Abstract
Uveitis is a diverse group of potentially sight-threatening intraocular inflammatory diseases and pathology derives from sustained production of pro-inflammatory cytokines in the optical axis. Although topical or systemic steroids are effective therapies, their adverse effects preclude prolonged usage and are impetus for seeking alternative immunosuppressive agents, particularly for patients with refractory uveitis. In this study, we synthesized a 16 amino acid membrane-penetrating lipophilic suppressor of cytokine signaling 1 peptide (SOCS1-KIR) that inhibits JAK/STAT signaling pathways and show that it suppresses and ameliorates experimental autoimmune uveitis (EAU), the mouse model of human uveitis. Fundus images, histological and optic coherence tomography analysis of eyes showed significant suppression of clinical disease, with average clinical score of 0.5 compared to 2.0 observed in control mice treated with scrambled peptide. We further show that SOCS1-KIR conferred protection from ocular pathology by inhibiting the expansion of pathogenic Th17 cells and inhibiting trafficking of inflammatory cells into the neuroretina during EAU. Dark-adapted scotopic and photopic electroretinograms further reveal that SOCS1-KIR prevented decrement of retinal function, underscoring potential neuroprotective effects of SOCS1-KIR in uveitis. Importantly, SOCS1-KIR is non-toxic, suggesting that topical administration of SOCS1-Mimetics can be exploited as a non-invasive treatment for uveitis and for limiting cytokine-mediated pathology in other ocular inflammatory diseases including scleritis
Keywords: Uveitis, experimental autoimmune uveitis (EAU), suppressor of cytokine signaling, ocular inflammation, SOCS1, SOCS1 mimetic, Biologics
1. Introduction
Uveitis is a diverse group of potentially sight-threatening intraocular inflammatory diseases that account for more than 10% of severe visual handicaps in the United States. These potentially blinding diseases include sympathetic ophthalmia, birdshot retinochoroidopathy, Behcet’s disease, Vogt-Koyanagi-Harada syndrome, pars planitis, ocular sarcoidosis and are characterized by repeated cycles of remission and recurrent intraocular inflammation [1]. The disease may occur in the front of the eye (anterior uveitis), back of the eye (posterior uveitis) or throughout the eye (pan uveitis) and can be of infectious or autoimmune etiology [1]. Periocular or intravitreal corticosteroid therapies are effective for anterior uveitis but are to a large extent ineffective for posterior or pan uveitis due to limited penetration into the posterior segment. Moreover, steroids can induce a variety of adverse effects including mechanical injury to intraocular structures and occasional endophthalmitis. Nonetheless, prolonged use of corticosteroids for treatment of chronic uveitis is associated with the development of serious side effects such as glaucoma or cataract and is impetus for developing alternative therapies [2]. Although the etiology of human autoimmune uveitis is still not well understood, experimental autoimmune uveitis (EAU), the animal model of human posterior uveitis, has provided valuable insights into the pathophysiology of uveitis [3] and therapeutic approaches to the treatment [4, 5]. Studies on the immunopathogenic mechanisms that mediate EAU and analysis of the blood of uveitis patients revealed an association between Th1 and Th17 responses and the development of uveitis [6–8]. These observations have led to the prevailing view that Th1 and Th17 cells may be the etiologic agents of human uveitis [6–8]. It is therefore of note that the development of both T cell subsets requires STAT1 and STAT3, respectively. Studies showing that the activation of STAT pathways downstream of JAK kinases regulates mechanisms that mediate ocular pathology during EAU thus suggest that JAK kinases and STAT pathways are potential therapeutic targets that can be exploited to mitigate uveitis [6, 8].
The JAK/STAT pathway plays critical roles in lymphocyte development, maintenance of immune homeostasis and regulation of host immunity to infectious agents and tumors [9–11]. However, unbridled activation of the JAK/STAT signaling pathway has also been implicated in the development of most systemic and organ-specific autoimmune diseases, underscoring the importance of stringent regulation of this evolutionarily conserved signaling pathway [12]. Suppressors of cytokine signaling 1 (SOCS1) and SOCS3 regulate the intensity and duration of JAK/STAT signaling and thus play important roles in maintaining the delicate balance between efficient activation of the JAK/STAT pathway and timely termination of STAT signals when the instigating threat subsides [13–15]. Inhibitory effects of SOCS1/SOCS3 derive in part from direct interactions of their SOCS box with cytokine receptors and/or activated JAKs, targeting them for proteasome-mediated degradation and thereby preventing further recruitment of STATs to the activated cytokine/receptor complex [12]. In a recent study, we showed that rats and mice with targeted over-expression of SOCS1 in the retina are partially protected from EAU [16]. In addition, lymphocytes from patients with scleritis are markedly defective in up-regulating SOCS1 expression, suggesting that the inability to induce SOCS1 expression may contribute to the persistent ocular inflammation in patients with this blinding disease [16]. Although augmenting SOCS1 activity has long been considered a potential therapeutic approach for the treatment of autoimmune diseases such as scleritis, a major impediment to the therapeutic use of SOCS1 is its relatively short half-life [17]. Consequently there has been significant interest in developing strategies to efficiently deliver exogenous SOCS1 into cells [17].
SOCS1 and SOCS3 possess a kinase inhibitory region (KIR) that is capable of potently inhibiting kinase function, even in the absence of the SOCS box [12]. In this study, we have exploited this fact and synthesized a 16 amino acid peptide bearing a cell membrane penetrating lipophilic group (SOCS1-KIR) that interacts with JAK2 auto-phosphorylation loop and inhibits JAK kinase activity [18]. This lipophilic SOCS1-KIR peptide without the SOCS box, which significantly curtails the half-life of SOCS protein, has the advantage of sustaining inhibitory activity. We show here that topical administration of SOCS1-KIR suppresses uveitis and confers protection from ocular pathology during EAU, in part, by inhibiting the trafficking of inflammatory cells into the retina.
2. Material and methods
2.1. Mice
C57BL/6J mice (6–8 weeks old) were purchased from Jackson Laboratory (Bar Harbor, ME) and animal care and use was in compliance with NIH guidelines.
2.2 Peptide Synthesis
SOCS1-KIR (DTHFRTFRSHSDYRRI) and SOCS1 scrambled (KHRTDSRHSDRIYTFRF) were synthesized to >95% purity at GenScript (Piscataway, NJ). An N terminal palmitoyl-lysine group was attached to the peptide.
2.2. Induction of EAU and Histology
We induced EAU by active immunization with bovine interphotoreceptor retinoid-binding protein (IRBP, 150 μg) and human IRBP peptide (300μg amino acid residues 1–20) in a 0.2 ml emulsion (1:1 v/v with complete Freund’s adjuvant (CFA) containing mycobacterium tuberculosis strain H37RA (2.5 mg/ml). Mice also received Bordetella pertussis toxin (0.2μg/mouse) concurrent with immunization. For each study 8 mice were used per group and they were matched by age and sex. On the day of immunization and every day until day 13 post-immunization, mice were treated with either 1 eye-drop/per eye (10μg in 5μl) of the scrambled peptide (control peptide) or SOCS1-KIR. Clinical disease was established and scored by fundoscopy and histology as described previously [19]. Eyes for histological evaluation were harvested 21 days post-immunization, fixed in 10% buffered formalin and serially sectioned in the vertical pupillary-optic nerve plane. All sections were stained with hematoxylin and eosin.
2.3. Imaging mouse fundus
Funduscopic examinations were performed at day 14 and 21 after EAU induction using a modified Karl Storz veterinary otoendoscope coupled with a Nikon D90 digital camera, as previously described [20]. Briefly, following systemic administration of systemic anesthesia [intraperitoneal injection of ketamine (1.4 mg/mouse) and xylazine (0.12 mg/mouse)], the pupil was dilated by topical administration of 1% tropicamide ophthalmic solution (Alcon Inc, Fort Worth, Texas). To avoid a subjective bias, evaluation of the fundus photographs was conducted without knowledge of the mouse identity by a masked observer. At least six images (2 posterior central retinal view, 4 peripheral retinal views) were taken from each eye by positioning the endoscope and viewing from superior, inferior, lateral and medial fields and each individual lesion was identified, mapped and recorded. The clinical grading system for retinal inflammation was as previously established [21].
2.4. Imaging mouse retina by Spectral-domain Optical Coherence Tomography (SD-OCT)
Optical coherence tomography (OCT) is a noninvasive procedure that allows visualization of internal microstructure of various eye structures in living animals. An SD-OCT system with 1180 nm center wavelength broadband light source (Bioptigen, NC) was used for in vivo non-contact imaging of eyes from scrambled peptide or SOCS1-KIR mice. Before OCT imaging was performed, each animal was anesthetized and the pupils dilated. The anesthetized mouse was immobilized using adjustable holder that could be rotated easily allowing for horizontal or vertical scan scanning. Each scan was performed at least twice, with realignment each time. The dimension of the scan (in depth and transverse extent) was adjusted until the optimal signal intensity and contrast was achieved.
2.5. Electroretinogram (ERG) Recordings
Before the ERG recordings, mice were dark-adapted overnight, and experiments were performed under dim red illumination. Mice were anesthetized with a single intraperitoneal injection of ketamine (1.4 mg/mouse) and xylazine (0.12 mg/mouse) and pupils were dilated with Midrin P containing of 0.5% tropicamide and 0.5% phenylephrine hydrochloride (Santen Pharmaceutical Co., Osaka, Japan). ERGs were recorded using an electroretinography console (Espion E2; Diagnosys LLC, Lowell, MA, USA) that generated and controlled the light stimulus. Dark-adapted ERG was recorded with single-flash delivered in a Ganzfeld dome with intensity of −4 to 1 log cd·s/m2 delivered in 6 steps. Light-adapted ERG was obtained with a 20 cd/m2 background, and light stimuli started at 0.3 to 100 cd·s/m2 in 6 steps. Gonioscopic prism solution (Alcon Labs, Fort Worth, TX, USA) was used to provide good electrical contact and to maintain corneal moisture. A reference electrode (gold wire) was placed in the mouth, and a ground electrode (subcutaneous stainless steel needle) was positioned at the base of the tail. Signals were differentially amplified and digitized at a rate of 1 kHz. Amplitudes of the major ERG components (a- and b-wave) were measured (Espion software; Diagnosys LLC) using automated and manual methods. Immediately after ERG recording, imaging of the fundus was performed as previously described above.
2.6. Analysis of T-lymphocytes
Cells were isolated from the retina, spleen or lymph nodes (LN) as described [8] and cell surface protein expression was detected and quantified by FACS. For intracellular cytokine detection, cells were re-stimulated for 5h with PMA (20ng/ml)/ionomycin (1μM). Golgi-stop was added in the last hour and intracellular cytokine staining was performed using BD Biosciences Cytofix/Cytoperm kit as recommended (BD Pharmingen, San Diego, CA). FACS analysis was performed on a Becton-Dickinson FACSCalibur (BD Biosciences) using Ag-specific monoclonal antibodies and corresponding isotype control Abs (PharMingen, San Diego, CA) as described [6].
2.7. Lymphocyte proliferation assay and ELISA
Cells isolated from the lymph nodes and spleen of mice with EAU were re-stimulated in vitro with IRBP or ConA in the presence of the scrambled peptide or SOCS1-KIR. After 48 h, cultures were pulsed with 3H-thymidine (0.5 μCi/10 μl/well) for 12 additional hours and analyzed as described [4]. The presented data are the mean cpm. ± s.e.m. of the responses of five replicate cultures. ELISA was performed using mouse IL-10 quantikine elisa kit according to the manufacturer’s instructions (R&D Systems).
2.8. Quantitative (qPCR) analysis
Total RNA was extracted from lymphocytes and retinal cells using the TRIzol reagent according to the procedures recommended by the manufacturer (Life Technologies, Gaithersburg, MD). All RNA samples were digested with RNase-free DNase 1 (Life Technologies) for 30 minutes, purified by phenol/chloroform extractions, and precipitated in 0.4 M LiCl. RNA (10 μg), a commercial synthesis system (SuperScript III Reverse Transcriptase; Life Technologies), and oligo(dT) were used for first-strand synthesis as previously described. First-strand synthesis containing each mRNA sample but without reverse transcriptase was performed to control for possible DNA contamination; failure to obtain RT-PCR products with any of the PCR amplimers confirmed the absence of contaminating DNA. All cDNA preparations used were suitable for PCR amplification on the basis of efficient amplification of a β-actin sequence. Real-time PCR was performed on a fast real-time PCR system (ABI 7500) and PCR parameters were as recommended by the manufacturer (TaqMan Universal PCR Kit; Applied Biosystems). Primers and probes used were purchased from Applied Biosystems. The mRNA expression levels were normalized to the levels of GAPDH housekeeping gene.
2.9 Statistical Analysis
Statistical analyses were performed by independent two-tailed Student’s t test. Probability values of ≤0.05 were considered statistically significant. The data are presented as mean + SEM.
3. Results and Discussion
3.1. SOCS1-KIR confers protection from severe uveitis
Lipophilic peptide corresponding to the kinase inhibitory region (KIR) of SOCS1 or SOCS3 (SOCS-KIR) have recently been shown to efficiently penetrate mammalian cell membranes and inhibit inflammation [22, 23]. In this study, we synthesized a 16 amino acids lipophilic SOCS1 peptide (SOC1-KIR) as previously described [18] and investigated whether topical administration of SOCS1-KIR can be used to inhibit experimental autoimmune uveitis (EAU), the animal model of human uveitis. EAU is readily induced in susceptible mouse strains by active immunization with emulsion of ocular proteins in Complete Freund’s Adjuvant (CFA) or adoptive transfer of activated uveitogenic T cells [24, 25]. In this study, we induced EAU by active immunization of C57BL/6 mice with IRBP, a 140-kDa retinal glycoprotein produced by photoreceptor cells [24]. On the day of immunization and every day until day 13 post-immunization, mice were treated with either 1 eye-drop/per eye (10μg in 5μl) of a scrambled 16 amino acids peptide (control peptide) or SOCS1-KIR. The peptide was administrated at a relatively low dose by local eye drops compared with systemic administration in order to minimize possibility of drug resistance or potential systemic adverse effect and to avoid the degradation or clearance of the drug before reaching its target tissue. Disease progression was monitored by fundoscopy and confirmed histologically by accessing the extent of lymphocyte infiltration of the neuroretina and ocular pathology. Fundus images of mice that received control peptide showed severe inflammation with blurred optic disc margins and enlarged juxtapapillary area, retinal vasculitis with moderate cuffing, vitreitis, choroiditis and yellow-whitish retinal and choroidal infiltrates (Fig. 1). In contrast, disease in mice treated SOCS1-KIR was significantly inhibited with average clinical score of 0.5 compared to 2.0 observed in the control eyes (Fig. 1).
Figure 1. SOCS1-KIR suppresses the development of EAU.
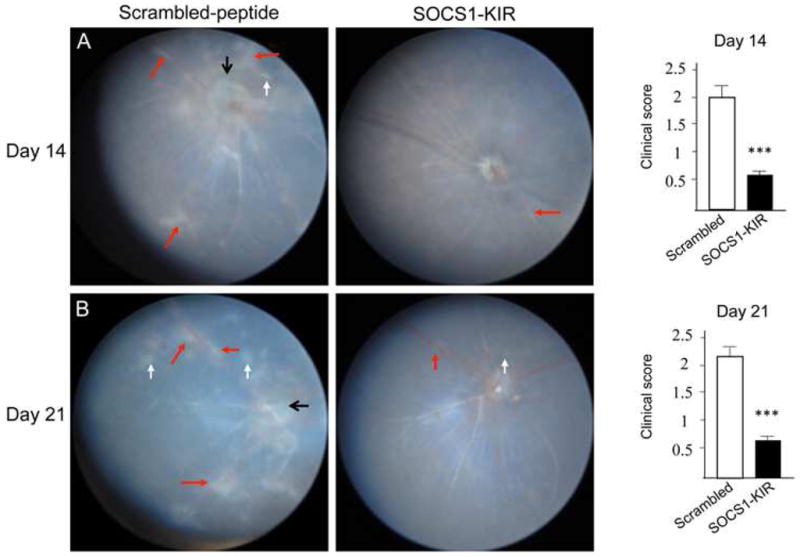
Fundus image of retina at day 14 (A) or day 21 (B) after EAU induction were taken using an otoendoscopic imaging system, and clinical score and assessment of disease severity were based on changes at the optic nerve disc or retinal vessels and retinal and choroidal infiltrates. Fundus images reveal inflammation with blurred optic disc margins and enlarged juxtapapillary area (black arrows), retinal vasculitis with moderate cuffing (red arrows), and yellow-whitish retinal and choroidal infiltrates (white arrows).
3.2. SOCS1-KIR inhibits ocular inflammation and protects the retina from tissue injury
Autoimmune disease of the eye is mediated, in part, by the massive numbers of inflammatory cells that infiltrate the uvea and neuroretina during intraocular inflammation and the hallmark of severe uveitis is the formation of retinal in-folding and development of optic neuritis. We performed optical coherence tomography (OCT) and histological analysis of day-14 (disease onset) or day-21 (peak of EAU) eyes to determine whether treatment with SOCS1-KIR would attenuate or prevent pathology deriving from severe uveitis. OCT analysis revealed substantial presence of inflammatory cells in the optic nerve head and vitreous suggestive of the development of papillitis in mice that received the scrambled peptides (Fig. 2A). In contrast, the mice that were treated with SOCS1-KIR did not exhibit these hallmark features of severe uveitis (Fig. 2A). Histological analysis further revealed the development of retinal folds in mice treated with the scrambled peptide while no evidence of retina folding was observed in the eyes of mice that received the SOCS1-Mimetic (Fig. 2B). The development of these cardinal pathological features of severe uveitis in the eyes of the control but not eyes of SOCS1-KIR treated mice suggest that the topical administration of SOCS1 Mimetic was effective in mitigating uveitis.
Figure 2. SOCS1-KIR inhibits uveitis and confers protection from ocular pathology.
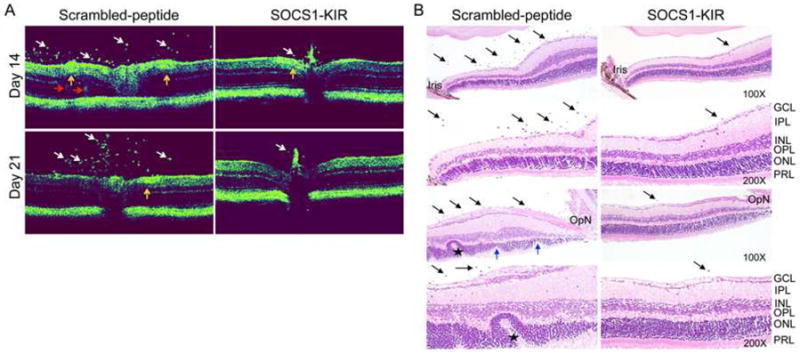
(A) Optical coherence tomography (OCT) analysis showing layered structure of the retina and ocular inflammation. Representative OCT images showing markedly increased inflammatory cells (white arrows) in the vitreous of mice treated with scrambled peptide. In addition, increased retinal vasculitis (yellow arrows), granulomatous lesions (red arrows), disturbance of the retinal layer structure, discontinuous and obscuring of the external limiting membrane (ELM) were observed in mice treated with scrambled peptide compared to SOCS1-KIR. (B) Histological analysis showing substantial numbers of inflammatory cells in the vitreous (black arrows), retinal folds (asterisk), photoreceptor cell lose (blue arrows) in retina of mice treated with scrambled peptide compared to SOCS1-KIR. The sections were stained with hematoxylin and eosin. GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; PRL, photoreceptor layer; OpN, optic nerve.
3.3. SOCS1-KIR prevents decrement of retinal function during uveitis
Inflammation of the retina during EAU induces changes in the electroretinogram (ERG) indicative of alterations in visual function [26]. The ERG measures electrical potential change of the retina in response to light stimulation arising predominantly from changes in neural activity of photoreceptor and second-order neuron in the retina. Thus, it is a well-established tool for uncovering gross physiologic changes in the intact retina and is routinely used to analyze layer-by-layer changes of retinal function in patients and animals. The two major ERG waves are the photoreceptor-derived a-wave (which represents first-order neurons/photoreceptor signaling) and the b-wave (which represents mostly second-order neurons/bipolar cell signaling) that derives from bipolar cells in the inner nuclear layer (INL). Furthermore, the ERG under the light-adaptive stimuli reflects only cone-driven signaling, while the dark-adapted b-wave responses represented mainly the rod-driven signaling by modification on ERG procedures. We therefore measured photopic (light adaptation) and scotopic (dark adaptation) ERG responses of the immunized mice to assess whether SOCS1-KIR could rescue the visual function loss associated with EAU [26]. Because the amplitude of light-adapted ERG a-wave of experimental mice is relatively small and prone to inaccuracies we did not use the light adapted a wave to quantify ERG changes. Analysis of light adapted ERG on day 14 and day 20 post-immunization show significantly lower b-wave amplitudes in eyes of mice treated with the scrambled peptide (Fig. 3) suggesting significant preservation of cone signaling functions in SOCS1-KIR-treated mice. The maximal ERG response is evoked in dark-adapted conditions, representing mainly rod responses and similar to the light-adapted responses, mice treated with the scrambled peptide responded with significantly lower b-wave amplitudes further underscoring the neuroprotective effects of SOCS1-KIR during EAU. It is important to note that the functional changes observed by ERG reflect corresponding pathological lesions noted by our OCT and fundus data.
Figure 3. SOCS1-KIR protects retinal function during uveitis.
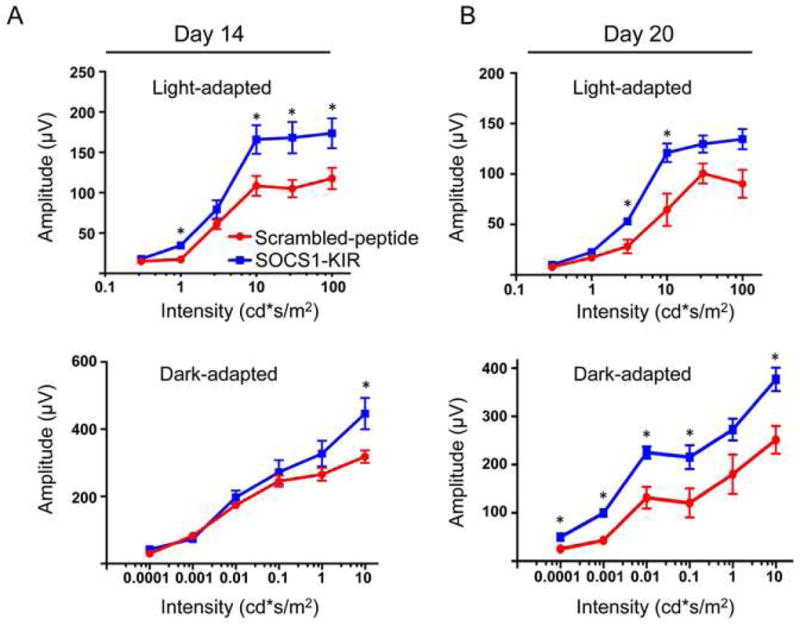
ERG analysis of the retina of mice treated with scrambled peptide or SOCS1-KIR on day 14 (A) or day 20 (B) after EAU induction. The averages of light- or dark-adapted ERG b-wave amplitudes are plotted as a function of flash luminance and values are means ± SEM from 4 animals in each group.
3.4. SOCS1-KIR suppresses infiltration of inflammatory cells into the retina
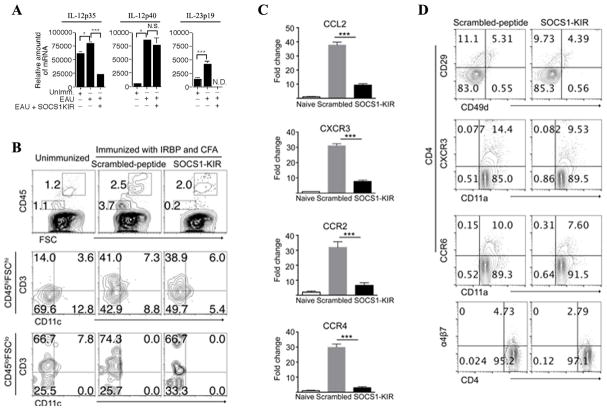
It is of note that IL-23 and IL-12 produced by microglial cells contribute to EAE and multiple sclerosis (MS) by facilitating the expansion and effector functions of Th1 and Th17 cells [27, 28]. We therefore examined whether the suppression of EAU in mice treated with SOCS1-KIR derived in part from inhibition of IL-23 and IL-12 production in the retina and/or inhibition of the recruitment of inflammatory cells into the retina. We isolated retina of mice treated with scrambled peptide or SOCS1-KIR on day 21 post-immunization, digested the tissue with collagenase and RNA isolated from the cells was analyzed by qPCR to assess the transcript levels of subunits that comprise IL-23 (IL-12p40/IL-23p19) or IL-12 (IL-12p35/IL-12p40) cytokine. In line with published data on the EAE model, we detected significantly lower levels of IL-23p19 and IL-12p35 transcripts in retina of SOCS1-KIR-treated mice (Fig. 4A), suggesting that the mimetic peptide suppressed the expression of IL-12 and IL-23 during EAU. Microglia cells are predominantly CD45Lo while lymphoid cells are CD45Hi cells [29] and so we analyzed the levels of cells expressing this cell surface marker in the retina during EAU by FACS. Overall we found a substantial reduction in the levels of inflammatory cells in the retinae of mice treated with SOCS1-KIR (Fig. 4B). While we observe a modest decrease in the levels of lymphoid cells in mice treated with SOCS1-KIR (Fig. 4B), the data indicates that SOCS1-KIR selectively targets microglial cells for inhibition as indicated by a >18 fold decrease in the frequency of microglia cells in SOCS1-KIR treated retinal cells (compare 3.7% to 0.2%). In the context of neuroinflammation, it is notable that astrocytes and microglia do not easily succumb to endoplasmic reticulum (ER) stress. Rather, ER stress induces activation of a JAK1/STAT3 axis that activates microglia, up-regulates the expression of IL-6, oncostatin M (OSM), several chemokines and promotes neuroinflammation [30]. This feed-forward inflammatory loop involving astrocytes and microglia that drive neuroinflammation is thought to be relevant in EAE/MS and it is highly dependent on JAK1/STAT3 signaling pathways [30]. Targeting of the JAK/STAT pathway by SOCS1-KIR may thus mitigate ocular inflammation through inhibition of microglia activation, differentiation and proliferation. To further understand mechanisms that might contribute to the reduced recruitment of inflammatory cells into the retinae of the mice treated with SOCS1-KIR, we examined the expression of chemokines and chemokine receptors in mouse retina. RNA from the retina of the control or SOCS1-KIR-treated mice was isolated, converted to cDNA and the mRNA levels of molecules that mediate lymphocyte trafficking were quantified by RT-qPCR analysis. We show here that the expression of several chemokine receptors (CCR2, CXCR3, CCR4) and chemokine (CCL2) that mediate the recruitment of inflammatory cells to sites of inflammation is markedly down regulated in the retinas of SOCS1-KIR treated mice (Fig. 4C). To confirm these observations, we re-stimulated the lymphocytes from EAU mice with IRBP peptide and treated them with scrambled peptide or SOCS1-KIR in vitro. Flow cytometric analysis revealed significant diminution of the levels of T cells expressing CCR6 and CXCR3, two chemokine receptors that are preferentially expressed by activated Th17 and Th1 cells, respectively (Fig. 4D). Compared to lymphocytes treated with the scrambled peptide we also found reduction of CD29, an integrin associated with very late antigen receptors. Furthermore, the level of α4β7 is also reduced in lymphocytes treated with SOCS1-KIR (Fig. 4D). The substantial decrease in T cells expressing chemokine receptors and integrins that promote trafficking of inflammatory cells into the retina, provide in part, mechanistic explanation for the lower amounts of inflammatory cells detected in the eyes of mice treated with SOCS1-KIR. Together these results suggest that SOCS1-KIR may confer protection from EAU by inhibiting the expression of molecules that promote trafficking of inflammatory cells into the immune privileged uvea and retina.
Figure 4. SOCS1-KIR suppresses trafficking of inflammatory cells into the retina.
(A) Retinae of mice treated with scrambled peptide or SOCS1-KIR were isolated on day 21 post-immunization, digested with collagenase and analyzed for the expression of IL-23 (IL-12p40, IL-23p19) or IL-12 (IL-12p35, IL-12p40) by qRT-PCR. (B) Flow cytometric analysis of inflammatory cells in the retina on day 21 after EAU induction. Numbers in quadrants indicate percentage of CD45+, CD3+ and/or CD11c+ cells in the retinae during EAU. Cells isolated from the spleen and LN of mice with EAU were re-stimulated in vitro with IRBP in medium containing SOCS1 KIR or scrambled peptides and analyzed for the expression integrins and chemokine receptors by qPCR (C) or FACS (D). Plots in (D) were gated on CD4+ T cells and numbers in quadrants indicate percent of CD4+ T cells expressing CD29, CD49d, CXCR3, CD11a, CCR6, or a4β7. Data are representative of three independent experiments.
3.5. SOCS1-KIR suppressed EAU by inhibiting pathogenic T cells and up-regulating IL-10
To better understand the effects of SOCS1-KIR on lymphocytes, we isolated T cells from the lymph nodes and spleen of mice with EAU and re-stimulated the cells in vitro with IRBP in the presence of the scrambled peptide or SOCS1-KIR. As expected T cells from unimmunized mice did not mount proliferative responses to IRBP. On the other hand, uveitogenic T cells cultured with the scrambled peptide proliferated vigorous in response to IRBP while SOCS1-KIR significantly inhibited the IRBP-specific T cell proliferative response (Fig. 5A). Because Th17 cells are a major effector T cell subset implicated in the pathogenesis of EAU and ocular inflammatory diseases including scleritis and human uveitis [6], it was of interest to determine the extent to which the expansion of Th17 cells was inhibited by SOCS1-KIR. As shown in Fig. 5B, SOCS1-KIR induced more than 2 fold reduction IL-17-producing Th17 cells and the inhibitory effect is dose dependent (Fig. 5B; right-most panel). Most striking is the effect of SOCS1-KIR on IL-6, a pro-inflammatory cytokine that serves a biological marker of inflammation. Compared to LN cells stimulated with IRBP in presence of scrambled peptides, we observed more than a 6 fold decrease in the level of IL-6-producing cells (Fig. 5C). In contrast to IL-17 or IL-6, IL-10 is an anti-inflammatory cytokine that antagonizes pro-inflammatory T cell subsets such as Th1 and Th17 cells and increase in IL-10 secretion in the retina correlates with recovery from uveitis [4]. Analysis of supernatants derived from the cultured cells revealed a >5 folds increase in IL-10 secretion by the cells cultured in SOCS1-KIR (Fig. 5D). Taken together, these results thus suggest that SOCS1-KIR might inhibit ocular inflammation by promoting the expansion of IL-10-producing regulatory cells while inhibiting the proliferation or effector functions of the pro-inflammatory T cells that mediate uveitis.
Figure 5. SOCS1-KIR inhibits proliferation of uveitogenic T cells and up-regulates IL-10.
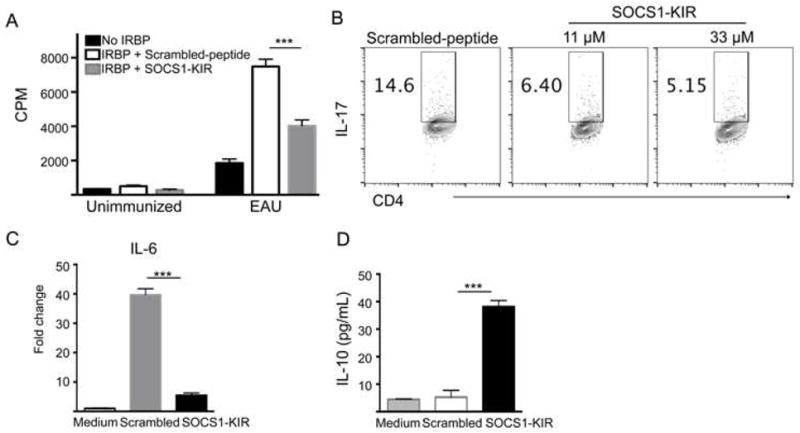
Cells isolated from the spleen and LN of mice with EAU were re-stimulated in vitro with IRBP in medium containing SOCS1 KIR or scrambled peptide and analyzed by Thymidine-incorporation assay (A), intracellular cytokine staining assay (B), qPCR (C) or ELISA (D). Proliferative responses were analyzed in six replicate cultures and data presented as CPM and indicate the mean values of six replicate cultures (A) and numbers in quadrants indicate percentage of IL-17-expressing CD4+ T cells (B). Results represent at least three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 (Student’s t test (two tailed)).
3.6. SOCS1-KIR enhances ocular immunity with no effects on systemic immune response
SOCS1-KIR inhibits ocular inflammation and it was of interest to know whether its immune suppressive functions extend to the peripheral immune system or mainly localized to the eye. We isolated cells from the spleen or lymph nodes from the mice immunized with IRBP and analyzed the effects of SOCS1-KIR by FACS, qPCR or lymphocyte proliferation assay. Intracellular staining analysis of the relative abundance of IL-17-, IFN-γ– and/or IL-10-producing cells reveals no substantial difference between mice treated with the scrambled peptide or SOCS1-KIR (Fig. 6A). qPCR analysis of the expression of immune modulatory molecules (chemokines, chemokine receptors, inflammatory cytokines and pro-apoptotic proteins) in the spleen, blood and lymph nodes also reveals no substantial difference between mice treated with scrambled peptide or SOCS1-KIR (Fig. 6B–D) and this is in contrast to the down-regulatory effects observed in the retina of mice treated with SOCS1-KIR. Furthermore, analysis by thymidine incorporation assay reveals no apparent difference between the proliferative responses to IRBP of peripheral lymphocytes of mice treated with scrambled peptide or SOCS1-KIR (Fig. 6E). Taken together, these results suggest that topical administration of SOCS1-KIR is effective therapy for uveitis and importantly, its effects are restricted to the eye.
Figure 6. SOCS1-KIR has no effects on systemic immune response.
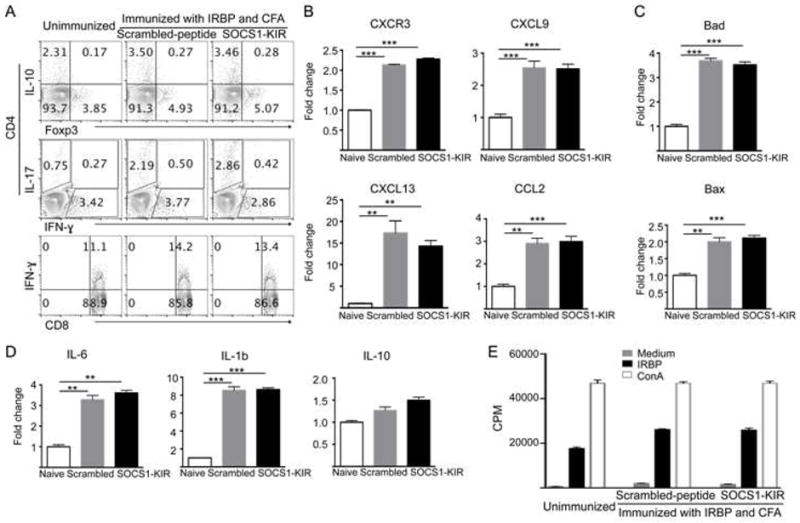
(A) LN cells from unimmunized mice or mice immunized with IRBP and treated with SOCS1-KIR or scrambled peptide were analyzed by the intracellular cytokine staining assay. Numbers in quadrants indicate percent of CD4+ or CD8+ T cells expressing IL-17 or IFN-γ, Foxp3 or IL-10. (B–D) LN cells from unimmunized mice or mice immunized with IRBP and treated with SOCS1-KIR or scrambled peptide were analyzed by qPCR for the expression of chemokine or chemokine receptors (B), apoptotic proteins (C) and cytokines (D). (E) LN cells from mice described in (A) were re-stimulated with IRBP or ConA and proliferative responses were analyzed by the thymidine incorporation assay. Results represent at least three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 (Student’s t test (two tailed).
4. Conclusion
We show here that SOCS1-KIR is an effective treatment for EAU. It suppressed EAU and conferred protection from ocular pathology by inhibiting the expansion and trafficking of pathogenic Th17 cells into the retina. ERG data show that SOCS1-KIR rescued mice from decrement of retinal function associated with uveitis, underscoring the neuroprotective effect of SOCS1-KIR. Of clinical importance, SOCS1-KIR is non-toxic and mitigates uveitis without inducing systemic immune responses. Eye drop administration is non-invasive and caused relatively no discomfort to the mice, suggesting that SOCS1-KIR would be palatable to patients and may complement anti-inflammatory agents currently used to treat uveitis. We envision that SOCS1-KIR may also be useful in suppressing cytokine-induced fibrosis during glaucoma surgery, as well as, limiting inflammation during ocular surgery or post-operatively after cataract surgery. A drug such as SOCS1-KIR that requires only a single eye drop/day would be highly desirable to elder patients required to self-administer a regiment of anti-inflammatory drugs several days after cataract surgery to prevent post-operative inflammation or cystoid macular edema. Compliance is a major concern due to the daunting challenge of keeping count of the number of times each drug is administered several times a day. Nonetheless, the data presented here suggest that topical administration of SOCS1-Mimetic can be exploited as non-invasive effective therapy for mitigating human uveitis and other ocular inflammatory diseases.
Highlights.
Topical SOCS1-KIR inhibits ocular inflammation and confers protection from severe uveitis by inhibiting Th17 expansion and trafficking of inflammatory cells into retina during EAU.
SOCS1-KIR is neuroprotective and prevents decrement of retina function during uveitis.
SOCS1-KIR is a non-toxic, non-invasive therapy with no effects on systemic immunity.
SOCS1-KIR may have utility beyond the treatment of uveitis, particularly in limiting cytokine-mediated pathology in other ocular inflammatory diseases including scleritis.
Acknowledgments
We thank Dr. Haohua Qian and Yichao Li (Visual function core, National Eye Institute, National institute of health) for OCT and ERG technical assistance. Research was supported by the Intramural Research Program of the National Eye Institute (NEI), National Institutes of Health (NIH): Projects # EY000350-15 and EY000280-23.
Abbreviations
- SOCS
suppressor of cytokine signaling
- SOCS1-KIR
the kinase inhibitory region of SOCS1
- EAU
experimental autoimmune uveitis
- IRBP
interphotoreceptor retinoid-binding protein
- JAK
janus kinase
- STAT
signal transducers and activators of transcription
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nussenblatt RB. The natural history of uveitis. International ophthalmology. 1990;14:303–8. doi: 10.1007/BF00163549. [DOI] [PubMed] [Google Scholar]
- 2.Nussenblatt RB. Bench to bedside: new approaches to the immunotherapy of uveitic disease. Int Rev Immunol. 2002;21:273–89. doi: 10.1080/08830180212067. [DOI] [PubMed] [Google Scholar]
- 3.Caspi RR. Understanding autoimmune uveitis through animal models. The Friedenwald Lecture. Invest Ophthalmol Vis Sci. 2014;52:1872–9. doi: 10.1167/iovs.10-6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang RX, Yu CR, Dambuza IM, Mahdi RM, Dolinska MB, Sergeev YV, Wingfield PT, Kim SH, Egwuagu CE. Interleukin-35 induces regulatory B cells that suppress autoimmune disease. Nat Med. 2014;20:633–41. doi: 10.1038/nm.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang RX, Yu CR, Mahdi RM, Egwuagu CE. Novel IL27p28/IL12p40 cytokine suppressed experimental autoimmune uveitis by inhibiting autoreactive Th1/Th17 cells and promoting expansion of regulatory T cells. J Biol Chem. 2012;287:36012–21. doi: 10.1074/jbc.M112.390625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amadi-Obi A, Yu CR, Liu X, Mahdi RM, Clarke GL, Nussenblatt RB, Gery I, Lee YS, Egwuagu CE. T(H)17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. 2007;13:711–8. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- 7.Luger D, Silver PB, Tang J, Cua D, Chen Z, Iwakura Y, Bowman EP, Sgambellone NM, Chan CC, Caspi RR. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J Exp Med. 2008 doi: 10.1084/jem.20071258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu X, Lee YS, Yu CR, Egwuagu CE. Loss of STAT3 in CD4+ T cells prevents development of experimental autoimmune diseases. J Immunol. 2008;180:6070–6. doi: 10.4049/jimmunol.180.9.6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Villarino AV, Kanno Y, Ferdinand JR, O’Shea JJ. Mechanisms of Jak/STAT signaling in immunity and disease. J Immunol. 194:21–7. doi: 10.4049/jimmunol.1401867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Shea JJ, Holland SM, Staudt LM. JAKs and STATs in immunity, immunodeficiency, and cancer. N Engl J Med. 2013;368:161–70. doi: 10.1056/NEJMra1202117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Egwuagu CE, Larkin J., III Therapeutic targeting of STAT pathways in CNS autoimmune diseases. JAKSTAT. 2013;2:e24134. doi: 10.4161/jkst.24134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alexander WS, Hilton DJ. The role of suppressors of cytokine signaling (SOCS) proteins in regulation of the immune response. Annu Rev Immunol. 2004;22:503–29. doi: 10.1146/annurev.immunol.22.091003.090312. [DOI] [PubMed] [Google Scholar]
- 13.Alexander WS, Starr R, Fenner JE, Scott CL, Handman E, Sprigg NS, Corbin JE, Cornish AL, Darwiche R, Owczarek CM, et al. SOCS1 is a critical inhibitor of interferon gamma signaling and prevents the potentially fatal neonatal actions of this cytokine. Cell. 1999;98:597–608. doi: 10.1016/s0092-8674(00)80047-1. [DOI] [PubMed] [Google Scholar]
- 14.Kubo M, Hanada T, Yoshimura A. Suppressors of cytokine signaling and immunity. Nat Immunol. 2003;4:1169–76. doi: 10.1038/ni1012. [DOI] [PubMed] [Google Scholar]
- 15.Seki Y, Inoue H, Nagata N, Hayashi K, Fukuyama S, Matsumoto K, Komine O, Hamano S, Himeno K, Inagaki-Ohara K, et al. SOCS-3 regulates onset and maintenance of T(H)2-mediated allergic responses. Nat Med. 2003;9:1047–54. doi: 10.1038/nm896. [DOI] [PubMed] [Google Scholar]
- 16.Yu CR, Mahdi RR, Oh HM, Amadi-Obi A, Levy-Clarke G, Burton J, Eseonu A, Lee Y, Chan CC, Egwuagu CE. Suppressor of cytokine signaling-1 (SOCS1) inhibits lymphocyte recruitment into the retina and protects SOCS1 transgenic rats and mice from ocular inflammation. Invest Ophthalmol Vis Sci. 2011;52:6978–86. doi: 10.1167/iovs.11-7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jo D, Liu D, Yao S, Collins RD, Hawiger J. Intracellular protein therapy with SOCS3 inhibits inflammation and apoptosis. Nat Med. 2005;11:892–8. doi: 10.1038/nm1269. [DOI] [PubMed] [Google Scholar]
- 18.Jager LD, Dabelic R, Waiboci LW, Lau K, Haider MS, Ahmed CM, Larkin J, 3rd, David S, Johnson HM. The kinase inhibitory region of SOCS-1 is sufficient to inhibit T-helper 17 and other immune functions in experimental allergic encephalomyelitis. J Neuroimmunol. 2011;232:108–18. doi: 10.1016/j.jneuroim.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takase H, Yu CR, Liu X, Fujimoto C, Gery I, Egwuagu CE. Induction of suppressors of cytokine signaling (SOCS) in the retina during experimental autoimmune uveitis (EAU): potential neuroprotective role of SOCS proteins. J Neuroimmunol. 2005;168:118–27. doi: 10.1016/j.jneuroim.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 20.Paques M, Guyomard JL, Simonutti M, Roux MJ, Picaud S, Legargasson JF, Sahel JA. Panretinal, high-resolution color photography of the mouse fundus. Invest Ophthalmol Vis Sci. 2007;48:2769–74. doi: 10.1167/iovs.06-1099. [DOI] [PubMed] [Google Scholar]
- 21.Xu H, Koch P, Chen M, Lau A, Reid DM, Forrester JV. A clinical grading system for retinal inflammation in the chronic model of experimental autoimmune uveoretinitis using digital fundus images. Exp Eye Res. 2008;87:319–26. doi: 10.1016/j.exer.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 22.Flowers LO, Johnson HM, Mujtaba MG, Ellis MR, Haider SM, Subramaniam PS. Characterization of a peptide inhibitor of Janus kinase 2 that mimics suppressor of cytokine signaling 1 function. J Immunol. 2004;172:7510–8. doi: 10.4049/jimmunol.172.12.7510. [DOI] [PubMed] [Google Scholar]
- 23.Waiboci LW, Ahmed CM, Mujtaba MG, Flowers LO, Martin JP, Haider MI, Johnson HM. Both the suppressor of cytokine signaling 1 (SOCS-1) kinase inhibitory region and SOCS-1 mimetic bind to JAK2 autophosphorylation site: implications for the development of a SOCS-1 antagonist. J Immunol. 2007;178:5058–68. doi: 10.4049/jimmunol.178.8.5058. [DOI] [PubMed] [Google Scholar]
- 24.Caspi RR, Roberge FG, Chan CC, Wiggert B, Chader GJ, Rozenszajn LA, Lando Z, Nussenblatt RB. A new model of autoimmune disease. Experimental autoimmune uveoretinitis induced in mice with two different retinal antigens. J Immunol. 1988;140:1490–5. [PubMed] [Google Scholar]
- 25.Egwuagu CE, Charukamnoetkanok P, Gery I. Thymic expression of autoantigens correlates with resistance to autoimmune disease. J Immunol. 1997;159:3109–12. [PubMed] [Google Scholar]
- 26.Chen J, Qian H, Horai R, Chan CC, Falick Y, Caspi RR. Comparative analysis of induced vs. spontaneous models of autoimmune uveitis targeting the interphotoreceptor retinoid binding protein. PLoS One. 2013;8:e72161. doi: 10.1371/journal.pone.0072161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, Chu N, Hu A, Gran B, Rostami A, Zhang GX. Increased IL-23p19 expression in multiple sclerosis lesions and its induction in microglia. Brain. 2007;130:490–501. doi: 10.1093/brain/awl273. [DOI] [PubMed] [Google Scholar]
- 28.Yoshida Y, Yoshimi R, Yoshii H, Kim D, Dey A, Xiong H, Munasinghe J, Yazawa I, O’Donovan MJ, Maximova OA, et al. The transcription factor IRF8 activates integrin-mediated TGF-beta signaling and promotes neuroinflammation. Immunity. 2013;40:187–98. doi: 10.1016/j.immuni.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Loo G, De Lorenzi R, Schmidt H, Huth M, Mildner A, Schmidt-Supprian M, Lassmann H, Prinz MR, Pasparakis M. Inhibition of transcription factor NF-kappaB in the central nervous system ameliorates autoimmune encephalomyelitis in mice. Nat Immunol. 2006;7:954–61. doi: 10.1038/ni1372. [DOI] [PubMed] [Google Scholar]
- 30.Meares GP, Liu Y, Rajbhandari R, Qin H, Nozell SE, Mobley JA, Corbett JA, Benveniste EN. PERK-dependent activation of JAK1 and STAT3 contributes to endoplasmic reticulum stress-induced inflammation. Mol Cell Biol. 2014;34:3911–25. doi: 10.1128/MCB.00980-14. [DOI] [PMC free article] [PubMed] [Google Scholar]