | 0.97 (0.94, 1.00) | 0.0661 |
| SF-36 Physical Functioning | 76.6 (22.7) | 69.3 (24.2) | 0.99 (0.98, 1.00) | 0.0023 |
| SF-36 Mental Functioning | 74.6 (20.2) | 67.8 (22.5) | 0.99 (0.98, 0.99) | 0.0017 |
| Perceived Stress | 20.4 (7.9) | 22.1 (8.8) | 1.02 (1.00, 1.05) | 0.0496 |
| Communication and attitudinal self-efficacy | 43.6 (4.6) | 41.9 (5.2) | 0.93 (0.90, 0.97) | 0.0011 |
| Impact of events-Avoidance score | 11.2 (9.6) | 13.9 (10.2) | 1.03 (1.01, 1.05) | 0.0120 |
| Impact of events-Intrusive score | 6.6 (7.8) | 9.7 (9.5) | 1.04 (1.02, 1.07) | 0.0008 |
Multivariate predictors of reporting barriers
In the multivariate backwards selection model, only two predictors were retained as significant at the 0.05 level. Having any comorbidity (OR = 1.65, 95% CI = 1.04, 2.61) and a higher intrusive thoughts and feelings score (OR = 1.25 for a 5-unit increase, 95% CI = 1.10, 1.41) were significantly associated with reporting one or more barriers to diagnostic resolution in the first 90 days. The two-way interaction was not significant (P = 0.15).
Discussion
This study assessed the demographic and psychological predictors of barriers to diagnostic resolution of a cancer screening abnormality among participants enrolled in a PN intervention study. Previous research has found that having at least one barrier to care significantly increases the chance of loss to follow-up.9,10 PN has been shown to reduce barriers,23,24 as well as loss to follow-up.25 Thus, knowing who is more likely to have a barrier to recommended care can allow medical facilities to direct scarce PN resources to those patients, increasing compliance with CoC mandates while improving the receipt of timely and quality care.
In this study, more than one third of patients reported one or more barriers to diagnostic resolution. The most commonly reported barriers were at the patient (misconception/beliefs about a test or treatment) and system-level (communication difficulty with their health care provider, problems with scheduling). Previous research has found that barriers to diagnostic resolution do occur at the patient, provider, and system levels.26–29 A positive aspect of the patient-level barriers is that these factors are attitudinal and potentially modifiable through educational resources and emotional support from patient navigators.
Furthermore, patients who were non-white, uninsured, and unemployed/retired, had lower education and income, and received treatment at FQHCs were more likely to report one or more barriers to diagnostic resolution. Previous research found similar results, identifying measures of SES (i.e., income, insurance status) and other demographic factors (i.e., race, education) as important determinants of timely follow-up after abnormal screening tests.26–30
Univariate and multivariate analyses found that psychological functioning (i.e., high anxiety, depression, intrusive thoughts and feelings) and social support (i.e., low friend support) were associated with reporting one or more barriers to diagnostic resolution. Furthermore, it is worth noting the impact of high intrusive thoughts and feelings on reporting barriers to diagnostic resolution. Our findings are similar to previous research that found abnormal cancer screening tests cause significant anxiety and have persistent detrimental psychological effects both in women with cancer and in those with benign diagnostic results.31,32 Patient navigators talk with patients about potential negative psychological effects of abnormal cancer screenings, particularly intrusive thoughts and feelings, and encourage patients to rely on their social networks and the navigator for emotional support, if needed. Patient navigators can also identify psychological barriers to diagnostic resolution and can work with local psychological services to help patients address these problematic symptoms.
Multivariate analyses also found that patients with a comorbidity more likely to report one or more barriers to diagnostic resolution. Previous research found similar results, that the presence of comorbidities is associated with diagnostic delay.33,34 In addition, past studies have found that physicians reach the correct diagnosis of cancer slower among patients with more comorbidities.35,36 Potential reasons why the presence of a comorbidity is associated with barriers to care may include a difficulty scheduling medical appointments, the burden of the diagnosed chronic condition, and the prioritization of medical care for one condition over another by the patient and/or physician.37 Patient navigators can assist physicians and nurses in identifying and properly addressing chronic conditions in addition to encouraging and assisting patients in seeking follow-up care for their abnormal cancer screening result or cancer treatment.
Practical Implications
PN works with individuals to overcome barriers to timely, appropriate high-quality care, resulting in better and timely care. The results of this study may assist healthcare facilities with limited resources to develop PN programs. For example, simple screening surveys could be developed and administered upon registration to collect information on the factors identified in this study that correlate with reporting a barrier. Patients who meet these criteria could then be assigned for PN. Thus, not all patients would proactively receive PN, a strategy that would ensure compliance with the unfunded CoC mandate, conserve resources, and deliver timely, quality care to all patients. Ultimately, this strategy could result in lowered health care costs since the number of patients at greatest risk for loss to follow-up and diagnosis at a later and more costly stage of disease would be reduced.
Strengths/Limitations
This study possesses several strengths, including the focus on three common cancers and investigation of a large sample of PN participants from a mix of clinic types and the operationalization of the psychological dimension in study participants. The current study had several limitations also. First, we used cross sectional analyses of data from the intervention arm and there is no comparison group. However, given our focus of examining patient factors as barriers to diagnostic resolution, data from the PN group alone are sufficient. Most participants were white women with breast cancer, limiting generalizability across populations and cancer types. In addition, because we studied navigation in Ohio-based clinics, we do not know whether these findings generalize to other parts of the country with different populations and clinic arrangements.
Conclusion
PN can reduce barriers to care and promote the receipt of timely and quality care. In order to implement PN programs, each of the CoC’s 1,500+ accredited institutions will have to determine how to comply with this mandate. We provide information as to which patients might benefit from PN. Targeted efforts can identify individuals most likely to have a barrier to care and at risk for loss to follow-up (e.g., low SES, younger age) in order to assign them to PN and improve their care. Moreover, a multidisciplinary approach to PN that involves assessing barriers to care and then tailoring navigation to specific barrier type (e.g., patient, physician, or system-level) may help health care facilities provide comprehensive care across the cancer care continuum and enhance patients’ experiences with the healthcare system.
Acknowledgments
Funding: This work is supported by Special Initiative Research Scholar Grant (112190-SIRSG-05-253-01) from the American Cancer Society and a supplement from the National Cancer Institute Center to Reduce Health Disparities to Award Number (P30CA016058). Dr. Krok-Schoen is funded by a grant from the National Cancer Institute (P50 CA105632).
Footnotes
Conflict of interest statement: There are no conflicts of interest for any of the authors. All authors have read and approved the manuscript. This manuscript is not under consideration elsewhere.
References
- 1.American Cancer Society. Cancer Facts & Figures 2014. Atlanta, GA: American Cancer Society, Inc; [Google Scholar]
- 2.Gehlert S, Colditz GA. Cancer disparities: unmet challenges in the elimination of disparities. Cancer Epidemiol Biomarkers Prev. 2011;20:1809–1814. doi: 10.1158/1055-9965.EPI-11-0628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54:78–93. doi: 10.3322/canjclin.54.2.78. [DOI] [PubMed] [Google Scholar]
- 4.Battaglia TA, Roloff K, Posner MA, Freund KM. Improving follow-up to abnormal breast cancer screening in an urban population. A patient navigation intervention. Cancer. 2007;109:S359–367. doi: 10.1002/cncr.22354. [DOI] [PubMed] [Google Scholar]
- 5.Freund KM, Battaglia TA, Calhoun E, et al. Impact of patient navigation on timely cancer care: the patient navigation research program. J Natl Cancer Inst. 2014;106:dju115. doi: 10.1093/jnci/dju115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ell K, Vourlekis B, Lee PJ, Xie B. Patient navigation and case management following an abnormal mammogram: a randomized clinical trial. Prev Med. 2008;44:26–33. doi: 10.1016/j.ypmed.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Donaldson EA, Holtgrave DR, Duffin RA, et al. Patient navigation for breast and colorectal cancer in 3 community hospital settings. Cancer. 2012;118:4851–4859. doi: 10.1002/cncr.27487. [DOI] [PubMed] [Google Scholar]
- 8.Paskett ED, Katz ML, Post DM, et al. The Ohio Patient Navigation Research Program: does the American Cancer society model improve time to resolution in patients with abnormal screening tests? Cancer Epidemiol Biomarkers Prev. 2012;21:1620–1628. doi: 10.1158/1055-9965.EPI-12-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eggleston KS, Coker AL, Das IP, Cordray ST, Luchok KJ. Understanding barriers for adherence to follow-up care for abnormal pap tests. J Women Health. 2007;16:311–330. doi: 10.1089/jwh.2006.0161. [DOI] [PubMed] [Google Scholar]
- 10.Tejeda S, Darnell JS, Cho YI, et al. Patient barriers to follow-up care for breast and cervical cancer abnormalities. J Womens Health. 2013;22:507–517. doi: 10.1089/jwh.2012.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wagner EH, Bennett SM, Austin BT, et al. Finding common ground: patient-centeredness and evidence-based chronic illness care. J Altern Complement Med. 2005;11:S7–15. doi: 10.1089/acm.2005.11.s-7. [DOI] [PubMed] [Google Scholar]
- 12.Heaney CA, Israel B. Social networks and social support. In: Glanz K, Rimer BK, Viswanath K, editors. Health Behavior and Health Education Theory, Research, and Practice. 4. San Francisco, CA: Jossey-Bass; 2008. pp. 189–207. [Google Scholar]
- 13.Rosenstock I. The health belief model and preventive health behavior. Health Educ Monogr. 1974;2:354–386. [Google Scholar]
- 14.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 15.Radloff LS. The CES-D scale a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 16.Procidano ME, Heller K. Measures of perceived social support from friends and from family: three validation studies. Am J Commun Psychol. 1983;11:1–24. doi: 10.1007/BF00898416. [DOI] [PubMed] [Google Scholar]
- 17.Anderson LA, Dedrick RF. Development of the Trust in Physician Scale: a measure to assess interpersonal trust in patient-physician relationships. Psychol Rep. 1990;67:1091–100. doi: 10.2466/pr0.1990.67.3f.1091. [DOI] [PubMed] [Google Scholar]
- 18.Ware JE, Snow KK, Kosinski M, Gandek B. SF-36 Health Survey Manual and Interpretation Guide. Boston, MA: New England Medical Center, The Health Institute; 1993. [Google Scholar]
- 19.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:358–396. [PubMed] [Google Scholar]
- 20.Wolf MS, Chang CH, Davis T, Makoul G. Development and validation of the Communication and Attitudinal Self-efficacy scale for cancer (CASE-cancer) Pat Educ Counsel. 2005;57:333–341. doi: 10.1016/j.pec.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 21.Horowitz MJ, Wilner N, Alvarez W. The impact of event scale: A measure of subjective stress. Psychosom Med. 1979;41(3):209–218. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Freund KM, Battaglia TA, Calhoun E, et al. The National Cancer Institute Patient Navigation Research Program: methods, protocol, and measures. Cancer. 2008;113(12):3391–3399. doi: 10.1002/cncr.23960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raich PC, Whitley EM, Thorland W, Valverde P, Fairclough D. Patient navigation improves cancer diagnostic resolution: An individually randomized clinical trial in an underserved population. Cancer Epidemiol Biomarkers Prev. 2012;21(10):1629–1638. doi: 10.1158/1055-9965.EPI-12-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koh C, Nelson JM, Cook PF. Evaluation of a patient navigation program. Clin J Oncol Nurs. 2011;15(1):41–48. doi: 10.1188/11.CJON.41-48. [DOI] [PubMed] [Google Scholar]
- 25.Wagner EH, Ludman EJ, Bowles EJA, et al. Nurse navigators in early cancer care: A randomized, controlled trial. J Clin Oncol. 2014;32(1):12–18. doi: 10.1200/JCO.2013.51.7359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maly RC, Leake B, Mojica CM, Liu Y, Diamant AL, Thind A. What influences diagnostic delay in low-income women with breast cancer? J Womens Health. 2011;20(7):1017–1023. doi: 10.1089/jwh.2010.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kerlikowske K. Timeliness of follow-up after abnormal screening mammography. Breast Cancer Res Treat. 1996;40(1):53–64. doi: 10.1007/BF01806002. [DOI] [PubMed] [Google Scholar]
- 28.Yabroff KR, Breen N, Vernon SW, Meissner HI, Freedman AN, Ballard-Barbash R. What factors are associated with diagnostic follow-up after abnormal mammograms? Findings from a U.S. national survey. Cancer Epidemiol Biomarkers Prev. 2004;13(5):723–732. [PubMed] [Google Scholar]
- 29.Ell K, Vourlekis B, Lee PJ, Xie B. Patient navigation and case management following an abnormal mammogram: a randomized clinical trial. Prev Med. 2008;44(1):26–33. doi: 10.1016/j.ypmed.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 30.Strzelczyk JJ, Dignan MB. Disparities in adherence to recommended follow-up on screening mammography: Interaction of sociodemographic factors. Ethn Dis. 2001;12(1):77–86. [PubMed] [Google Scholar]
- 31.Montegomery M, McCrone SH. Psychological distress associated with the diagnostic phase for suspected breast cancer: systematic review. J Advan Nurs. 2010;66(11):2372–2390. doi: 10.1111/j.1365-2648.2010.05439.x. [DOI] [PubMed] [Google Scholar]
- 32.Keyzer-Dekker CM, De Vries J, van Esch L, et al. Anxiety after an abnormal screening mammogram is a serious problem. Breast. 2012;21(1):83–88. doi: 10.1016/j.breast.2011.08.137. [DOI] [PubMed] [Google Scholar]
- 33.Gwyn K, Bondy ML, Cohen DS, et al. Racial differences in diagnosis, treatment, and clinical delays in a population-based study of patients with newly diagnosed breast carcinoma. Cancer. 2004;100(8):1595–1604. doi: 10.1002/cncr.20169. [DOI] [PubMed] [Google Scholar]
- 34.George P, Chandwani S, Gabel M, et al. Diagnosis and surgical delays in African American and white women with early-stage breast cancer. J Womens Health. 2015 doi: 10.1089/jwh.2014.4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bjerager M, Palshof T, Dahl R, Vedsted P, Olesen F. Delay in diagnosis of lung cancer in general practice. Br J Gen Pract. 2006;56(532):863–868. [PMC free article] [PubMed] [Google Scholar]
- 36.Teppo H, Alho OP. Comorbidity and diagnostic delay in cancer of the larynx, tongue and pharynx. Oral Oncol. 2009;45(8):692–695. doi: 10.1016/j.oraloncology.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 37.Ogle KS, Swanson GM, Woods N, Azzouz F. Cancer and comorbidity. Cancer. 2000;88(3):653–663. doi: 10.1002/(sici)1097-0142(20000201)88:3<653::aid-cncr24>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]