Abstract
At late mitosis, the mother cell divides by the formation of a cleavage furrow, leaving two daughter cells connected by a thin intercellular bridge. During ingression of the cleavage furrow, the central spindle microtubules are compacted to form the structure known as the midbody (MB). The MB is situated within the intercellular bridge, with the abscission site sometimes occurring on one side of the MB. As a result of this one-sided (asymmetric) abscission, only one daughter cell can inherit the post-mitotic MB. Interestingly, recent studies have identified post-mitotic MBs as novel signaling platforms regulating stem cell fate and proliferation. Additionally, MBs were proposed to serve a role of polarity cues during the neurite outgrowth and apical lumen formation. Thus, abscission and MB inheritance is clearly a highly regulated cellular event that can affect development and various other cellular functions. In this review we discuss the latest findings regarding post-mitotic MB functions, as well as the machinery regulating MB inheritance and accumulation.
Keywords: cytokinesis, abscission, autophagy, recycling endosomes, midbody, midbody inheritance
INTRODUCTION
The last step of cell division is the separation of two daughter cells via a process known as cytokinesis. After replication of the genetic material, the mother cell divides by the formation of the cleavage furrow, leaving two daughter cells connected by a thin intracellular bridge (ICB) [1, 2]. The resolution of this bridge (known as abscission) results in the separation of the two daughter cells. Importantly, abscission has emerged as one of the major regulators of cell differentiation, tissue morphogenesis and carcinogenesis. Due to the importance of abscission, recent work from our laboratory and other laboratories has begun to identify and define the abscission machinery. As the result of this work, abscission is now recognized to involve localized remodeling of endocytic machinery and the cytoskeleton.
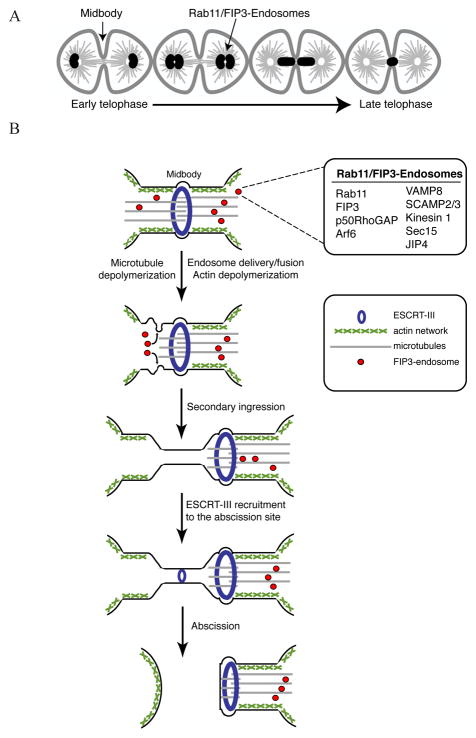
Recycling endosomes have emerged as important players in mediating abscission [3]. Specialized Rab11/FIP3-containing endosomes are transported to the cleavage furrow during late telophase (Fig. 1A) [4–7]. These Rab11/FIP3-endosomes initiate abscission by regulating localized disassembly of the cortical actin cytoskeleton (Fig. 1B) [5]. This leads to “secondary ingression”, which is the narrowing of the ICB. Further, the recruitment of ESCRT complexes to the narrowed ICB completes the abscission and the final separation of daughter cells (Fig. 1B) [4, 8, 9].
Figure 1.
(A) Schematic diagram of Rab11/FIP3-endosome dynamics during telophase. (B) The latest model of endosome and ESCRT roles during abscission and asymmetric midbody inheritance.
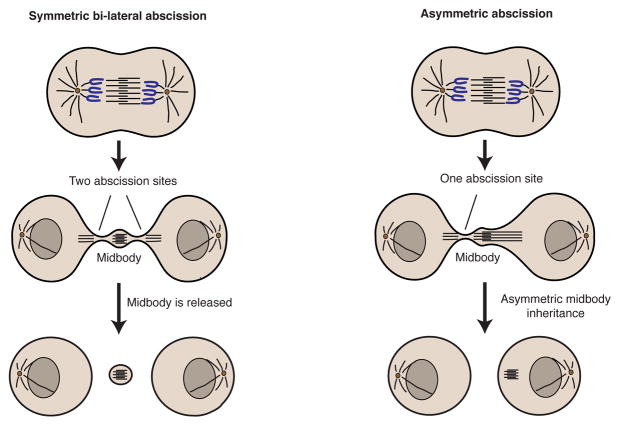
Abscission can sever the ICB on one (asymmetric abscission) or both (symmetric abscission) sides of the midbody (MB) (Fig. 2) [4, 5, 8]. In the case of asymmetric abscission, one of the daughter cells retains the MB (MB inheritance). Importantly, these post-mitotic MB derivatives have been found in undifferentiated, stem cell-like populations in vitro and in vivo [10–12]. Hence, MBs may function as signaling platforms that are asymmetrically inherited during cell division and determine the “stemness” of the daughter cell. Additionally, MBs may act as polarity cues during epithelia or neuronal morphogenesis [11, 13, 14]. Thus, it is clear that MBs have important post-mitotic roles and are major regulators of cell polarization and fate. In this review we will discuss the latest findings regarding post-mitotic MB functions, as well as the machinery regulating MB inheritance and accumulation.
Figure 2.
The mechanisms of midbody inheritance and degradation.
MIDBODY AND REGULATION OF CELL FATE AND DIFFERENTIATION
Midbody inheritance and release during development
Despite the fact that MB was originally described by Walther Flemming over 100 years ago, its function remains enigmatic to this day. Originally it was thought that these microtubule-rich MBs serve as diffusion barriers limiting cytoplasmic exchange during telophase. Since then, many important mitotic roles of MBs have been uncovered, such as the recruitment of Aurora B and Plk1 kinases that play important roles in orchestrating cellular abscission. Additionally, MBs regulate the final membrane scission step by serving as a staging station for microtubule severing enzyme spastin, as well as ESCRT complexes, which then translocate from the MB to the abscission site (Fig. 1B) [8, 15, 16]. It is generally assumed that MBs are transient structures that regulate mitosis and, after completion of mitotic division, are either released into the extracellular space or are rapidly degraded via autophagy. Interestingly, evidence gathered over the last decade suggests that MBs are also involved in non-mitotic functions. First, proteomic analysis demonstrated that MBs are very complex structures containing hundreds of proteins, some of them with known signaling roles during cell growth and differentiation [17, 18]. One protein found in MBs, prominin-1 (CD133), is a well-defined stem cell marker in both normal and tumorous epithelial tissue [19]. Prominin-1 is a pentaspanning membrane protein that accumulates at the MBs in dividing neuronal progenitor cells [20]. Importantly, prominin-1-rich MBs are released from progenitor cells [19], suggesting that the removal of prominin-1 is required for differentiation. Another MB protein, Core Binding Factor β (CBFβ), was shown to interact with Runt related (RUNX) transcription factors and regulate differentiation and proliferation in various tissues [21]. Since CBFβ can be asymmetrically inherited by one of the daughter cells, it is tempting to speculate that the inheritance and accumulation of CBFβ-rich MBs differentially affect the proliferation and/or differentiation in one of the daughter cells.
Recent studies have revealed the highly dynamic and complex processes that underlie MBs inheritance and release. In the event of asymmetric abscission a daughter cell inherits the MB, whereas during symmetric abscission MB is released into the extracellular milieu (Fig. 2). During normal development, cells usually release MBs. Stem cells and cancer cells, however, are more prone to undergoing asymmetric abscission with one of the daughter cells inheriting the MB [10, 12]. Furthermore, daughter cells with the older “mother” centriole tend to inherit the MB and possess a more stem-like phenotype [10]. Consistently, it was demonstrated that stem cells in mouse seminiferous tubules, neural progenitor cells (labeled by Sox2) and hair follicle stem cells (labeled by keratin 15) contain more MBs [10]. The induction of differentiation in NS5 and Neuro2a cells leads to cells switching from asymmetric to symmetric abscission, thus leading to increased MB release [12]. While multiple studies have clearly established the correlation between MBs inheritance and maintenance of stem-like characteristics, how exactly MBs may be regulating “stemness” remains unclear. It is possible that by virtue of inheriting MBs cells acquire a set of signaling and/or transcription factors that regulate cell proliferation and differentiation. Association of signaling/transcription factors with MB during mitosis would ensure asymmetric inheritance of these signaling molecules, thus leading to differential fate of daughter cells. Importantly, it is now well established that asymmetric cell division is a key step during neuronal development. It is likely that similar asymmetric division-dependent mechanisms also play a role during development of other tissues. However, the identities of signaling molecules that are inherited in MB-dependent fashion and the signaling pathways involved remain to be elucidated.
Intriguingly, a recent study suggested that not all stem cells retain MBs. It was shown that during asymmetric division of Drosophila germline stem cells (GSCs), male GSCs that inherit mother centriole exclude MBs, while female GSCs with daughter centrioles usually retain MBs [22]. These new findings are not consistent with a uniform MB inheritance mechanism and suggest that the role of MB inheritance/accumulation in regulating cell “stemness” likely depends on the cell and tissue type, as well as conditions that induce cell differentiation. Thus, additional studies, including comparative proteomic analysis of MB compositions in different tissues and cell types, will be needed to fully understand the roles of MB inheritance during development.
Midbodies and cellular signaling
One of the more intriguing suggestions is that MBs act as an asymmetrically positioned signaling scaffold that transfer signaling molecules to one daughter cell. To date, more than 300 MB proteins have been shown by either immunofluorescence or mass spectrometry [17, 18, 23]. Importantly, a large number of kinases and phosphatases are present in the MBs [23]. The idea that MBs may be play key signaling roles is enticing for several reasons. First, MBs are plastic. Ultrastructure analysis reveals that the composition of post-mitotic MBs is different from mitotic MBs, and various sizes of secreted MBs can be recovered from different cell lines [12, 20]. The difference of MB structure suggests that the MB components can be actively remodeled, perhaps to allow the strategic placement of signaling components to interact with cytoplasmic or extracellular growth factors and receptors. Second, post-mitotic MBs are long-lived structures that can persist in the cytoplasm for hours [24]. This provides ample time for MBs to interact with other signaling components in the cytoplasm.
Given that the components of several major signaling pathways have been found to localize to the MBs, we review a few examples of these pathways that may depend, at least in part, on MBs for post-mitotic signaling [23]. The Wnt signaling pathway is well studied for its role in embryogenesis such as body axis patterning, cell proliferation, migration and cell fate specification. Wnt signaling components are asymmetrically distributed to the daughter cell that has been exposed to Wnt ligand and serve to dictate stem cell fate [25, 26]. Several key components of the Wnt signaling pathway such as Frizzled 2, β-catenin and Dishevelled, are found in MBs during mitosis [27, 28] and may persist following mitosis. The accumulation of post-mitotic MBs may, therefore, polarize the Wnt signaling pathway in the more stem-like daughter cell to maintain “stemness”. Interestingly, the Wnt adaptor protein, Dvl, has been shown to bind the LIR motif of LC3 prior to undergoing autophagy [29]. Possibly Dvl contained in MBs may be necessary to target MBs to LC3 autophagosomes.
Chemokine signaling pathways are important for mobilization and homing of normal mesenchymal stem cells [30]. Moreover, the chemokine receptor CXCR4 has been suggested to maintain self-renewal and multipotency of neural stem cells [31]. In the context of cancer, CXCR4 is considered a biomarker for cancer stem cells and its expression reflects poor prognosis. CXCR4 is also known to promote metastasis in several solid tumor types [32, 33]. The heterotrimeric Giα proteins (molecular switches that bind to chemokine receptor) and several phospholipase C isoforms (downstream effector of chemokine receptor) have been found in MBs during cell division [34, 35]. While localization of these proteins in MBs serves to mediate cytokinesis, the potential involvement of chemokine receptor signaling in mediating stem cell renewal and chemotaxis post-mitosis should also be considered.
Finally, numerous studies have reported that the presence of MEK1/2 and ERK1/2 in MBs of mitotic cells [36, 37]. While MAP kinase is a well-known central regulator of cellular proliferation, activation of the MAP kinase signaling has also been shown to promote tumorigenicity by enhancing cancer stem cell phenotype [38, 39] and regulate stem cell fate [40]. In all, the vast signaling information potentially contained in MBs may have important implications in many fundamental biological processes post-mitosis. Due to simultaneous localization of these signaling proteins in various cellular compartments, deciphering the importance of these MB-dependent signaling pathways may not be straightforward and will likely require innovative experimental approaches..
Midbodies and cancer
Among normal dividing cells, stem cells and cancer cells, cancer cells contain the highest level of MB accumulation [10, 12]. In cultured cancer cells, a sub-population accumulates high levels of MBs and shows enhanced colony formation [10]. Similarly, cancer cells that are artificially induced to accumulate MBs show increased tumorigenic potential in vitro [10]. Moreover, sorted Side Populations (SP) from MCF-7 breast cancer cell line display greater MB accumulation when compared to the non-SP cells [10]. SP cells are a subset of cancer stem cells that express high level of ATP-Binding Cassette transporter, which endow these cells with ability to efflux chemotherapeutic drugs [41]. Taken together, these studies suggest that MB accumulation in cancer cells may contribute to the stem-like characteristics.
Intriguingly, the “stemness” of cancer cell is a highly dynamic process that can be lost or regained. Specifically, soluble factors from tumor microenvironment have been shown to convert differentiated cancer cell to a more primitive, stem-like state [42]. Given that secreted MBs can be engulfed by cancer cells [43] it is plausible that cancer cells that come in contact with secreted MBs at the extracellular milieu potentially could uptake and accumulate MBs to acquire “stemness”. It is likely that this process is highly regulated, although the pathways that mediate MB uptake have not yet been characterized.
The current consensus is that MB accumulation is associated with enhanced cellular proliferation, whereas MB disposal (by autophagic degradation or release into the extracellular milieu) is linked to cellular differentiation [12]. Unlike normal stem cells, cancer stem cells have uncontrolled proliferation due to their inability to regulate cell-cell contact inhibition and impaired growth inhibitory responses [44, 45]. Thus, MB inheritance and accumulation in cancers may contribute to the high proliferation and de-differentiation of these cancer cells. Similar to normal stem cells, cancer stem cells tend to have very active constitutive autophagy to maintain the self-renewal state [46]. Hence, the accumulation of MBs in cancer stem cells would require the cell to evade constitutive autophagy. Recent findings reveal that autophagy of cellular organelles and protein aggregates is a highly selective process [47]. The selectivity of autophagy is dependent upon the availability of cargo receptors and adaptors. Therefore, MBs in cancer stem cells may contain specific autophagic adaptors that distinguish them from more global autophagy pathways. If this is the case, MB accumulation could occur even in the cells with high general autophagic activity. More studies are needed, especially using animal models and primary tumor cells, to clearly define a connection between MB accumulation and cancer progression.
Midbody, the regulator of cell polarity
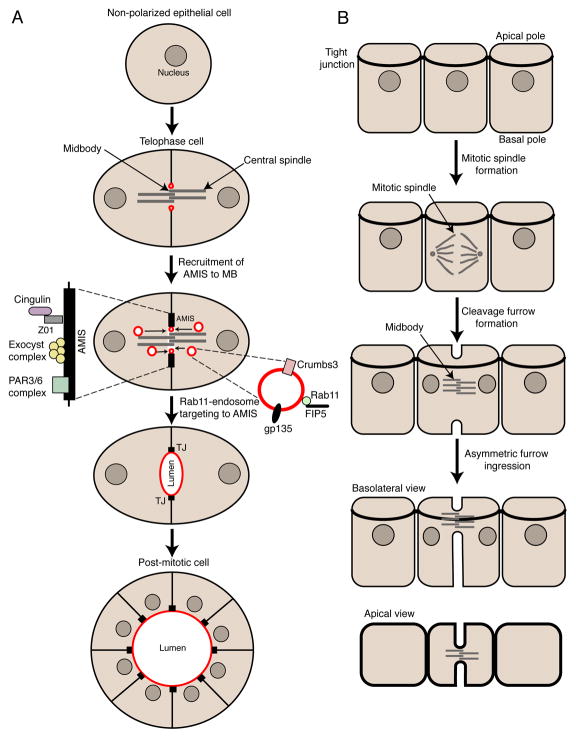
Mitotic cell division and polarization have often been viewed as opposing events in the cell cycle, given that cells undergoing mitotic division lose their polarity. However, recent studies have demonstrated that during division some cells can retain their polarity. The best-known example of polarized cell division is the division of epithelial cells. During metaphase-to-telophase progression epithelial cells retain some of polarized structures, such as tight junctions, adherens junctions and the apical plasma membrane domain. This apicobasal polarity is crucial for establishing the plane of mitotic spindle, a key element regulating epithelial tissue structure and morphogenesis. Interestingly, MB formation during epithelial cell cytokinesis is also polarized. While the cleavage furrow initiates coincidently at the apical and basal surfaces, the rate of furrow ingression is more rapid from the basal surface to consistently cause MB formation near tight junctions (Fig. 3A) [48, 49]. This asymmetric MB placement is required to maintain the integrity of tight junctions, thus preserving the barrier properties of epithelial sheets [49, 50].
Figure 3.
(A) Schematic representation of cleavage furrow ingression and midbody formation during polarized cell division. (B) Midbody role during the establishment of apical lumen formation site.
Recent studies analyzing the de novo polarization of epithelial cells during tissue morphogenesis have discovered that cell division often precedes the initiation of epithelial polarization. It has become increasingly clear that MBs play an active role in determining the location and timing of the apical lumen formation. During epithelia polarization and apical lumen formation in 3D tissue culture systems, MB formation is the first “symmetry-breaking” event and is prerequisite for the establishment of the nascent apical lumen site (Fig. 3B) [13, 51, 52]. MB formation during telophase triggers the establishment of the apical lumen initiation site (AMIS) that forms as ring around the MB (Fig. 3B) [13]. Upon the formation of the AMIS, Rab11-containing endosomes are transported via central spindle microtubules to the site of the future apical lumen. These Rab11-endosomes carry apical plasma membrane resident proteins (e.g. gp135 and Crumbs3) that are required for apical plasma membrane formation and apical lumen expansion (Fig. 3B) [13, 51]. The machinery mediating the initiation and targeting of AMIS formation around the MB remains unclear. Given that AMIS contains many tight junction proteins (e.g. ZO1, cingulin, Par3/6) and the Exocyst complex [53], it can be hypothesized that machinery mediating AMIS recruitment is similar to the machinery regulating MB association with the tight junctions during division of polarized epithelia cells.
The role of MBs in serving as a polarity cue is not limited to epithelial cells in 3D tissue culture. In fact, the abscission site regulates neuronal polarity by marking the site for the sprouting of the first neurite during Drosophila development [11]. MBs and mitotic abscission regulate development of the zebrafish neural rod, since oriented cell divisions (known as C-divisions) have a dominant influence in establishing the site of nascent neural lumen [54]. MBs also play a role of “cortical-site” marker for rotation of mitotic spindle during the second division in early C. elegans development [14]. The key to normal C. elegans embryo development is mitotic spindle orientation during the first two cell divisions, leading to a formation of four cells with distinct cellular fates. In C. elegans, the first division occurs along the long axis of the embryo [14]. The formation of this axis is regulated by an external polarity cue, such as the site of fertilization. During the second division the mitotic spindle rotates by 90 degrees leading to the formation of dorso-ventral axis and establishment of germline and endo-mesoderm lineages [14]. This 90-degree rotation depends on the MB generated earlier during the first division, since mitotic spindle is oriented by attaching to the MB-induced actin-rich “cortical-site” [14]. Interestingly, unlike regulation of apical or neuronal polarity, formation of this cortical-site does not depend on internalization of the MB, suggesting that MBs can also function as an extracellular polarity cues [14]. Together these studies demonstrate that MBs can act as polarity cues in many developmental contexts. In this way MBs could be likened to the yeast bud-scar, a structure left from the budding of daughter cell, which is integral for the formation of new budding sites in yeasts. While bud-scars and MBs are very different structures, both are cell division remnants. Thus, the concept of division playing the role of a polarity cue is conserved from yeasts to multi-cellular organisms.
MECHANISMS REGULATING MIDBODY ACCUMULATION
Asymmetric midbody inheritance and uptake
With the emergence of MBs as important regulators of cell proliferation and polarity, it is not surprising that MB inheritance and accumulation are tightly controlled cellular events. One of the ways that cells can obtain the MBs is through inheritance from the mitotic division. Importantly, MB inheritance is always an asymmetric event, since only one daughter cell can keep the MB from mitotic division. Our understanding of the molecular machinery determining which daughter cell inherits the MB is only beginning to emerge. It was shown that the daughter cell with the mother centriole usually inherits the MB [10]. Interestingly, Rab11/FIP3-endosomes accumulate around centrioles during metaphase and telophase before translocating to the cleavage furrow and establishing the abscission site (Fig. 1A) [4, 5]. This translocation often occurs in asymmetric fashion with Rab11/FIP3-endosomes from one daughter cell moving to the cleavage furrow earlier than the endosomes from the other daughter cell [4, 5]. Thus, it was proposed that the timing of the Rab11/FIP3-translocation to the cleavage furrow establishes the abscission site and ultimately determines which cell will keep the MB, although the machinery governing this asymmetric endosomal delivery is not defined. One possibility is that the mother centriole inhibits Rab11/FIP3-endosome translocation to the cleavage furrow, thus allowing the abscission to occur on the side of the cell containing the daughter centriole. Significantly, recent studies have demonstrated that Evi5 proteins accumulates at the mother centriole, presumably via binding to cenexin, a component of centriole appendages that are absent at the newly made daughter centriole [55]. Evi5 is a Rab11 GAP known to inactivate Rab11, as well as to directly compete with FIP3 for binding to Rab11 [56, 57]. Evi5 likely inactivates Rab11 at the mother centriole to inhibit Rab11/FIP3 complex formation. That would lead to the delay in translocation of Rab11/FIP3-endosomes from the mother centriole to the cleavage furrow, thereby favoring the abscission on the side of the cell containing the daughter centriole.
While MB can be asymmetrically inherited by one of the daughter cells, often abscission occurs at both sides of the MB, leading to the release of MB into the extracellular milieu [3]. This MB release or shedding is common in differentiating cells and could be one of the ways of eliminating MB-based signaling during differentiation [12]. Interestingly, a recent study used time-lapse imaging to demonstrate that in HeLa cells MBs can be internalized by neighboring interphase cells [43]. While the significance of this uptake remains unclear, it is possible that MB internalization mediates lateral trans-cellular transfer of signaling molecules. Alternatively, MB internalization and subsequent degradation may be needed to eliminate MB-based extracellular cues that affect development. Many questions, however, remain unanswered. How common is MB uptake in HeLa cells or other cell types? Can MBs be engulfed in vivo? Can these internalized MBs signal and affect cell differentiation or are they immediately degraded? Finally, is there specific molecular machinery that governs MB internalization by post-mitotic cells? Further studies will be needed before the significance and impact of MB internalization is fully understood.
Midbody degradation
In addition to MB inheritance and uptake, the other way to affect intracellular MB accumulation is by regulating its degradation. Several recent studies demonstrated that MBs are degraded via selective macroautophagy [10, 12, 58]. In accordance, the knockdown of various autophagy regulators results in the increase in MBs within the cell [10]. Typically autophagic degradation of organelles is initiated by the recruitment of LC3-rich double membrane structures known as phagophores [59]. The extension of these phagophores leads to the formation of the isolation membrane that allows selective degradation of engulfed cellular structures. It is now well-established that ubiquitin is required for this process and can induce the recruitment of phagophores via binding to autophagy receptors [59, 60]. What remains controversial is how phagophores are specifically recruited to MBs. Recently it has been reported that p62 autophagy receptor mediates MB degradation [58]. Surprisingly, subsequent studies from different group suggested that NBR1 autophagy receptor, rather than p62, mediates MB autophagic degradation [10]. Additionally, it was proposed that NBR1 is recruited to the MBs by binding to known MB protein CEP55 [10]. The reasons for these discrepancies remain unclear. It is possible that different cell types may differentially use either p62 or CEP55/NBR1 to degrade MBs. Alternatively, the true receptor required for selective MB degradation may not yet be known. Indeed, the knock-down of either p62 or NBR1 (or both) results in only moderate delay in MB degradation. The potential effects of other well-established autophagy receptors (e.g. NDP52, OPTN and NIX) in regulating MB degradation should be tested. Furthermore, a recently identified family of putative autophagy receptors, known as Tripartite Motif (TRIM) proteins [61] may play a role in mediating MB degradation. Thus, with an ever-increasing number of autophagosome receptors, further studies will be required to understand the machinery underlying MB degradation.
CONCLUSIONS AND FUTURE DIRECTIONS
While MBs were originally identified more than a century ago, we are only beginning to grasp the complexity of their function and accumulation. It is now established that MBs can serve as extracellular and intracellular polarity cues during early embryogenesis as well as during epithelia and neuron polarization. The molecular machinery governing the positioning of the MBs and how MBs transmit cues to differentiating neurons or epithelia remains unknown. Importantly, MBs may also function as intracellular signaling scaffolds that regulate the proliferation and fate of post-mitotic cells. Since MBs can be released extra-cellularly and taken up by other non-mitotic cells, we postulate that MBs can function as vehicles to laterally transfer complex sets of signaling molecules and/or receptors between the cells, thus profoundly affecting signaling as a whole.
While the importance of many of these MB functions remains controversial, it appears that MBs can, at least in some cases, directly affect the differentiation and proliferation of cancer and stem cells. However, MBs do not appear to have a universally conserved role in determining fate of all cells. Thus, the effects of MBs on cell differentiation are assuredly dependent on cell and tissue type as well as stage of the development. More studies in this emerging research field will eventually establish the roles of MBs during development and carcinogenesis.
Acknowledgments
We apologize to our colleagues for not being able to cite all work related to cytokinesis due to the focused nature of this review and its requirement for brevity. We are grateful to Drs. Chad Pearson, Jeffrey Moore, and Kalen Dionne for their critical reading of this manuscript. Research in Dr. Rytis Prekeris’ laboratory is supported by the National Institute of Health (R01 DK064380) and the Cancer League of Colorado Foundation. Research in Dr. Xiao-Jing Wang’s laboratory is supported by the National Institute of Health (R01 DE015953 and DE024371). Dr. Lai Kuan Dionne is supported by an NIH T32 training grant (T32 CA174648) and Cancer League of Colorado Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES AND RECOMMENDED READING
Papers of particular interest have been highlighted as:
* of special interest
** of outstanding interest
- 1.Pollard TD. Mechanics of cytokinesis in eukaryotes. Curr Opin Cell Biol. 2010;22(1):50–6. doi: 10.1016/j.ceb.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fededa JP, Gerlich DW. Molecular control of animal cell cytokinesis. Nat Cell Biol. 2012;14(5):440–7. doi: 10.1038/ncb2482. [DOI] [PubMed] [Google Scholar]
- 3.Schiel JA, Childs C, Prekeris R. Endocytic transport and cytokinesis: from regulation of the cytoskeleton to midbody inheritance. Trends Cell Biol. 2013;23(7):319–27. doi: 10.1016/j.tcb.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schiel JA, et al. Endocytic membrane fusion and buckling-induced microtubule severing mediate cell abscission. J Cell Sci. 2011;124(Pt 9):1411–24. doi: 10.1242/jcs.081448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5**.Schiel JA, et al. FIP3-endosome-dependent formation of the secondary ingression mediates ESCRT-III recruitment during cytokinesis. Nat Cell Biol. 2012;14(10):1068–78. doi: 10.1038/ncb2577. Showed that the FIP3-endosome delivery to the cleavage furrow mediates actin dissasembly, secondary inbgreassion formation and ESCRT-III mediates abscission. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simon GC, et al. Sequential Cyk-4 binding to ECT2 and FIP3 regulates cleavage furrow ingression and abscission during cytokinesis. Embo J. 2008 doi: 10.1038/emboj.2008.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson GM, et al. The FIP3-Rab11 Protein Complex Regulates Recycling Endosome Targeting to the Cleavage Furrow during Late Cytokinesis. Mol Biol Cell. 2004 doi: 10.1091/mbc.E04-10-0927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elia N, et al. Dynamics of endosomal sorting complex required for transport (ESCRT) machinery during cytokinesis and its role in abscission. Proc Natl Acad Sci U S A. 2011;108(12):4846–51. doi: 10.1073/pnas.1102714108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guizetti J, et al. Cortical constriction during abscission involves helices of ESCRT-III-dependent filaments. Science. 2011;331(6024):1616–20. doi: 10.1126/science.1201847. [DOI] [PubMed] [Google Scholar]
- 10**.Kuo TC, et al. Midbody accumulation through evasion of autophagy contributes to cellular reprogramming and tumorigenicity. Nat Cell Biol. 2011;13(10):1214–23. doi: 10.1038/ncb2332. Demonstrates that midbodies accumulate asymmterically at the mother centriole containign cell and midbodies are degraded via CEP55/NBR1-mediated autophagicytosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11*.Pollarolo G, et al. Cytokinesis remnants define first neuronal asymmetry in vivo. Nat Neurosci. 2011;14(12):1525–33. doi: 10.1038/nn.2976. Showns that midbody marks the site of first neurite formatioin in vivo. [DOI] [PubMed] [Google Scholar]
- 12**.Ettinger AW, et al. Proliferating versus differentiating stem and cancer cells exhibit distinct midbody-release behaviour. Nat Commun. 2011;2:503. doi: 10.1038/ncomms1511. Demonstrates that midbodies are inherited in stem cells and that midbdies are degraded or released during cell differentiation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13**.Li D, et al. FIP5 phosphorylation during mitosis regulates apical trafficking and lumenogenesis. EMBO Rep. 2014;15(4):428–37. doi: 10.1002/embr.201338128. Show that midbody formation is the first symetry-breaking event that established the site for apical lumen formation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14**.Singh D, Pohl C. Coupling of rotational cortical flow, asymmetric midbody positioning, and spindle rotation mediates dorsoventral axis formation in C. elegans. Dev Cell. 2014;28(3):253–67. doi: 10.1016/j.devcel.2014.01.002. Demonstrates that midbody plays a key role in regylating the mitotic spindle orientation during C. elegans early developemnt. [DOI] [PubMed] [Google Scholar]
- 15.Carlton JG, Agromayor M, Martin-Serrano J. Differential requirements for Alix and ESCRT-III in cytokinesis and HIV-1 release. Proc Natl Acad Sci U S A. 2008;105(30):10541–6. doi: 10.1073/pnas.0802008105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Connell JW, et al. Spastin couples microtubule severing to membrane traffic in completion of cytokinesis and secretion. Traffic. 2009;10(1):42–56. doi: 10.1111/j.1600-0854.2008.00847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17*.Skop AR, et al. Dissection of the mammalian midbody proteome reveals conserved cytokinesis mechanisms. Science. 2004;305(5680):61–6. doi: 10.1126/science.1097931. Proteomic analysis of post-mitotic midbodies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen TC, et al. From midbody protein-protein interaction network construction to novel regulators in cytokinesis. J Proteome Res. 2009;8(11):4943–53. doi: 10.1021/pr900325f. [DOI] [PubMed] [Google Scholar]
- 19.Marzesco AM, et al. Release of extracellular membrane particles carrying the stem cell marker prominin-1 (CD133) from neural progenitors and other epithelial cells. J Cell Sci. 2005;118(Pt 13):2849–58. doi: 10.1242/jcs.02439. [DOI] [PubMed] [Google Scholar]
- 20*.Dubreuil V, et al. Midbody and primary cilium of neural progenitors release extracellular membrane particles enriched in the stem cell marker prominin-1. J Cell Biol. 2007;176(4):483–95. doi: 10.1083/jcb.200608137. Shows that known stem cell marker prominin-1 accumulates in the midbdodies and is released during differentiation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopez-Camacho C, et al. Core binding factor beta (CBFbeta) is retained in the midbody during cytokinesis. J Cell Physiol. 2014;229(10):1466–74. doi: 10.1002/jcp.24588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salzmann V, et al. Centrosome-dependent asymmetric inheritance of the midbody ring in Drosophila germline stem cell division. Mol Biol Cell. 2014;25(2):267–75. doi: 10.1091/mbc.E13-09-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang Z, et al. MiCroKiTS 4.0: a database of midbody, centrosome, kinetochore, telomere and spindle. Nucleic Acids Res. 2015;43(Database issue):D328–34. doi: 10.1093/nar/gku1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crowell EF, Tinevez JY, Echard A. A simple model for the fate of the cytokinesis midbody remnant: implications for remnant degradation by autophagy. Bioessays. 2013;35(5):472–81. doi: 10.1002/bies.201200132. [DOI] [PubMed] [Google Scholar]
- 25.Clevers H, Loh KM, Nusse R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science. 2014;346(6205):1248012. doi: 10.1126/science.1248012. [DOI] [PubMed] [Google Scholar]
- 26.Habib SJ, et al. A localized Wnt signal orients asymmetric stem cell division in vitro. Science. 2013;339(6126):1445–8. doi: 10.1126/science.1231077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fumoto K, et al. Wnt5a signaling controls cytokinesis by correctly positioning ESCRT-III at the midbody. J Cell Sci. 2012;125(Pt 20):4822–32. doi: 10.1242/jcs.108142. [DOI] [PubMed] [Google Scholar]
- 28.Kaplan DD, et al. Identification of a role for beta-catenin in the establishment of a bipolar mitotic spindle. J Biol Chem. 2004;279(12):10829–32. doi: 10.1074/jbc.C400035200. [DOI] [PubMed] [Google Scholar]
- 29.Gao C, et al. Autophagy negatively regulates Wnt signalling by promoting Dishevelled degradation. Nat Cell Biol. 2010;12(8):781–90. doi: 10.1038/ncb2082. [DOI] [PubMed] [Google Scholar]
- 30.Andreas K, Sittinger M, Ringe J. Toward in situ tissue engineering: chemokine-guided stem cell recruitment. Trends Biotechnol. 2014;32(9):483–92. doi: 10.1016/j.tibtech.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 31.Li M, et al. Chemokine receptor CXCR4 signaling modulates the growth factor-induced cell cycle of self-renewing and multipotent neural progenitor cells. Glia. 2011;59(1):108–18. doi: 10.1002/glia.21080. [DOI] [PubMed] [Google Scholar]
- 32.Muller A, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410(6824):50–6. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 33.Graham NA, Graeber TG. Complexity of metastasis-associated SDF-1 ligand signaling in breast cancer stem cells. Proc Natl Acad Sci U S A. 2014;111(21):7503–4. doi: 10.1073/pnas.1405991111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cho H, Kehrl JH. Localization of Gi alpha proteins in the centrosomes and at the midbody: implication for their role in cell division. J Cell Biol. 2007;178(2):245–55. doi: 10.1083/jcb.200604114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naito Y, Okada M, Yagisawa H. Phospholipase C isoforms are localized at the cleavage furrow during cytokinesis. J Biochem. 2006;140(6):785–91. doi: 10.1093/jb/mvj209. [DOI] [PubMed] [Google Scholar]
- 36.Kasahara K, et al. Src signaling regulates completion of abscission in cytokinesis through ERK/MAPK activation at the midbody. J Biol Chem. 2007;282(8):5327–39. doi: 10.1074/jbc.M608396200. [DOI] [PubMed] [Google Scholar]
- 37.Willard FS, Crouch MF. MEK, ERK, and p90RSK are present on mitotic tubulin in Swiss 3T3 cells: a role for the MAP kinase pathway in regulating mitotic exit. Cell Signal. 2001;13(9):653–64. doi: 10.1016/s0898-6568(01)00185-1. [DOI] [PubMed] [Google Scholar]
- 38.Balko JM, et al. Activation of MAPK pathways due to DUSP4 loss promotes cancer stem cell-like phenotypes in basal-like breast cancer. Cancer Res. 2013;73(20):6346–58. doi: 10.1158/0008-5472.CAN-13-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morel AP, et al. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS One. 2008;3(8):e2888. doi: 10.1371/journal.pone.0002888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bernet JD, et al. p38 MAPK signaling underlies a cell-autonomous loss of stem cell self-renewal in skeletal muscle of aged mice. Nat Med. 2014;20(3):265–71. doi: 10.1038/nm.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu C, Alman BA. Side population cells in human cancers. Cancer Lett. 2008;268(1):1–9. doi: 10.1016/j.canlet.2008.03.048. [DOI] [PubMed] [Google Scholar]
- 42.Borovski T, et al. Cancer stem cell niche: the place to be. Cancer Res. 2011;71(3):634–9. doi: 10.1158/0008-5472.CAN-10-3220. [DOI] [PubMed] [Google Scholar]
- 43.Crowell EF, et al. Engulfment of the midbody remnant after cytokinesis in mammalian cells. J Cell Sci. 2014;127(Pt 17):3840–51. doi: 10.1242/jcs.154732. [DOI] [PubMed] [Google Scholar]
- 44.Driessens G, et al. Defining the mode of tumour growth by clonal analysis. Nature. 2012;488(7412):527–30. doi: 10.1038/nature11344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trosko JE, et al. Ignored hallmarks of carcinogenesis: stem cells and cell-cell communication. Ann N Y Acad Sci. 2004;1028:192–201. doi: 10.1196/annals.1322.023. [DOI] [PubMed] [Google Scholar]
- 46.Pan H, et al. Autophagic control of cell ‘stemness’. EMBO Mol Med. 2013;5(3):327–31. doi: 10.1002/emmm.201201999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johansen T, Lamark T. Selective autophagy mediated by autophagic adapter proteins. Autophagy. 2011;7(3):279–96. doi: 10.4161/auto.7.3.14487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48*.Morais-de-Sa E, Sunkel C. Adherens junctions determine the apical position of the midbody during follicular epithelial cell division. EMBO Rep. 2013;14(8):696–703. doi: 10.1038/embor.2013.85. Shows that midbdoy is asymmetrically localized dueing furrow ingreassion in polarized epithelial cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Herszterg S, et al. Interplay between the dividing cell and its neighbors regulates adherens junction formation during cytokinesis in epithelial tissue. Dev Cell. 2013;24(3):256–70. doi: 10.1016/j.devcel.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 50.Morais-de-Sa E, Sunkel CE. Connecting polarized cytokinesis to epithelial architecture. Cell Cycle. 2013;12(23):3583–4. doi: 10.4161/cc.26910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li D, Kuehn EW, Prekeris R. Kinesin-2 mediates apical endosome transport during epithelial lumen formation. Cell Logist. 2014;4(1):e28928. doi: 10.4161/cl.28928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang T, et al. Cytokinesis defines a spatial landmark for hepatocyte polarization and apical lumen formation. J Cell Sci. 2014;127(Pt 11):2483–92. doi: 10.1242/jcs.139923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bryant DM, et al. A molecular network for de novo generation of the apical surface and lumen. Nat Cell Biol. 2010;12(11):1035–45. doi: 10.1038/ncb2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Buckley CE, et al. Mirror-symmetric microtubule assembly and cell interactions drive lumen formation in the zebrafish neural rod. EMBO J. 2013;32(1):30–44. doi: 10.1038/emboj.2012.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hehnly H, et al. The centrosome regulates the Rab11- dependent recycling endosome pathway at appendages of the mother centriole. Curr Biol. 2012;22(20):1944–50. doi: 10.1016/j.cub.2012.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Westlake CJ, et al. Identification of Rab11 as a small GTPase binding protein for the Evi5 oncogene. Proc Natl Acad Sci U S A. 2007;104(4):1236–41. doi: 10.1073/pnas.0610500104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Laflamme C, et al. Evi5 promotes collective cell migration through its Rab-GAP activity. J Cell Biol. 2012;198(1):57–67. doi: 10.1083/jcb.201112114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58*.Pohl C, Jentsch S. Midbody ring disposal by autophagy is a post-abscission event of cytokinesis. Nat Cell Biol. 2009;11(1):65–70. doi: 10.1038/ncb1813. Demonstrates that p62 is involved in targeting modbdies for autophagososmal degradation. [DOI] [PubMed] [Google Scholar]
- 59.Mizushima N. Autophagy: process and function. Genes Dev. 2007;21(22):2861–73. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- 60.Abrahamsen H, Stenmark H, Platta HW. Ubiquitination and phosphorylation of Beclin 1 and its binding partners: Tuning class III phosphatidylinositol 3-kinase activity and tumor suppression. FEBS Lett. 2012;586(11):1584–91. doi: 10.1016/j.febslet.2012.04.046. [DOI] [PubMed] [Google Scholar]
- 61.Mandell MA, et al. TRIM proteins regulate autophagy and can target autophagic substrates by direct recognition. Dev Cell. 2014;30(4):394–409. doi: 10.1016/j.devcel.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]