Abstract
De novo thrombotic microangiopathy (TMA) after renal transplant is rare. Cytomegalovirus (CMV)-related posttransplant TMA has only been reported in 6 cases. We report an unusual case of a 75-year old woman who developed de novo TMA in association with CMV viremia. The recurrence of TMA with CMV viremia, resolution with treatment for CMV and the lack of correlation with a calcineurin inhibitor (CNI) in our case supports CMV as the cause of the TMA. What is unique is that the use of eculizumab without plasmapheresis led to prompt improvement in renal function. After a failure to identify a genetic cause for TMA and the clear association with CMV, eculizumab was discontinued. This case provides insight into the pathogenesis and novel treatment of de novo TMA, highlights the beneficial effects of complement inhibitors in this disease and shows that they can be safely discontinued once the inciting etiology is addressed.
Keywords: Atypical hemolytic uremic syndrome, cytomegalovirus, eculizumab, renal transplant thrombotic microangiopathy
Introduction
Thrombotic microangiopathy is a disease of the microvasculature characterized by arteriolar thickening, endothelial swelling, intraluminal platelet thrombi and red blood cell (RBC) fragmentation as blood flows across the partially occluded microcirculation. Consumption of platelets and RBCs causes thrombocytopenia, microangiopathic hemolytic anemia and varying degrees of tissue ischemia and organ dysfunction. It is a well-recognized complication of bone marrow, liver, heart transplant and can be particularly devastating after a kidney transplant. It may occur de novo or recur in the allograft. Two clinical entities have been described: thrombotic thrombocytopenic purpura (TTP) and hemolytic uremic syndrome (HUS).
Hemolytic uremic syndrome is a TMA featuring a triad of hemolytic anemia, thrombocytopenia, and acute renal failure (ARF). Atypical HUS (aHUS) is distinguished by the absence of Shiga toxin producing E. coli infection and results from complement-mediated damage to the microvascular endothelium. Mutations in genes encoding proteins of the alternative pathway account for 50%–70% of cases. The heterozygous mutations in aHUS are mostly rare or unique and deleterious1.
Cytomegalovirus is a ubiquitous herpesvirus transmitted through multiple ways, including solid-organ transplantation. In an immunocompromised host, the virus causes disease by primary infection or after reactivation. CMV is a risk factor for decreased renal allograft survival due to a multitude of effects such as acute and chronic rejection, atherosclerosis, transplant renal artery stenosis2 and opportunistic infections.
We report an unusual case of de novo post-transplant TMA/aHUS in association with CMV viremia successfully treated with eculizumab.
Case report
A 75-year old Caucasian woman with ESRD from diabetes underwent a 4-antigen mismatched, CMV donor seronegative into recipient seropositive, deceased donor renal transplant (DDRT) in November 2011. Induction immunosuppression included methylprednisolone 500 mg intraoperatively and rabbit antithymocyte globulin (rATG, Thymoglobulin) 5 mg/kg over 3 days, followed by maintenance azathioprine 150 mg daily (changed from mycophenolic acid due to nausea and vomiting), tacrolimus 2 mg daily (once daily tacrolimus due to slow graft function) and prednisone 5mg daily. Serum creatinine (SCr) was 1.4 mg/dL at discharge.
In April 2012, the patient developed ARF (SCr = 9.2 mg/dl), requiring hemodialysis (Figure 1). Allograft biopsy revealed ischemic glomeruli, thrombosis and interstitial hemorrhage consistent with acute TMA. Systemic evidence of TMA was absent (Hemoglobin=11 g/dL, Lactate dehydrogenase= 214 U/L, Platelet count = 137K/cumm, no schistocytes on peripheral smear). The C4d staining and anti-donor specific antibodies (DSA) testing were negative ruling out antibody-mediated rejection (AMR). A disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS 13) was 75% and Shiga toxin E. coli was negative. Thus, a diagnosis of aHUS was made. A single dose of Eculizumab (1200 mg) was given with prompt improvement in urine output and SCr (Figure 1). Infectious workup demonstrated CMV viremia (2,582 copies/mL), Citrobacter bacteremia and Enterococcus pyelonephritis, which was treated with valganciclovir (VGCV; renally dosed at 450mg every 48 hours) meropenem and vancomycin. An echocardiogram was negative for endocarditis. Belatacept was substituted for tacrolimus for possible CNI-induced TMA. Once CMV viremia cleared by 21 days, the VGCV dose was reduced to prophylactic levels (450 mg po daily) for three months and then discontinued. Creatinine stabilized at 1.8 mg/dl.
Figure 1.
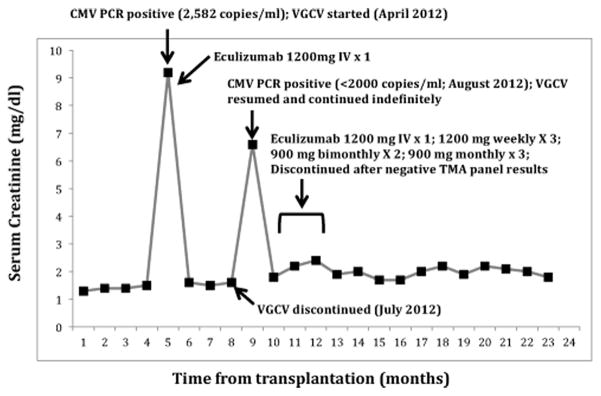
Acute renal failure secondary to thrombotic microangiopathy after CMV viremia, at five months and nine months posttransplant, successfully treated with valganciclovir and eculizumab.
One month after VGCV discontinuation, she became oliguric and SCr abruptly rose to 6.6 mg/dL. Allograft biopsy showed recurrent TMA, which was again renal-confined. The C4d staining and DSA were negative. A whole blood CMV PCR was positive but unable to quantify because of its low value (< 2000 copies/mL). The VGCV was resumed at therapeutic doses (450 mg po every 48 hours). Belatacept was discontinued and eculizumab 1200 mg administered. Within 12 hours, urine output increased to over 2 liters per day and the creatinine improved to 2.0 mg/dL over three weeks. The CMV viremia resolved within two weeks. Eculizumab was continued for another three months until one-year anniversary of her transplant and then discontinued. Valganciclovir was continued at prophylactic doses (450 mg po daily). Analysis of blood for the TMA panel showed normal alleles for factor B, factor H, factor I, membrane cofactor protein (MCP; CD46), C3, FHR1-FHR3 genes and thrombomodulin. Repeat biopsy two months later showed chronic TMA features.
Three years after transplant and more than two years after initial treatment, the patient has remained clinically stable with a serum creatinine of 1.8mg/dL with negligible proteinuria (100 mg/day). Her immunosuppressive regimen consists of azathioprine, 100 mg po daily and prednisone, 5 mg po daily. We plan to continue prophylactic doses of VGCV (450 mg po daily) indefinitely to prevent CMV recurrence.
Discussion
The incidence of de novo TMA is 0.8 to 15% with graft loss occurring in over one third of cases3. It localizes to the graft in about 30% cases4. The time from transplant to diagnosis of TMA ranges from a few days to years after transplantation. Risk factors include use of immunosuppressive drugs5, viral infections6–8, ADAMTS 13 inhibitors and malignancy9. The lesion may be associated with AMR where a kidney biopsy helps distinguish the two. In addition, evidence suggests a genetic susceptibility to de novo TMA in patients with complement gene abnormalities, similar to aHUS10,11.
Although the pathogenesis is incompletely understood, investigators speculate that an initial insult by ischemia-reperfusion may be undesirably enhanced by viral infections, immunosuppressive drugs or dysregulated complement activation12.
CMV as a trigger for posttransplant TMA has only been reported in 6 cases (Reviewed in Table I). Evidence suggests that CMV can directly damage endothelial cells and cause platelet adhesion by inducing the expression of adhesion molecules and release of von Willebrand factor13. This pathogenic sequence of events where endothelial damage can lead to microvascular thrombosis can help establish why CMV and TMA may be closely related. However, it has been shown that quantitative CMV-PCR may not correlate with renal allograft pathology or with detection of CMV inclusions in renal tissue14,15.
Table I.
REVIEW OF LITERATURE
| Cases | Presentation | Treatment | Outcome |
|---|---|---|---|
| Hochstetler et al [20] |
|
IV immunoglobulin | Complete recovery |
| Jeejeebhoy & Zaltzman [21] | De novo TMA associated with primary CMV disease 6 weeks post transplant | Condition worsened with daily PE but responded to IV Ganciclovir | Complete recovery |
| Waiser et al [6] | De novo HUS 5 days after patient developed biopsy proven CMV esophagitis | IV Ganciclovir | Complete recovery |
| Waiser et al [6] | De novo HUS within 3 days after onset of primary CMV disease | IV Ganciclovir | Complete recovery |
| Olie et al [22] | TMA post transplant in a patient with familial HUS with factor H mutation 10 months post transplant successfully treated. Recurrent TMA with CMV relapse | IV Ganciclovir; PE | Complete recovery |
| De Keyzer et al [23] | De novo TMA 25 years post transplant in association with CMV esophagitis, gastritis and pneumonitis | Daily PE, IV Ganciclovir, CMVIg and IV foscarnet | Allograft loss; 2nd transplant |
The numbers in parenthesis refer to the number in the References
The recurrence of TMA with CMV viremia and resolution of the acute TMA with treatment for CMV and the lack of correlation with a CNI in our case supports CMV as the cause of the TMA. What is unique is that the use of eculizumab without plasmapheresis led to prompt improvement in renal function.
Eculizumab is a humanized monoclonal antibody against C5, which inhibits the cleavage of C5 into C5a and C5b, thus preventing the formation of the membrane attack complex (MAC). CMV has been reported to cause direct activation of the classical pathway mediated by binding of C1q to CMV infected cells, resulting in MAC formation and ultimately cellular lysis and death. The virus itself, however evades the complement system by incorporating complement regulatory proteins (CD55 and CD59) into its virion16.
We believe that prompt administration of eculizumab prevented complement-mediated destruction of host cells by the virus, thus creating a window of opportunity for the clearance of viremia by VGCV. This is consistent with our observation that improvement in urine output and renal function preceded the clearance of viremia. After a failure to identify a genetic cause for aHUS and in the absence of CMV recurrence, eculizumab was discontinued.
Eculizumab is FDA approved for the treatment of paroxysmal nocturnal hemoglobinuria and since 2009, has been recognized as a life-saving option for patients with aHUS17. Although well tolerated and relatively safe, the optimal therapeutic regimen for this drug still remains unclear as much as the necessity for life-long administration and as such warrants large scale prospective randomized trials. Because we did not identify a complement genetic abnormality and due to the convincing role of CMV in the pathogenesis we believed we could safely discontinue eculizumab but continue to control CMV through the use of indefinite valganciclovir which is expensive, approximately $10,000 per year but much less than the cost of eculizumab at approximately $500,000 per year. However, a genetic mutation as the cause for the aHUS is only found in 50% of cases18. Interestingly, a recent report of 10 aHUS patients with an identified complement abnormality showed that eculizumab could be discontinued in 7/10 patients19.
Acknowledgments
Funding Sources
This research was supported by NIH 5T32 DK007126 (AJ) and in part by the Alan A. and Edith L. Wolff endowment, the Eileen M. Brooks Fund, and Donald F. Roach to Daniel C. Brennan
Footnotes
Authorship
- Conception or design, or analysis and interpretation of data, or both.
- Drafting the article or revising it.
- Providing intellectual content of critical importance to the work described.
- Final approval of the version to be published.
Ethics
The patient authorized Washington University to disclose to media representatives and/or public affairs staff members protected health information and information about herself, her condition or treatment for purposes of publicity, promotion, education or publication in print, broadcast and electronic media. A signed consent form is on file at Washington University School of Medicine.
Conflict of Interest
Daniel Brennan has research support from Bristol-Myers Squibb and Alexion, and is on the speakers bureau for Alexion.
Christina Klein is on the speakers bureau for Alexion.
References
- 1.Java A, Atkinson J, Salmon J. Defective complement inhibitory function predisposes to renal disease. Annu Rev Med. 2013;64:307–24. doi: 10.1146/annurev-med-072211-110606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Audard V, Matignon M, Hemery F, et al. Risk factors and long-term outcome of transplant renal artery stenosis in adult recipients after treatment by percutaneous transluminal angioplasty. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2006;6:95–9. doi: 10.1111/j.1600-6143.2005.01136.x. [DOI] [PubMed] [Google Scholar]
- 3.Reynolds JC, Agodoa LY, Yuan CM, Abbott KC. Thrombotic microangiopathy after renal transplantation in the United States. Am J Kidney Dis. 2003;42:1058–68. doi: 10.1016/j.ajkd.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Schwimmer J, Nadasdy TA, Spitalnik PF, Kaplan KL, Zand MS. De novo thrombotic microangiopathy in renal transplant recipients: a comparison of hemolytic uremic syndrome with localized renal thrombotic microangiopathy. Am J Kidney Dis. 2003;41:471–9. doi: 10.1053/ajkd.2003.50058. [DOI] [PubMed] [Google Scholar]
- 5.Zarifian A, Meleg-Smith S, O’Donovan R, Tesi RJ, Batuman V. Cyclosporine-associated thrombotic microangiopathy in renal allografts. Kidney Int. 1999;55:2457–66. doi: 10.1046/j.1523-1755.1999.00492.x. [DOI] [PubMed] [Google Scholar]
- 6.Waiser J, Budde K, Rudolph B, Ortner MA, Neumayer HH. De novo hemolytic uremic syndrome postrenal transplant after cytomegalovirus infection. Am J Kidney Dis. 1999;34:556–9. doi: 10.1016/s0272-6386(99)70085-5. [DOI] [PubMed] [Google Scholar]
- 7.Murer L, Zacchello G, Bianchi D, et al. Thrombotic microangiopathy associated with parvovirus B 19 infection after renal transplantation. J Am Soc Nephrol. 2000;11:1132–7. doi: 10.1681/ASN.V1161132. [DOI] [PubMed] [Google Scholar]
- 8.Petrogiannis-Haliotis T, Sakoulas G, Kirby J, et al. BK-related polyomavirus vasculopathy in a renal-transplant recipient. N Engl J Med. 2001;345:1250–5. doi: 10.1056/NEJMoa010319. [DOI] [PubMed] [Google Scholar]
- 9.Gohh RY, Williams ME, Crosson AW, Federman M, Zambetti FX. Late renal allograft failure secondary to thrombotic microangiopathy associated with disseminated malignancy. Am J Nephrol. 1997;17:176–80. doi: 10.1159/000169094. [DOI] [PubMed] [Google Scholar]
- 10.Kavanagh D, Richards A, Atkinson JP. Complement regulatory genes and hemolytic uremic syndromes. Annu Rev Med. 2008;59:293–309. doi: 10.1146/annurev.med.59.060106.185110. [DOI] [PubMed] [Google Scholar]
- 11.Abarrategui-Garrido C, Melgosa M, Pena-Carrion A, et al. Mutations in proteins of the alternative pathway of complement and the pathogenesis of atypical hemolytic uremic syndrome. Am J Kidney Dis. 2008;52:171–80. doi: 10.1053/j.ajkd.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 12.Ponticelli C, Banfi G. Thrombotic microangiopathy after kidney transplantation. Transpl Int. 2006;19:789–94. doi: 10.1111/j.1432-2277.2006.00354.x. [DOI] [PubMed] [Google Scholar]
- 13.Rahbar A, Soderberg-Naucler C. Human cytomegalovirus infection of endothelial cells triggers platelet adhesion and aggregation. J Virol. 2005;79:2211–20. doi: 10.1128/JVI.79.4.2211-2220.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liapis H, Storch GA, Hill DA, Rueda J, Brennan DC. CMV infection of the renal allograft is much more common than the pathology indicates: a retrospective analysis of qualitative and quantitative buffy coat CMV-PCR, renal biopsy pathology and tissue CMV-PCR. Nephrol Dial Transplant. 2003;18:397–402. doi: 10.1093/ndt/18.2.397. [DOI] [PubMed] [Google Scholar]
- 15.Grim SA, Pereira E, Guzman G, Clark NM. CMV PCR as a diagnostic tool for CMV gastrointestinal disease after solid organ transplantation. Transplantation. 2010;90:799–801. doi: 10.1097/TP.0b013e3181eceac9. [DOI] [PubMed] [Google Scholar]
- 16.Loenen WA, Bruggeman CA, Wiertz EJ. Immune evasion by human cytomegalovirus: lessons in immunology and cell biology. Semin Immunol. 2001;13:41–9. doi: 10.1006/smim.2001.0294. [DOI] [PubMed] [Google Scholar]
- 17.Zuber J, Fakhouri F, Roumenina LT, Loirat C, Fremeaux-Bacchi V. Use of eculizumab for atypical haemolytic uraemic syndrome and C3 glomerulopathies. Nature reviews Nephrology. 2012;8:643–57. doi: 10.1038/nrneph.2012.214. [DOI] [PubMed] [Google Scholar]
- 18.Noris M, Caprioli J, Bresin E, et al. Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype. Clinical journal of the American Society of Nephrology : CJASN. 2010;5:1844–59. doi: 10.2215/CJN.02210310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ardissino G, Testa S, Possenti I, et al. Discontinuation of eculizumab maintenance treatment for atypical hemolytic uremic syndrome: a report of 10 cases. Am J Kidney Dis. 2014;64:633–7. doi: 10.1053/j.ajkd.2014.01.434. [DOI] [PubMed] [Google Scholar]
- 20.Hochstetler LA, Flanigan MJ, Lager DJ. Transplant-associated thrombotic microangiopathy: the role of IgG administration as initial therapy. Am J Kidney Dis. 1994;23:444–50. doi: 10.1016/s0272-6386(12)81010-9. [DOI] [PubMed] [Google Scholar]
- 21.Jeejeebhoy FM, Zaltzman JS. Thrombotic microangiopathy in association with cytomegalovirus infection in a renal transplant patient: a new treatment strategy. Transplantation. 1998;65:1645–8. doi: 10.1097/00007890-199806270-00018. [DOI] [PubMed] [Google Scholar]
- 22.Olie KH, Goodship TH, Verlaak R, et al. Posttransplantation cytomegalovirus-induced recurrence of atypical hemolytic uremic syndrome associated with a factor H mutation: successful treatment with intensive plasma exchanges and ganciclovir. Am J Kidney Dis. 2005;45:e12–5. doi: 10.1053/j.ajkd.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 23.De Keyzer K, Van Laecke S, Peeters P, Vanholder R. De novo thrombotic microangiopathy induced by cytomegalovirus infection leading to renal allograft loss. Am J Nephrol. 2010;32:491–6. doi: 10.1159/000321328. [DOI] [PubMed] [Google Scholar]


