Abstract
Background
Patient gender plays a significant role in patient-physician communication, patient illness understanding and aggressiveness of end of life (EoL) care. However, little is known about the extent to which gender differences in the effects of EoL discussions on EoL care contribute to gender differences in EoL care. The present study aims to determine if gender differences exist in receipt of intensive care unit (ICU) care near death and in the association between EoL discussions and receipt of ICU EoL care.
Methods
Multi-site, prospective, cohort study of patients (N=353) with metastatic cancers, identified as terminally ill at study enrollment and interviewed a median of 4.1 months before their deaths. Postmortem chart reviews and caregiver interviews documented ICU stays in the last week of life.
Results
Patients who received ICU care at the EoL were more likely to be male than those who did not (73% male vs. 52% male, p=0.02). Adjusting for potential confounds, male patients reporting an EoL discussion were less likely to have an ICU stay in the last week of life than male patients with no EoL discussion (AOR=0.26, 95% CI 0.07–0.91; p=0.04). There was no association between EoL discussions and ICU stays near death among female patients.
Conclusions
Men with advanced cancers are more likely than women to receive aggressive, non-beneficial, ICU care near death. Gender differences in effects of EoL discussions on EoL care likely contribute to, and may even explain, gender differences in receipt of ICU care in the last week of life.
INTRODUCTION
In recent years a great deal of attention has focused on the harms and limited benefits of overly aggressive care of patients at the end of life (EoL).1–5 Aggressive EoL care such as intensive care unit (ICU) stays has been shown to impair patients’ quality of life, not cure disease or significantly enhance survival, and comes at great public and personal expense.5–7 Patients with life-limiting illnesses such as advanced cancer often receive sub-optimal care at the EoL.2,3,8 Patients with advanced cancer often receive aggressive treatment at the EoL including ICU admission, initiation of new chemotherapy regimens in the last month of life, and delayed access to hospice.4 Recently, Teno et al.9 found that ICU use in the last month of life has been increasing steadily over the last decade. In order to improve EoL care, it is important to know who is at risk of aggressive, non-beneficial EoL care and what factors might reduce that risk.
In order to provide high quality EoL care for patients with serious illness, physicians must be able to communicate effectively with patients and their family members about disease status and prognosis, align the care plan with the patient’s values and goals, and provide support for medical decision making in a way that respects patient preferences.1 Unfortunately multiple studies have shown that patients with advanced cancer often have limited understanding of the incurability of their disease,10–14 life expectancy and survival,10,11,15 the impact of different treatments on cure16 and quality of life,11,13 and alternatives to treatment.17,18 In contrast, EoL discussions have been shown to facilitate both receipt of care that is consistent with patient preferences,6 and EoL care that is less aggressive5 and costly.7 Yet, these studies5 have not examined potential gender differences in the association between EoL discussions and intensity of EoL care.
Gender affects the communication of information between physicians and patients with advanced cancer, the EoL care that patients receive, and patient preferences for EoL care. Compared to men, for example, women with metastatic colorectal cancer are less likely to want prognostic information and more likely to prefer a passive role in decision making.19 Despite these differences, little is known about the role of gender in EoL communication and how gender differences in EoL communication affect EoL care for patients with advanced cancer. In a study using Surveillance, Epidemiology, and End Results (SEER) registry data, Earle et al.4 found that men are more likely than women to receive chemotherapy in the last two weeks of life and less likely to receive hospice. Lal et al.20 also found that men at a tertiary care cancer center had significantly more hospital admissions than women. Prior research in other clinical contexts has shown gender differences in EoL care preferences with women being less likely to prefer life-sustaining technology and other aggressive treatments, and more likely to have do-not-resuscitate (DNR) orders and prefer a dignified death than older men.21–25 Despite this prior work, there is limited understanding and a need to examine gender disparities in ICU utilization at the EoL and factors that may influence such a gender disparity.
Recently, we have shown that among patients with advanced cancer, women were more likely than men to recognize that their cancer was incurable and at an advanced stage, and to report having discussed life expectancy with their oncologist.26 After controlling for patient-reported discussion of life expectancy, however, the gender disparity in patients’ understanding that their cancer was incurable reduced to a level of statistical non-significance.26 These results, coupled with prior research demonstrating that EoL discussions between patients and oncologists about the care patients would want to receive if dying are associated with lower rates of ICU admission and other forms of aggressive care in the last week of life,5 motivated our interest in determining: a) if there are significant gender differences in rates of receipt of ICU care for advanced cancer patients in the last week of life and, to the extent that there are such differences, b) if EoL discussions between patients and their oncologists explain the effect of gender on the intensity of care patients receive at the EoL. We hypothesized that men would have higher rates of EoL ICU care but that patient report of an EoL discussion would attenuate the male-female disparity in rates of ICU care in the last week of life.
METHODS
Study Sample
Study participants (N=353) were patients recruited as part of the Coping with Cancer (CwC) study, a prospective, multi-institutional cohort study of patients with advanced cancer and their caregivers funded by the National Cancer Institute (CA106370) and the National Institute of Mental Health (MH63892). Participants were recruited between September 2002 and February 2008 at six comprehensive cancer centers across the United States: Yale Cancer Center (New Haven, CT), Veterans Affairs (VA) Connecticut Healthcare System Comprehensive Cancer Clinics (West Haven, CT), Parkland Hospital (Dallas, TX), Simmons Comprehensive Cancer Center (Dallas, TX), Dana-Farber Cancer Institute (Boston, MA), and New Hampshire Oncology-Hematology (Hookset, NH). Criteria for patient eligibility included diagnosis of advanced cancer (presence of distant metastases and disease refractory to first line chemotherapy); age 20 years or older; availability of an informal caregiver willing to participate in the study; ability to complete the interview; and fluency in English or Spanish. Patients who were significantly cognitively impaired (by neurobehavioral cognitive status examination with more than 5 errors)27 were excluded. Review boards of all participating institutions approved study procedures; all participants provided written informed consent.
Research and clinical staff reviewed outpatient clinic lists weekly to identify eligible participants. Of the 939 eligible patients, 661 (70.4%) participated in the study. The most common reasons for nonparticipation were “not interested” (n=106), “caregiver refuses” (n=32), and “too upset” (n=21). Participants and non-participants did not differ significantly in age, gender, race/ethnicity, or years of education. Eligible participants completed a baseline assessment and were followed prospectively until the time of death.
Because our analysis focuses on predictors of EoL care, the sample for the present study was restricted to deceased individuals with postmortem data and complete baseline assessments of the predictor variables of interest. Postmortem data were available for 368 (95.8%) of 384 patients who had died by the close of the study; baseline assessments of DNR order completion, preference for palliative EoL care, and patient-reported EoL discussions were present for 353 (95.9%) of these patients. The present study sample comprised these 353 patients with advanced cancer who provided a baseline interview a median of 4.1 months prior to their deaths (for which they received $25 compensation).
Measures
Baseline socio-demographic and health status characteristics
Patients’ age, gender, race/ethnicity, years of education, marital status, and health insurance status were reported by the patient at the baseline assessment. Disease information was obtained from medical charts. Performance status was determined by trained interviewers using the Karnofsky scale.28 Karnofsky performance status ratings included: 100=patient has no symptoms, carries out all normal activities; 50=patient requires medical care and much assistance with self care; 0=patient is dead. The Charlson Comorbidity Index29 evaluated the number and severity of the patient's co-morbid illnesses.
Do-Not-Resuscitate (DNR)
Patients were asked “Have you completed a Do-Not-Resuscitate (DNR) order?” Responses were coded as “1=yes” and “2=no”.
Preference for Palliative EoL Care
Patients were asked to answer the following question from the SUPPORT study8: “If you could choose, would you prefer: 1) a course of treatment that focused on extending life as much as possible, even if it meant more pain and discomfort, or 2) a plan of care that focused on relieving pain and discomfort as much as possible, even if that meant not living as long?” Patients who indicated a preference to relieve pain or discomfort as much as possible were coded as “1=yes” for having a preference for palliative EoL care. Patients who indicated either a preference to extend life as much as possible or “don’t know” in response to this question were coded as “2=no” for not having a preference for palliative EoL care.
EoL Discussions
Patients were asked “Have you and your doctor discussed any particular wishes you have about the care you would want to receive if you were dying?” Responses were coded as “1=yes” and “2=no”. Answering “yes” to this question has been associated with receipt of less aggressive EoL care.5
Intensive EoL Care
Patient receipt of intensive EoL care, defined here as care provided in an ICU in the last week of life, was documented via postmortem medical chart reviews and confirmed by caregiver interviews within three weeks of patients’ deaths. In the present study, a large majority, 27 (73.0%) of 37 patients, who received care in an ICU in the last week of life died there.
Statistical Methods
Patient characteristics and responses to baseline interview questions were described in terms of means and frequencies. Bivariate associations between patient characteristics and responses to questions on the one hand, and patient gender and patient receipt of intensive EoL care on the other, were assessed using t-tests for continuous variables and chi-square tests for categorical variables. Multiple logistic regression analysis was used to test the hypothesis that gender modifies an association between EoL discussions and receipt of intensive EoL care. Care in an ICU at the EoL was regressed on the main and interactive effects of gender and EoL discussions, adjusting for potential confounds identified as patient characteristics and responses to questions found to be at least marginally significantly associated (p<0.10) with both gender and intensive EoL care. Statistical analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, NC).
RESULTS
Table 1 presents characteristics of the study sample, broken down by gender and receipt of intensive EoL care. Of the total sample (N=353), 54% percent of patients were male, 64% were non-Hispanic white, 19% were black, 16% were Hispanic, 55% were married, and 57% had health insurance. Compared to men, women had higher Karnofsky performance status scores (65.8 vs. 62.2, p=0.028), were less likely to be married (43% vs. 64%, p<0.001) or recruited at the VA hospital (0% vs. 8%, p=0.003). Women were more likely to have a DNR order (48% vs. 37%, p=0.039) and express a preference for palliative EoL care (70% vs. 58%, p=0.017) than men. Patients who received ICU care at the EoL were more likely to be male (73% vs. 52%, p=0.015), and less likely to prefer palliative EoL care (35% vs. 67%, p<0.001) and to report having had an EoL discussion (19% vs. 38%, p=0.02), than patients who did not receive intensive EOL care.
Table 1.
Patient Characteristics by Gender and Receipt of ICU Care at the End of Life (EoL) (N=353)
| Gender | ICU @ EoL | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Male | Female | Yes | No | ||||||||
| N (%) | 353 | 100.0% | 191 | 54.1% | 162 | 45.9% | 37 | 10.5% | 316 | 89.5% | ||
| Characteristic | Mean | SD | Mean | SD | Mean | SD | P value | Mean | SD | Mean | SD | P value |
| Age in years | 58.3 | 12.6 | 58.9 | 12.7 | 57.6 | 12.5 | 0.348 | 56.3 | 14.1 | 58.6 | 12.4 | 0.304 |
| Education in years | 12.4 | 4.1 | 12.6 | 4.0 | 12.2 | 4.1 | 0.333 | 12.6 | 2.6 | 12.4 | 4.2 | 0.698 |
| Karnofsky Performance Score | 63.9 | 15.6 | 62.2 | 15.0 | 65.8 | 16.0 | 0.028 | 62.0 | 10.5 | 64.1 | 16.0 | 0.308 |
| Charlson Comobidity Index | 8.4 | 2.6 | 8.4 | 2.7 | 8.5 | 2.6 | 0.719 | 8.4 | 3.4 | 8.4 | 2.5 | 0.992 |
| n | % | n | % | n | % | p | n | % | n | % | p | |
| Male gender | 191 | 54.1% | 27 | 73.0% | 164 | 51.9% | 0.015 | |||||
| Race/ethnicity | 0.201 | 0.142 | ||||||||||
| White | 224 | 63.5% | 121 | 63.4% | 103 | 63.6% | 20 | 54.1% | 204 | 64.6% | ||
| Black | 68 | 19.3% | 37 | 19.4% | 31 | 19.1% | 12 | 32.4% | 56 | 17.7% | ||
| Hispanic | 56 | 15.9% | 28 | 14.7% | 28 | 17.3% | 4 | 10.8% | 52 | 16.5% | ||
| Other | 5 | 1.4% | 5 | 2.6% | 0 | 0.0% | 1 | 2.7% | 4 | 1.3% | ||
| Married | 190 | 54.8% | 121 | 64.4% | 69 | 43.4% | 0.000 | 24 | 64.9% | 166 | 53.5% | 0.191 |
| Health Insurance | 198 | 57.4% | 108 | 58.4% | 90 | 56.3% | 0.690 | 22 | 61.1% | 176 | 57.0% | 0.633 |
| Recruitment Site | 0.003 | 0.419 | ||||||||||
| Yale Cancer Center | 76 | 21.7% | 43 | 22.6% | 33 | 20.5% | 8 | 21.6% | 68 | 21.7% | ||
| West Haven VA Cancer Center | 16 | 4.6% | 16 | 8.4% | 0 | 0.0% | 0 | 0.0% | 16 | 5.1% | ||
| Simmons Comprehensive Cancer Center | 35 | 10.0% | 13 | 6.8% | 22 | 13.7% | 2 | 5.4% | 33 | 10.5% | ||
| Parkland Hospital | 154 | 43.9% | 81 | 42.6% | 73 | 45.3% | 16 | 43.2% | 138 | 43.9% | ||
| Partners (DFCI, MGH) Cancer Centers | 9 | 2.6% | 4 | 2.1% | 5 | 3.1% | 1 | 2.7% | 8 | 2.5% | ||
| New Hampshire Oncology-Hematology | 61 | 17.4% | 33 | 17.4% | 28 | 17.4% | 10 | 27.0% | 51 | 16.2% | ||
| Type of Cancer | 0.000 | 0.543 | ||||||||||
| Lung | 78 | 22.5% | 50 | 26.7% | 28 | 17.5% | 13 | 35.1% | 65 | 21.0% | ||
| Colon | 45 | 13.0% | 27 | 14.4% | 18 | 11.3% | 4 | 10.8% | 41 | 13.2% | ||
| Pancreatic | 28 | 8.1% | 13 | 7.0% | 15 | 9.4% | 3 | 8.1% | 25 | 8.1% | ||
| Other Gastrointestinal | 44 | 12.7% | 30 | 16.0% | 14 | 8.8% | 4 | 10.8% | 40 | 12.9% | ||
| Breast | 42 | 12.1% | 1 | 0.5% | 41 | 25.6% | 3 | 8.1% | 39 | 12.6% | ||
| Other | 110 | 31.7% | 66 | 35.3% | 44 | 27.5% | 10 | 27.0% | 100 | 32.3% | ||
| Do-Not-Resuscitate Order | 147 | 41.6% | 70 | 36.6% | 77 | 47.5% | 0.039 | 10 | 27.0% | 137 | 43.4% | 0.057 |
| Preference for Palliative EOL care | 225 | 63.7% | 111 | 58.1% | 114 | 70.4% | 0.017 | 13 | 35.1% | 212 | 67.1% | 0.000 |
| EOL Discussion | 128 | 36.3% | 68 | 35.6% | 60 | 37.0% | 0.780 | 7 | 18.9% | 121 | 38.3% | 0.020 |
Variables missing data: Karnofsky (5), Charlson (6), Married (6), Health Insurance (8), Recruitment Site (2), Cancer Diagnosis (6).
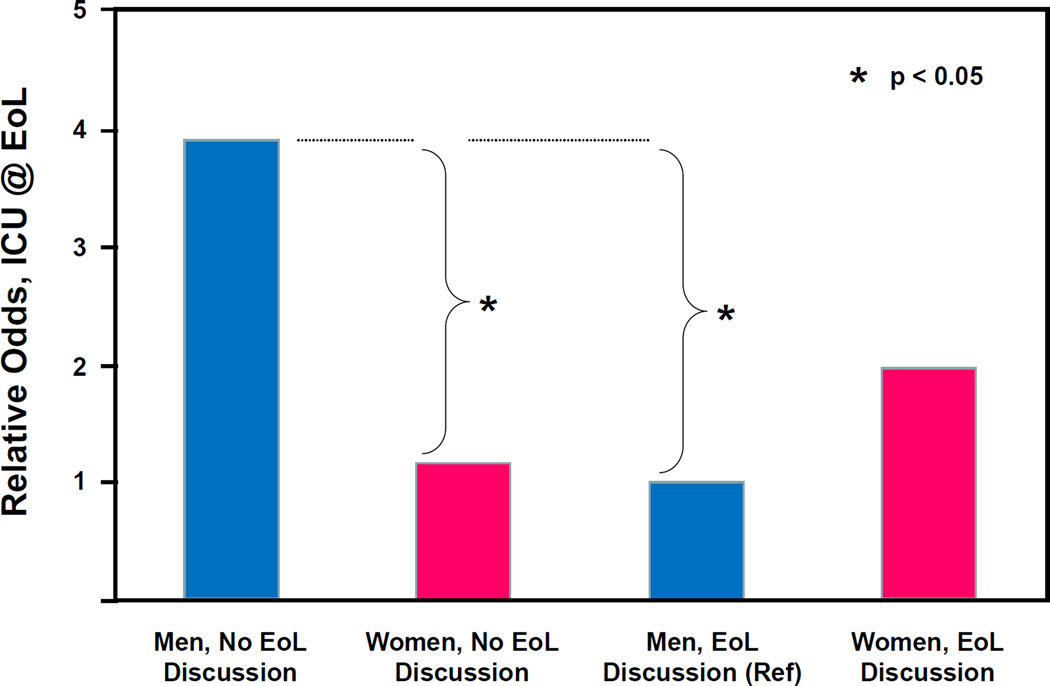
In the multiple logistic regression analysis, we found a significant interaction between gender and EoL discussion adjusting for baseline DNR order completion and preference for palliative EoL care. The relative odds of receipt of intensive EOL care associated with having an EoL discussion was significantly lower for men than for women (AOR=0.15, 95% CI 0.02–0.93; p=0.041). Table 2 presents, and Figure 1 depicts, the relative odds of receipt of intensive EoL care by gender and EoL discussion that are the results of this analysis. Among patients who at baseline reported not having EoL discussions with their oncologists, men were significantly more likely than women to receive intensive EoL care (OR=3.42, 95% CI 1.32–8.87; p=0.011). Among patients reporting having an EoL discussion, there was no significant difference in receipt of intensive EoL care between men and women (OR=0.51, 95% CI 0.11–2.45; p=0.399). For men, those reporting an EoL discussion were significantly less likely to receive intensive EoL care than those not reporting an EoL discussion (OR=0.26, 95% CI 0.07–0.91; p=0.036). For women, there was no significant difference in receipt of intensive EoL care between those who reported an EoL discussion at baseline and those who did not (OR=1.72, 95% CI 0.43–6.81; p=0.440).
Table 2.
Effects of Gender and EoL Discussions on Receipt of ICU Care at the End of Life (EoL)1 (N=353)
| Effect of: | For group: | On: | ICU @ EoL | |||
|---|---|---|---|---|---|---|
| AOR2 | (95% CI) | χ2 | df | p | ||
| Gender, male vs. female | without EoL Discussion | 3.42 | (1.32 – 8.87) | 6.40 | 1 | 0.011 |
| with EoL Discussion | 0.51 | (0.11 – 2.45) | 0.71 | 1 | 0.399 | |
| EoL Discussion, with vs. without | Gender, male | 0.26 | (0.07 – 0.91) | 4.42 | 1 | 0.036 |
| Gender, female | 1.72 | (0.43 – 6.81) | 0.60 | 1 | 0.440 | |
Based on multiple logistic regression model for ICU @ EoL indicating a significant interaction between the effects of gender and EoL discussions (interaction χ2=4.17, df=1, p=0.041).
Adjusted Odds Ratio (AOR) – odds ratio adjusted for baseline Do-Not-Resuscitate (DNR) order completion and for baseline preference for palliative EoL care.
Figure 1.
Relative Odds1 of Receipt of ICU Care at the End of Life (EoL) by Gender and EoL Discussion (N=353)
1 Relative odds for ICU @ EoL, adjusted for baseline DNR order completion and for baseline preference for palliative EoL care, using men reporting EoL discussions at baseline as a reference group.
DISCUSSION
In this prospective, multisite study of patients with advanced cancer, we found that men were three times more likely than women to receive ICU care at the EoL. This is consistent with results from prior work.30 Our data suggest that this difference in ICU care use near the EoL may be attributed to a gender difference in the association between EoL discussions and EoL care. For patients who reported not having EoL discussions with their oncologists, male patients were significantly more likely than female patients to receive ICU care in their last week of life, adjusting for baseline DNR order completion and preference for palliative EoL care. For patients who reported having EoL discussions, there was no significant gender difference in receipt of ICU EoL care.
Our results highlight both how male patients may be at higher risk than female patients of receiving non-beneficial care at the EoL and the way in which communication may decrease this risk. Given prior studies that suggest more than 25% of older patients with cancer have an ICU stay in the last month of life,9 the identification of which patients are at higher risk for such overly-aggressive care is critical. We found that compared to men who did not have an EoL discussion with their physician, men who did have one were much less likely to receive ICU care at the EoL. In contrast, women who reported an EoL discussion did not receive less ICU care at the EoL than women who did not have an EoL discussion. Thus, EoL discussions were associated with decreased ICU use at the EoL for men dying with advanced cancer, but these discussions did not result in less ICU care at the EoL for women dying with advanced cancer.
Our findings of gender differences in the association between EoL discussions and receipt of ICU care at the EoL occurred in the presence of similar rates of reported EoL discussion for men (36%) and women (37%). Possible explanations for these results include the existence of gender differences in the content of patient-physician discussions about EoL care and/or in the way that patients use the information from these discussions to make EoL decisions. Our prior work, using an independent sample of patients with advanced cancer, revealed that women were more likely than men to understand that they had incurable disease.26 Since other studies have shown that men are more likely to desire prognostic information than women,19,31 perhaps communicating this information is more critical to EoL decision making for men than women. In this way, men may benefit even more from explicit physician discussion about EoL care, particularly because they are less likely than women to initiate discussion about death and dying.32
Consistent with findings from prior studies, we also found that women were more likely than men to prefer palliative care at the EoL and to have a DNR order.21,22 Not surprisingly, patients who preferred palliative care at the EoL were also less likely than those who did not to receive ICU care at the EoL. However, in the absence of EoL discussions, women were far less likely than men to receive ICU care at the EoL even after controlling for patient preferences. It is not known whether there are differences in physician recommendations for EoL care related to patient gender that could be a contributing factor to this disparity. Johnson et al.33 found that among hospitalized patients with an estimated prognosis of six months or less, women were more likely than men to believe that their physician had recommended comfort care. Although discussion content in that study was assessed by patient self-report and thus it is unclear whether there were actual differences in physician recommendations based on patients’ gender, their findings suggest that gender differences may at least exist in patient interpretation of physician recommendations. Additional research is thus needed to better elucidate the way in which gender influences both the information that is exchanged between physicians and patients in EoL discussions and the role of communication in facilitating EoL decision making.
Our results suggest that EoL discussions can decrease receipt of overly-aggressive care at the EoL for male patients. Further, our findings indicate that prior research showing an association between EoL discussions and less aggressive EoL care5 may have been driven by the effects of these discussions in men. It is possible that gender differences in receipt of other measures of overly-aggressive care at the EoL (i.e., use of chemotherapy, emergency room visits, hospital admissions, inpatient death, and lack of hospice referral) may be partially explained by gender differences in associations between EoL discussions and aggressive EoL care. This merits further investigation.
Our findings should be interpreted in the context of potential limitations. First, because we do not have information about the content of the EoL discussions, we cannot comment on what specific aspects of these discussions reduce utilization of ICU care and whether gender differences in discussion content contributes to the observed gender difference in the association between EoL discussions and EoL ICU care. Future research that includes recording and analyzing these discussions would thus provide important data on the existence of gender differences in discussion content. Second, the present study does not include any information about physicians’ characteristics, e.g., physician gender, which may play a role in patient-physician communication. This is another area that warrants future investigation. Third, because we collected patient data on the occurrence of EoL discussions and preferences for EoL care at a single time point, we are unable to comment on how EoL patient-physician communication and patient preferences for EoL care may have changed over time. Finally, future research might investigate reasons why patients are admitted to an ICU near the time of their deaths, and how these reasons are influenced by patient gender and gender differences in EoL patient-physician communication.
CONCLUSIONS
Receipt of ICU care at the EoL has been associated with greater physical and emotional distress and poorer quality of life at the EoL for patients with advanced cancer. Our finding that EoL discussions facilitated decreased receipt of ICU care for male patients lends further support to the importance of having these discussions among men. Additional efforts to increase the frequency of EoL discussions among male patients with advanced cancer may thus help these patients avoid aggressive, burdensome care that is not consistent with their values and goals. At the same time, further research is needed to explore why these discussions are more effective for male patients and to identify other factors that are shaping their preferences for and receipt of more aggressive forms of EoL care.
Acknowledgments
Funding/Support: Drs. Maciejewski and Prigerson were supported by research grant CA 106370 (PI: Prigerson) from the National Cancer Institute. Dr. Sharma was supported in part by Grant Number K12 HD055884 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Competing Interests: No authors have any competing interests.
Ethics Statement: Review boards of all participating institutions approved study procedures; all participants provided written informed consent.
References
- 1.National Consensus Project for Quality Palliative Care: Clinical Practice Guidelines for Quality Palliative Care, Executive Summary. J Palliat Med. 2004;7(5):611–627. doi: 10.1089/jpm.2004.7.611. [DOI] [PubMed] [Google Scholar]
- 2.Dy SM, Asch SM, Lorenz KA, et al. Quality of end-of-life care for patients with advanced cancer in an academic medical center. J Palliat Med. 2011;14(4):451–457. doi: 10.1089/jpm.2010.0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walling AM, Asch SM, Lorenz KA, et al. The quality of supportive care among inpatients dying with advanced cancer. Support Care Cancer. 2012;20(9):2189–2194. doi: 10.1007/s00520-012-1462-3. [DOI] [PubMed] [Google Scholar]
- 4.Earle CC, Landrum MB, Souza JM, Neville BA, Weeks JC, Ayanian JZ. Aggressiveness of cancer care near the end of life: is it a quality-of-care issue? J Clin Oncol. 2008;26(23):3860–3866. doi: 10.1200/JCO.2007.15.8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wright AA, Zhang B, Ray A, et al. Associations Between End-of-Life Discussions, Patient Mental Health, Medical Care Near Death, and Caregiver Bereavement Adjustment. JAMA. 2008;300(14):1665–1673. doi: 10.1001/jama.300.14.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mack JW, Weeks JC, Wright AA, Block SD, Prigerson HG. End-of-life discussions, goal attainment, and distress at the end of life: predictors and outcomes of receipt of care consistent with preferences. J Clin Oncol. 2010;28(7):1203–1208. doi: 10.1200/JCO.2009.25.4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang B, Wright AA, Huskamp HA, et al. Health care costs in the last week of life: associations with end-of-life conversations. Arch Intern Med. 2009;169(5):480–488. doi: 10.1001/archinternmed.2008.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The SUPPORT Principal Investigators. A Controlled Trial to Improve Care for Seriously III Hospitalized Patients. JAMA. 1995;274(20):1591–1598. [PubMed] [Google Scholar]
- 9.Teno JM, Gozalo PL, Bynum JP, et al. Change in end-of-life care for Medicare beneficiaries: site of death, place of care, and health care transitions in 2000, 2005, and 2009. JAMA. 2013;309(5):470–477. doi: 10.1001/jama.2012.207624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pronzato P, Bertelli G, Losardo P, Landucci M. What do advanced cancer patients know of their disease? Support Care Cancer. 1994;2(4):242–244. doi: 10.1007/BF00365729. [DOI] [PubMed] [Google Scholar]
- 11.Eidinger RN, Schapira DV. Cancer patients' insight into their treatment, prognosis, and unconventional therapies. Cancer. 1984;53(12):2736–2740. doi: 10.1002/1097-0142(19840615)53:12<2736::aid-cncr2820531233>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 12.Mackillop WJ, Stewart WE, Ginsburg AD, Stewart SS. Cancer patients perceptions of their disease and its treatment. Br J Cancer. 1988;58(3):355–358. doi: 10.1038/bjc.1988.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chow E, Andersson L, Wong R, et al. Patients with advanced cancer: a survey of the understanding of their illness and expectations from palliative radiotherapy for symptomatic metastases. Clin Oncol (R Coll Radiol) 2001;13(3):204–208. doi: 10.1053/clon.2001.9255. [DOI] [PubMed] [Google Scholar]
- 14.Gaston CM, Mitchell G. Information giving and decision-making in patients with advanced cancer: a systematic review. Soc Sci Med. 2005;61(10):2252–2264. doi: 10.1016/j.socscimed.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 15.Weeks JC, Cook EF, O'Day SJ, et al. Relationship Between Cancer Patients' Predictions of Prognosis and Their Treatment Preferences. JAMA. 1998;279(21):1709–1714. doi: 10.1001/jama.279.21.1709. [DOI] [PubMed] [Google Scholar]
- 16.Weeks JC, Catalano PJ, Cronin A, et al. Patients' expectations about effects of chemotherapy for advanced cancer. N Engl J Med. 2012;367(17):1616–1625. doi: 10.1056/NEJMoa1204410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jansen S, Otten W, Stiggelbout A. Factors affecting patients’ perceptions of choice regarding adjuvant chemotherapy for breast cancer. Breast Cancer Res Treat. 2006;99(1):35–45. doi: 10.1007/s10549-006-9178-z. [DOI] [PubMed] [Google Scholar]
- 18.Jansen SJT, Otten W, van de Velde CJH, Nortier JWR, Stiggelbout AM. The impact of the perception of treatment choice on satisfaction with treatment, experienced chemotherapy burden and current quality of life. Br J Cancer. 2004;91(1):56–61. doi: 10.1038/sj.bjc.6601903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elkin EB, Kim SHM, Casper ES, Kissane DW, Schrag D. Desire for Information and Involvement in Treatment Decisions: Elderly Cancer Patients' Preferences and Their Physicians' Perceptions. J Clin Oncol. 2007;25(33):5275–5280. doi: 10.1200/JCO.2007.11.1922. [DOI] [PubMed] [Google Scholar]
- 20.Lal LS, Miller LA, Arbuckle R, et al. Disparities in outpatient antidepressant prescribing patterns and determinants of resource utilization at a tertiary care cancer center. J Support Oncol. 2009;7(6):237–244. [PubMed] [Google Scholar]
- 21.Bookwala J, Coppola KM, Fagerlin A, Ditto PH, Danks JH, Smucker WD. Gender differences in older adults' preferences for life-sustaining medical treatments and end-of-life values. Death Stud. 2001;25(2):127–149. doi: 10.1080/07481180126202. [DOI] [PubMed] [Google Scholar]
- 22.Wenger NS, Pearson ML, Desmond KA, et al. Epidemiology of do-not-resuscitate orders. Disparity by age, diagnosis, gender, race, and functional impairment. Arch Intern Med. 1995;155(19):2056–2062. [PubMed] [Google Scholar]
- 23.Carmel S, Mutran E. Preferences for different life-sustaining treatments among elderly persons in Israel. J Gerontol B Psychol Sci Soc Sci. 1997;52(2):S97–S102. doi: 10.1093/geronb/52b.2.s97. [DOI] [PubMed] [Google Scholar]
- 24.Frankl D, Oye RK, Bellamy PE. Attitudes of hospitalized patients toward life support: a survey of 200 medical inpatients. Am J Med. 1989;86(6):645–648. [PubMed] [Google Scholar]
- 25.Garrett JM, Harris RP, Norburn JK, Patrick DL, Danis M. Life-sustaining treatments during terminal illness: who wants what? J Gen Intern Med. 1993;8(7):361–368. doi: 10.1007/BF02600073. [DOI] [PubMed] [Google Scholar]
- 26.Fletcher K, Prigerson HG, Paulk E, et al. Gender differences in the evolution of illness understanding among patients with advanced cancer. J Support Oncol. 2013;11(3):126–132. doi: 10.12788/j.suponc.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23(10):433–441. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 28.Karnofsky DA, Abelmann WH, Craver LF, Burchenal JH. The use of the nitrogen mustards in the palliative treatment of carcinoma. With particular reference to bronchogenic carcinoma. Cancer. 1948;1(4):634–656. [Google Scholar]
- 29.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 30.Earle CC, Neville BA, Landrum MB, Ayanian JZ, Block SD, Weeks JC. Trends in the aggressiveness of cancer care near the end of life. J Clin Oncol. 2004;22(2):315–321. doi: 10.1200/JCO.2004.08.136. [DOI] [PubMed] [Google Scholar]
- 31.Marwit SJ, Datson SL. Disclosure preferences about terminal illness: an examination of decision-related factors. Death Stud. 2002;26(1):1–20. doi: 10.1080/07481180210144. [DOI] [PubMed] [Google Scholar]
- 32.Skulason B, Hauksdottir A, Ahcic K, Helgason AR. Death talk: gender differences in talking about one's own impending death. BMC Palliat Care. 2014;13(1):8. doi: 10.1186/1472-684X-13-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson MF, Lin M, Mangalik S, Murphy DJ, Kramer AM. Patients' perceptions of physicians' recommendations for comfort care differ by patient age and gender. J Gen Intern Med. 2000;15(4):248–255. doi: 10.1111/j.1525-1497.2000.07004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]