Abstract
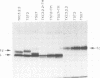
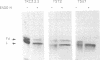
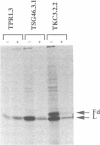
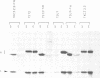
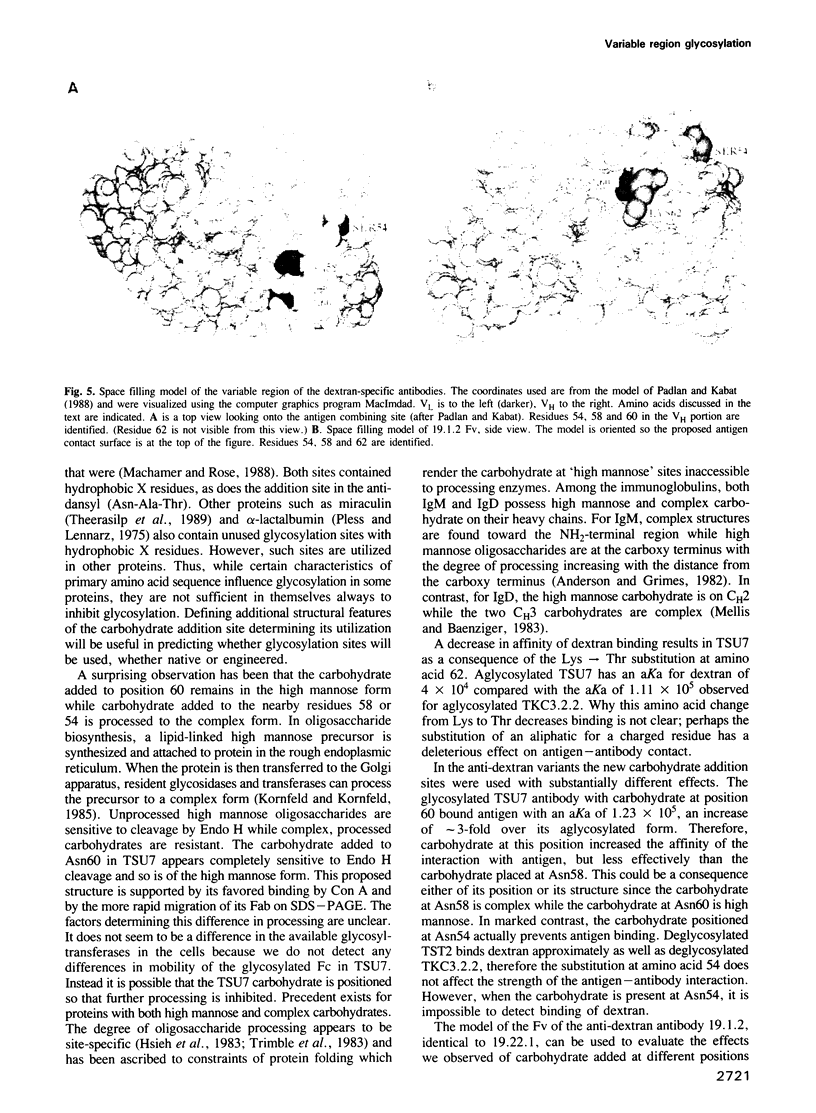
The presence of N-linked carbohydrate at Asn58 in the VH of the antigen binding site of an antibody specific for alpha(1----6)dextran (TKC3.2.2) increases its affinity for dextran 10- to 50-fold. Site-directed mutagenesis has now been used to create novel carbohydrate addition sequences in the CDR2 of a non-glycosylated anti-dextran at Asn54 (TST2) and Asn60 (TSU7). These antibodies are glycosylated and the carbohydrates are accessible for lectin binding. The amino acid change in TSU7 (Lys62----Thr62) decreases the affinity for antigen; however, glycosylation of TSU7 increased its affinity for antigen 3-fold, less than the greater than 10-fold increase in affinity seen for glycosylated TKC3.2.2. The difference in impact of glycosylation could result either from the position of the carbohydrate or from its structure; unlike the other antibodies, TSU7 attaches a high mannose, rather than complex, carbohydrate in CDR2. In contrast, glycosylation of TST2 at amino acid 54 inhibits dextran binding. Thus slight changes in the position of the N-linked carbohydrate in the CDR2 of this antibody result in substantially different effects on antigen binding. Unlike what was observed for the anti-dextrans, a carbohydrate addition site placed in a similar position in an anti-dansyl is not utilized.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akolkar P. N., Sikder S. K., Bhattacharya S. B., Liao J., Gruezo F., Morrison S. L., Kabat E. A. Different VL and VH germ-line genes are used to produce similar combining sites with specificity for alpha(1----6)dextrans. J Immunol. 1987 Jun 15;138(12):4472–4479. [PubMed] [Google Scholar]
- Alzari P. M., Lascombe M. B., Poljak R. J. Three-dimensional structure of antibodies. Annu Rev Immunol. 1988;6:555–580. doi: 10.1146/annurev.iy.06.040188.003011. [DOI] [PubMed] [Google Scholar]
- Bause E., Lehle L. Enzymatic N-glycosylation and O-glycosylation of synthetic peptide acceptors by dolichol-linked sugar derivatives in yeast. Eur J Biochem. 1979 Nov;101(2):531–540. doi: 10.1111/j.1432-1033.1979.tb19748.x. [DOI] [PubMed] [Google Scholar]
- Chothia C., Lesk A. M., Tramontano A., Levitt M., Smith-Gill S. J., Air G., Sheriff S., Padlan E. A., Davies D., Tulip W. R. Conformations of immunoglobulin hypervariable regions. Nature. 1989 Dec 21;342(6252):877–883. doi: 10.1038/342877a0. [DOI] [PubMed] [Google Scholar]
- Dangl J. L., Parks D. R., Oi V. T., Herzenberg L. A. Rapid isolation of cloned isotype switch variants using fluorescence activated cell sorting. Cytometry. 1982 May;2(6):395–401. doi: 10.1002/cyto.990020607. [DOI] [PubMed] [Google Scholar]
- Deisenhofer J. Crystallographic refinement and atomic models of a human Fc fragment and its complex with fragment B of protein A from Staphylococcus aureus at 2.9- and 2.8-A resolution. Biochemistry. 1981 Apr 28;20(9):2361–2370. [PubMed] [Google Scholar]
- Gavel Y., von Heijne G. Sequence differences between glycosylated and non-glycosylated Asn-X-Thr/Ser acceptor sites: implications for protein engineering. Protein Eng. 1990 Apr;3(5):433–442. doi: 10.1093/protein/3.5.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh P., Rosner M. R., Robbins P. W. Selective cleavage by endo-beta-N-acetylglucosaminidase H at individual glycosylation sites of Sindbis virion envelope glycoproteins. J Biol Chem. 1983 Feb 25;258(4):2555–2561. [PubMed] [Google Scholar]
- Jones P. T., Dear P. H., Foote J., Neuberger M. S., Winter G. Replacing the complementarity-determining regions in a human antibody with those from a mouse. 1986 May 29-Jun 4Nature. 321(6069):522–525. doi: 10.1038/321522a0. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Leatherbarrow R. J., Rademacher T. W., Dwek R. A., Woof J. M., Clark A., Burton D. R., Richardson N., Feinstein A. Effector functions of a monoclonal aglycosylated mouse IgG2a: binding and activation of complement component C1 and interaction with human monocyte Fc receptor. Mol Immunol. 1985 Apr;22(4):407–415. doi: 10.1016/0161-5890(85)90125-7. [DOI] [PubMed] [Google Scholar]
- Marshall R. D. The nature and metabolism of the carbohydrate-peptide linkages of glycoproteins. Biochem Soc Symp. 1974;(40):17–26. [PubMed] [Google Scholar]
- Mellis S. J., Baenziger J. U. Structures of the O-glycosidically linked oligosaccharides of human IgD. J Biol Chem. 1983 Oct 10;258(19):11557–11563. [PubMed] [Google Scholar]
- Morrison S. L., Johnson M. J., Herzenberg L. A., Oi V. T. Chimeric human antibody molecules: mouse antigen-binding domains with human constant region domains. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6851–6855. doi: 10.1073/pnas.81.21.6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto A., Gaya A., Jansa M., Moreno C., Vives J. Direct measurement of antibody affinity distribution by hapten-inhibition enzyme immunoassay. Mol Immunol. 1984 Jun;21(6):537–543. doi: 10.1016/0161-5890(84)90070-1. [DOI] [PubMed] [Google Scholar]
- Nose M., Wigzell H. Biological significance of carbohydrate chains on monoclonal antibodies. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6632–6636. doi: 10.1073/pnas.80.21.6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padlan E. A., Kabat E. A. Model-building study of the combining sites of two antibodies to alpha (1----6)dextran. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6885–6889. doi: 10.1073/pnas.85.18.6885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh R. B., Tse A. G., Dwek R. A., Williams A. F., Rademacher T. W. Tissue-specific N-glycosylation, site-specific oligosaccharide patterns and lentil lectin recognition of rat Thy-1. EMBO J. 1987 May;6(5):1233–1244. doi: 10.1002/j.1460-2075.1987.tb02359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pless D. D., Lennarz W. J. A lipid-linked oligosaccharide intermediate in glycoprotein synthesis. Characterization of [Man-14C]glycoproteins labeled from [Man-14C]oligosaccharide-lipid and GDP-[14C]Man. J Biol Chem. 1975 Sep 10;250(17):7014–7019. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S. U., Morrison S. L. Production and properties of chimeric antibody molecules. Methods Enzymol. 1989;178:459–476. doi: 10.1016/0076-6879(89)78034-4. [DOI] [PubMed] [Google Scholar]
- Sox H. C., Jr, Hood L. Attachment of carbohydrate to the variable region of myeloma immunoglobulin light chains. Proc Natl Acad Sci U S A. 1970 Jul;66(3):975–982. doi: 10.1073/pnas.66.3.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton B. J., Phillips D. C. The three-dimensional structure of the carbohydrate within the Fc fragment of immunoglobulin G. Biochem Soc Trans. 1983 Apr;11(2):130–132. [PubMed] [Google Scholar]
- Tao M. H., Morrison S. L. Studies of aglycosylated chimeric mouse-human IgG. Role of carbohydrate in the structure and effector functions mediated by the human IgG constant region. J Immunol. 1989 Oct 15;143(8):2595–2601. [PubMed] [Google Scholar]
- Theerasilp S., Hitotsuya H., Nakajo S., Nakaya K., Nakamura Y., Kurihara Y. Complete amino acid sequence and structure characterization of the taste-modifying protein, miraculin. J Biol Chem. 1989 Apr 25;264(12):6655–6659. [PubMed] [Google Scholar]
- Trimble R. B., Maley F., Chu F. K. GlycoProtein biosynthesis in yeast. protein conformation affects processing of high mannose oligosaccharides on carboxypeptidase Y and invertase. J Biol Chem. 1983 Feb 25;258(4):2562–2567. [PubMed] [Google Scholar]
- Trimble R. B., Maley F. Optimizing hydrolysis of N-linked high-mannose oligosaccharides by endo-beta-N-acetylglucosaminidase H. Anal Biochem. 1984 Sep;141(2):515–522. doi: 10.1016/0003-2697(84)90080-0. [DOI] [PubMed] [Google Scholar]
- Wallick S. C., Kabat E. A., Morrison S. L. Glycosylation of a VH residue of a monoclonal antibody against alpha (1----6) dextran increases its affinity for antigen. J Exp Med. 1988 Sep 1;168(3):1099–1109. doi: 10.1084/jem.168.3.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis of DNA fragments cloned into M13 vectors. Methods Enzymol. 1983;100:468–500. doi: 10.1016/0076-6879(83)00074-9. [DOI] [PubMed] [Google Scholar]