Abstract
Introduction
N-Myc downstream-regulated gene 1 (NDRG1) expression is increased in placentas of human pregnancies with intrauterine growth restriction and in hypoxic cultured primary trophoblasts. We previously showed that elevated NDRG1 decreases trophoblast apoptosis induced by hypoxia. Separately, we found that pomegranate juice (PJ) decreases cell death induced by hypoxia in trophoblasts. Here, we test the hypothesis that PJ protects trophoblasts from hypoxia-induced apoptosis by modulating NDRG1 expression.
Methods
Quantitative rtPCR was used to investigate the effects of PJ treatment on mRNA levels of 22 candidate genes involved in apoptosis, oxidative stress, and differentiation in trophoblasts. Western blotting and immunofluorescence were used to analyze NDRG1 protein levels. siRNA-mediated NDRG1 knockdown was used to investigate the role of NDRG1 in response to PJ in hypoxic BeWo choriocarcinoma cells and hypoxic cultured primary human trophoblasts.
Results
The mRNA levels of eight genes were altered, with NDRG1 showing the largest response to PJ and thus, we pursued the role of NDRG1 here. PJ significantly increased NDRG1 protein expression in primary trophoblasts and in BeWo cells. Knockdown of NDRG1 in hypoxic BeWo cells in the presence of PJ yielded increased apoptosis. In contrast, knockdown of NDRG1 in hypoxic primary trophoblasts in the presence of PJ did not increase apoptosis.
Discussion
We conclude that the PJ-mediated decrease in cell death in hypoxia is partially mediated by NDRG1 in BeWo cells but not in primary trophoblasts. The disparate effects of NDRG1 between BeWo cells and primary trophoblasts indicate caution is required when extrapolating from results obtained with cell lines to primary trophoblasts.
1. Introduction
Normal placental development and function are keys to a successful pregnancy. Pre-eclampsia and intrauterine growth restriction (IUGR) are often associated with placental dysfunction, which is in part due to maldevelopment and in part to increased placental oxidative stress. Pre-eclampsia and IUGR also associate with short-term and long-term adverse health consequences for both mother and offspring [1]. Thus, dissection of the mechanisms by which villous trophoblast responds to oxidative stress is critical for the identification of prophylactic or therapeutic approaches to ameliorate injury.
N-myc downstream-regulated gene1 (NDRG1) belongs to a family of proteins (NDRG1-4) implicated in many cellular processes, including differentiation, proliferation and invasion [2,3]. NDRG1 is expressed in diverse cell types and functionally interacts with p53, HIF-1α, N-Myc, c-Myc, and AP-1 [2,3]. NDRG1 appears to have complex roles, being implicated in cell-cycle regulation, vesicular transport and in cellular responses to stress [2,4,5]. Missense mutations in NDRG1 cause hereditary motor and sensory neuropathy, an autosomal-recessive form of Charcot-Marie-Tooth disease [6]. Several lines of evidence suggest NDRG1 is important in placental development and the response of placental trophoblasts to stress. First, pups of Ndrg1-null mice display an IUGR phenotype and an increased rate of hypoxia-induced death of female embryos [7]. Second, NDRG1 is expressed in vivo in human placental villous trophoblasts and its expression is elevated in pregnancies complicated by IUGR [8,9]. Third, we [8] and others [10] found that NDRG1 expression in cultured primary villous trophoblasts is induced by hypoxia and the hypoxia mimetic, cobalt chloride (CoCl2), but not by non-hypoxic stressors. Similarly, NDRG1 expression is also increased by hypoxia and CoCl2 in BeWo cells [10], a commonly used model thought to mimic villous trophoblasts. BeWo cells are a human choriocarcinoma derived cell line that exhibits many characteristics of primary villous trophoblasts, including the ability to fuse to form multinucleated syncytia and to secrete placental lactogen and chorionic gonadotropin [11,12]. The function of NDRG1 in BeWo cells is uninvestigated. However, using lentiviral-mediated siRNA knockdown of NDRG1 in primary trophoblasts, we found that reduction of NDRG1 in hypoxia increases apoptosis [8], indicating that NDRG1 can provide protection from stress-induced trophoblast death.
Pomegranate juice (PJ) is a food replete with polyphenols with antioxidant activity and other biological effects [13-17]. We showed previously that PJ reduces oxidative stress in human placental villi in vitro and in vivo and that PJ limits apoptosis in both villous explants and cultures of primary human trophoblasts exposed to hypoxia and other inducers of cell death [18,19]. Importantly, we found that at least part of the mechanism by which PJ-mediates attenuation of hypoxia-induced apoptosis in cultured trophoblasts involves down-regulation of p53 [18,19]. Thus, like NDRG1, PJ can provide protection from stress-induced trophoblast death. We used quantitative rtPCR to screen 22 candidate genes predicted to participate in the trophoblast responses to stimuli and found that NDRG1 mRNA levels were markedly enhanced in trophoblasts exposed to PJ, compared to control. We thus tested the hypothesis that PJ protects trophoblasts from hypoxia-induced apoptosis by modulating the expression of NDRG1.
2. Materials and Methods
2.1. Culture of primary human trophoblasts (PHTs) and BeWo choriocarcinoma cells
The Institutional Review Board of Washington University School of Medicine in St. Louis approved this study. Primary human cytotrophoblasts were isolated from normal term placentas of uncomplicated pregnancies at 39 weeks’ gestation after delivery by repeat C-section under epidural anesthesia, as described previously [8,20].
Cytotrophoblasts were plated at a density of 300,000 cells/cm2 and cultured at 37°C under 5% CO2 and 95% ambient air (with ~20% oxygen, termed as standard conditions) in Dulbecco’s Modified Eagle’s Medium (Life Technologies, Grand Island, NY) with 20 mM HEPES (Sigma, St. Louis, MO), 100 units/ml penicillin and 100 μg/ml streptomycin (hereafter referred to as DMEM) with 10% fetal bovine serum (FBS; Hyclone, Logan, UT). Cells were allowed to attach for 4 h, rinsed three times with DMEM to remove syncytial fragments and unattached cells [21] and then replenished with fresh, phenol-red-free DMEM (Life Technologies) with 10% charcoal stripped FBS (csFBS). To determine the effects of PJ on NDRG1 expression in standard conditions, cells were cultured for a total of 24 h under standard conditions with glucose or PJ added for the final 3, 8, or 24 h, as indicated. To determine the effects of PJ on NDRG1 and cleaved Parp expression under hypoxia, medium was replenished and cells were pretreated with PJ (1% vol/vol; POM Wonderful LLC, Los Angeles, CA) or with glucose (1% vol/vol of a 7.5 mM glucose solution) for 8 h under standard conditions and then transferred to hypoxia with hypoxia-equilibrated medium containing either PJ or glucose, and culture was continued an additional 16 h. All hypoxia experiments were conducted in an anaerobic glove box chamber that allowed pre-gassing of medium and handling of cultures without exposure to ambient conditions. For CoCl2 treatment, cells were cultured for a total of 24 h standard conditions with 200 μM of CoCl2 (Sigma) present for the last 4 h of culture. BeWo cells were maintained in DMEM until exposure to PJ, glucose or transfection reagents, as specified in the corresponding figure legends.
2.2. Quantitative rtPCR
RNA was obtained from primary trophoblasts using Tri-Reagent (Molecular Research Center, Inc., Cincinnati, OH) according to the manufacturer’s instructions. Purified RNA was treated for 1 h at 37 °C with DNas e I (DNA-free, Ambion, Austin, TX) to remove contaminating DNA. Reverse transcription to generate cDNA was performed using the High capacity cDNA reverse transcription reagents kit (Applied Biosystems) in a 50-μl reaction mix that contained 1 μg of total RNA, 1X RT buffer, 0.5 mM of each dNTP, 2.5 μM random hexamers, 0.4 units/μl RNAase inhibitor, and 1.25 units/μl Multi-Scribe reverse transcriptase, at 25 °C for 10 min, 37 °C for 120 min, and 85 °C for 10 min. For the PCR reaction, two microliters of cDNA was used with a 300 nM each of the forward and reverse gene-specific primers (Table 2) and SYBR green PCR Master Mix (Applied Biosystems) in a total reaction volume of 20 μl. Dissociation curves were evaluated for all reactions to ensure amplification of a single product with the appropriate melting temperature. Samples were normalized to parallel reactions, which contained primers specific for YWHAZ [22,23]. The fold changes of the examined genes were determined using the 2−ΔΔCt method [24].
Table 2.
Primers used for rtPCR.
| Gene | Primer | Sequence |
|---|---|---|
| BAD | F | ATC ATG GAG GCG CTG GGG CT |
| R | CTG GGC TCC TCC CCC ATC CC | |
| BECLIN | F | CCA GGA TGG TGT CTC TCG CA |
| R | CTG CGT CTG GGC ATA ACG CA | |
| P53 | F | AGA GAC CGG CGC ACA GAG GA |
| R | GGC TGG GGA GAG GAG CTG GT | |
| MDM2 | F | CCC TGG TTA GAC CAA AGC CAT |
| R | GGC ACG CCA AAC AAA TCT CC | |
| BAK | F | GCT CGC CAT CAT CGG GGA CG |
| R | CCA CTC TCA AAC AGG CTG GTG GC | |
| BID | F | CCT ACC CTA GAG ACA TGG AGA AG |
| R | TTT CTG GCT AAG CTC CTC ACG | |
| PUMA | F | GAC CTC AAC GCA CAG TAC GAG |
| R | AGG AGT CCC ATG ATG AGA TTG T | |
| NOXA | F | ACC AAG CCG GAT TTG CGA TT |
| R | ACT TGC ACT TGT TCC TCG TGG | |
| BNIP3 | F | GTT CCA GCC TCG GTT TCT ATT |
| R | AGC CCT GTT GGT ATC TTG TG | |
| BAX | F | AGG ATG CGT CCA CCA AGA AG |
| R | GCA GCT CCA TGT TAC TGT CCA G | |
| MCL1 | F | AGG CTG GGA TGG GTT TGT GGA GT |
| R | ACC TGC AAA AGC CAG CAG CA CA | |
| NDRG1 | F | CCG CCA GCA CAT TGT GAA T |
| R | GGC TGT TGT AGG CAT TGA TGA A | |
| VEGFA | F | GGA GCG TGT ACG TTG GTG CCC |
| R | AGC AAG GCC CAC AGG GAT GGG | |
| SFLT2 | F | ACA ATC AGA GGT GAG CAC TGC AA |
| R | TCC GAG CCT GAA AGT TAG CAA | |
| FLT1 | F | TGG CAG CGA GAA ACA TTC TTT TAT C |
| R | CAG CAA TAC TCC GTA AGA CCA CAC | |
| PLGF | F | CAG ACT GCC ACC TGT GCG GC |
| R | GGT GCG GGG TCT CTC TCC TCC | |
|
BETA-
HCG |
F | CGG GAC ATG GGC ATC CAA |
| R | GCG CAC ATC GCC GTA GTT | |
| SYNCYTIN | F | GAA GGC CCT TCA TAA CCA ATG A |
| R | GAT ATT TGG CTA AGG AGG TGA TGT C | |
| COX2 | F | TGA TTG CCC GAC TCC CTT GGG T |
| R | TG GTG AAA GCT GGC CCT CGC | |
| INOS | F | CGT GTT CCC CCA GCG GAG TG |
| R | ACG TTG GCA GGG TCC CCT CT | |
| ENOS | F | TCC CTA CTC CCA CCA GCG CC |
| R | GTG CAG GGC CCA TCC TGC TG | |
| PON2 | F | TCG GGG GAC ATC TGG GTA GGC |
| R | TGC GGA GAA CCT CTG ACG AGG G | |
| YWHAZ | F | ACT TTT GGT ACA TTG TGG CTT CAA |
| R | CCG CCA GGA CAA ACC AGT AT |
F-forward; R- reverse
2.3. Immunofluorescence
PHTs were cultured in standard conditions in the presence of PJ- or glucose-containing DMEM for 24 h and then fixed with methanol for 20 min at −20°C. After blocking for 1 h at room temperature with PBS with 5% bovine serum albumin (Sigma), fixed cells were incubated with either no primary antibody or a mixture of anti-NDRG1 (1:200; Catalog # 426200, Life Technologies) and anti-E-cadherin (1:200; Catalog # 18-0223, Life Technologies) antibodies overnight at 4°C. Cells were washed three times with PBS and incubated with a 1:500 dilution of DRAQ5 (Biostatus Unlimited, Leicestershire, UK) to stain DNA, and with appropriate secondary antibodies, as described [25]. One-micron-thick optical-section images were acquired by confocal microscopy using 600X total magnification and identical acquisition settings for all samples, as described [25]. The percent of cells expressing detectable NDRG1 was scored in five random fields, containing over 50 nuclei each, using trophoblasts from three different placentas, with the observer blinded to the treatment condition.
2.4. NDRG1 knockdown in BeWo cells and PHTs
BeWo cells were plated at a density of 50,000/cm2 in DMEM for 24 h and medium exchanged with OPTI-MEM reduced serum medium with 10% csFBS containing HEPES, sodium bicarbonate and L-glutamine (Life Technologies), and then transfected with three siNDRG1 RNAs (Ambion, Grand Island, NY; ID: s20334, s20335, s20336, designated as siNDRG1 A, B, and C, respectively) at 10 nM using DharmaFECT 1 (Ambion). 10 nM scrambled siRNA (Ambion: catalog # 4390843) was used as control. The duration of treatment with the siRNA was 24 h under standard conditions, after which the cells were washed, fresh DMEM with 10% csFBS was added, and culture was continued under standard conditions in the presence of PJ or glucose for 8 h. Media was then exchanged with pre-equilibrated, hypoxia-exposed media, and cells were cultured an additional 16 h in ≥1% O2 in the continued presence of PJ or glucose. Proteins were extracted after a total duration of 48 h of culture.
PHTs were plated at a density of 300,000/cm2 in DMEM under standard conditions and washed 4 h after plating to eliminate syncytial fragments and cell debris. Cells were then transferred to OPTI-MEM with 10% csFBS and transfected with 50 nM siNDRG1 A with DharmaFECT 1 for 16 h under standard conditions. After washing, fresh DMEM with 10% csFBS was added and culture was continued under standard conditions in the presence of either PJ or glucose for 8 h. Medium was then exchanged with pre-equilibrated, hypoxia-exposed medium, and PHTs were cultured an additional 16 h in ≤1% O2, in the continued presence of PJ or glucose. Proteins were extracted after a total duration of 48 h of culture.
2.5. Western blotting
Protein isolation and Western blotting were done as described previously [26]. PHTs and BeWo cells cultured in 35 mm dishes were rinsed with PBS, lysed with 200 μl of RIPA buffer (1% Nonidet P-40, 0.5% deoxycholate, and 0.1% SDS in PBS) containing protease and phosphatase inhibitors (Sigma), sonicated and centrifuged. Soluble proteins were separated by SDS-PAGE and transferred to polyvinylidene difluoride membranes, blocked for 1 h with 5% nonfat dry milk in PBST (PBS with 0.05% Tween 20) and incubated overnight with either rabbit polyclonal anti-NDRG1 (1:1000; Catalog # 42-6200; Life Technologies), monoclonal rabbit anti-cleaved Parp (1:1000; Catalog #9541S; Cell Signaling Technology, Danvers, MA), monoclonal mouse anti-cleaved cytokeratin 18 (1:1000; Catalog # 12140322001, Roche Diagnostics, Indianapolis, IN), monoclonal mouse anti-p53 (1:1000; Catalog # SC-126, Santa Cruz Biotechnology, Dallas, TX) or polyclonal goat anti-actin (1:1000; Catalog # SC-1616, Santa Cruz). Blots were washed, incubated for >2 h with horseradish peroxidase-conjugated donkey anti-rabbit, anti-mouse or anti-goat IgG antibodies (1:10,000, Santa Cruz), washed, and target proteins detected by enhanced chemiluminescence (Amersham Biosciences, Piscataway, NJ). NDRG1, cl-Parp, and cl-Cyt18, p53, and actin protein levels were determined using ImageJ software (rsbweb.nih.gov/ij/) after normalization to actin.
2.6. Data analyses and statistics
All experiments with primary trophoblasts used cells isolated from four or more different placentas. Each experiment with BeWo cells was repeated at least three times. Statistical tests used are described in the text or Figure legends and were performed using KaleidaGraph software (Synergy Software, Reading, PA), with P ≥ 0.05 considered significant.
3. Results
3.1. PJ affects gene expression in cultured primary human trophoblasts
Our previous study indicated that PJ decreases hypoxia-induced oxidative stress and apoptosis in placental trophoblasts [18,26]. To identify potential pathway(s) involved in PJ-mediated protection of trophoblasts, we cultured primary trophoblasts under ambient oxygen and treated the cells with either glucose (as control) or pomegranate juice (PJ) for 24 h. We then used quantitative rtPCR to compare expression of 22 candidate genes that were chosen on the basis of their known involvement in apoptosis, oxidative stress, and trophoblast differentiation (Table 1). PJ significantly altered the mRNA levels of eight genes. NDRG1 showed a marked, ~9 fold increase, in mRNA expression. BID, BAK, BETA-HCG and INOS levels were increased by 1-2 fold, and we found decreased levels of ENOS, NOXA and SYNCYTIN.
Table 1.
PJ effects on candidate gene mRNA expression in cytotrophoblasts.
| Gene | na | Fold expression in glucoseb |
Fold expression in PJb |
P-value |
|---|---|---|---|---|
| Apoptosis | ||||
| BAD | 3 | 1.0(0.01) | 0.6(0.54) | 0.26 |
| BECLIN | 6 | 1.0(0.04) | 1.0(1.29) | 0.92 |
| P53 | 8 | 1.0(0.004) | 1.0(0.15) | 0.86 |
| MDM2 | 7 | 1.0(0.03) | 1.7(1.07) | 0.40 |
| BAK | 7 | 1.0(0.02) | 1.6(0.65) | 0.03 |
| BID | 8 | 1.0(0.05) | 2.3(1.24) | <0.01 |
| PUMA | 7 | 1.0 0.01) | 1.4(1.28) | 0.38 |
| NOXA | 9 | 1.0(0.003) | 0.3(0.22) | <0.01 |
| BNIP3 | 9 | 1.0(0.16) | 1.6(2.13) | 0.44 |
| BAX | 9 | 1.0(0.02) | 0.9(0.43) | 0.47 |
| MCL1 | 7 | 1.0(0.02) | 1.1(0.58) | 0.49 |
| Up-regulated in hypoxia | ||||
| NDRG1 | 9 | 1.0(0.02) | 9.4(7.06) | <0.01 |
| VEGFA | 7 | 1.0(0.12) | 2.2(1.85) | 0.13 |
| SFLT2 | 5 | 1.0(0.02) | 0.8(0.60) | 0.65 |
| FLT1 | 7 | 1.0(0.07) | 0.7(0.73) | 0.38 |
| PLGF | 6 | 1.0(0.00) | 1.6(1.67) | 0.36 |
| Differentiation | ||||
| BETA-HCG | 5 | 1.1(0.14) | 2.4(0.68) | <0.01 |
| SYNCYTIN | 7 | 1.0(0.004) | 0.5(0.23) | <0.01 |
| Oxidative stress response | ||||
| COX2 | 6 | 1.0(0.01) | 7.7(7.78) | 0.07 |
| INOS | 6 | 1.0(0.01) | 1.6(0.44) | <0.01 |
| ENOS | 9 | 1.0(0.01) | 0.3(0.27) | <0.01 |
| PON2 | 7 | 1.0(0.01) | 1.3(0.77) | 0.27 |
Candidate genes are grouped into four categories based on presumed function. Gene expression was analyzed by quantitative rtPCR and normalized to YWHAZ.
Number of different placentas from which cytotrophoblasts were isolated for quantitative rtPCR.
Mean(Standard Deviation)
cStudent's t-test vs. glucose
PJ: pomegranate juice.
3.2. PJ enhances NDRG1 protein expression in primary trophoblasts and BeWo cells under conditions of ambient oxygen
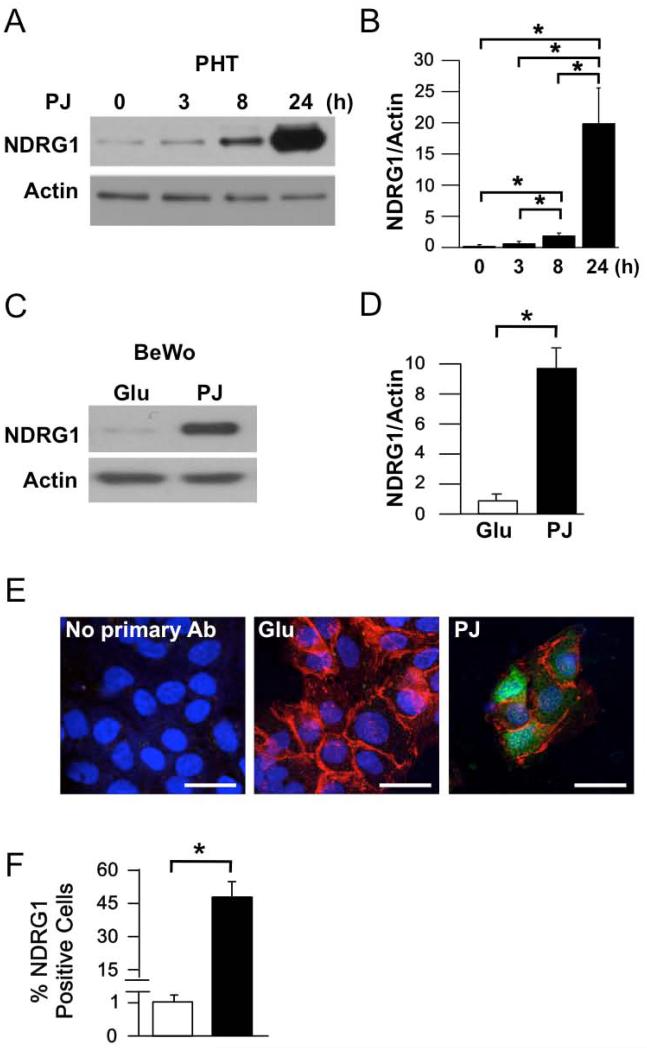
NDRG1 protein expression is increased in placental trophoblast by hypoxia and other stressors [8-10], as well as in placentas from pregnancies with IUGR [9], compared to placentas from uncomplicated pregnancies. We thus examined the effects of PJ on NDRG1 protein expression in the primary cytotrophoblasts under ambient oxygen. In agreement with the observed increase in mRNA levels, PJ increased NDRG1 protein levels in a time-dependent manner in cytotrophoblasts (Fig. 1A, B). Similarly, PJ increased NDRG1 protein levels in BeWo cells (Fig. 1C, D).
Figure 1. Effect of PJ on NDRG1 protein expression in primary human trophoblasts (PHTs) and BeWo choriocarcinoma cells.
Cells were cultured in 20% O2 for 24 h in the presence of glucose (Glu) or pomegranate juice (PJ). (A) Representative Western blot of NDRG1 in PHTs treated with PJ for the times indicated. (B) Quantification of NDRG1 levels, as represented in A (n=5 PHTs, *P ≤ 0.05 by ANOVA). (C) Representative Western blot of NDRG1 in BeWo cells treated with Glu or PJ for 24 h. (D) Quantification of NDRG1 levels in BeWo cells (n=3 experiments, *P ≤ 1.5 by Student's t-test). (E) Immunofluorescence of NDRG1 in PHTs stained for plasma membrane E-cadherin (red), nuclear DNA (blue), and NDRG1 (green). Scale bars: 10 μm. (F) Quantification of immunofluorescence, as represented in E. Percent of cells in which NDRG1 was detectable were 0% for no primary antibody 1.1±2% and 47.2±8.0% for glucose and PJ treated cells, respectively. (Mean±standard deviation, n=4 PHTs, *P ≤ 0.05 by Student's t-test).
Recent studies show that, in addition to increasing NDRG1 protein levels, hypoxia can result in subcellular redistribution of NDRG1 in trophoblasts, yielding substantial nuclear localization in addition to cytoplasmic localization [10]. We thus used immunofluorescence to assay NDRG1 expression and localization in PJ-treated primary trophoblasts. As expected, NDRG1 expression was significantly increased by PJ, with NDRG1 detectable in both the cytoplasm and nuclei of about half of the cells after exposure to PJ (Fig. 1E, F).
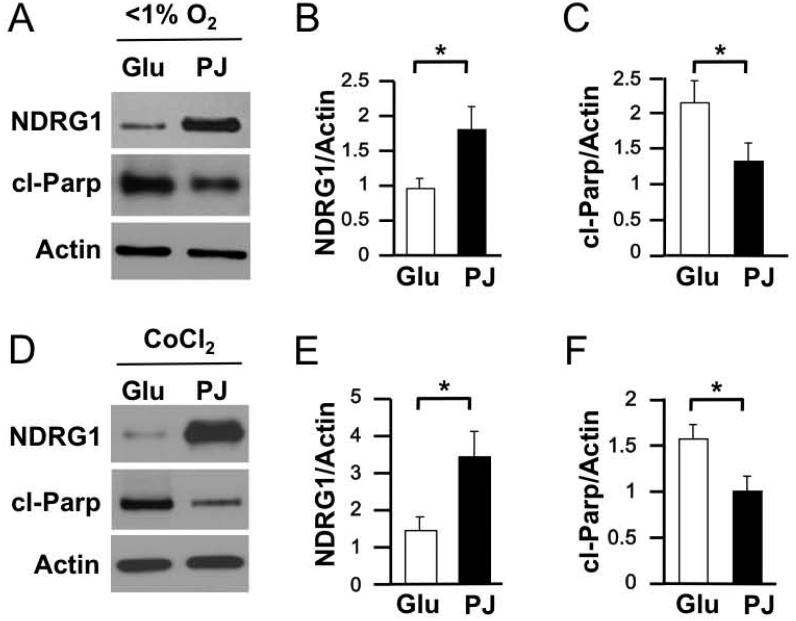
3.3. PJ increases NDRG1 expression in primary trophoblasts under hypoxia
We next asked whether PJ enhances NDRG1 expression in primary trophoblasts under hypoxia. Indeed, PJ significantly increased NDRG1 expression in the hypoxic primary trophoblasts compared to hypoxic trophoblasts exposed to glucose control (Fig. 2A, B). We also found that PJ increased NDRG1 expression in primary trophoblasts exposed to CoCl2, a hypoxia mimetic (Fig. 2D, E). Using cleaved Parp (cl-Parp) as a nuclear marker for apoptosis, we found that PJ reduced apoptosis in both hypoxic (Fig. 2A, C) and CoCl2-treated (Fig. 2D, F) cytotrophoblasts, compared to controls, confirming our previous results with cultured syncytiotrophoblasts [18,26].
Figure 2. Expression of NDRG1 and cl-Parp in PHTs in response to PJ in the presence of oxidative stressors.
PHTs were cultured in DMEM with glucose or PJ in 20% O2 for 8 h and then subjected to ≤1% O2 for 16 h or cultured initially in 20% O2 for 20 h and then exposed to CoCl2 for 4 h. (A, D) Representative Western blots of NDRG1 and cl-Parp in PHTs exposed to hypoxia (A) or CoCl2 (D). (B, C, E, F) Quantification of NDRG1 (B, E) and cl-Parp (C, F) in PHTs exposed to hypoxia (B, C) or cobalt chloride (E, F), as represented in A and D. (n=4 PHTs and n=3 BeWo experiments, *P ≤ 0.05 by Student's t-test).
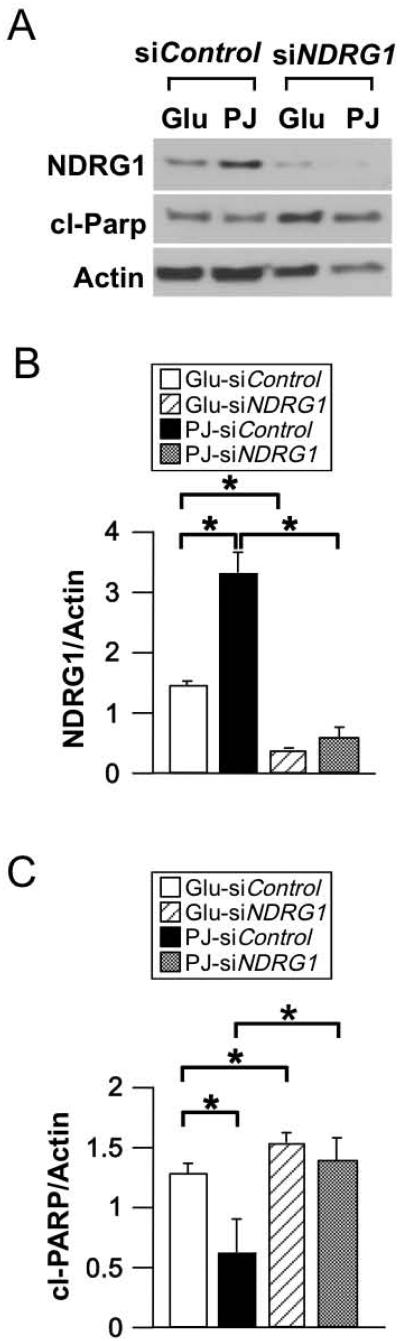
3.4. Silencing of NDRG1 in hypoxic BeWo cells in the presence of PJ increases apoptosis
We next asked if PJ-mediated induction of NDRG1 contributes to the ability of PJ to reduce hypoxia-induced apoptosis in BeWo cells. First, we noted that PJ increased NDRG1 levels in hypoxic BeWo cells, compared to glucose control (Fig. 3A, B). Next, we tested three candidate siRNAs to reduce NDRG1 expression (see Methods), and found siNDRG1 A most effective, yielding ~80% reduction of NDRG1 protein levels in hypoxic BeWos treated with PJ or glucose (Fig. 3A, B). Consistent with our findings with primary trophoblasts, PJ reduced apoptosis of BeWo cells cultured in hypoxia compared to glucose control (Fig. 3A, C). Moreover, in both glucose- and PJ-treated hypoxic BeWo cells, knockdown of NDRG1 significantly increased apoptosis (Fig. 3A,C). Together, these data indicate that PJ reduces hypoxia-induced apoptosis in BeWo cells, and, notably, that the PJ-mediated increase of NDRG1 contributes to the PJ-mediated decrease in apoptosis of hypoxic BeWos.
Figure 3. Effect of knockdown of NDRG1 on apoptosis in BeWo cells subjected to hypoxia in the presence of PJ or glucose.
BeWo cells were incubated for 24 h in 20% O2 with siNDRG1 or scrambled siRNA control. Cells were then pretreated in DMEM with either PJ or glucose in 20% O2 for 8 h and then exposed to ≤1% O2 for 16 h while continuing PJ or glucose treatment. (A) Representative Western blots for NDRG1 in transfected BeWo cells. (B, C) Quantification of NDRG1 (B) and cl-Parp (C), as represented in A. (n=3 experiments, *P ≤ 0.05 by Student's t-test).
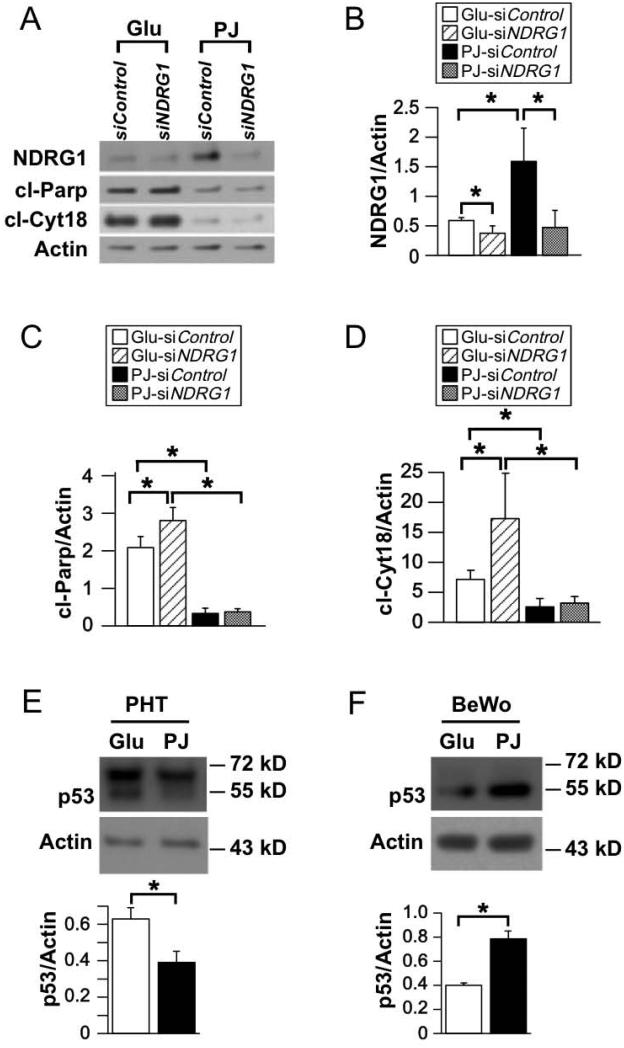
3.5. Silencing of NDRG1 in hypoxic primary trophoblasts in the presence of PJ did not increase apoptosis
We then asked if the PJ-mediated increase in NDRG1 levels played a role in reducing apoptosis in hypoxic primary trophoblasts. In these experiments, we used 50 nM siNDRG1 A, as this concentration was required to generate sufficient knockdown of NDRG1. We achieved >50% reduction in NDRG1 protein in the presence of glucose and ~70% in the presence of PJ (Fig. 4A, B). Consistent with our previous results using lentiviral-mediated knockdown [8], siRNA-mediated knockdown of NDRG1 in control (glucose) treated hypoxic trophoblasts resulted in increased apoptosis, as indicated by increased levels of cleaved Parp and of cleaved cytokeratin 18, a cytoplasmic marker for apoptosis (Fig. 4C, D). However, in PJ-treated hypoxic primary trophoblasts, knockdown of NDRG1 did not result in increased apoptosis compared to siRNA-control treated primary trophoblasts. Thus, in contrast to BeWo cells, the PJ-mediated increase of NDRG1 in hypoxic primary trophoblasts does not appear to contribute to the PJ-mediated reduction of apoptosis.
Figure 4. Effect of knockdown of NDRG1 on apoptosis in primary trophoblasts subjected to hypoxia in the presence of PJ or glucose.
Trophoblasts were cultured for 4 h in DMEM, transfected with siNDRG1 or scrambled siRNA control, treated with PJ or glucose for 8 h and then exposed to ≤1% O2 for 24 h while continuing PJ or glucose treatment. (A) Representative Western blots for NDRG1, cl-Parp, and cl-Cyt18 in transfected PHTs. (B-D) Quantification of NDRG1 (B), cl-Parp (C), and cl-Cyt18 (D), as represented in A. (n=4 PHTs, *P≤0.05 by Student’s t-test). (E, F) Representative Western blots (top) and quantification (bottom) for p53 in PHTs (E) and BeWos (F). (n=3 PHTs and n=6 experiments BeWos, *P ≤ 0.05 by Student’s t-test)
Down regulation of p53 is a potential mechanism by which PJ protects trophoblasts from hypoxia-induced injury [18,19]. Thus, we compared the affect of PJ on p53 in cytotrophoblasts and BeWo cells. We found that PJ significantly decreased p53 levels in hypoxic cytotrophoblasts but increased p53 levels in hypoxic BeWo cells (Fig. 4E, F).
4. Discussion
The data show that PJ selectively modulates gene expression in human cytotrophoblasts, with increased expression of BAD, BAK, INOS, BETA-HCG, and NDRG1 and decreased expression of NOXA, SYNCYTIN, and ENOS. Notably, PJ had the most marked effect on gene expression of NDRG1, which translated to substantially higher levels of NDRG1 in both the cytoplasm and nucleus. These results indicate that PJ can influence gene mRNA and protein levels in trophoblasts exposed to either standard culture conditions of 20% oxygen or hypoxia with ≤1% oxygen. Using siRNA-based knockdown of NDRG1, we found that PJ-induced expression of NDRG1 was important for the reduced apoptosis in hypoxic BeWo cells. However, PJ-mediated induction of NDRG1 was not detectably involved in PJ-mediated reduction of apoptosis in hypoxic primary trophoblasts. These data suggest that caution must be used in extrapolating results from trophoblast cell line models to primary trophoblasts.
We are investigating PJ as a potential therapeutic agent to protect human trophoblasts from exogenous insults. We previously found that PJ can reduce oxidative stress in vivo and in vitro, reduce apoptosis in primary trophoblasts exposed to hypoxia and other stressors, and that these protective effects involve, at least in part, reduction of p53 mRNA and protein [18,26]. PJ has been shown to affect the mRNA levels of PON1 in hepatocytes [16] and of PON2 in macrophages [17], indicating diverse biological activities of PJ. Our current finding that PJ significantly alters the mRNA levels of NDRG1 and other genes in trophoblasts are consistent with this idea, and suggest that the activity of PJ on trophoblasts involves more than simply acting as an antioxidant.
NDRG1 is expressed by many cell types, including trophoblasts, and NDRG1 function is complex, as noted in the Introduction. Using primary trophoblasts and BeWo choriocarcinoma cells, here we find that hypoxic stress increases NDRG1 levels in both primary trophoblasts and BeWo cells, in agreement with previous results by us [8] and others [10]. PJ treatment resulted in an increase of NDRG1 protein in both hypoxic primary trophoblasts and BeWo cells, suggesting additive effects of PJ and hypoxia. This finding, along with the above mentioned data showing that increased NDRG1 can reduce hypoxia-induced apoptosis, led us to test the hypothesis that the PJ-mediated induction of NDRG1 would further ameliorate apoptosis in hypoxic primary trophoblasts. Surprisingly, however, this was not the case: in these cells, siRNA-mediated knockdown of NDRG1 did not alter the ability of PJ to mediate protection from apoptosis. Thus, in hypoxic primary trophoblasts further induction of NDRG1 by PJ is not important for PJ-mediated protection from apoptosis.
In contrast, siRNA-mediated knockdown of NDRG1 significantly increased apoptosis in hypoxic BeWo cells treated with PJ. Thus, in this trophoblast cell line PJ-mediated induction of NDRG1 does contribute to PJ-mediated reduction of apoptosis. One contributor to the different role of NDRG1 in hypoxic, PJ-treated BeWos and primary trophoblasts may be technical, as knockdown was about 10% more efficient in BeWo cells than in primary cells. A second possibility is that NDRG1 plays qualitatively, or quantitatively, different roles in BeWo cells and primary trophoblasts. Notably, though both hypoxia and PJ enhance NDRG1 expression in both BeWo and primary trophoblasts, the fold increase differs between the two trophoblast lineages. Nonetheless, we can conclude that PJ alter NDRG1 mRNA and protein expression in both villous and choriocarcinoma trophoblast phenotypes.
Although often a good model system for villous trophoblasts, others have noted cases where BeWos differ from primary cells. For example, BeWos and primary trophoblasts differ in their DNA methylation [27,28], expression of the vitamin D receptor [27,29], and in their responses to activation by TLR ligands [30,31]. Here, we found that p53 responds to PJ treatment in opposite ways: PJ decreases p53 levels in hypoxic primary cytotrophoblasts but increases p53 levels in hypoxic BeWo cells. Together, these results indicate the need for strong caution when extrapolating from results obtained with cell lines to primary trophoblasts.
Highlights.
We studied the effects of pomegranate juice (PJ) on NDRG1 expression in human primary trophoblasts and BeWo cells.
PJ increased NDRG1 expression in primary trophoblasts and in BeWo cells in both standard and hypoxic conditions.
NDRG1 played a role in the PJ-mediated protection of BeWo cells from hypoxia.
NDRG1 did not detectably contribute to the PJ-mediated protection of primary trophoblasts from hypoxia.
Acknowledgments
We thank Dr. Deborah J. Frank for critical reading of the manuscript. This work was supported by NIH grant RO1 29190 to DMN and an unrestricted gift to the Washington University School of Medicine by POM Wonderful LLC, Los Angeles, CA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors declare they do not have any conflicts of interest. POM Wonderful LLC had no role in the design or interpretation of the experiments described or in reviewing, editing, or approving this work.
References
- [1].Longtine MS, Nelson DM. Placental dysfunction and fetal programming: The importance of placental size, shape, histopathology, and molecular composition. Semin Reprod Med. 2011;29:187–96. doi: 10.1055/s-0031-1275515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ellen TP, Ke Q, Zhang P, Costa M. NDRG1, a growth and cancer related gene: Regulation of gene expression and function in normal and disease states. Carcinogenesis. 2008;29:2–8. doi: 10.1093/carcin/bgm200. [DOI] [PubMed] [Google Scholar]
- [3].Melotte V, Qu X, Ongenaert M, Van Criekinge W, De Bruine AP, Baldwin HS, et al. The n-myc downstream regulated gene (NDRG) family: Diverse functions, multiple applications. FASEB J. 2010;24:4153–66. doi: 10.1096/fj.09-151464. [DOI] [PubMed] [Google Scholar]
- [4].Askautrud HA, Gjernes E, Gunnes G, Sletten M, Ross DT, Borresen-Dale AL, et al. Global gene expression analysis reveals a link between NDRG1 and vesicle transport. PLoS One. 2014;9:e87268. doi: 10.1371/journal.pone.0087268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kovacevic Z, Sivagurunathan S, Mangs H, Chikhani S, Zhang D, Richardson DR. The metastasis suppressor, N-myc downstream regulated gene 1 (NDRG1), upregulates p21 via p53-independent mechanisms. Carcinogenesis. 2011;32:732–40. doi: 10.1093/carcin/bgr046. [DOI] [PubMed] [Google Scholar]
- [6].Tazir M, Bellatache M, Nouioua S, Vallat JM. Autosomal recessive Charcot-Marie-Tooth disease: From genes to phenotypes. J Peripher Nerv Syst. 2013;18:113–29. doi: 10.1111/jns5.12026. [DOI] [PubMed] [Google Scholar]
- [7].Larkin J, Chen B, Shi XH, Mishima T, Kokame K, Barak Y, et al. NDRG1 deficiency attenuates fetal growth and the intrauterine response to hypoxic injury. Endocrinology. 2014;155:1099–106. doi: 10.1210/en.2013-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chen B, Nelson DM, Sadovsky Y. N-myc down-regulated gene 1 modulates the response of term human trophoblasts to hypoxic injury. J Biol Chem. 2006;281:2764–72. doi: 10.1074/jbc.M507330200. [DOI] [PubMed] [Google Scholar]
- [9].Choi SJ, Oh SY, Kim JH, Sadovsky Y, Roh CR. Increased expression of N-myc downstream-regulated gene 1 (NDRG1) in placentas from pregnancies complicated by intrauterine growth restriction or preeclampsia. Am J Obstet Gynecol. 2007;196:45. doi: 10.1016/j.ajog.2006.08.029. e1-7. [DOI] [PubMed] [Google Scholar]
- [10].Shi XH, Larkin JC, Chen B, Sadovsky Y. The expression and localization of N-myc downstream-regulated gene 1 in human trophoblasts. PLoS One. 2013;8:e75473. doi: 10.1371/journal.pone.0075473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Drewlo S, Baczyk D, Dunk C, Kingdom J. Fusion assays and models for the trophoblast. Methods in Molecular Biology. 2008;475:363–82. doi: 10.1007/978-1-59745-250-2_21. [DOI] [PubMed] [Google Scholar]
- [12].Orendi K, Gauster M, Moser G, Meiri H, Huppertz B. The choriocarcinoma cell line BeWo: Syncytial fusion and expression of syncytium-specific proteins. Reproduction. 2010;140:759–66. doi: 10.1530/REP-10-0221. [DOI] [PubMed] [Google Scholar]
- [13].Adams LS, Seeram NP, Aggarwal BB, Takada Y, Sand D, Heber D. Pomegranate juice, total pomegranate ellagitannins, and punicalagin suppress inflammatory cell signaling in colon cancer cells. J Agric Food Chem. 2006;54:980–5. doi: 10.1021/jf052005r. [DOI] [PubMed] [Google Scholar]
- [14].Adams LS, Zhang Y, Seeram NP, Heber D, Chen S. Pomegranate ellagitannin-derived compounds exhibit antiproliferative and antiaromatase activity in breast cancer cells in vitro. Cancer Prev Res (Phila) 2010;3:108–13. doi: 10.1158/1940-6207.CAPR-08-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Albrecht M, Jiang W, Kumi-Diaka J, Lansky EP, Gommersall LM, Patel A, et al. Pomegranate extracts potently suppress proliferation, xenograft growth, and invasion of human prostate cancer cells. J Med Food. 2004;7:274–83. doi: 10.1089/jmf.2004.7.274. [DOI] [PubMed] [Google Scholar]
- [16].Khateeb J, Gantman A, Kreitenberg AJ, Aviram M, Fuhrman B. Paraoxonase 1 (PON1) expression in hepatocytes is upregulated by pomegranate polyphenols: A role for ppar-gamma pathway. Atherosclerosis. 2010;208:119–25. doi: 10.1016/j.atherosclerosis.2009.08.051. [DOI] [PubMed] [Google Scholar]
- [17].Shiner M, Fuhrman B, Aviram M. Macrophage paraoxonase 2 (PON2) expression is up-regulated by pomegranate juice phenolic anti-oxidants via PPARγ and AP-1 pathway activation. Atherosclerosis. 2007;195:313–21. doi: 10.1016/j.atherosclerosis.2007.01.007. [DOI] [PubMed] [Google Scholar]
- [18].Chen B, Tuuli MG, Longtine MS, Shin JS, Lawrence R, Inder T, et al. Pomegranate juice and punicalagin attenuate oxidative stress and apoptosis in human placenta and in human placental trophoblasts. Am J Physiol Endocrinol Metab. 2012;302:E1142–52. doi: 10.1152/ajpendo.00003.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chen B, Longtine MS, Nelson DM. Punicalagin, a polyphenol in pomegranate juice, downregulates p53 and attenuates hypoxia-induced apoptosis in cultured human placental syncytiotrophoblasts. Am J Physiol Endocrinol Metab. 2013;305:E1274–80. doi: 10.1152/ajpendo.00218.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kliman HJ, Nestler JE, Sermasi E, Sanger JM, Strauss JF. Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology. 1986;118:1567–82. doi: 10.1210/endo-118-4-1567. [DOI] [PubMed] [Google Scholar]
- [21].Guilbert LJ, Winkler-Lowen B, Sherburne R, Rote NS, Li H, Morrish DW. Preparation and functional characterization of villous cytotrophoblasts free of syncytial fragments. Placenta. 2002;23:175–83. doi: 10.1053/plac.2001.0756. [DOI] [PubMed] [Google Scholar]
- [22].Meller M, Vadachkoria S, Luthy DA, Williams MA. Evaluation of housekeeping genes in placental comparative expression studies. Placenta. 2005;26:601–7. doi: 10.1016/j.placenta.2004.09.009. [DOI] [PubMed] [Google Scholar]
- [23].Murthi P, Fitzpatrick E, Borg AJ, Donath S, Brennecke SP, Kalionis B. GAPDH, 18S rRNA and YWHAZ are suitable endogenous reference genes for relative gene expression studies in placental tissues from human idiopathic fetal growth restriction. Placenta. 2008;29:798–801. doi: 10.1016/j.placenta.2008.06.007. [DOI] [PubMed] [Google Scholar]
- [24].Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative c(t) method. Nat Protoc. 2008;3:1101–8. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- [25].Longtine MS, Chen B, Odibo AO, Zhong Y, Nelson DM. Caspase-mediated apoptosis of trophoblasts in term human placental villi is restricted to cytotrophoblasts and absent from the multinucleated syncytiotrophoblast. Reproduction. 2012;143:107–21. doi: 10.1530/REP-11-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Chen B, Longtine MS, Sadovsky Y, Nelson DM. Hypoxia downregulates p53 but induces apoptosis and enhances expression of BAD in cultures of human syncytiotrophoblasts. Am J Physiol Cell Physiol. 2010;299:C968–76. doi: 10.1152/ajpcell.00154.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Novakovic B, Sibson M, Ng HK, Manuelpillai U, Rakyan V, Down T, et al. Placenta-specific methylation of the vitamin D 24-hydroxylase gene: Implications for feedback autoregulation of active vitamin D levels at the fetomaternal interface. J Biol Chem. 2009;284:14838–48. doi: 10.1074/jbc.M809542200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Pospechova K, Rozehnal V, Stejskalova L, Vrzal R, Pospisilova N, Jamborova G, et al. Expression and activity of vitamin D receptor in the human placenta and in choriocarcinoma BeWo and JEG-3 cell lines. Mol Cell Endocrinol. 2009;299:178–87. doi: 10.1016/j.mce.2008.12.003. [DOI] [PubMed] [Google Scholar]
- [29].Novakovic B, Gordon L, Wong NC, Moffett A, Manuelpillai U, Craig JM, et al. Wide-ranging DNA methylation differences of primary trophoblast cell populations and derived cell lines: Implications and opportunities for understanding trophoblast function. Mol Hum Reprod. 2011;17:344–53. doi: 10.1093/molehr/gar005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Komine-Aizawa S, Majima H, Yoshida-Noro C, Hayakawa S. Stimuli through toll-like receptor (TLR) 3 and 9 affect human chorionic gonadotropin (hCG) production in a choriocarcinoma cell line. J Obstet Gynaecol Res. 2008;34:144–51. doi: 10.1111/j.1447-0756.2008.00752.x. [DOI] [PubMed] [Google Scholar]
- [31].Tangeras LH, Stodle GS, Olsen GD, Leknes AH, Gundersen AS, Skei B, et al. Functional toll-like receptors in primary first-trimester trophoblasts. J Reprod Immunol. 2014;106:89–99. doi: 10.1016/j.jri.2014.04.004. [DOI] [PubMed] [Google Scholar]