Abstract
Antibodies have clearly demonstrated their utility as therapeutics, providing highly selective and effective drugs to treat diseases in oncology, hematology, cardiology, immunology and autoimmunity, and infectious diseases. More recently, a pressing need for equally specific and targeted imaging agents for assessing disease in vivo, in preclinical models and patients, has emerged. This review summarizes strategies for developing and optimizing antibodies as targeted probes for use in non-invasive imaging using radioactive, optical, magnetic resonance, and ultrasound approaches. Recent advances in engineered antibody fragments and scaffolds, conjugation and labeling methods, and multimodality probes are highlighted. Importantly, antibody-based imaging probes are seeing new applications in detection and quantitation of cell surface biomarkers, imaging specific responses to targeted therapies, and monitoring immune responses in oncology and other diseases. Antibody-based imaging will provide essential tools to facilitate the transition to truly precision medicine.
Keywords: Antibody fragments, molecular imaging, immunoPET, image-guided surgery
1. Introduction
Over the past decades, antibodies have evolved to become a mainstay of biotherapeutics, bolstered by progress in target discovery and validation, advances in antibody isolation, design, and engineering, and successes in clinical translation and commercialization (Scott et al., 2012). Antibodies can rightly be considered among the first molecularly-targeted therapeutics. In parallel, the continued development of antibody-based therapeutics requires a detailed understanding of the normal function of potential targets, and how modifications contribute to, or are correlated with, disease. Antibodies have already provided invaluable tools for biomedical investigations. The precise discrimination offered by antibodies has formed the basis of workhorse assays used in research labs worldwide. Antibodies are essential components in ELISAs, flow cytometry, immunocyto- and immunohistochemistry (ICC and IHC), immunofluorescence, Western blotting, immunoprecipitation, protein microarray analysis, and many other techniques. Many antibody-based laboratory tests have been standardized and validated for routine clinical use (Fleuren et al., 2014); for example, Hercep Test™ (semi-quantitative HER2-specific IHC) is used to guide decisions on the use of trastuzumab, pertuzumab, and ado-trastuzumab emtansine and serves as a prime example of a companion diagnostic. The need for precision in vitro diagnostics is accelerating in parallel with the development of new molecularly-targeted therapeutics.
This review focuses on the growing applications of antibodies for in vivo diagnostics; specifically, the development of antibody-targeted agents for non-invasive imaging. While imaging modalities such as x-ray computed tomography (CT), magnetic resonance imaging (MRI), and ultrasound are current backbones in diagnostic medicine, these methods are largely restricted to providing anatomical and physiological information. There is an increasing need to analyze specific targets and biomarkers in vivo, including distinct molecules, events, and processes. Molecular imaging allows detection of these targets, usually via use of radioactive and/or optical probes, and offers numerous advantages, including the ability to detect biomarkers with nanomolar sensitivity (James and Gambhir, 2012). Furthermore, non-invasive imaging technologies permit visualization and quantitation over the entire living organism. This is particularly critical when studying disease processes that are disseminated or systemic, such as cancer metastasis or immune responses. In animal studies, molecular imaging allows repeat assessment of the same subject over time, reducing variability. In the clinical setting, non-invasive imaging stands to have an increasing impact, circumventing the sampling limitations inherent to tissue biopsy.
Thus, it is not surprising that there is renewed interest in the utility of imaging agents based on antibodies. The ability of antibodies to engage specific targets with nanomolar or picomolar affinity provides a foundation for developing highly sensitive imaging agents. The relatively large size of antibodies means that a variety of cargoes, for imaging or therapeutic applications, can be appended without perturbing their innate specificity and targeting properties (in contrast to the challenges faced in modifying small molecules or peptides for imaging purposes). The resounding successes of antibody therapeutics validate their ability to find and engage their targets in vivo. Furthermore, corresponding expertise and infrastructure are now in place for the routine large-scale production and purification of antibodies for clinical use. Finally, advances in protein engineering allow customization of biophysical and biological properties of antibodies to enhance their efficacy.
Importantly, current interest in antibody imaging is driven by the need for specific molecular information to guide the development and use of targeted therapeutics. Non-invasive imaging using antibodies provides a powerful and general approach for assessing cell surface phenotype in vivo. Detection and quantification of tissue- or tumor-specific markers in vivo can be used to identify and localize tumors, providing information on the nature and extent of disease. There are also numerous ways in which molecular imaging can contribute to development and applications of targeted therapeutics. An obvious example would be the assessment of target expression and availability in vivo, which would not only be informative for patient selection, but could also provide an early indication of potential normal organ toxicities. Whole-body evaluation of target expression would be of value due to the heterogeneity observed in metastatic cancers, including intralesion and lesion-to-lesion variations. Imaging can be employed for direct assessment of pharmacokinetics, biodistribution, and targeted delivery of therapeutic antibodies labeled with radionuclides or dyes. Molecular imaging can also provide highly specific readouts of response to therapy, either directly or indirectly. Direct elimination of targeted cells or downregulation of biomarkers can be assessed. Alternatively, antibody-based imaging of a cell surface biomarker can provide a downstream readout of intracellular events, providing insights into pathways and mechanism. In summary, the potential applications of imaging cell-surface phenotype, based on the specificity afforded by antibodies, are many.
The applications of antibodies for molecular imaging, primarily in the context of oncology, and often focusing on a single imaging modality, have been recently reviewed (Warram et al., 2014; Wu, 2014). Here we highlight recent advances in the modification and use of antibodies for a variety of in vivo molecular imaging applications (Figure 1). Contributions to the field of antibody engineering and conjugation chemistry will be discussed, including established methods and emerging improvements. Examples of using antibody-based imaging modalities, including single photon emission computed tomography (SPECT), positron emission tomography (PET), MRI, optical imaging, ultrasonography, and innovative combinations of the above, will be summarized. In addition, the importance of antibody-based imaging in both theranostics and non-clinical applications will be discussed, with a spotlight on imaging the immune system.
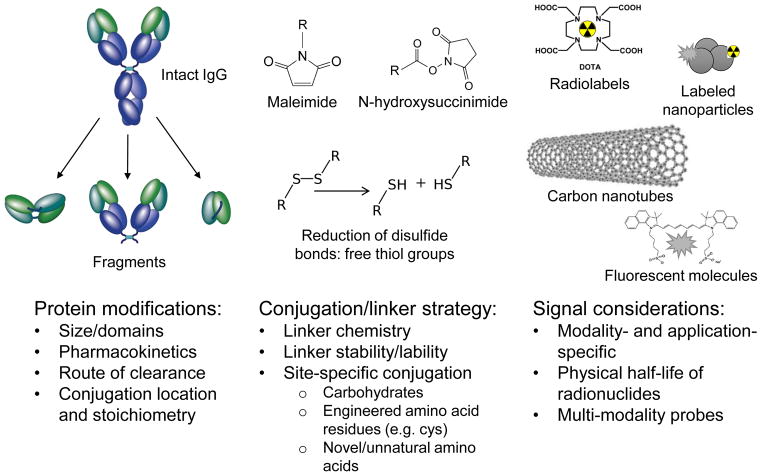
Figure 1. Development of antibodies for in vivo imaging: a multifaceted endeavor.
Numerous characteristics must be optimized in order to employ antibodies for targeting and imaging in vivo. A typical imaging probe will consist of a targeting moiety, linker, and signaling agent.
2. Development of Antibodies and Fragments for In Vivo Imaging
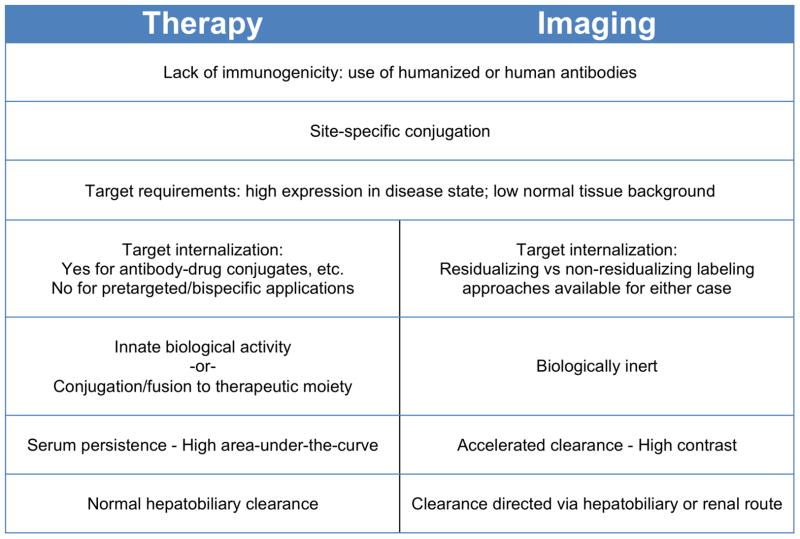
Decades of research and development of antibodies for therapeutic applications has led to a wealth of information and experience in areas that are also highly relevant to the generation of imaging agents (Scott et al., 2012). For example, the potential immunogenicity of murine antibodies in patients has led to robust methods for routine humanization of antibodies or directly accessing fully human antibodies via technologies such as phage display. Availability of antibodies that cross-react with human and murine or primate target proteins can facilitate toxicological studies. The steady expansion of the use of antibody-drug conjugates and radioimmunoconjugates for therapy has increased attention to conjugation methods and spurred interest in site-specific conjugation (Agarwal and Bertozzi, 2015; Smaglo et al., 2014). Manufacturability and stability must be addressed up front for clinical applications. On the other hand, imaging applications impose different requirements on the agents employed (Figure 2). For example, there is considerable overlap in desired characteristics of targets (high expression in disease state; low expression in normal tissue) but not all therapeutic targets or epitopes will be ideal for imaging, and vice versa. Whereas a significant effort has gone into enhancing effector functions of therapeutic antibodies (Fc engineering; glyco-engineering), it is preferable that an imaging agent be biologically inert. Optimal pharmacokinetics and distribution also differ; whereas therapeutic antibodies need to achieve significant concentrations in target tissue over time (high area-under-the-curve), imaging agents instead need to achieve high contrast at early times. As a result, the long plasma persistence of most therapeutic antibodies is a distinct disadvantage for imaging purposes. Here we discuss specific approaches to modification of antibodies for in vivo imaging including protein engineering and modification, conjugation strategies, and the types of tracers and tags that can be introduced for detection by various imaging modalities.
Figure 2. Desired characteristics of imaging vs. therapeutic antibodies.
Properties such as effector function, half-life, and conjugation sites are modified to suit either therapy or imaging applications.
2.1. Engineering to Optimize Antibodies for Imaging Applications
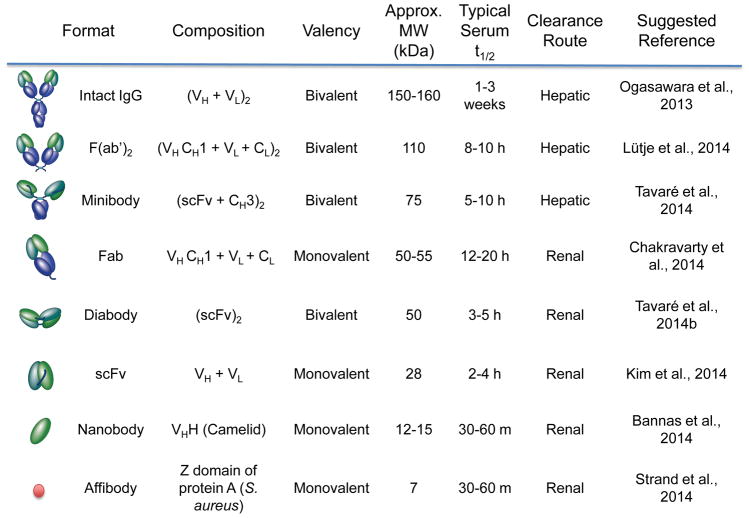
Intact antibodies function well as therapeutics due to their long serum half-life (1–3 weeks), increasing exposure of the affected tissues to the antibody; furthermore, biological activity from the effector domain (fragment crystallizing; Fc) is often essential for therapeutic function (Wu, 2014). In contrast, the long half-life of intact antibodies hampers their use as imaging agents: several days are required for blood and background clearance in order to achieve a good signal:noise ratio. For radiolabeled antibodies, this means that the organism will be exposed to radioactivity for an extended period of time. Furthermore, biological activity is undesirable in an imaging agent, in the interest of studying the system without perturbing it and reducing the risk of unwanted side effects. Many of these issues have been addressed by enzymatic cleavage or reformatting antibodies into smaller antibody fragments with a variety of molecular weights, valencies, clearance routes, and conjugation strategies (Figure 3). Retention of the Fv (fragment variable) domain preserves antigen binding, but fragments typically lack the Fc region, eliminating complement- and effector cell-mediated immune interactions. Removal of the Fc region also prevents recycling through the neonatal Fc receptor (FcRn) pathway, facilitating visualization of targeted tissues via rapid blood clearance and improved contrast. Smaller fragments also enable the use of radionuclides that decay more rapidly (e.g. 18F); the combination of short physical and biological half-lives of a radiotracer can result in reduced radiation exposure. Furthermore, use of lower-molecular weight antibody fragments and proteins (below ~60 kDa) accelerates elimination via renal clearance. Several recent reviews have described in detail the biochemical and biophysical properties of antibody fragments (James and Gambhir, 2012; Knowles and Wu, 2012; Wu, 2014).
Figure 3. Antibody fragments.
Summary of salient properties of intact antibodies, enzymatic fragments, recombinant fragments and smaller scaffolds.
Using single-chain variable fragments (scFv; 25kDa) and portions of the constant region (CH) as building blocks, additional fragments can be constructed such as diabodies (Db; dimers of scFv, 50kDa), minibodies (Mb; dimers of scFv-CH3, 80kDa), and scFv-Fc (dimers of scFv fused to Fc, 105kDa) (James and Gambhir, 2012; Olafsen and Wu, 2010). Comparisons of the pharmacokinetics and targeting of intact and antibody fragments (enzymatically-derived and recombinant) have been conducted; recent examples include targeted imaging of prostate-specific membrane antigen (PSMA) using 111In-radiolabeled F(ab′)2 and Fab (Fragment antigen binding) fragments, and 89Zr-labeled minibodies and diabodies with small animal SPECT or PET (Lütje et al., 2014c; Viola-Villegas et al., 2014). Continued interest in smaller antibody fragments and protein scaffolds is evidenced by several imaging studies with nanobodies (Bannas et al., 2014; De Vos et al., 2014; Massa et al., 2014) and affibodies (Kim et al., 2014; Strand et al., 2014; Zielinski et al., 2012). Nanobodies (~15kDa) are single-domain variable heavy chain (VHH) fragments, which are derived from heavy chain-only antibodies found in camelids (Schoonooghe et al., 2012), while affibodies (~7kDa) are based on Staphylococcus aureus protein A (Zielinski et al., 2012). The small sizes of these fragments enable them to bind to epitopes that antibody Fv-based fragments cannot access (Schoonooghe et al., 2012) and makes them suitable for applications in which extremely rapid clearance is desired.
The route of clearance can be influenced by the size, charge, and hydrophobicity/hydrophilicity of the fragment as well as any fused or conjugated moieties. Fragments with molecular weights below the renal threshold (~60 kDa) clear through the kidneys; larger fragments are eliminated through the liver. Thus, format selection depends upon the final imaging application (e.g. use of a renally-cleared probe if liver metastases are to be visualized). Half-lives of small scaffolds such as single-domain antibodies or affibodies can be extended by conjugation with polyethylene glycol (PEG) or fusion to albumin or an albumin-binding molecule. The addition of PEG to a conjugated label enabled imaging of liver metastases in mice with a fluorescently-labeled intact anti-carcinoembryonic antigen (CEA) antibody; a higher signal:background ratio was achieved in the liver compared to the fluorescent immunoconjugate alone (Maawy et al., 2014). A novel approach for controlling clearance and image contrast is offered by blocking the FcRn salvage receptor with Fc-engineered antibodies, or Abdegs. These IgG mutants have higher affinity for the FcRn recycling receptor than wildtype IgG, thus preventing other IgG – such as imaging probes - from binding FcRn. The net result is accelerated clearance and degradation of the imaging IgG. Swiercz et al. showed that an injection of Abdegs 8 hours after administration of 124I-pertuzumab decreased background and improved tumor-to-blood ratios. The authors also note that the faster clearance time of the labeled probe decreased the levels of radioactivity exposure, an important consideration in clinical practice (Swiercz et al., 2014; Ward et al., 2015).
Progress continues in modifying antibodies at specific amino acid residues to enable site-specific conjugation (Agarwal and Bertozzi, 2014). In contrast to conjugation at random surface residues, which produces a heterogeneous product and may introduce the label into the antigen-binding site (ABS), site-specific labeling creates a homogenous product in which the label can be intentionally located away from the ABS. A common approach is the addition of a cysteine residue at the carboxyl-terminus, which creates a site for specific labeling via a thio-ether bond (Massa et al., 2014; Tavaré et al., 2014a, 2014b). Alternatively, bio-orthogonally reactive unnatural amino acids, which are not naturally encoded in the genetic code but can still be incorporated artificially into proteins, can be inserted to provide specific sites for conjugation (Axup et al., 2012; Kim et al., 2013, 2012).
Conserved glycosylation sites in antibodies can be utilized for site-specific conjugation as well. Rochefort et al. took advantage of the N-glycosylation site in the CH2 domain by culturing anti-CA19-9 antibody-producing hybridoma cells with peracetylated N-azidoacetylmannosamine. The resulting antibody incorporated the azido-sugar at the N-glycosylation site and was subsequently conjugated via click chemistry to either phosphine-PEG-biotin or DyLight-650-phosphine, and showed specific targeting both in vitro and in vivo (Rochefort et al., 2014).
2.2. Labeling Strategies
Chemical conjugation is a critical step in attaching a detectable tracer to an antibody or fragment. Ideally, labeling should proceed quickly and with high efficiency, at established tracer:antibody ratios, and the immunoconjugate should be stable under in vivo conditions. Conjugation can be either direct (e.g., radionuclide or dye is attached directly to the antibody at certain amino acids) or indirect (e.g. radiometal labeling via attachment of a chelating moiety, such as 1,4,7,10-tetraazacyclododecane-N, N′, N″, N‴-tetraacetic acid; DOTA). Both direct and indirect labeling may lead to incorporation of the tracer at random sites on the protein, potentially impacting immunoreactivity if the ABS is modified. This can be addressed by engineering a specific site for tracer conjugation away from the ABS, as discussed above.
Background signal can be reduced by pretargeting, in which the antibody is modified so that it can capture a ligand-bound radioisotope or tag, injected only after the antibody has had time to pre-localize to its target in vivo. This approach can dramatically improve contrast and also reduces non-specific toxicity, a consideration when radioactive detection is employed (Zeglis et al., 2013). Alternatively, activatable optical tracers may be employed which are initially quenched, and only produce a signal when bound to their cognate ligand (Ogawa et al., 2009; Sano et al., 2013, 2012; Spring et al., 2014). The choice of conjugation strategy – site-specific or random - can alter the biodistribution and pharmacokinetics of the labeled antibody. In an immunoPET study, Tavaré et al. demonstrated that labeling an anti-ALCAM cys-diabody site-specifically with 64Cu using maleimide-DOTA resulted in increased tumor uptake and improved tumor-to-blood ratios compared to random labeling using N-hydroxysuccinimide-DOTA (Tavaré et al., 2014b). Conversely, antibodies can be used to alter the targeting and biodistribution of larger cargoes such as multifunctional nanoparticles. A bispecific fragment composed of anti-mPEG Fab and anti-EGFR or anti-HER2 scFv was combined with mPEG-conjugated nanoparticles carrying a fluorescent dye, an MRI contrast, or liposomal doxorubicin. The anti-mPEG portion of the fragment increased accumulation of the nanoparticles in tumors and enhanced optical or MRI tumor imaging as well as anti-tumor drug efficacy (Kao et al., 2014).
The use of click chemistry potentially simplifies conjugation of tracer molecules to imaging antibodies. Click chemistry reactions are modular and rapid, give high product yields and nontoxic byproducts, remain stable in physiological conditions and utilize benign solvents (Kolb et al., 2001). These reactions also enable modular assembly of antibody fragments with ease (Kim et al., 2012). The rapid reaction rates of several click chemistries (Rossin and Robillard, 2014) are especially advantageous for radionuclide labeling with short-lived isotopes. Furthermore, it is possible to conduct click reactions in vivo if the reactants are bio-orthogonal, with a pretargeting strategy. Zeglis et al. demonstrated pre-targeted in vivo labeling using A33 antibody modified with a trans-cyclooctene to enable an inverse-electron demand Diels-Adler reaction. Twelve hours after injection into mice with EGFR-expressing tumors, 64Cu-NOTA-tetrazine was administered for PET imaging and biodistribution studies. In comparison to A33 directly labeled with 64Cu or 89Zr, comparable uptakes and target:background ratios were obtained, but at much shorter times and reduced dose deposition (Zeglis et al., 2013). Evans et al. extended the concept by demonstrating pretargeted click labeling of cetuximab-trans-cyclooctene followed by 68Ga-DOTA-tetrazine administration. Pretargeting for 23 hours increased the tumor:liver ratio to 2.64, compared with a ratio of < 0.5 for free 68Ga-DOTA-tetrazine or 3 hour pretargeting (Evans et al., 2014).
2.3. Modality-Specific Tracers
The choice of imaging modality and tracer depends on a number of factors, including the required sensitivity, resolution, and whether quantitation and multiplexing are possible (James and Gambhir, 2012). For clinical applications, nuclear medicine-based imaging modalities (SPECT and PET), in which a scanner is used to detect gamma rays emitted by radionuclides attached to or incorporated into a tracer, are widely used. PET has higher spatial resolution and sensitivity than SPECT, but energy discrimination can be incorporated into single-photon imaging in order to detect photons with different energies, enabling multiplex detection of more than one target. An increasing number of radionuclides are commercially available, the choice of which depends upon the modality, the biomarker to be detected, the fragment to be used, and the physical characteristics of the radionuclide (Wu, 2011). For internalizing targets, a residualizing radiolabeling approach, where radioactive metabolites remain trapped inside the cell following internalization and metabolism, may be desirable to maximize activity accumulation in target cells; however, activity will also be retained in normal organs of clearance, increasing background. Many factors must be considered in the selection of the most appropriate radiolabel, as can be seen from studies that compare different radionuclides attached to the same probe. For instance, a recent study compared the use of 124I and 89Zr as labels for the A11 anti-PSCA (prostate stem cell antigen) minibody in a murine prostate cancer xenograft model. Although 89Zr-A11 demonstrated significantly higher PSCA-positive tumor uptake, activity also accumulated non-specifically in other organs, leading to increased background and lower contrast. 124I-A11 activity cleared quickly from nontumor tissue and, despite its comparatively lower accumulation in the tumor, had significantly higher tumor:blood contrast. Therefore, although PSCA is an internalizing target, 124I was determined to be the better choice of radionuclide for imaging in this model (Knowles et al., 2014b).
Ideally one wants to match the physical half-life of the radionuclide with the biological half-life of the antibody or fragment. Fluorine-18 is of great interest for same day imaging due to its high positron yield and favorable short half-life (109.8 min), which matches the fast clearance of smaller fragments such as scFvs, nanobodies and affibodies, but rapid labeling methods are essential. Methods using vinyl sulfone linker chemistry (Z. Wu et al., 2014) or aluminum fluoride (Lütje et al., 2014a; McBride et al., 2012) have decreased the time required for labeling from several hours to as little as 30 minutes, making 18F a viable option. For larger fragments such as intact antibodies and minibodies, 124I (t1/2 = 4.18 d) is better suited; however, conventional iodination yields a non-residualizing label and therefore is more appropriate for non-internalizing targets. 64Cu (t1/2 = 12.7 hr) and 89Zr (t1/2 = 78.4 hr) are well-matched for intermediate-sized fragments such as scFvs, diabodies, and minibodies, as well as intact antibodies. Use of 89Zr has dramatically expanded recently due to its half-life and ease of production; in addition, its positron emissions are lower energy than those of 124I, improving intrinsic resolution (van de Watering et al., 2014). 89Zr is usually attached indirectly to an antibody through conjugation of the chelator desferrioxamine (DFO) (van de Watering et al., 2014). However, 89Zr released from DFO accumulates in bone and can presents toxicity issues or obscure imaging of lesions in the bone. Efforts have been made to improve upon the chelating molecule (Deri et al., 2014; Guérard et al., 2014, 2013; Price et al., 2014). Scandium-44 (t1/2 = 3.9 h) continues to be explored in conjunction with intermediate-sized fragments such as Fabs (Chakravarty et al., 2014), and gallium-68 (t1/2 = 68 min) is gaining popularity as a label for smaller fragments such as nanobodies and affibodies (Honarvar et al., 2014; Xavier et al., 2013). 99mTc (t1/2 = 6.0 hr) continues to be the most commonly used SPECT radionuclide for a variety of uses in the clinic including conventional bone scans. Its broad availability has enabled numerous groups to label antibodies with 99mTc for the detection of varied biomarkers; recent examples include anti-VCAM-1 for inflamed atherosclerotic lesions in mice (Broisat et al., 2014), anti-RAGE F(ab′)2 for atherosclerotic plaques in pigs (Johnson et al., 2014), and anti-CD11b for myeloid-derived suppressor cells in colon cancer (Cheng et al., 2014).
Optical imaging provides an important alternative for molecularly-targeted imaging since it avoids the use of ionizing radiation (James and Gambhir, 2012). However, optical imaging with fluorescently labeled probes including antibodies has been more limited in application due to tissue scatter and absorption of photons in the visible range (400–700 nm) and background signals from autofluorescence (Suemizu et al., 2013). As a result, potential clinical uses of antibody-based optical imaging tracers are generally restricted to the visualization of superficial lesions (<1cm depth) but numerous applications have been demonstrated in endoscopy, laparoscopy, and the intraoperative setting. Targeted fluorescence imaging with IRDye 800CW conjugated to one of two mAb probes (anti-VEGF or –HER2) was recently demonstrated for intraoperative detection of disseminated intraperitoneal metastases in mouse models (Terwisscha van Scheltinga et al., 2011). Furthermore, newer dyes that emit in the near-infrared (NIR) region extend the utility of fluorescence detection since NIR photons (700–1000 nm) penetrate further through tissue and autofluorescence in vivo is significantly reduced. Maawy et al. compared depth of imaging, resolution, tumor:background ratio, photobleaching, and hemoglobin quenching of both visible and near-infrared dyes for imaging pancreatic cancer with an anti-CEA antibody (Maawy et al., 2013). In another study, affibody-DyLight-750 conjugates were employed to image HER2 expression levels in breast cancer xenografts in mice (Zielinski et al., 2012). Figure 4 shows the use of a NIR-labeled anti-PSCA diabody to assist resection of an intramuscular prostate cancer xenograft in mice (Behesnilian et al., 2015). Multichannel optical imaging can detect more than one biomarker simultaneously, potentially improving accuracy of detection; in one study, antibodies recognizing carcinoembryonic antigen (CEA), matrix metalloproteinase (MMP) 9, and MMP 14 (all markers of cancer progression) were conjugated to different wavelength quantum dots (Park et al., 2014). In a murine colon cancer model, when sprayed intrarectally, the MMP probes identified lesions more accurately than the CEA probe, illustrating the value of using multiple markers to diagnose disease.
Figure 4. Fluorescence-guided resection of tumors.
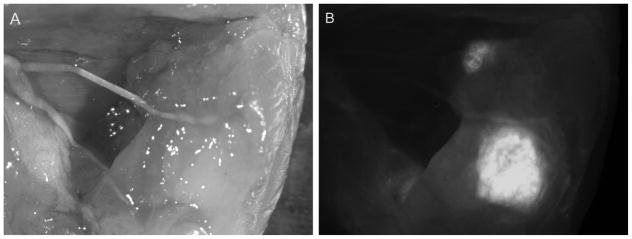
By probing with a fluorescently-labeled antibody for a tumor marker, surgeons can immediately determine whether a tumor has been fully resected, and identify local metastases that may not be visible by eye. Here, a far-red fluorescent dye (Cy5)-labeled anti-PSCA diabody was used to guide resection of an intramuscular 22rv1-PSCA prostate cancer xenograft in a murine model. Cy5-anti-PSCA diabody was injected intravenously and surgery was performed 6 hours post-injection. (A) A white-light image of the tumor bed post-resection, with residual tumor purposely left unresected. (B) Fluorescence imaging shows residual tumor clearly. (Behesnilian et al., 2015)
Antibodies have also been employed to impart specificity to magnetic resonance imaging and ultrasound imaging, although thus far, applications have been limited to preclinical settings. Targeted MRI requires the conjugation of contrast agents such as gadolinium (Gd)-complexes or superparamagnetic iron oxide (SPIO) nanoparticles to a specific probe (James and Gambhir, 2012). By attaching SPIOs to anti-CD80 and anti-CD206 antibodies, Al Faraj et al. were able to track macrophage M1 and M2 subsets, respectively, in a mouse model of chronic obstructive pulmonary disease (Al Faraj et al., 2014). The sensitivity of US can be improved by attaching antibodies to microbubbles (James and Gambhir, 2012) or carbon nanotubes in order to provide targeted contrast. In a murine model of ovarian cancer, endothelial cells associated with ovarian cancer were imaged using anti-CD276 targeted microbubbles (Lutz et al., 2014). Antibody-conjugated multiwalled carbon nanotubes were employed as both contrast agents and a drug delivery system by loading doxycycline onto a carbon nanotube conjugated with anti-PSCA antibodies for imaging and treatment in prostate cancer xenograft models (H. Wu et al., 2014).
Indeed, antibodies are particularly suited to the development of multi-modal and multifunctional imaging and theranostic agents (see Section 3.1), due to the ability to conjugate a variety of cargoes to larger proteins with minimal or manageable impact on pharmacokinetics, biodistribution, and clearance, as discussed earlier. Recent examples include dual-labeled antibodies such as probes (anti-EpCAM, anti-CEA, anti-PSMA) with dual PET and optical imaging functions, for imaging prostate cancer pre- and intra-surgery, and set the stage for clinical translation (Figure 5) (Hall et al., 2012; Lütje et al., 2014b; Rijpkema et al., 2014).
Figure 5. Dual-modality antibody imaging.
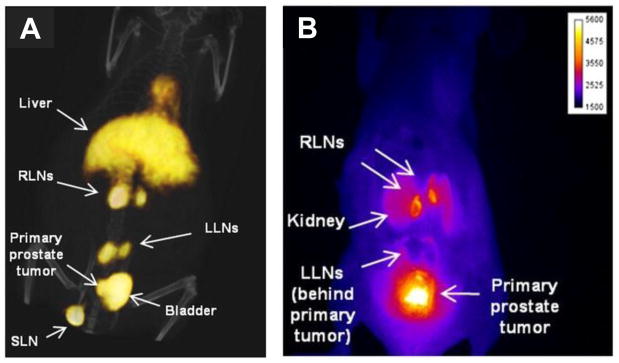
Non-invasive staging of lymph nodes (LN) followed by image-guided resection of only cancer-positive LNs offers more precision in identifying and removing metastases. Hall et al. used a dual-labeled anti-EpCAM mAb, conjugated with both IRDye 800CW and 64Cu-DOTA, to image prostate cancer LN metastases with PET/CT (A) and NIR fluorescence (B) imaging. PC3 cells were implanted in the prostate and imaging was performed 10–12 weeks later. Lumbar LNs (LLNs); renal LNs (RLNs); sciatic LN (SLN). This research was originally published in JNM. Hall MA et al. J Nucl Med. 2012;53:1427–37. © by the Society of Nuclear Medicine and Molecular Imaging, Inc.
3. Applications of Antibody-Based Imaging
3.1. Diagnostics and Theranostics
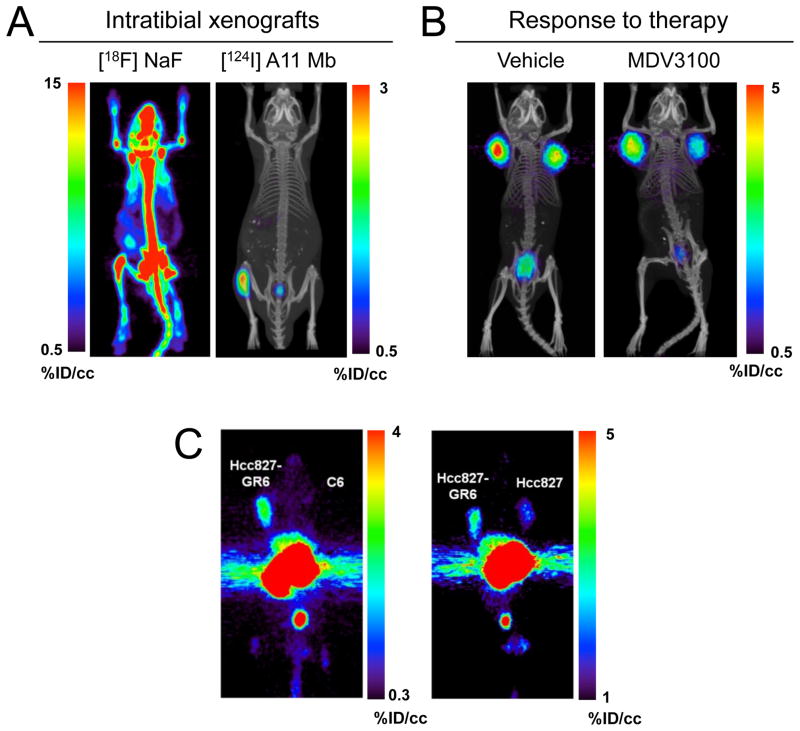
The precision with which antibodies can identify their targets has stimulated broad development of applications, particularly in oncology. Current mainstream diagnostic imaging approaches, while practical and effective, still leave room for improvement. For example, PET using 18F-fluorodeoxyglucose has proved invaluable for the detection of elevated glycolysis which is a hallmark of malignant transformation, but suffers from false negatives (from tumors with more indolent growth, or reliance on other metabolic pathways) or false positives (e.g. from infection or inflammation). 99mTc-MDP bone scintigraphy is a workhorse method for detecting metastases based on bone remodeling. However, it is non-specific: other biological processes (fractures, arthritis, inflammation) also generate positive signals, and soft tissue lesions are not addressable. Knowles et al. recently compared PET imaging using 18F-NaF bone scans to a tumor-specific 124I-PSCA minibody for detecting intratibial prostate cancer in a mouse model (Knowles et al., 2014a), illustrating the significant advantages of targeting a tumor-specific marker in this setting (Figure 6).
Figure 6. Applications of antibody imaging in oncology.
Imaging of specific biomarkers can be used to accurately locate lesions, aid in choosing a treatment, and monitor response to therapy. (A) PET imaging using the 124I-anti-PSCA minibody A11 was able to detect intratibial LAPC-9 prostate cancer xenografts with higher sensitivity and specificity than a 18F-Fluoride bone scan. (B) Response to anti-androgen treatment was monitored by visualizing reduced 124I-A11 activity in the tumors of enzalutamide-treated mice, reflecting a therapy-induced downregulation of PSCA expression. (C) The Hcc827-GR6 non-small cell lung cancer line developed resistance to gefitinib through upregulation of MET expression. PET imaging MET using a 89Zr-DFO-labeled MET-specific human minibody clearly visualized the overexpression of MET in the gefitinib-resistant tumors. (Knowles et al., 2014a; Li et al., 2014)
Beyond biomarker-specific visualization of disease there is great interest in developing antibodies as theranostic agents which act as both diagnostic and therapeutic molecules (Fleuren et al., 2014). Theranostic uses of antibodies may be particularly pertinent in oncology, where distinct alterations can be exploited for molecularly-targeted therapy. In vivo imaging using antibodies provides a versatile approach for identification and characterization of tumors for diagnosis, staging and disease management. Non-invasive detection and quantitation of biomarkers is increasingly needed for profiling disease in specific patients, and may be essential for addressing heterogeneity in vivo. Furthermore, therapeutic antibodies themselves can be directly labeled in order to assess drug delivery and target saturation. In preclinical models, including breast cancer models expressing different levels of HER2 (Zielinski et al., 2012) and lung, pancreatic, and prostate cancer xenograft models expressing differential Axl levels (Liu et al., 2014; Nimmagadda et al., 2014), antibody-directed PET and optical imaging have demonstrated that graded expression can be visualized. Antibody-based imaging provides a direct method for imaging response to therapy, and imaging cell-surface markers can provide insight into biological mechanisms of response. Recent examples include imaging EGFR downregulation to provide a measure of trastuzumab efficacy in HER2/EGFR-expressing tumors (Ma et al., 2014). A similar strategy was used to detect response to the mTOR inhibitor rapalog in renal cancer xenografts by imaging expression of VEGF, a downstream marker of mTOR activation (Chang et al., 2013). Knowles et al. employed the 124I-PSCA-specific minibody to evaluate response to enzalutamide treatment in a prostate cancer model, demonstrating downregulation of the androgen signaling axis in vivo (Figure 6). Ogasawara et al. demonstrated that antibodies specific for phosphatidylserine, which is transiently expressed on the cell surface during apoptosis, can be used to assess cell death in response to treatment; the long serum half-life of intact anti-phosphatidylserine antibodies proved advantageous for detection of the wave of apoptosis that occurred after delivery of pro-apoptotic therapy (Ogasawara et al., 2013). Antibody-directed imaging might also provide a method for assessing the development of resistance to therapy. Li et al. recently used engineered minibodies and diabodies derived from a phage display anti-MET antibody to image elevated MET in non-small cell lung cancer xenografts from cell lines resistant to EGFR-targeted therapy via overexpression of MET (Figure 6) (Li et al., 2014).
Quantitative imaging has always played a central role in radioimmunotherapy, for assessing whether effective doses can be delivered to target tissues, while minimizing toxicity in normal tissues. PET imaging using 124I-radretumab was used to estimate the dose delivered to the blood, bone marrow, and tumor in patients with brain metastases in advance of using 131I-radretumab for radioimmunotherapy (Poli et al., 2013). Antibody uptake varied even between lesions from the same patient. A recent pretargeting implementation utilized an antibody bispecific for TROP-2 (expressed at low levels in some glandular cells, and upregulated in several epithelial cancers) and the peptide hapten histamine-succinyl-glycine (HSG) to target prostate cancer xenografts in mice. Preliminary biodistribution studies using radiolabeled 111In-di-HSG were conducted in order to establish optimal dose and timing. ImmunoPET with 68Ga-di-HSG to visualize the tumors was followed by radioimmunotherapy using 177Lu-di-HSG. Compared to direct radioimmunotherapy with anti-TROP-2 mAb (177Lu-hRS7), the pretargeting strategy increased median survival, with no significant renal or hematological toxicity compared to intact antibody (van Rij et al., 2013).
Photoimmunotherapy uses antibodies to deliver a photosensitizing molecule to target tissues, followed by excitation with low-energy light and production of reactive oxygen species toxic to surrounding cells. Anti-CEA photoimmunotherapy inhibited tumor growth in a xenograft model of gastric carcinoma (Shirasu et al., 2014). Efficacy may be improved in photoimmunotherapy, and perhaps other theranostic applications as well, by delivering a cocktail of antibody conjugates recognizing more than one antigen of interest. Combining antibodies recognizing both CD25 and EGFR resulted in greater tumor cell death in vivo than with either antibody alone (Nakajima et al., 2013). The authors note that this may be due to the “binding site barrier”, in which highly expressed antigen on the periphery of a tumor binds most of the antibody and prevents any from reaching the interior of the tumor. By using both antibodies for a highly expressed antigen (EGFR) and a low-expression antigen (CD25), greater penetration was possible (Nakajima et al., 2013).
3.3. Immunological Applications
An important application of molecular imaging is the detection and tracking of immune cells in vivo. Non-invasive imaging based on cell surface biomarkers can provide valuable information about the localization and migration of immune cells, in an inherently dynamic system. Systemic in vivo imaging presents an exciting solution to this problem, extending the temporal and spatial nature of information that can be collected. Importantly, tracking immune cell subsets, key to differential immune responses, could provide in vivo insight into diagnosis, mechanisms, and treatment of inflammation, infection, autoimmunity, and other diseases. For example, inflammation accompanies the development of many malignancies. Tracking systemic cell localization would be especially advantageous in cell-based immunotherapy, wherein it is critical that modified immune cells successfully traffic to and reside in tumors. Immunodulatory treatments (such as checkpoint inhibitor antibodies targeting CTLA-4 or PD-1/PD-L1 interactions) are dependent on the presence of appropriate immune cell subsets (such as CD8+ T lymphocytes). Immune system reconstitution following a hematopoietic stem cell transplant could be non-invasively monitored, as could graft rejection and host-vs-graft disease after organ transplantation.
To date, several non-antibody-based methodologies have been used to track immune cells throughout the body, including ex vivo labeling of immune cells with radionuclides for PET/SPECT imaging, targeted MRI, reporter gene technologies, nanoparticles, and intravital two-photon microscopy (Ahrens and Bulte, 2013; Mandl et al., 2012; Nair-Gill et al., 2008; Weissleder et al., 2014). Currently, the only FDA-approved method for cell tracking in humans is SPECT imaging of ex vivo 111In-oxine-radiolabeled white blood cells (Ahrens and Bulte, 2013). This method is non-specific and complex, as it requires the removal of leukocytes from the patient followed by labeling, reinfusion, and scanning. The probe also becomes diluted over time as cells divide (Nair-Gill et al., 2008). Reporter gene technology, in which cells are engineered to produce a protein marker that is the target of an imaging probe, is advantageous in that only cells that are genetically modified will produce a signal. The major limitation is that it requires genetic modification and thus is an invasive procedure (Nair-Gill et al., 2008). Nanoparticle-based imaging has been of particular interest for imaging macrophages due to their high endocytic activity (Weissleder et al., 2014), and intravital imaging has shown promise in preclinical models (Germain et al., 2012), but both could be significantly enhanced by incorporating antibodies in order to add specificity.
Preclinical imaging studies using antibodies for several types of immune cells have been reported. For SPECT imaging of myeloid-specific suppressor cells, which are markers of the inflammatory microenvironment in tumors, Cheng et al. used a 99mTc-labeled anti-CD11b antibody (Cheng et al., 2014). The presence of tumor-infiltrating T cells in subcutaneous colon tumors was analyzed with PET using a 64Cu-labeled anti-CTLA-4 mAb antibody (Higashikawa et al., 2014), and CD8+ T cells were imaged systemically with a 89Zr-labeled minibody (Figure 7) (Tavaré et al., 2014a). Griessinger et al. used the internalization of the T cell receptor complex to their advantage so that they could stably label T cells for PET imaging (Griessinger et al., 2015). In a study of eosinophil recruitment to the airways during asthma, optical imaging with AlexaFluor-labeled anti-Siglec-F antibody showed accumulation of Siglec-F-expressing eosinophils in the lungs of affected mice (Markus et al., 2014). Imaging of B cells was conducted in a huCD20 transgenic mouse model using 64Cu-labeled rituximab (Natarajan et al., 2012). Natural killer cells labeled ex vivo with quantum dot-conjugated anti-CD56 were injected intratumorally into mice and imaged to monitor their presence in the tumor (Lim et al., 2009).
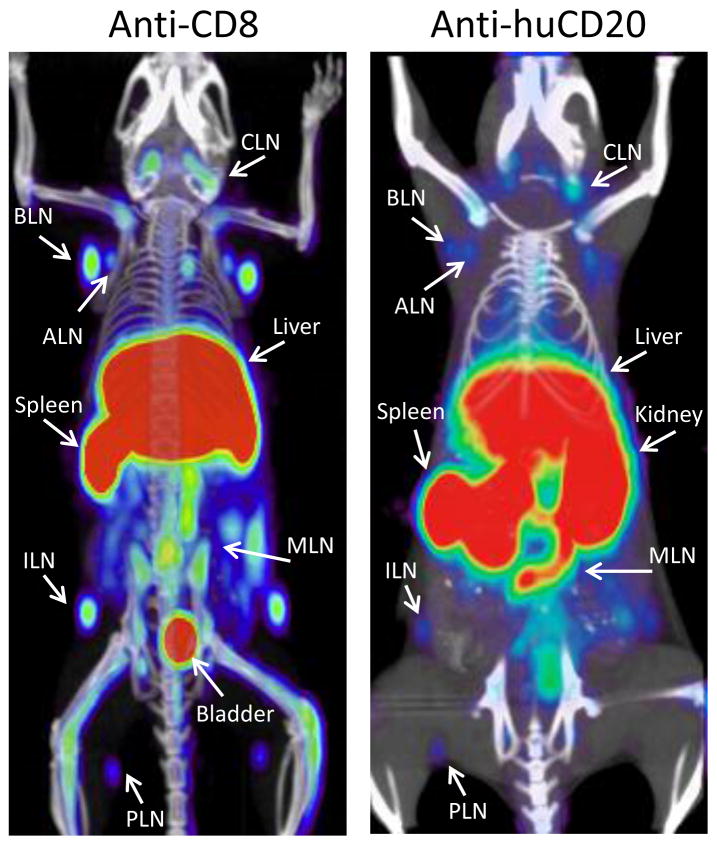
Figure 7. Imaging immune cell subsets.
Tracking CD8+ T cells could help detect and stage CD8+ lymphomas and aid in monitoring T cell immunotherapy. Here, imaging of CD8+ T cells with a 64Cu-NOTA-anti-CD8 minibody in wild type mice visualizes the spleen and lymph nodes (left panel). 89Zr-DFO-anti-huCD20 cys-minibody imaging of a transgenic mouse expressing huCD20 reveals B cells in the spleen and lymph nodes (right panel); this could also be applied to for the detection of B-cell lymphomas. (Tavaré et al., 2014a; Zettlitz et al., 2013)
Antibodies and fragments have been used in humans to study several autoimmune diseases. In a phase I proof of principle study using 99mTc-labeled anti-CD4-Fab to image CD4+ T cells in patients with rheumatoid arthritis, 70% of clinically affected (painful or swollen) joints were positive on the scan (Steinhoff et al., 2014). The authors suggest that the low detection rate may be due to non-correlation between symptoms and presence of CD4+ T cells in the synovial joints, and that other cells may be involved in joint inflammation. Even so, this represents a potentially useful diagnostic application. In Crohn’s disease, current treatments, including the antibody adalimumab, aim to neutralize the proinflammatory cytokine tumor necrosis factor alpha (TNFα), but only 50% of patients respond to adalimumab and those that do not respond are exposed to potentially harmful side effects (Atreya et al., 2014). Atreya et al. addressed this need to stratify patients by using fluorescently labeled adalimumab applied topically to inflamed regions of the bowel during a colonoscopy, followed by confocal laser endomicroscopy to quantify fluorescence. The response rate to subsequent adalimumab was correlated with the expression of TNFα. Results suggested that prescreening with fluorescently-labeled antibodies can successfully segregate patients into responders and nonresponders (Atreya et al., 2014).
4. Conclusion
Interest and progress in antibody-targeted imaging is accelerating, supported by a push from the therapeutic antibody field (e.g. underlying work on target selection and validation, humanized and human antibodies, and production for clinical and commercial use) as well as a pull from medical fields, where there are still significant unmet needs in molecular imaging. In parallel, developments in imaging instrumentation and novel molecular probes are providing innovative new applications employing PET, SPECT, MRI and US to provide highly specific information on biological processes in vivo, in both preclinical models and patients. With regard to clinical translation, radiolabeled antibody probes provide the most straightforward path, given the existing imaging infrastructure in nuclear medicine departments and established procedures for clinical production, radiolabeling, and regulatory review, as well as increased availability of long half-life PET radionuclides such as 89Zr. Regarding clinical uses of optical imaging, applications in optical surgical navigation (including robotic and laparascopic) and endoscopy hold promise. Antibody targeted micro-and nano-particles (for MRI, US, and other applications) are particularly relevant for intravascular targets; however, numerous hurdles remain in accessing tissue- and tumor-specific targets and significant additional work needs to be done prior to effective clinical translation (Prabhakar et al., 2013). Nonetheless, antibody-based biomarker imaging approaches are highly complementary to in vitro diagnostics, and should play an increasingly important role in providing essential information needed to effectively guide the development and implementation of molecularly targeted therapeutics as we move into the era of precision medicine.
Highlights.
Antibodies’ exquisite specificity enables targeted imaging of single biomarkers and cell types
Antibody-based imaging has proven successful in multiple preclinical models
Success with antibodies as therapeutics makes them highly translatable as imaging agents
Non-invasive, whole-body imaging is becoming a critical tool in the study of immunology
Acknowledgments
The authors wish to thank the following for sharing their unpublished work: Dr. Kirstin Zettlitz, Crump Institute for Molecular Imaging, Department of Molecular and Medical Pharmacology at UCLA and Dr. John Timmerman, Department of Medicine at the David Geffen School of Medicine at UCLA for the huCD20 transgenic mouse image; and Dr. Zettlitz and Drs. Andrew Behesnilian, Ziyue Jiang, and Robert Reiter, Department of Urology at the David Geffen School of Medicine at UCLA for the fluorescence guided surgery using anti-PSCA diabody. Funding was provided by NIH grants P30 CA092131, R01 CA174294, R21 AI114255, R21 CA190044, the Stanford Center for Cancer Excellence and Translation (U54 CA151459) and the UCLA Jonsson Comprehensive Cancer Center (P30 CA016042). Anna M. Wu is a shareholder and consultant to ImaginAb, Inc., and the Regents of the University of California have licensed technology to ImaginAb.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Works Cited
- Agarwal P, Bertozzi CR. Site-specific antibody-drug conjugates: the nexus of bioorthogonal chemistry, protein engineering, and drug development. Bioconjug Chem. 2015 doi: 10.1021/bc5004982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahrens ET, Bulte JWM. Tracking immune cells in vivo using magnetic resonance imaging. Nat Rev Immunol. 2013;13:755–63. doi: 10.1038/nri3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Faraj A, Shaik AS, Afzal S, Al Sayed B, Halwani R. MR imaging and targeting of a specific alveolar macrophage subpopulation in LPS-induced COPD animal model using antibody-conjugated magnetic nanoparticles. Int J Nanomedicine. 2014;9:1491–503. doi: 10.2147/IJN.S59394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atreya R, Neumann H, Neufert C, Waldner MJ, Billmeier U, Zopf Y, Willma M, App C, Münster T, Kessler H, Maas S, Gebhardt B, Heimke-Brinck R, Reuter E, Dörje F, Rau TT, Uter W, Wang TD, Kiesslich R, Vieth M, Hannappel E, Neurath MF. In vivo imaging using fluorescent antibodies to tumor necrosis factor predicts therapeutic response in Crohn’s disease. Nat Med. 2014;20:313–8. doi: 10.1038/nm.3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axup JY, Bajjuri KM, Ritland M, Hutchins BM, Kim CH, Kazane SA, Halder R, Forsyth JS, Santidrian AF, Stafin K, Lu Y, Tran H, Seller AJ, Biroc SL, Szydlik A, Pinkstaff JK, Tian F, Sinha SC, Felding-Habermann B, Smider VV, Schultz PG. Synthesis of site-specific antibody-drug conjugates using unnatural amino acids. Proc Natl Acad Sci USA. 2012;109:16101–6. doi: 10.1073/pnas.1211023109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannas P, Well L, Lenz A, Rissiek B, Haag F, Schmid J, Hochgräfe K, Trepel M, Adam G, Ittrich H, Koch-Nolte F. In vivo near-infrared fluorescence targeting of T cells: comparison of nanobodies and conventional monoclonal antibodies. Contrast Media Mol Imaging. 2014;9:135–42. doi: 10.1002/cmmi.1548. [DOI] [PubMed] [Google Scholar]
- Behesnilian AS, Jiang ZK, Sonn G, Zettlitz KA, Wu AM, Reiter RE. Real-time fluorescence-guided surgery of prostate cancer xenografts with anti-prostate stem cell antigen diabody. J Nucl Med. 2015;56(suppl):2A. doi: 10.1158/1078-0432.CCR-15-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broisat A, Toczek J, Dumas LS, Ahmadi M, Bacot S, Perret P, Slimani L, Barone-Rochette G, Soubies A, Devoogdt N, Lahoutte T, Fagret D, Riou LM, Ghezzi C. 99mTc-cAbVCAM1-5 imaging is a sensitive and reproducible tool for the detection of inflamed atherosclerotic lesions in mice. J Nucl Med. 2014;55:1678–84. doi: 10.2967/jnumed.114.143792. [DOI] [PubMed] [Google Scholar]
- Chakravarty R, Goel S, Valdovinos HF, Hernandez R, Hong H, Nickles RJ, Cai W. Matching the decay half-life with the biological half-life: immunoPET imaging with 44Sc-labeled Cetuximab Fab fragment. Bioconjug Chem. 2014;25:2197–2204. doi: 10.1021/bc500415x. doi:dx.doi.org/10.1021/bc500415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang AJ, Sohn R, Lu ZH, Arbeit JM, Lapi SE. Detection of rapalog-mediated therapeutic response in renal cancer xenografts using 64Cu-bevacizumab immunoPET. PLoS One. 2013;8:e58949. doi: 10.1371/journal.pone.0058949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng D, Zou W, Li X, Xiu Y, Tan H, Shi H, Yang X. Preparation and evaluation of 99mTc-labeled anti-CD11b antibody targeting inflammatory microenvironment for colon cancer imaging. Chem Biol Drug Des. 2014 doi: 10.1111/cbdd.12459. [DOI] [PubMed] [Google Scholar]
- De Vos J, Mathijs I, Xavier C, Massa S, Wernery U, Bouwens L, Lahoutte T, Muyldermans S, Devoogdt N. Specific targeting of atherosclerotic plaques in ApoE−/− mice using a new camelid sdAb binding the vulnerable plaque marker LOX-1. Mol Imaging Biol. 2014;16:690–8. doi: 10.1007/s11307-014-0731-6. [DOI] [PubMed] [Google Scholar]
- Deri MAM, Ponnala S, Zeglis BMB, Pohl G, Dannenberg JJ, Lewis JS, Francesconi LC. Alternative chelator for 89Zr radiopharmaceuticals: radiolabeling and evaluation of 3,4,3-(LI-1,2-HOPO) J Med Chem. 2014;57:4849–4860. doi: 10.1021/jm500389b. doi:dx.doi.org/10.1021/jm500389b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans H, Nguyen Q, Carroll LS, Kaliszczak M, Twyman FJ, Spivey AC, Aboagye EO. A bioorthogonal 68Ga-labelling strategy for rapid in vivo imaging. Chem Commun. 2014;50:9557–60. doi: 10.1039/c4cc03903c. [DOI] [PubMed] [Google Scholar]
- Fleuren EDG, Versleijen-Jonkers YMH, Heskamp S, van Herpen CML, Oyen WJG, van der Graaf WTA, Boerman OC, Van Herpen CML, Van Der Graaf WTA. Theranostic applications of antibodies in oncology. Mol Oncol. 2014;8:799–812. doi: 10.1016/j.molonc.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain RN, Robey EA, Cahalan MD. A decade of imaging cellular motility and interaction dynamics in the immune system. Science. 2012;336:1676–81. doi: 10.1126/science.1221063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griessinger CM, Maurer A, Kesenheimer C, Kehlbach R, Reischl G, Ehrlichmann W, Bukala D, Harant M, Cay F, Brück J, Nordin R, Kohlhofer U, Rammensee H-G, Quintanilla-Martinez L, Schaller M, Röcken M, Pichler BJ, Kneilling M. 64Cu antibody-targeting of the T-cell receptor and subsequent internalization enables in vivo tracking of lymphocytes by PET. Proc Natl Acad Sci USA. 2015;112 doi: 10.1073/pnas.1418391112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guérard F, Lee YS, Brechbiel MW. Rational design, synthesis, and evaluation of tetrahydroxamic acid chelators for stable complexation of zirconium(IV) Chem Eur J. 2014;20:5584–91. doi: 10.1002/chem.201304115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guérard F, Lee YS, Tripier R, Szajek LP, Deschamps JR, Brechbiel MW. Investigation of Zr(IV) and 89Zr(IV) complexation with hydroxamates: progress towards designing a better chelator than desferrioxamine B for immuno-PET imaging. Chem Commun. 2013;49:1002–1004. doi: 10.1039/c2cc37549d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MA, Pinkston KL, Wilganowski N, Robinson H, Ghosh P, Azhdarinia A, Vazquez-Arreguin K, Kolonin AM, Harvey BR, Sevick-Muraca EM. Comparison of mAbs targeting epithelial cell adhesion molecule for the detection of prostate cancer lymph node metastases with multimodal contrast agents: quantitative small-animal PET/CT and NIRF. J Nucl Med. 2012;53:1427–37. doi: 10.2967/jnumed.112.106302. [DOI] [PubMed] [Google Scholar]
- Higashikawa K, Yagi K, Watanabe K, Kamino S, Ueda M, Hiromura M, Enomoto S. 64Cu-DOTA-anti-CTLA-4 mAb enabled PET visualization of CTLA-4 on the T-cell infiltrating tumor tissues. PLoS One. 2014;9:e109866. doi: 10.1371/journal.pone.0109866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honarvar H, Strand J, Perols A, Orlova A, Selvaraju RK, Karlström AE, Tolmachev V. Position for Site-Specific Attachment of a DOTA Chelator to Synthetic Affibody Molecules Has a Different Influence on the Targeting Properties of 68Ga-Compared to 111In-Labeled Conjugates. Mol Imaging. 2014;13:1–12. doi: 10.2310/7290.2014.00034. [DOI] [PubMed] [Google Scholar]
- James ML, Gambhir SS. A molecular imaging primer: modalities, imaging agents, and applications. Physiol Rev. 2012;92:897–965. doi: 10.1152/physrev.00049.2010. [DOI] [PubMed] [Google Scholar]
- Johnson LL, Tekabe Y, Kollaros M, Eng G, Bhatia K, Li C, Krueger CG, Shanmuganayagam D, Schmidt AM. Imaging RAGE expression in atherosclerotic plaques in hyperlipidemic pigs. EJNMMI Res. 2014;4:26. doi: 10.1186/s13550-014-0026-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao CH, Wang JY, Chuang KH, Chuang CH, Cheng TLTC, Hsieh YC, Tseng YL, Chen BM, Roffler SR. One-step mixing with humanized anti-mPEG bispecific antibody enhances tumor accumulation and therapeutic efficacy of mPEGylated nanoparticles. Biomaterials. 2014;35:9930–40. doi: 10.1016/j.biomaterials.2014.08.032. [DOI] [PubMed] [Google Scholar]
- Kim CH, Axup JY, Dubrovska A, Kazane SA, Hutchins BA, Wold ED, Smider VV, Schultz PG. Synthesis of bispecific antibodies using genetically encoded unnatural amino acids. JACS. 2012;134:9918–21. doi: 10.1021/ja303904e. doi:dx.doi.org/10.1021/ja303904e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH, Axup JY, Schultz PG. Protein conjugation with genetically encoded unnatural amino acids. Curr Opin Chem Biol. 2013;17:412–9. doi: 10.1016/j.cbpa.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HY, Wang X, Wahlberg B, Edwards WB. Discovery of hapten-specific scFv from a phage display library and applications for HER2-positive tumor imaging. Bioconjug Chem. 2014;25:1311–22. doi: 10.1021/bc500173f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles SM, Tavare R, Zettlitz KA, Rochefort MM, Salazar FB, Jiang ZK, Reiter RE, Wu AM. Applications of immunoPET: using 124I-anti-PSCA A11 minibody for imaging disease progression and response to therapy in mouse xenograft models of prostate cancer. Clin Cancer Res. 2014a;20:6367–6378. doi: 10.1158/1078-0432.CCR-14-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles SM, Wu AM. Advances in immuno-positron emission tomography: antibodies for molecular imaging in oncology. J Clin Oncol. 2012;30:3884–92. doi: 10.1200/JCO.2012.42.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles SM, Zettlitz KA, Tavaré R, Rochefort MM, Salazar FB, Stout DB, Yazaki PJ, Reiter RE, Wu AM. Quantitative immunopet of prostate cancer xenografts with 89Zr- and 124I-labeled anti-PSCA A11 minibody. J Nucl Med. 2014b;55:452–9. doi: 10.2967/jnumed.113.120873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb HC, Finn MG, Sharpless KB. Click chemistry: diverse chemical function from a few good reactions. Angew Chemie. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Li K, Tavaré R, Zettlitz KA, Mumenthaler SM, Mallick P, Zhou Y, Marks JD, Wu AM. Anti-MET immunoPET for non-small cell lung cancer using fully human antibody fragments. Mol Cancer Ther. 2014;13:2607–17. doi: 10.1158/1535-7163.MCT-14-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim YT, Cho MY, Noh YW, Chung JW, Chung BH. Near-infrared emitting fluorescent nanocrystals-labeled natural killer cells as a platform technology for the optical imaging of immunotherapeutic cells-based cancer therapy. Nanotechnology. 2009;20:475102. doi: 10.1088/0957-4484/20/47/475102. [DOI] [PubMed] [Google Scholar]
- Liu S, Li D, Guo J, Canale N, Li X, Liu R, Krasnoperov V, Gill PS, Conti PS, Shan H, Li Z. Design, synthesis and validation of Axl-targeted monoclonal antibody probe for microPET imaging in human lung cancer xenograft. Mol Pharm. 2014;11:3974–9. doi: 10.1021/mp500307t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lütje S, Franssen GM, Sharkey RM, Laverman P, Rossi EA, Goldenberg DM, Oyen WJG, Boerman OC, McBride WJ. Anti-CEA antibody fragments labeled with [18F]AlF for PET imaging of CEA-expressing tumors. Bioconjug Chem. 2014a;25:335–41. doi: 10.1021/bc4004926. [DOI] [PubMed] [Google Scholar]
- Lütje S, Rijpkema M, Franssen GM, Fracasso G, Helfrich W, Eek A, Oyen WJ, Colombatti M, Boerman OC. Dual-modality image-guided surgery of prostate cancer with a radiolabeled fluorescent anti-PSMA monoclonal antibody. J Nucl Med. 2014b;55:995–1001. doi: 10.2967/jnumed.114.138180. [DOI] [PubMed] [Google Scholar]
- Lütje S, van Rij CM, Franssen GM, Fracasso G, Helfrich W, Eek A, Oyen WJ, Colombatti M, Boerman OC. Targeting human prostate cancer with 111In-labeled D2B IgG, F(ab′)2 and Fab fragments in nude mice with PSMA-expressing xenografts. Contrast Media Mol Imaging. 2014c doi: 10.1002/cmmi.1596. [DOI] [PubMed] [Google Scholar]
- Lutz AM, Bachawal SV, Drescher CW, Pysz MA, Willmann JK, Gambhir SS. Ultrasound molecular imaging in a human CD276 expression-modulated murine ovarian cancer model. Clin Cancer Res. 2014;20:1313–22. doi: 10.1158/1078-0432.CCR-13-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T, Sun X, Cui L, Gao L, Wu Y, Liu H, Zhu Z, Wang F, Liu Z. Molecular imaging reveals trastuzumab-induced epidermal growth factor receptor downregulation in vivo. J Nucl Med. 2014;55:1002–1007. doi: 10.2967/jnumed.114.137000. [DOI] [PubMed] [Google Scholar]
- Maawy AA, Hiroshima Y, Kaushal S, Luiken GA, Hoffman RM, Bouvet M. Comparison of a chimeric anti-carcinoembryonic antigen antibody conjugated with visible or near-infrared fluorescent dyes for imaging pancreatic cancer in orthotopic nude mouse models. J Biomed Opt. 2013;18:126016. doi: 10.1117/1.JBO.18.12.126016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maawy AA, Hiroshima Y, Zhang Y, Luiken GA, Hoffman RM, Bouvet M. Polyethylene glycol (PEG) linked to near infrared (NIR) dyes conjugated to chimeric anti-carcinoembryonic antigen (CEA) antibody enhances imaging of liver metastases in a nude-mouse model of human colon cancer. PLoS One. 2014;9:e97965. doi: 10.1371/journal.pone.0097965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandl JN, Liou R, Klauschen F, Vrisekoop N, Monteiro JP, Yates AJ, Huang AY, Germain RN. Quantification of lymph node transit times reveals differences in antigen surveillance strategies of naïve CD4+ and CD8+ T cells. Proc Natl Acad Sci USA. 2012;109:1–7. doi: 10.1073/pnas.1211717109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus MA, Dullin C, Mitkovski M, Prieschl-Grassauer E, Epstein MM, Alves F. Non-invasive optical imaging of eosinophilia during the course of an experimental allergic airways disease model and in response to therapy. PLoS One. 2014;9:e90017. doi: 10.1371/journal.pone.0090017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massa S, Xavier C, De Vos J, Caveliers V, Lahoutte T, Muyldermans S, Devoogdt N. Site-specific labeling of cysteine-tagged camelid single-domain antibody-fragments for use in molecular imaging. Bioconjug Chem. 2014;25:979–88. doi: 10.1021/bc500111t. [DOI] [PubMed] [Google Scholar]
- McBride WJ, D’Souza CA, Sharkey RM, Goldenberg DM. The radiolabeling of proteins by the [18F]AlF method. Appl Radiat Isot. 2012;70:200–4. doi: 10.1016/j.apradiso.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair-Gill ED, Shu CJ, Radu CG, Witte ON. Non-invasive imaging of adaptive immunity using positron emission tomography. Immunol Rev. 2008;221:214–28. doi: 10.1111/j.1600-065X.2008.00585.x. [DOI] [PubMed] [Google Scholar]
- Nakajima T, Sano K, Choyke PL, Kobayashi H. Improving the efficacy of photoimmunotherapy (PIT) using a cocktail of antibody conjugates in a multiple antigen tumor model. Theranostics. 2013;3:357–65. doi: 10.7150/thno.5908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan A, Gowrishankar G, Nielsen CH, Wang S, Iagaru A, Goris ML, Gambhir SS. Positron emission tomography of 64Cu-DOTA-rituximab in a transgenic mouse model expressing human CD20 for clinical translation to image NHL. Mol Imaging Biol. 2012;14:608–16. doi: 10.1007/s11307-011-0537-8. [DOI] [PubMed] [Google Scholar]
- Nimmagadda S, Pullambhatla M, Lisok A, Hu C, Maitra A, Pomper MG. Imaging Axl expression in pancreatic and prostate cancer xenografts. Biochem Biophys Res Commun. 2014;443:635–40. doi: 10.1016/j.bbrc.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogasawara A, Tinianow JN, Vanderbilt AN, Gill HS, Yee S, Flores JE, Williams SP, Ashkenazi A, Marik J. ImmunoPET imaging of phosphatidylserine in pro-apoptotic therapy treated tumor models. Nucl Med Biol. 2013;40:15–22. doi: 10.1016/j.nucmedbio.2012.09.001. [DOI] [PubMed] [Google Scholar]
- Ogawa M, Regino CAS, Seidel J, Green MV, Xi W, Williams M, Kosaka N, Choyke PL, Kobayashi H. Dual-modality molecular imaging using antibodies labeled with activatable fluorescence and a radionuclide for specific and quantitative targeted cancer detection. Bioconjug Chem. 2009;20:2177–84. doi: 10.1021/bc900362k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olafsen T, Wu AM. Antibody vectors for imaging. Semin Nucl Med. 2010;40:167–81. doi: 10.1053/j.semnuclmed.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y, Ryu Y, Jung Y, Wang T, Baek Y, Yoon Y, Bae SM, Park J, Hwang S, Kim J, Do E, Kim S, Chung E, Kim KH, Kim S, Myung SJ. Spraying quantum dot conjugates in the colon of live animals enabled rapid and multiplex cancer diagnosis using endoscopy. ACS Nano. 2014;8:8896–8910. doi: 10.1021/nn5009269. [DOI] [PubMed] [Google Scholar]
- Poli GL, Bianchi C, Virotta G, Bettini A, Moretti R, Trachsel E, Elia G, Giovannoni L, Neri D, Bruno A. Radretumab radioimmunotherapy in patients with brain metastasis: a 124I-L19SIP dosimetric PET study. Cancer Immunol Res. 2013;1:134–43. doi: 10.1158/2326-6066.CIR-13-0007. [DOI] [PubMed] [Google Scholar]
- Prabhakar U, Maeda HK, Jain R, Sevick-Muraca EM, Zamboni W, Farokhzad OC, Barry ST, Gabizon A, Grodzinski P, Blakey DC. Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Cancer Res. 2013;73:2412–2417. doi: 10.1158/0008-5472.CAN-12-4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price EW, Zeglis BM, Lewis JS, Adam MJ, Orvig C. H6phospa-trastuzumab: bifunctional methylenephosphonate-based chelator with 89Zr, 111In and 177Lu. Dalt Trans. 2014;43:119–31. doi: 10.1039/c3dt51940f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijpkema M, Oyen WJ, Bos D, Franssen GM, Goldenberg DM, Boerman OC. SPECT- and fluorescence image-guided surgery using a dual-labeled carcinoembryonic antigen-targeting antibody. J Nucl Med. 2014;55:1–6. doi: 10.2967/jnumed.114.142141. [DOI] [PubMed] [Google Scholar]
- Rochefort MM, Girgis MD, Ankeny JS, Tomlinson JS. Metabolic exploitation of the sialic acid biosynthetic pathway to generate site-specifically labeled antibodies. Glycobiology. 2014;24:62–9. doi: 10.1093/glycob/cwt090. [DOI] [PubMed] [Google Scholar]
- Rossin R, Robillard MS. Pretargeted imaging using bioorthogonal chemistry in mice. Curr Opin Chem Biol. 2014;21:161–9. doi: 10.1016/j.cbpa.2014.07.023. [DOI] [PubMed] [Google Scholar]
- Sano K, Mitsunaga M, Nakajima T, Choyke PL, Kobayashi H. In vivo breast cancer characterization imaging using two monoclonal antibodies activatably labeled with near infrared fluorophores. Breast Cancer Res. 2012;14:R61. doi: 10.1186/bcr3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano K, Nakajima T, Ali T, Bartlett DW, Wu AM, Kim I, Paik CH, Choyke PL, Kobayashi H. Activatable fluorescent cys-diabody conjugated with indocyanine green derivative: consideration of fluorescent catabolite kinetics on molecular imaging. J Biomed Opt. 2013;18:101304. doi: 10.1117/1.JBO.18.10.101304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoonooghe S, Laoui D, Van Ginderachter JA, Devoogdt N, Lahoutte T, De Baetselier P, Raes G. Novel applications of nanobodies for in vivo bio-imaging of inflamed tissues in inflammatory diseases and cancer. Immunobiology. 2012;217:1266–72. doi: 10.1016/j.imbio.2012.07.009. [DOI] [PubMed] [Google Scholar]
- Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat Rev Cancer. 2012;12:278–87. doi: 10.1038/nrc3236. [DOI] [PubMed] [Google Scholar]
- Shirasu N, Yamada H, Shibaguchi H, Kuroki M, Kuroki M. Potent and specific antitumor effect of CEA-targeted photoimmunotherapy. Int J Cancer. 2014:1–14. doi: 10.1002/ijc.28907. [DOI] [PubMed] [Google Scholar]
- Smaglo BG, Aldeghaither D, Weiner LM. The development of immunoconjugates for targeted cancer therapy. Nat Rev Clin Oncol. 2014:1–12. doi: 10.1038/nrclinonc.2014.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spring BQ, Abu-Yousif AO, Palanisami A, Rizvi I, Zheng X, Mai Z, Anbil S, Sears RB, Mensah LB, Goldschmidt R, Erdem SS, Oliva E, Hasan T. Selective treatment and monitoring of disseminated cancer micrometastases in vivo using dual-function, activatable immunoconjugates. Proc Natl Acad Sci USA. 2014;111:E933–42. doi: 10.1073/pnas.1319493111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhoff K, Pierer M, Siegert J, Pigla U, Laub R, Hesse S, Seidel W, Sorger D, Seese A, Kuenstler JU, Pietzsch HJ, Lincke T, Rullmann M, Emmrich F, Sabri O. Visualizing inflammation activity in rheumatoid arthritis with Tc-99 m anti-CD4-mAb fragment scintigraphy. Nucl Med Biol. 2014;41:350–4. doi: 10.1016/j.nucmedbio.2013.12.018. [DOI] [PubMed] [Google Scholar]
- Strand J, Varasteh Z, Eriksson O, Abrahmsen L, Orlova A, Tolmachev V. Gallium-68-labeled affibody molecule for PET imaging of PDGFRβ expression in vivo. Mol Pharm. 2014;11:3957–64. doi: 10.1021/mp500284t. [DOI] [PubMed] [Google Scholar]
- Suemizu H, Kawai K, Higuchi Y, Hashimoto H, Ogura T, Itoh T, Sasaki E, Nakamura M. A versatile technique for the in vivo imaging of human tumor xenografts using near-infrared fluorochrome-conjugated macromolecule probes. PLoS One. 2013;8:e82708. doi: 10.1371/journal.pone.0082708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiercz R, Chiguru S, Tahmasbi A, Ramezani SM, Hao G, Challa DK, Lewis MA, Kulkarni PV, Sun X, Ober RJ, Mason RP, Ward ES. Use of Fc-engineered antibodies as clearing agents to increase contrast during PET. J Nucl Med. 2014;55:1204–1207. doi: 10.2967/jnumed.113.136481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavaré R, McCracken MN, Zettlitz KA, Knowles SM, Salazar FB, Olafsen T, Witte ON, Wu AM. Engineered antibody fragments for immuno-PET imaging of endogenous CD8+ T cells in vivo. Proc Natl Acad Sci USA. 2014a;111:1108–13. doi: 10.1073/pnas.1316922111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavaré R, Wu WH, Zettlitz KA, Salazar FB, McCabe KE, Marks JD, Wu AM. Enhanced immunoPET of ALCAM-positive colorectal carcinoma using site-specific 64Cu-DOTA conjugation. Protein Eng Des Sel. 2014b;27:317–24. doi: 10.1093/protein/gzu030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terwisscha van Scheltinga AGT, van Dam GM, Nagengast WB, Ntziachristos V, Hollema H, Herek JL, Schröder CP, Kosterink JGW, Lub-de Hoog MN, de Vries EGE. Intraoperative near-infrared fluorescence tumor imaging with vascular endothelial growth factor and human epidermal growth factor receptor 2 targeting antibodies. J Nucl Med. 2011;52:1778–85. doi: 10.2967/jnumed.111.092833. [DOI] [PubMed] [Google Scholar]
- Van de Watering FCJ, Rijpkema M, Perk L, Brinkmann U, Oyen WJG, Boerman OC. Zirconium-89 labeled antibodies: a new tool for molecular imaging in cancer patients. Biomed Res Int. 2014;2014:1–13. doi: 10.1155/2014/203601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rij CM, Lütje S, Frielink C, Sharkey RM, Goldenberg DM, Franssen GM, McBride WJ, Rossi EA, Oyen WJG, Boerman OC. Pretargeted immuno-PET and radioimmunotherapy of prostate cancer with an anti-TROP-2 x anti-HSG bispecific antibody. Eur J Nucl Med Mol Imaging. 2013;40:1377–83. doi: 10.1007/s00259-013-2434-7. [DOI] [PubMed] [Google Scholar]
- Viola-Villegas NT, Sevak KK, Carlin SD, Doran MG, Evans HW, Bartlett DW, Wu AM, Lewis JS. Noninvasive imaging of PSMA in prostate tumors with 89Zr-Labeled huJ591 engineered antibody fragments: the faster alternatives. Mol Pharm. 2014;11:3965–73. doi: 10.1021/mp500164r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward ES, Devanaboyina SC, Ober RJ. Targeting FcRn for the modulation of antibody dynamics. Mol Immunol. 2015 doi: 10.1016/j.molimm.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warram JM, de Boer E, Sorace AG, Chung TK, Kim H, Pleijhuis RG, van Dam GM, Rosenthal EL. Antibody-based imaging strategies for cancer. Cancer Metastasis Rev. 2014;33:809–22. doi: 10.1007/s10555-014-9505-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissleder R, Nahrendorf M, Pittet MJ. Imaging macrophages with nanoparticles. Nat Mater. 2014;13:125–38. doi: 10.1038/nmat3780. [DOI] [PubMed] [Google Scholar]
- Wiehr S, Bühler P, Gierschner D, Wolf P, Rolle AM, Kesenheimer C, Pichler BJ, Elsässer-Beile U. Pharmacokinetics and PET imaging properties of two recombinant anti-PSMA antibody fragments in comparison to their parental antibody. Prostate. 2014;74:743–55. doi: 10.1002/pros.22794. [DOI] [PubMed] [Google Scholar]
- Wu AM. Antibodies for the delivery of radionuclides. Drug Delivery in Oncology: From Basic Research to Cancer Therapy. 2011:411–439. [Google Scholar]
- Wu AM. Engineered antibodies for molecular imaging of cancer. Methods. 2014;65:139–47. doi: 10.1016/j.ymeth.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Shi H, Zhang H, Wang X, Yang Y, Yu C, Hao C, Du J, Hu H, Yang S. Prostate stem cell antigen antibody-conjugated multiwalled carbon nanotubes for targeted ultrasound imaging and drug delivery. Biomaterials. 2014;35:5369–80. doi: 10.1016/j.biomaterials.2014.03.038. [DOI] [PubMed] [Google Scholar]
- Wu Z, Li L, Liu S, Yakushijin F, Yakushijin K, Horne D, Conti PS, Li Z, Kandeel F, Shively JE. Facile preparation of a thiol-reactive 18F-labeling agent and synthesis of 18F-DEG-VS-NT for PET imaging of a neurotensin receptor-positive tumor. J Nucl Med. 2014;55:1178–1184. doi: 10.2967/jnumed.114.137489. [DOI] [PubMed] [Google Scholar]
- Xavier C, Vaneycken I, D’huyvetter M, Heemskerk J, Keyaerts M, Vincke C, Devoogdt N, Muyldermans S, Lahoutte T, Caveliers V. Synthesis, preclinical validation, dosimetry, and toxicity of 68Ga-NOTA-anti-HER2 Nanobodies for iPET imaging of HER2 receptor expression in cancer. J Nucl Med. 2013;54:776–84. doi: 10.2967/jnumed.112.111021. [DOI] [PubMed] [Google Scholar]
- Zeglis BM, Sevak KK, Reiner T, Mohindra P, Carlin SD, Zanzonico P, Weissleder R, Lewis JS. A pretargeted PET imaging strategy based on bioorthogonal Diels-Alder click chemistry. J Nucl Med. 2013;54:1389–96. doi: 10.2967/jnumed.112.115840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zettlitz KA, Tavaré R, Salazar FB, Steward KK, Yamada RE, Timmerman JM, Wu AM. Engineered antibody fragments for ImmunoPET imaging of the B-cell compartment in a transgenic mouse model expressing human CD20. Abstract #LBA10. Presented; September 19, 2013; World Molecular Imaging Congress.2013. [Google Scholar]
- Zielinski R, Hassan M, Lyakhov I, Needle D, Chernomordik V, Garcia-Glaessner A, Ardeshirpour Y, Capala J, Gandjbakhche A. Affibody-DyLight conjugates for in vivo assessment of HER2 expression by near-infrared optical imaging. PLoS One. 2012;7:e41016. doi: 10.1371/journal.pone.0041016. [DOI] [PMC free article] [PubMed] [Google Scholar]