Abstract
Objectives
We sought to determine whether biomarkers ST2, GDF-15, NT-proBNP, and high-sensitivity Troponin I are elevated in HIV-infected patients and associated with cardiovascular dysfunction and all-cause mortality.
Background
HIV-infected individuals have high rates of cardiovascular disease. Markers of myocardial stress may identify at-risk patients and provide additional prognostic information.
Methods
Biomarkers and echocardiograms were assessed in 332 HIV-infected patients and 50 age- and gender-matched controls. Left ventricular systolic dysfunction (LVSD) was defined as ejection fraction <50%, diastolic dysfunction (DD) as ≥stage 1, and pulmonary hypertension as PASP ≥35mmHg. Mortality data was obtained from the National Death Index.
Results
HIV patients were a median 49 years and 80% male. Compared with controls, HIV patients had higher percent estimates of all biomarkers except ST2 and interleukin-6. Among HIV patients, 45% had DD; only ST2 was associated with DD (RR 1.36, p=0.047). LVSD was rare in this cohort (5%). Pulmonary hypertension was present in 27% of HIV patients and associated with GDF-15 (RR 1.18, p=0.04), NT-proBNP (RR 1.18, p=0.007), and Cystatin C (RR 1.54, p=0.03). Thirty-eight deaths occurred among HIV subjects over a median 6.1 years. In adjusted analysis, all-cause mortality was independently predicted by ST2 (HR 2.04, p=0.010), GDF-15 (HR 1.42, p=0.0054), hsCRP (HR 1.25, p=0.023) and D-dimer (HR 1.49, p=0.029). Relationships were unchanged when analyses were restricted to virally-suppressed HIV-infected patients on antiretroviral therapy.
Conclusions
Among HIV-infected individuals, ST2 and GDF-15 are associated with both cardiovascular dysfunction and all-cause mortality and may be useful at identifying those at-risk for developing cardiovascular events and death.
Keywords: HIV, death, cardiovascular dysfunction, biomarkers, mortality
Introduction
Through the evolution of the human immunodeficiency virus (HIV) epidemic, cardiovascular disease (CVD) has emerged as a major cause of morbidity and mortality among HIV-infected individuals. In contemporary, observational studies of HIV patients, the proportion of total deaths from CVD has ranged from 6.5% to 15%, with HIV infection alone conferring a 61% increased risk compared with uninfected individuals.(1, 2)
Previously, this elevated risk of CVD, present even among treated and virally suppressed individuals, was largely attributed to the consequences of antiretroviral therapy (ART) use and the increased burden of traditional risk factors. However, in the Strategies for Management of Anti-Retroviral Therapy (SMART) trial, chronic inflammation and viral replication were identified as causative factors, which have since prompted further investigation into the role of HIV-induced inflammation and immune activation as possible mediators of cardiovascular risk.(1, 3)
An important step in establishing a relationship between HIV-associated immunologic perturbations and CVD is demonstrating that specific markers of these pathways predict subsequent events. However, most studied biomarkers, including high sensitivity C-reactive protein (hsCRP), D-dimer, Interleukin-6 (IL-6) and Cystatin C, are predominantly released outside of the myocardium and may not represent the direct relationship between HIV infection and CVD.
Among individuals without HIV, novel biomarkers primarily expressed or secreted by cardiovascular tissue in response to pathological stress have been predictive of cardiovascular events and mortality. These include soluble ST2, growth differentiation factor-15 (GDF-15), N-terminal-pro-B-type-natriuretic-peptide (NT-proBNP), and high-sensitivity troponin I (hsTnI).(4) However, only NT-proBNP has been evaluated in the HIV population.(5)
The purpose of this study was to determine whether ST2, GDF-15, NT-proBNP, and hsTnI are elevated in HIV-infected individuals compared with uninfected controls and are associated with cardiovascular dysfunction and mortality. We also sought to establish whether these cardiac biomarkers provide independent assessment of risk compared with previously studied biomarkers hsCRP, IL-6, D-dimer, and Cystatin C.
Methods
Participants
Individuals with HIV infection were consecutively enrolled between September 2004 and March 2011 from the Study of the Consequences of the Protease Inhibitor Era (SCOPE), a large clinic-based cohort at San Francisco General Hospital. All participants of SCOPE were documented to be HIV-infected. The cohort includes: 1) untreated patients, defined as no ART in the preceding 6 months; 2) treated patients with detectable viremia, as defined as >24 weeks of ART with the most recent 2 HIV RNA levels >75 copies/ml; and 3) treated patients who achieved full viral suppression, as defined as >24 weeks of ART with 2 most recent HIV RNA levels <75 copies/ml. The only inclusion criterion was HIV infection and there were no exclusion criteria. Enrollment of the uninfected control group was targeted towards individuals with similar age, gender, and smoking status as the SCOPE population. Controls were not known to have CVD at the time of enrollment and tested negative for HIV. Written informed consent was provided by all study participants. The study was approved by the University of California, San Francisco Committee on Human Research.
Measurements
Clinical and Sociodemographic Characteristics
At enrollment, all participants completed a detailed interview, and information on traditional risk factors, medication use, and sociodemographic factors were collected. HIV-related disease characteristics collected included ART, duration of infection, history of opportunistic infections, and nadir CD4 count.
Echocardiography
As described previously(6), a 2D transthoracic echocardiogram was performed on each participant within 6 months of enrollment by a single sonographer blinded to participant’s HIV status. The presence of diastolic dysfunction (DD) was determined using the guidelines from the American Society of Echocardiography.(7) Left ventricular end-diastolic and end-systolic volumes and left ventricular ejection fraction (LVEF) were assessed using the modified Simpson’s rule and indexed to body surface area. Pulmonary artery systolic pressure (PASP) was quantified by using the modified Bernoulli equation to obtain the calculated pressure gradient and then added to the mean right atrial pressure, which was estimated from the diameter of the inferior vena cava, degree of inspiratory collapse, and hepatic vein Doppler profile.(8) All calculations and interpretations were performed off-line by 2 cardiologists who were blinded to participants’ HIV infection and clinical status.
Laboratory Assays
Eight biomarkers were selected because of reported associations with CVD or death in either the general population and/or HIV-infected individuals. The biomarkers included: ST2 (fibrosis); GDF-15 (apoptosis); NT-proBNP (myocyte stretch); hsTnI (myocardial injury); hsCRP and IL-6 (inflammation); Cystatin C (renal dysfunction); and D-dimer (thrombosis).(4, 5, 9–12) Thresholds of risk for ST2, GDF-15, and NT-proBNP were pre-defined based on prior studies associating values exceeding these cut-points with CVD. These thresholds were: ST2 >35 ng/ml, GDF-15 >1200 pg/ml, and NT-proBNP >300 pg/ml.(13–16) Detectable troponin was defined as ≥0.006 ng/ml, which is the minimal detection limit. Details regarding assay brands, ranges and sensitivities are shown in Table 1S (supplement).
Follow-up and Outcomes
All patients were followed as part of the SCOPE study. The main echocardiographic outcomes were DD, defined as ≥ stage 1 findings, and pulmonary hypertension (PHTN), classified as PASP >35mmHg.(8) Systolic dysfunction, defined as a LVEF of <50%, was not considered a primary outcome due to the low prevalence in the HIV population.(17) For all-cause mortality, participants were followed through December 2012 or until time of death as determined by the National Death Index. Two independent physicians adjudicated cardiovascular death using patient ICD-9 codes provided by the National Death Index. To be considered a cardiovascular death, patients required an ICD-9 code related to cardiovascular pathology in ≥1 of the first 3 ICD-9 codes reported on the death document.
Adjustment Variables
Covariates of interest included demographic characteristics, CVD risk factors, and HIV-related risk factors. Multiple imputation with the Markov chain Monte Carlo method was used to impute missing covariates, with 5 imputations to yield ~95% relative efficiency.
Statistical Analysis
We compared demographic and clinical characteristics between groups using the Mann-Whitney U test for continuous variables and Fisher’s exact test for categorical variables. Spearman coefficients were used to evaluate correlations between biomarkers.
We used multivariable linear regression to evaluate the associations of HIV infection and other factors with each candidate biomarker, in separate models for each outcome. We used Huber-White standard errors which are designed to be robust to non-normally distributed residuals. We used Tobit regression to evaluate associations with hsTnI, due to the high percentage of below detectable values. Because the biomarkers were right-skewed, each outcome was log-transformed for analysis; results were back-transformed to produce estimated percentage differences. To determine whether HIV infection was independently associated with each biomarker, multivariable models were adjusted for age, gender, and estimated glomerular filtration rate, calculated using the MDRD formula.
Poisson regression with a robust variance estimator was used to assess the associations of biomarkers with the dichotomous outcomes of DD and PHTN, and Cox proportional hazards regression was used to obtain hazard ratios for all-cause mortality. We also used unadjusted generalized additive models to produce a spline plot depicting the probability of mortality over the range of each biomarker.
To determine whether each biomarker was independently associated with the outcome, multivariable models were sequentially adjusted for: 1) demographics and 2) cardiovascular and HIV-related risk factors. Factors forced in the full model included age, gender, and race/ethnicity. We used stepwise backward selection with a significance level of α=0.05 to remove candidate biomarkers that were not associated with each outcome. As an alternative approach, we used Bayesian model averaging and retained predictors with posterior probabilities >35%. We constructed ROC curves and calculated Harrell’s C-index of concordance for survival models to assess model discrimination.
Bayesian model averaging was performed using the BMA package for the R statistical computing language (R Development Core Team, Vienna, Austria). All other analyses were conducted using the SAS system, version 9.3 (SAS Institute, Inc., Cary, NC).
Results
Study Group Characteristics
A total of 332 HIV-infected patients and 50 age- and gender-matched controls were studied. Baseline characteristics are shown in Table 1. HIV-infected patients were more often African American, and had a higher prevalence of hypertension, Hepatitis C infection and previous intravenous drug use. Among HIV-infected participants, the median duration of infection was 14 years, 79% were currently on ART, and 60% had undetectable viral loads. The median current CD4 count was 469 cells/mm3 and median nadir CD4 count was 160 cells/mm3.
Table 1.
Baseline Characteristics of HIV-Infected Participants and Controls
| Characteristic | HIV+ (N = 332) | Control (N = 50) | P-value |
|---|---|---|---|
| Age (y) | 49 (42–54) | 46 (40–57) | 0.60 |
| Male | 265 (80%) | 44 (88%) | 0.45 |
| Race | <0.001 | ||
| Caucasian | 186 (56%) | 33 (66%) | |
| African American | 105 (32%) | 4 (8%) | |
| Latino | 22 (7%) | 5 (10%) | |
| Other | 19 (6%) | 8 (16%) | |
| Cigarette smoking | 0.32 | ||
| Current | 117 (35%) | 12 (24%) | |
| Past | 98 (30%) | 17 (35%) | |
| Never | 117 (35%) | 20 (41%) | |
| Coronary artery disease | 13 (4%) | 0 | 0.23 |
| Diabetes mellitus | 27 (8%) | 4 (8%) | 0.99 |
| Hypertension | 112 (34%) | 3 (6%) | <0.001 |
| Hyperlipidemia | 97 (29%) | 12 (24%) | 0.50 |
| LDL cholesterol (mg/dL) | 101 (76–128) | 106 (89–114) | 0.57 |
| HDL cholesterol (mg/dL) | 47 (39–55) | 46 (44–62) | 0.23 |
| Triglycerides (mg/dL) | 149 (100–258) | 92 (66–166) | 0.003 |
| Total cholesterol (mg/dL) | 185 (152–216) | 177 (169–191) | 0.57 |
| eGFR (ml/min/1.73m2) | 77 (63–91) | 70 (62–77) | 0.17 |
| Systolic blood pressure (mmHg) | 120 (111–130) | 122 (114–128) | 0.40 |
| Body mass index (kg/m2) | 25 (23–29) | 26 (24–29) | 0.52 |
| Hepatitis C infection | 87 (26%) | 1 (2%) | <0.001 |
| History of intravenous drug use | 131 (39%) | 1 (2%) | <0.001 |
| Duration of HIV infection (y) | 14 (10–18) | ||
| ARV use: | |||
| Current | 261 (79%) | ||
| Past | 20 (6%) | ||
| Never | 51 (15%) | ||
| HAART use | 265 (80%) | ||
| HAART duration (y)* | 5.4 (2.2–7.5) | ||
| NRTI use | 280 (84%) | ||
| NRTI duration (y)* | 7.1 (2.3–9.8) | ||
| NNRTI use | 157 (47%) | ||
| NNRTI duration (y)* | 3.3 (1.3–5.5) | ||
| PI use | 242 (73%) | ||
| PI duration (y) | 5.3 (2.4–7.8) | ||
| Current Abacavir use | 99 (30%) | ||
| Current CD4 (cells/mm3) | 469 (294–668) | ||
| Nadir CD4 (cells/mm3) | 160 (41–300) | ||
| Plasma HIV RNA (copies/ml) : | |||
| < 75 | 198 (60%) | ||
| 75–1999 | 60 (18%) | ||
| 2000–9999 | 29 (9%) | ||
| >10000 | 44 (13%) | ||
| History of opportunistic infection | 93 (28%) |
Data are presented as Median (IQR) or numbers (percent).
Duration of antiretroviral use among ever users.
Abbreviations: ARV, antiretroviral; eGFR, estimated glomerular filtration rate; IQR, interquartile range; HAART, highly active antiretroviral therapy; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
Echocardiographic Parameters and Study Outcomes
Echocardiographic measures and study outcomes are summarized in Table 2. Median LVEF was 61% in both HIV-infected patients and controls. Fourteen HIV-infected patients, whereas no controls, had a LVEF of less than 50% (p=0.23). DD was present in 45% of HIV-infected participants versus 28% of controls (p=0.02). The median left ventricular mass was greater in HIV participants compared with controls (77.3 gm/m2 vs 66.4 gm/m2, p <0.001). Eighty-nine HIV-infected patients, whereas only 1 control, met criteria for PHTN (p<0.001). PASP was significantly higher in HIV-infected patients compared with controls (31.0 mmHg vs 22.5 mmHg, p<0.001). Results were similar when the analysis was restricted to treated, virally-suppressed HIV-infected patients (supplemental Table 2S).
Table 2.
Echocardiographic Measures and Study Outcomes for HIV-Infected Participants and Controls
| HIV+ (N = 332) | Control (N = 50) | P-value | |
|---|---|---|---|
| Ejection Fraction (%) | 61.0 (56.5–66.0) | 61.5 (59.0–67.0) | 0.35 |
| Systolic Dysfunction | 14 (5%) | 0 | 0.23 |
| Diastolic Dysfunction | 138 (45%) | 14 (28%) | 0.02 |
| Stage 1 | 128 (93%) | 14 (100%) | 0.60 |
| LV Mass Index (gm/m2) | 77.3 (66.2–88.7) | 66.4 (63.0–72.6) | <0.001 |
| Pulmonary Hypertension | 89 (27%) | 1 (2.0%) | <0.001 |
| PASP (mmHg) | 31.0 (25.0–36.0) | 22.5 (19.0–25.0) | <0.001 |
| Mortality Rate* | 10.3% (7.4%–14.2%) | 4.0% (1.3%–12.3%) | 0.094 |
Data are presented as Median (IQR) or numbers (percent).
Mortality is reported as five-year rate (95% CI)
Abbreviations: LV, left ventricular; PASP, pulmonary artery systolic pressure; IQR, interquartile range
Thirty-eight deaths occurred in the HIV-infected cohort over a median follow-up of 6.1 years (IQR 3.0, 7.9), and 3 deaths occurred in the control group over a median 7.7 years (IQR 7.6, 7.8) of follow-up, corresponding to a 5-year mortality rate of 10.3% in HIV-infected persons and 4.0% in controls (p=0.094). HIV-infected patients who died compared to those who survived had a higher prevalence of Hepatitis C and opportunistic infections, longer duration of HIV infection, lower current CD4 counts, and higher HIV viral loads (supplemental Table 3S).
Biomarker Concentrations
Compared with controls, HIV-infected participants had higher levels of all biomarkers with the exception of ST2, IL-6 and the prevalence of detectable hsTnI (Table 3). After controlling for age, gender, and eGFR, HIV infection was associated with greater percent estimates of all candidate biomarkers except for ST2 and IL-6. Additionally, more HIV-infected patients compared with controls exceeded pre-defined CVD risk thresholds for ST2 (31% vs. 14%, p=0.01), GDF-15 (40% vs. 0%, p<0.001), and NT-proBNP (9.5% vs. 0%, p=0.02).(13–16) Biomarkers levels were only moderately intra-correlated (r≤0.35 for all) (supplemental Table 4S). Results were similar when the analysis was restricted to treated, virally-suppressed HIV-infected patients (supplemental Table 5S).
Table 3.
Concentration of Biomarkers for HIV-Infected Participants and Controls
| Biomarker | HIV+ (N = 332) | Control (N = 50) | P-value | HIV+ versus Control | |||
|---|---|---|---|---|---|---|---|
| Unadjusted %Estimate | P-value | Adjusted %Estimate* | P-value | ||||
| ST2 (ng/ml) | 28.5 (23.1, 37.6) | 29.2 (23.8, 33.0) | 0.34 | 7.1 (−3.0, 18.2) | 0.18 | 8.8 (−1.1, 19.7) | 0.08 |
| NT-proBNP (pg/ml) | 38.6 (19.2, 108.3) | 17.7 (11.9, 32.2) | <0.001 | 151.0 (96.7, 220.4) | <0.001 | 130.2 (82.3. 190.7) | <0.001 |
| GDF-15 (pg/ml) | 971 (571, 1805) | 336 (263, 431) | <0.001 | 237.7 (189.5, 293.8) | <0.001 | 231.5 (183.9, 286.9) | <0.001 |
| hsCRP (mg/L) | 2.3 (0.8, 5.6) | 1.9 (0.4, 4.5) | 0.05 | 54.9 (6.1, 126.3) | 0.02 | 53.8 (4.7, 126.0) | 0.03 |
| IL-6 (pg/ml) | 3.1 (1.5, 5.8) | 2.3 (1.4, 3.8) | 0.06 | 22.8 (−7.5, 63.1) | 0.16 | 20.3 (−9.6, 60.1) | 0.21 |
| Cystatin C (mg/L) | 0.78 (0.66, 0.93) | 0.61 (0.47, 0.64) | <0.001 | 39.4 (27.6, 52.3) | <0.001 | 39.5 (27.6, 52.4) | <0.001 |
| D-Dimer (ng/mL) | 231 (147, 389) | 183 (114, 265) | 0.002 | 40.3 (18.9, 65.7) | <0.001 | 36.0 (17.5, 57.4) | <0.001 |
| Detectable hsTnI (ng/ml) | 85 (27%) | 7 (14%) | 0.06 | 94.6 (11.1, 240.9) | 0.02 | 90.1 (6.4, 239.4) | 0.03 |
Data are presented as Median (IQR) or Percent (95% Confidence Interval)
Adjusted for Age, Gender and Estimated Glomerular Filtration Rate
Association of Biomarkers with Study Outcomes in HIV-Infected Participants
Diastolic Dysfunction
ST2 was the only biomarker significantly associated with DD, with each doubling of ST2 conferring a 43% increased risk (see Table 4). ST2 remained independently related to DD after adjustment for CVD and HIV-related risk factors (+36%, p=0.047), while other biomarkers showed weaker associations that were not statistically significant.
Table 4.
Associations between Individual Biomarkers with Diastolic Dysfunction, Pulmonary Hypertension, and All-cause Mortality in HIV-Infected Participants
| Outcome | Demographic adjusted RR (95% CI) | Fully adjusted* RR (95% CI) |
|---|---|---|
| Diastolic Dysfunction | ||
| ST2 | 1.43 (1.06, 1.92), p=0.02 | 1.36 (1.00, 1.85), p=0.047 |
| NT-proBNP | 1.01 (0.93, 1.11), p=0.75 | 1.00 (0.91, 1.10), p=0.96 |
| hsCRP | 1.06 (0.97, 1.16), p=0.23 | 1.08 (0.98, 1.18), p=0.12 |
| GDF-15 | 1.08 (0.96, 1.22), p=0.18 | 1.05 (0.92, 1.19), p=0.47 |
| Cystatin C | 1.19 (0.90, 1.57), p=0.22 | 1.11 (0.80, 1.54), p=0.52 |
| IL-6 | 1.09 (0.99, 1.20), p=0.08 | 1.09 (0.98, 1.20), p=0.11 |
| D-dimer | 1.17 (0.99, 1.38), p=0.07 | 1.17 (0.98, 1.39), p=0.09 |
| Detectable Troponin | 1.19 (0.83, 1.73), p=0.35 | 1.10 (0.75, 1.60), p=0.63 |
| Pulmonary Hypertension | ||
| ST2 | 1.18 (0.83, 1.67), p=0.35 | 1.21 (0.84, 1.75), p=0.29 |
| NT-proBNP | 1.15 (1.04, 1.26), p=0.004 | 1.18 (1.04, 1.32), p=0.007 |
| hsCRP | 1.07 (0.96, 1.19), p=0.25 | 1.03 (0.90, 1.17), p=0.71 |
| GDF-15 | 1.19 (1.03, 1.37), p=0.02 | 1.18 (1.01, 1.39), p=0.04 |
| Cystatin C | 1.33 (0.97, 1.83), p=0.08 | 1.54 (1.04, 2.29), p=0.03 |
| IL-6 | 1.02 (0.91, 1.14), p=0.74 | 1.03 (0.91, 1.17), p=0.62 |
| D-dimer | 1.11 (0.90, 1.37), p=0.32 | 1.06 (0.85, 1.33), p=0.59 |
| Detectable Troponin | 0.76 (0.45, 1.26), p=0.28 | 0.84 (0.49, 1.42), p=0.51 |
|
| ||
| All-cause mortality | Demographic adjusted HR (95% CI) | Fully adjusted** HR (95% CI) |
|
| ||
| ST2 | 2.30 (1.40, 3.77), p=0.0011 | 2.04 (1.19, 3.50), p=0.010 |
| NT-proBNP | 1.28 (1.10, 1.48), p=0.0012 | 1.20 (1.00, 1.44), p=0.054 |
| hsCRP | 1.19 (1.00, 1.43), p=0.055 | 1.25 (1.03, 1.51), p=0.023 |
| GDF-15 | 1.52 (1.22, 1.89), p<0.001 | 1.42 (1.11, 1.82), p=0.0054 |
| Cystatin C | 1.71 (1.07, 2.74), p=0.025 | 1.18 (0.56, 2.50), p=0.66 |
| IL-6 | 1.23 (0.99, 1.54), p=0.063 | 1.05 (0.86, 1.28), p=0.64 |
| D-dimer | 1.84 (1.35, 2.50), p<0.001 | 1.49 (1.04, 2.12), p=0.029 |
| Detectable Troponin | 1.36 (0.68, 2.72), p=0.38 | 1.09 (0.52, 2.30), p=0.81 |
For Diastolic Dysfunction and Pulmonary Hypertension, fully adjusted Poisson models control for age, gender, ethnicity, CVD risk factors (history of DM, HTN, Prior CAD, Prior MI, CHF, CKD, family history of CAD, Prior Stroke, current tobacco use, HDL, LDL), CD4 count, and HIVRNA.
For All-cause mortality, fully adjusted Cox models control for age, gender, ethnicity, HIVRNA, HCV, history of OI, use of aspirin or clopidogrel, and current CD4 count.
Pulmonary Hypertension
In demographic adjusted analysis, each doubling of NT-proBNP was associated with a 15% increased risk of PHTN (p=0.004), and each doubling of GDF-15 was associated with a 19% increased risk (p=0.02). Both NT-proBNP and GDF-15 remained independently related to PHTN after multivariable adjustment (see Table 4). Cystatin C was associated with PHTN only in fully adjusted analysis (RR 1.54, 95% CI 1.04-2.29, p=0.03). No combination of biomarkers strengthened the association with PHTN.
All-Cause Mortality
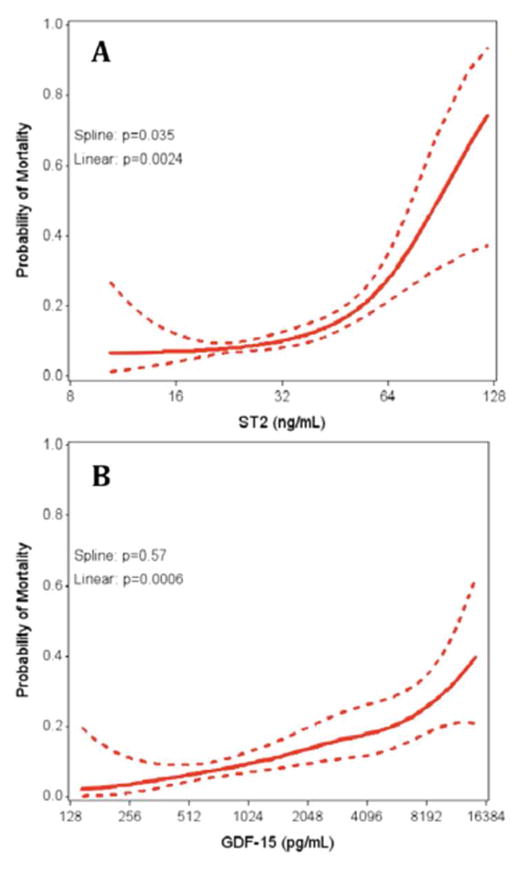
In fully adjusted analysis, ST2, GDF-15, hsCRP and D-dimer were each associated with all-cause mortality (Table 4). ST2 demonstrated the strongest association with all-cause mortality, with each doubling of ST2 conferring a 104% increased risk of death. No combination of biomarkers was simultaneously associated with mortality. When controlling for ST2, GDF-15, hsCRP and D-dimer simultaneously, the hazard ratios for each marker were attenuated, and no associations with mortality remained statistically significant. Graphical examination of the association of ST2 and GDF-15 with all-cause mortality is shown in Figure 1. A strong linear association was seen for GDF-15 (p<0.001), where higher GDF-15 levels were associated with a greater probability of mortality. Increasing ST2 was also associated with all-cause mortality (p=0.0024), but the slope appeared steeper at higher values of ST2 (test for non-linearity: p=0.035), with an inflection point occurring near 50 ng/mL.
Figure 1. Association of ST2 and GDF-15 Levels with All-Cause Mortality in HIV-Infected Participants.
Increasing ST2 (A) and GDF-15 (B) levels are associated with increasing risk of all-cause mortality. Spline p values <0.05 suggest non-linear associations. Solid lines denote predicted probability of mortality and dotted lines represent 95% confidence interval bounds.
For all-cause mortality, model discrimination was modest in unadjusted analysis for ST2 (c=0.61; 95%CI: 0.51, 0.71) and GDF-15 (c=0.67; 95%CI: 0.58, 0.76). Fully adjusted models that included demographic and clinical variables demonstrated strong discrimination for all-cause mortality (c=0.85; 95% CI 0.79, 0.91), with no change in AUC when either biomarker was added (supplemental Figure 1S).
Of the 38 HIV patients who died, 12 were from cardiovascular causes. Among these patients, the median ST2 level was 38.1 ng/ml (IQR 26.6, 51.2) and median GDF-15 level was 1777.4 pg/ml (IQR 942.8, 4528.4). Nine of the 12 patients with cardiovascular death exceeded pre-defined CVD risk thresholds for either ST2 and/or GDF-15,(13–16) with 5 of these patients having elevated levels of both ST2 and GDF-15.
Association of Biomarkers with Study Outcomes in Treated, Virally-Suppressed HIV-Infected Participants
For each study outcome, we assessed for the interaction between individual biomarkers and detectable viremia and ART use. The only significant interaction occurred between ST2 and detectable viral load for the outcome of mortality (p=0.49) (supplemental Table 6S). When the analysis was restricted to HIV participants on ART and with undetectable viral loads, there was no significant change in the associations between biomarkers and study outcomes (supplemental Table 7S).
Discussion
In the current study, we examined the relationship between cardiovascular dysfunction and all-cause mortality in ambulatory HIV-infected individuals with 4 cardiac biomarkers (ST2, GDF-15, NT-proBNP, hsTnI) and 4 non-specific biomarkers previously associated with HIV-related cardiovascular events (hsCRP, IL-6, D-dimer, Cystatin C). Of these, ST2 was the only biomarker associated with DD, a finding that was independent of traditional and HIV-related risk factors, whereas GDF-15, NT-proBNP and Cystatin C were independently associated with PHTN. In addition, ST2, GDF-15, hsCRP and D-dimer were each predictive of all-cause mortality. Only ST2 and GDF-15 were associated with both cardiovascular dysfunction and all-cause mortality and were elevated in the majority of HIV-infected patients who suffered from cardiovascular death. Importantly, the associations between biomarkers and outcomes were unchanged when restricted to treated, virally-suppressed HIV participants.
CVD in the HIV population accounts for a large proportion of morbidity and mortality, and as HIV-infected patients continue to live longer, the burden of CVD will simultaneously rise.(1) As such, CVD has become an important health issue for primary providers, and the ability to identify HIV-infected patients at risk is now an essential component in their ongoing management. The pathophysiology of CVD in HIV, however, is complex and multifactorial, likely involving the interplay between an increased burden of traditional risk factors, adverse effects of ART, immune activation, and chronic viral-mediated inflammation.(1) As a result, traditional cardiovascular risk algorithms developed for the non-HIV infected population do not accurately predict cardiovascular risk in HIV-infected patients, as they fail to account for factors unique to HIV infection.(18)
Incorporating serum biomarkers reflecting specific disease pathways into risk-stratification algorithms has previously demonstrated improvements in the ability to predict cardiovascular events among non-HIV persons beyond established risk factors.(4) High-sensitivity CRP, as an example, has demonstrated the ability to strengthen the prediction of cardiovascular risk in the general population and is associated with subclinical atherogenesis, cardiovascular events, and mortality in HIV-infected individuals.(9, 10) However, hsCRP is primarily secreted by the liver and likely reflects an indirect mechanism involved in the development of CVD.
In contrast, cardiac-specific biomarkers represent more terminal pathways in the pathophysiology of CVD and may improve the prediction of cardiovascular-related outcomes. For example, among ambulatory individuals from the Framingham Heart Study, ST2, GDF-15, hsTnI, and BNP were each associated with incident heart failure, major cardiovascular events, and death, whereas hsCRP only showed an association with death.(4)
ST2 is a member of the interleukin-1 receptor family and is induced in cardiac myocytes and fibroblasts in response to mechanical strain. Signaling between IL-33 and ST2 is thought to be cardioprotective, primarily through inhibiting the development of cardiac fibrosis. Circulating ST2 is a sensitive marker of cardiac stress and has prognostic value in patients with myocardial infarction and heart failure.(13, 19) To date, the only published analysis of ST2 in HIV-infected individuals is from a small study comparing ST2 levels in 26 HIV-infected patients with non-infected controls either with or without active atopic dermatitis.(20)
Although ST2 levels in HIV-infected patients were similar to that of controls in our study, HIV patients were more likely to exceed a ST2 risk threshold associated with adverse events in heart failure patients.(13, 14) In addition, despite our HIV cohort having a younger age, more optimal lipid profiles, and a lower prevalence of diabetes, median ST2 levels among HIV patients were higher than levels seen in the Framingham population (28.5 ng/ml vs 21.05 ng/ml).(4)
More impressive, however, was the relationship between ST2 with DD and all-cause mortality in HIV-infected individuals. In a previous study by our group, we determined that HIV-infected patients have a higher prevalence of DD and increased left ventricular mass indices compared with non-infected individuals, independent of traditional risk factors.(6) These findings have been replicated in subsequent studies among different HIV populations.(17) Furthermore, a high prevalence of cardiac steatosis and fibrosis as shown by cardiac MRI has been reported in treated HIV-infected patients compared with non-infected controls.(21) Myocardial fibrosis and DD are known risk-factors for mortality in the general population, and in a separate study by our group, HIV-infected patients who suffered sudden cardiac death were nearly eight times more likely to have DD compared with those suffering from AIDS-related deaths.(22) Thus, it is plausible that elevated ST2 in HIV-infected patients is a mediator of fibrosis, DD, and possibly death. Further investigation will be needed to more completely delineate this association.
GDF-15 is strongly induced in cardiac myocytes in response to cardiovascular inflammation, tissue injury, and pressure-overload states, and is involved in the regulation of cell differentiation and tissue repair. Among individuals without HIV, levels of GDF-15 are markedly elevated in the setting of acute coronary syndromes and heart failure, and correlate with disease severity and mortality risk.(23, 24) In addition, GDF-15 levels identify ambulatory patients with and without CVD at-risk for future events and is predictive of the development of PHTN.(15, 25)
To our knowledge, GDF-15 has not been described in the HIV population. In the present study, HIV-infected patients had GDF-15 concentrations significantly greater than that of controls, and remained independently associated with PHTN, increasing PASP, and mortality after controlling for traditional and HIV-related variables.
GDF-15 is up-regulated in pulmonary vascular endothelial cells in response to hypoxia and laminar stress and is thought to be involved in the regulation of apoptosis, cellular proliferation, and the development of PHTN.(26) As PHTN is a well-recognized complication of HIV-infection and heightens risk of mortality, it is plausible that increased GDF-15 expression in HIV-infected patients mediates this disease process.
Besides ST2 and GDF-15, this is also the first study to assess a high-sensitivity troponin I assay in HIV-infected patients, which expands upon limited existing data investigating the association between other troponin assays and HIV-related CVD.(27) Although the current study did not demonstrate a significant relationship between hsTnI levels and cardiovascular dysfunction or mortality, HIV-infected patients were more likely to have detectable hsTnI levels compared with controls. It is possible that detection of hsTnI in our study reflects subclinical coronary artery disease, which we were unable to assess.
Levels of NT-proBNP were associated with PHTN in the current study, but not with DD or mortality after full adjustment. Unlike the other cardiac-specific biomarkers, the relationship between NT-proBNP and CVD in HIV-infected individuals has been previously evaluated. In the SMART trial, NT-proBNP was independently associated with cardiovascular events after adjustment for baseline covariates, traditional risk factors, IL-6, hsCRP, and D-dimer.(5) Other studies have also explored the relationship between natriuretic peptides and cardiovascular function in HIV-infected individuals with conflicting results.(28) In a prior study, we found no association between BNP and DD in HIV-infected patients, but a similar positive relationship with PASP.(6) Thus, at this time, the association between NT-proBNP and HIV-related CVD remains unclear.
Our study demonstrated significant associations between D-dimer and hsCRP with all-cause mortality, consistent with previous studies.(9–11) However, neither of these was associated with cardiovascular dysfunction. Importantly, few prior studies have evaluated whether a relationship exists between these non-specific biomarkers and cardiovascular function as determined by echocardiography in HIV patients. In a previous study by our group, hsCRP was not found to be significantly associated with the presence of DD in HIV-infected individuals.(6) In addition, a recent meta-analysis of 2242 HIV-infected patients from 11 studies showed no association between hsCRP and DD.(17) Therefore, these non-specific biomarkers may only be a global marker of risk for all-cause mortality but not useful for identifying patients with occult cardiovascular dysfunction.
There are several strengths and limitations of our study. This is the first study to determine levels of GDF-15 and hsTnI in HIV-infected patients and the first to examine whether cardiac biomarkers are associated with echocardiographic determinants of cardiovascular dysfunction and all-cause mortality. Although only a single assessment of each biomarker was used for the prediction of the study outcomes, prior studies have demonstrated that baseline biomarker measurements in HIV-infected individuals are stable over time and remain strongly and independently associated with future events.(12) Due to the small number of deaths, only a descriptive analysis of cardiovascular mortality was performed. In addition, the diagnosis of PHTN was made using echocardiographic measurements, which lacks specificity compared with invasive hemodynamic studies and cannot discriminate between the etiologies of PHTN. Although we found similar results when we restricted our analysis to patients on ART with undetectable viremia, our results do not exclude the possibility that certain ART agents or classes may have important associations with the development of CVD. Lastly, we cannot exclude the possibility that associations between biomarkers and outcomes may in part be due to residual confounding or unmeasured confounders.
In conclusion, our study demonstrates that ST2 and GDF-15 are associated with both cardiovascular dysfunction and all-cause mortality in ambulatory HIV-infected patients. Future studies are necessary to elucidate the potential role of ST2 and GDF-15 in identifying patients with sub-clinical cardiovascular dysfunction and those at risk of developing cardiovascular events including death.
Supplementary Material
Clinical Perspectives.
Competency in Medical Knowledge
With the advent of highly active antiretroviral therapy, the HIV population is now growing older, and in parallel with this trend, cardiovascular disease has emerged as an important health concern. Novel biomarkers of myocardial stress have identified at-risk patients for cardiovascular events in the general population and may provide additional prognostic information for patients with HIV infection
Translational Outlook
As methods to better identify HIV-infected patients at risk for cardiovascular events are needed, further investigation into the role of cardiac biomarkers such as ST2 and GDF-15 for risk stratifying HIV-infected individuals is warranted.
Acknowledgments
Grant Support: NHLBI RO1 HL095130 (P.Y.H.), NHLBI RO1 HL091526 (P.Y.H.).
Abbreviations
- ART
Antiretroviral Therapy
- CAD
Coronary Artery Disease
- CVD
Cardiovascular Disease
- DD
Diastolic Dysfunction
- GDF-15
Growth Differentiation Factor-15
- hsCRP
High-Sensitivity C-Reactive Protein
- hsTnI
High-Sensitivity Troponin I
- HIV
Human Immunodeficiency Virus
- IL-6
Interleukin-6
- NT-proBNP
N-terminal-pro-B-type-Natriuretic-Peptide
- PASP
pulmonary artery systolic pressure
- PHTN
pulmonary hypertension
Footnotes
Disclosures: P.Y.H. has received honoraria from Gilead and Pfizer. J.S. is an employee of Critical Care Diagnostics. There was no relationship with industry in supporting this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stein JH, Hsue PY. Inflammation, immune activation, and CVD risk in individuals with HIV infection. JAMA. 2012;308:405–6. doi: 10.1001/jama.2012.8488. [DOI] [PubMed] [Google Scholar]
- 2.Boccara F, Lang S, Meuleman C, et al. HIV and coronary heart disease: time for a better understanding. J Am Coll Cardiol. 2013;61:511–23. doi: 10.1016/j.jacc.2012.06.063. [DOI] [PubMed] [Google Scholar]
- 3.El-Sadr WM, Lundgren JD, Neaton JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–96. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 4.Wang TJ, Wollert KC, Larson MG, et al. Prognostic utility of novel biomarkers of cardiovascular stress: the Framingham Heart Study. Circulation. 2012;126:1596–604. doi: 10.1161/CIRCULATIONAHA.112.129437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duprez D, Neuhaus J, Tracy R, et al. N-terminal-proB-type natriuretic peptide predicts cardiovascular disease events in HIV-infected patients. AIDS. 2011;25:651–7. doi: 10.1097/QAD.0b013e32834404a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsue PY, Hunt PW, Ho JE, et al. Impact of HIV infection on diastolic function and left ventricular mass. Circ Heart Fail. 2010;3:132–9. doi: 10.1161/CIRCHEARTFAILURE.109.854943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–33. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 8.Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 9.Duprez D, Neuhaus J, Kuller LH, et al. Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLoS One. 2012;7:e44454. doi: 10.1371/journal.pone.0044454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Luca A, de Gaetano Donati K, Colafigli M, et al. The association of high-sensitivity c-reactive protein and other biomarkers with cardiovascular disease in patients treated for HIV: a nested case--control study. [Accessed September 25, 2013];BMC Infect Dis. 2013 13:414. doi: 10.1186/1471-2334-13-414. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24004495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lucas G, Cozzi-Lepri A, Wyatt C, et al. Glomerular filtration rate estimated using creatinine, cystatin C or both markers and the risk of clinical events in HIV-infected individuals. HIV Med. 2014 Feb;15(2):116–23. doi: 10.1111/hiv.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neuhaus J, Jacobs DR, Baker JV, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010;201:1788–95. doi: 10.1086/652749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rehman SU, Mueller T, Januzzi JL. Characteristics of the novel interleukin family biomarker ST2 in patients with acute heart failure. J Am Coll Cardiol. 2008;52:1458–65. doi: 10.1016/j.jacc.2008.07.042. [DOI] [PubMed] [Google Scholar]
- 14.Ky B, French B, McCloskey K, et al. High-sensitivity ST2 for prediction of adverse outcomes in chronic heart failure. Circ Heart Fail. 2011;4:180–7. doi: 10.1161/CIRCHEARTFAILURE.110.958223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kempf T, Horn-Wichmann R, Brabant G, et al. Circulating Concentrations of Growth-Differentiation Factor 15 in Apparently Healthy Elderly Individuals and Patients with Chronic Heart Failure as Assessed by a New Immunoradiometric Sandwich Assay. Clin Chem. 2006;53:284–291. doi: 10.1373/clinchem.2006.076828. [DOI] [PubMed] [Google Scholar]
- 16.Januzzi JL, van Kimmenade R, Lainchbury J, et al. NT-proBNP testing for diagnosis and short-term prognosis in acute destabilized heart failure: an international pooled analysis of 1256 patients: the International Collaborative of NT-proBNP Study. Eur Heart J. 2006;27:330–7. doi: 10.1093/eurheartj/ehi631. [DOI] [PubMed] [Google Scholar]
- 17.Cerrato E, D’Ascenzo F, Biondi-Zoccai G, et al. Cardiac dysfunction in pauci symptomatic human immunodeficiency virus patients: a meta-analysis in the highly active antiretroviral therapy era. Eur Heart J. 2013;34:1432–6. doi: 10.1093/eurheartj/ehs471. [DOI] [PubMed] [Google Scholar]
- 18.Friis-Møller N, Thiébaut R, Reiss P, et al. Predicting the risk of cardiovascular disease in HIV-infected patients: the data collection on adverse effects of anti-HIV drugs study. Eur J Cardiovasc Prev Rehabil. 2010;17:491–501. doi: 10.1097/HJR.0b013e328336a150. [DOI] [PubMed] [Google Scholar]
- 19.Eggers KM, Armstrong PW, Califf RM, et al. ST2 and mortality in non-ST-segment elevation acute coronary syndrome. Am Heart J. 2010;159:788–94. doi: 10.1016/j.ahj.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 20.Miyagaki T, Sugaya M, Yokobayashi H, et al. High levels of soluble ST2 and low levels of IL-33 in sera of patients with HIV infection. J Invest Dermatol. 2011;131:794–6. doi: 10.1038/jid.2010.366. [DOI] [PubMed] [Google Scholar]
- 21.Holloway CJ, Ntusi N, Suttie J, et al. Comprehensive Cardiac Magnetic Resonance Imaging and Spectroscopy Reveal a High Burden of Myocardial Disease in HIV Patients. Circulation. 2013;128:814–822. doi: 10.1161/CIRCULATIONAHA.113.001719. [DOI] [PubMed] [Google Scholar]
- 22.Moyers BS, Secemsky EA, Vittinghoff E, et al. Effect of Left Ventricular Dysfunction and Viral Load on Risk of Sudden Cardiac Death in Patients With Human Immunodeficiency Virus. Am J Cardiol. 2014;113:1260–5. doi: 10.1016/j.amjcard.2013.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wollert KC, Kempf T, Peter T, et al. Prognostic value of growth-differentiation factor-15 in patients with non-ST-elevation acute coronary syndrome. Circulation. 2007;115:962–71. doi: 10.1161/CIRCULATIONAHA.106.650846. [DOI] [PubMed] [Google Scholar]
- 24.Kempf T, von Haehling S, Peter T, et al. Prognostic utility of growth differentiation factor-15 in patients with chronic heart failure. J Am Coll Cardiol. 2007;50:1054–60. doi: 10.1016/j.jacc.2007.04.091. [DOI] [PubMed] [Google Scholar]
- 25.Nickel N, Kempf T, Tapken H, et al. Growth differentiation factor-15 in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2008;178:534–41. doi: 10.1164/rccm.200802-235OC. [DOI] [PubMed] [Google Scholar]
- 26.Nickel N, Jonigk D, Kempf T, et al. GDF-15 is abundantly expressed in plexiform lesions in patients with pulmonary arterial hypertension and affects proliferation and apoptosis of pulmonary endothelial cells. Respir Res. 2011;12:62. doi: 10.1186/1465-9921-12-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jain N, Reddy DH, Verma SP, et al. Cardiac Abnormalities in HIV-Positive Patients: Results from an Observational Study in India. J Int Assoc Physicians AIDS Care (Chic) 2012 doi: 10.1177/1545109712456740. [DOI] [PubMed] [Google Scholar]
- 28.Lebech A-M, Gerstoft J, Hesse B, Petersen CL, Kjaer A. Right and left ventricular cardiac function in a developed world population with human immunodeficiency virus studied with radionuclide ventriculography. Am Heart J. 2004;147:482–8. doi: 10.1016/j.ahj.2003.09.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.