Abstract
Cellular mechanisms of membrane traffic and signal transduction are deeply interconnected. The present review discusses how membrane trafficking in the endocytic pathway impacts receptor-mediated signaling. Examples of recent progress are highlighted, focusing on the endocytosis-signaling nexus in mammals.
Introduction
Close relationships between endocytosis and receptor-mediated cellular signaling have been recognized since early investigations of ligand-induced down-regulation of epidermal growth factor receptors (EGFRs, reviewed in [1]), and the identification of endosomes as discrete membrane compartments containing internalized growth factors and activated growth factor receptors [2–5]. Subsequent studies have verified and extended this relationship in many systems, as reviewed previously (e.g., [6–9]). The present discussion seeks to minimize duplication by focusing on recent developments and restricting scope to results from mammalian systems.
We will begin with a brief review of mechanisms determining the molecular sorting of signaling receptors in endosomes, and the role of these mechanisms in modulating long-term cellular signaling responsiveness. We will then discuss the hypothesis that endosomes serve, additionally, as sites of active signal initiation. There are other interesting examples of intracellular signaling that do not require receptor endocytosis per se (such as nutrient sensing by endosomes); these are not discussed here but excellent reviews have appeared elsewhere (e.g., [10]).
Endosomes as sorting stations determining long-term cellular signaling responsiveness
Endocytosis of signaling receptors is widely recognized to confer long-term homeostatic control on cellular signaling responsiveness by adjusting the total cellular receptor complement, or surface-accessible complement, in accord with the cell’s history of cognate ligand exposure or overall activation state. Ligand-induced activation typically increases receptor endocytic rate, and internalized receptors engage molecular sorting machineries that specify subsequent transport via divergent lysosomal and recycling routes. These events, in turn, determine the degree to which cellular ligand responsiveness is attenuated (‘down-regulated’) or sustained (‘re-sensitized’) under conditions of prolonged or repeated ligand exposure.
Many signaling receptors internalize via clathrin-coated pits and a considerable amount is now known about this mechanism (reviewed in [11]). However, it has been recognized for many years that additional endocytic mechanisms exist [12], and one area of recent progress is toward identifying alternate mechanisms relevant to signaling receptors. One that has been described recently requires endophilin but not clathrin, and is called ‘fast endophilin-mediated endocytosis’ (FEME) to distinguish it from clathrin-mediated endocytosis (CME) [13]. FEME is outwardly similar to CME in that dynamin and local actin polymerization contribute to endocytic membrane scission, but FEME occurs through the formation of distinct tubulovesicular structures lacking clathrin, with endophilin providing the major force for membrane deformation [14]. FEME also differs from CME in its mechanism of cargo selection. CME is generally engaged by receptor association with clathrin adaptor proteins [11], whereas FEME appears to be engaged by binding of proline-rich sequences in the receptor to the SH3 domain of endophilin [13]. Identification of the FEME mechanism is an exciting development and a remarkable number of signaling receptors appear to engage it, but questions remain. For example, the D4 dopaminergic receptor (DRD4) is a putative FEME cargo but its SH3 domain-interacting sequences were found previously to inhibit, rather than promote, endocytosis of receptors. In addition, mutating these motifs to fully destabilize SH3 domain binding results in a ligand-independent endocytic phenotype [15]. These observations, not easily reconciled with the present understanding of cargo engagement with FEME, suggest that still more remains to be learned about diversity and specificity in mechanisms of signaling receptor endocytosis.
Progress has also been made recently toward more fully understanding how signaling receptors are sorted after endocytosis. Ubiquitin-directed engagement of the endosomal sorting complex required for transport (ESCRT) is an important mechanism driving lysosomal down-regulation and is highly conserved, including in yeast where many components of this machinery were first identified [16]. However, it has been suspected for some time that additional mechanisms operate in higher eukaryotes. This appears particularly likely for the GPCR family, which is ~1000-fold more diverse in mammals than in yeast.
Early evidence suggesting the existence of additional endosomal sorting machinery emerged through the study of GPCR down-regulation leading to identification of a putative ‘GPCR-associated sorting protein’ (GASP) that binds various GPCR cytoplasmic tails without requiring ubiquitination [17]. GASP-1 (or GPRASP1) is the founding member of a small protein family that is widely expressed in mammals but not found in yeast [18]. The precise cellular function(s) of GASPs remain poorly understood, but recent studies suggest interesting possibilities. GASP-1 binds Beclin2, a mammalian-restricted paralogue of Beclin1 (ATG6), through a Beclin2-specific N-terminal domain [19]. Beclin2 is otherwise similar to Beclin1, including in its ability to regulate the endosomal type III PI3-kinase (VPS34) and bind ATG14 that functions as a tethering protein in autophagosome-endolysosome fusion [20]. GASP-1 can also bind dysbindin as well as the stimulatory heterotrimeric G protein, Gs. These interactions appear to promote GPCR degradation by engaging the ESCRT through additional association with HRS, providing a path of alternate receptor connectivity to the ESCRT that does not require ubiquitination and is regulated by heterotrimeric G protein [21,22].
Studies of GPCR recycling provided further evidence for additional mechanisms of signaling receptor sorting in mammals. A PDZ and PX domain-containing protein called sorting nexin 27 (SNX27) was identified as a key protein that binds beta-adrenergic receptors in endosomes and promotes receptor recycling [23]. SNX27 associates with the WASH - Arp2/3 actin nucleation complex and this interacts, in turn, with the retromer complex. This ‘actin module-SNX27-retromer tubule module’ (ASRT) machinery assembles at the base of specialized membrane tubules that extend from the endosome limiting membrane and mediate cargo exit from endosomes [24]. SNX27 interacts not only with WASH but also with retromer directly through the arrestin-like protein VPS26, and the integrated ASRT machinery appears to mediate specific endosome-to-plasma membrane transport of various signaling receptors as well as other specialized membrane cargoes such as the Glut1 (SLC2A1) glucose transporter [25]. Physiological roles of this mechanism are only beginning to be explored but are likely considerable. For example, the ASRT machinery was shown recently to mediate a discrete route of localized membrane delivery to the postsynaptic plasma membrane that is required for functional surface expression of excitatory neurotransmitter receptors at synapses [26]. It is also interesting to note that human genetic studies have linked core components of retromer, as well as SNX27 and WASH components, to neurological and neurodegenerative syndromes (reviewed in [27]).
Endosomes as sites of receptor-mediated signal initiation
As noted above, it was proposed from the earliest investigations that endosomes may themselves function as active signaling sites. This idea, formalized in the ‘signaling endosome’ hypothesis, has been supported by many subsequent studies. However, two fundamental questions remain incompletely resolved. First, are endosomes bona fide sites of significant signal initiation under normal physiological conditions? Second, does the endosome signal confer functional effects different from the plasma membrane signal?
Perhaps the strongest support, overall, for an affirmative answer to both questions comes from the study of retrograde neurotrophin signaling (reviewed in [7]). Sympathetic neurons require stimulation by ligands released from peripheral targets that they innervate. Absent such signals, neurons undergo apoptotic cell death and are eliminated. One trophic signaling ligand, nerve growth factor (NGF), is a polypeptide that activates the TrkA tyrosine kinase receptor. TrkA activity is required to induce an anti-apoptotic transcriptional program mediated by a downstream MAP kinase cascade. Using a compartmentalized culture system, NGF applied selectively to distal axons was shown sufficient to elicit an anti-apoptotic transcriptional response in the neuronal cell body. Further, NGF was shown to internalize together with TrkA, and endosomes containing both cargoes were shown to move from the axon to cell body. The current model is that TrkA is continuously ligand-activated and phosphorylated in signaling endosomes during retrograde movement, effectively carrying the trophic signal from the cell periphery to nucleus. Considerable evidence supports this model, including recent work elaborating features of cytoskeletal control that are required for both retrograde endosome movement and the functional trophic signaling response [28]. However, this model is not beyond reproach. For example, some results suggest that chemical inhibition of TrkA kinase activity in the cell body is not sufficient to block the trophic signal initiated in distal axons; thus it has been suggested that the retrograde signal may be carried by a downstream kinase rather than by internalized TrkA itself [29].
Evidence regarding endosome signaling by other receptors is similar in broad outline, if less compelling in some of its detail. A number of studies followed the development of a mutant dynamin construct that inhibits clathrin-mediated endocytosis when over-expressed. It was first shown that this construct blocked activation of the ERK MAP kinase elicited by EGFRs [30]. Then similar results were reported for ERK activation elicited by several GPCRs, starting with beta-adrenergic receptors [31]. Such results were interpreted initially as direct evidence of receptor signaling from endosomes. Refuting this claim, mutant dynamin was later found to block ERK activation elicited by an endocytosis-defective GPCR [32] as well as receptor-independent activation of protein kinase C [33]. These observations suggested that dynamin has additional signaling-relevant effect(s), such as internalizing an essential downstream mediator or pathway regulator separate from receptors [32,33], but also highlighted the inherent limitations of indirect methods when applied to determining the subcellular location of key signaling reactions. This limitation persists in more recent studies as well. Still the accumulated weight of evidence, particularly with the development of more direct approaches (discussed later in this review), favors the hypothesis that some signaling reactions indeed occur in endosomes. Attention has shifted to the second question, whether endosome-initiated signals are functionally different from those emanating from the plasma membrane.
Wingless or Wnt signaling provides a particularly interesting example because different mechanisms appear to operate at each location [34]. A key event in canonical Wnt signaling is the ligand-dependent reduction of cytoplasmic GSK3 activity, which reduces the rate of beta-catenin turnover and subsequently drives the downstream transcriptional response in receiving cells. Endosomes were proposed to act as signal-activating devices by physically sequestering GSK3 from the cytoplasm into intralumenal vesicles [35]. This idea was been questioned since its proposal because previous studies showed that a portion of the Wnt receptor complex (low density lipoprotein receptor-related protein 6) can act directly as a pseudosubstrate inhibitor of GSK3 kinase activity [36]. Recent work may have resolved this conundrum by suggesting that both mechanisms are germane, with pseudosubstrate inhibition rapidly reducing cytoplasmic GSK3 activity from the plasma membrane and GSK3 sequestration in endosomes mediating a sustained component of the response [37].
A number of other recent studies provide interesting suggestions regarding the functional significance of endosome signaling. One study described biphasic EGFR signaling determined by the concentration of growth factor to which cells are exposed. At low ligand concentration, downstream MAP kinase signaling is weak and mediated by activated EGFRs present in the plasma membrane. At higher ligand concentration, receptors are ubiquitinated and internalized. This results in a supra-linear increase in downstream signaling strength by redistributing activated receptors to endosomes, where downstream signaling is more efficient [38]. Another study reported that chemically distinct polypeptide ligands can elicit different responses via the EGFR through ligand-specific differences in endosomal dynamics, and that endosomes can be considered ‘quantal’ signaling devices because they contain a relatively uniform number of activated receptors [39]. A third interesting example is a study of MAP kinase signaling initiated by GPCRs. Here, it was proposed that distinct spatiotemporal profiles of MAP kinase activation can be produced by receptor recycling through different endosome populations [40].
Recent progress in the study of endosome signaling and its consequences
Significant advances have been made recently toward determining the subcellular location of defined receptor-mediated signaling mechanisms directly. As noted above, this is a key limitation of many studies in this area, and directly detecting signal initiation would seem feasible for growth factor signaling because pathway activation is associated with receptor phosphorylation and physical association of receptors with a signaling adaptor. Indeed, energy transfer methods have yielded arguably direct evidence of receptor-adaptor association in endosomes (e.g., [41]). However, concerns have been raised regarding the degree to which such interactions occur under conditions of physiological (versus supra-physiological) protein expression and ligand concentration [6]. Recently gene editing has been used to express recombinant proteins at near-endogenous levels. The results support endosome-based signaling under physiological or near-physiological conditions, and over a wide range of ligand concentration. They also suggest that endosomes make a remarkably large contribution to overall EGFR signaling activity, apparently more than that emanating from the plasma membrane [42].
Determining the subcellular location of canonical GPCR signal initiation poses an additional challenge because this is a catalytic reaction, involving formation of an activated receptor-G protein complex that is very short-lived under physiological conditions [43]. Recent progress on this problem emerged through development of single domain antibody fragments (nanobodies) that recognize specific GPCRs or G proteins in a conformation-selective manner. These reagents, generated initially for in vitro structural studies [44], were adapted to act as ‘conformational biosensors’ of GPCR and G protein activation when expressed as cytoplasmic fusion proteins.
Using such nanobody-derived tools, an activated conformational state of the beta-2 adrenergic receptor and a conformational intermediate in the process of cognate G protein (Gs) activation were detected within seconds after application of agonist ligand at the plasma membrane, consistent with the conventional model. In addition, evidence of a discrete activation phase was detected at endosomes, commencing within minutes after the arrival of internalized receptors and persisting after the initial plasma membrane activation phase diminished [45].
By adapting a light-activated adenylyl cyclase enzyme to optogenetically drive production of the second messenger cyclic AMP (cAMP) selectively from the plasma membrane or from the endosome limiting membrane, it was then shown that endosome-based signal activation preferentially induces cAMP-dependent transcriptional responses relative to signal initiation from the plasma membrane [46]. Caveats remain (e.g., protein over-expression and potential perturbing effects of biosensors or optogenetic actuators) but, together, these results constitute arguably direct evidence of GPCR-mediated signal initiation from endosomes and provide initial insight to its functional significance.
Outlook
Accumulating evidence strongly supports the general idea that receptor-mediated signaling and membrane trafficking processes are intimately interconnected (Figure 1). One connection, which is now well established, is that endocytic trafficking modulates long-term cellular responsiveness by dynamically adjusting the number of receptors accessible to extracellular ligands in the plasma membrane (or relevant domains thereof, such as synapses). Multiple endosomal sorting machineries contribute to such control, at least in higher eukaryotes, and these remain to be fully elaborated. Because some signaling receptors can engage more than one sorting machinery and ‘switch’ itinerary [47], much also remains unknown about how discrete endosomal machineries are coordinated and regulated to execute the appropriate net sorting decision.
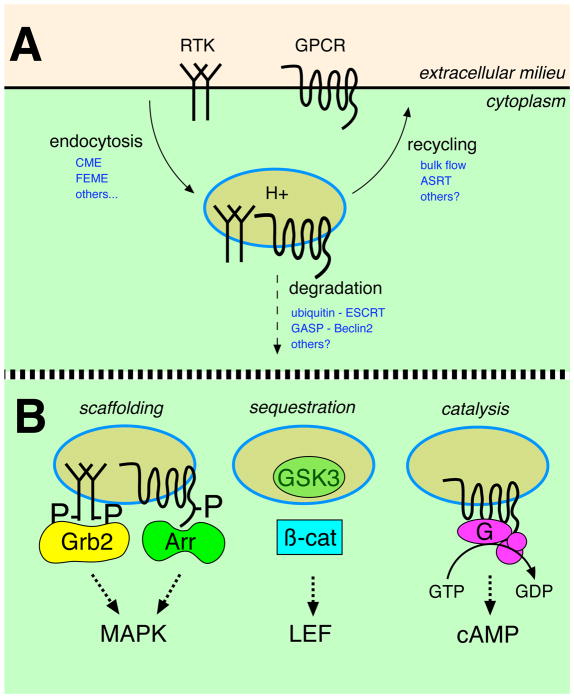
Figure 1. Simplified schematic of endocytic trafficking itineraries relevant to signaling receptors and proposed mechanisms of endosome-based signal activation.
(A) Signaling receptors such as receptor tyrosine kinase (RTK) growth factor receptors and members of the large G protein-coupled receptor (GPCR) family can internalize by various routes, including clathrin-mediated endocytosis (CME), fast endophilin-mediated endocytosis (FEME) and probably others. Internalized receptors are sorted by various mechanisms, a subset of which are indicated in the diagram using abbreviations explained in the text. These mechanisms determine the degree to which internalized receptors are delivered to lysosomes (degradation) or returned intact to the plasma membrane (recycling), divergent itineraries that down-regulate or sustain / resensitize net cellular signaling responsiveness, respectively. (B) Three biochemical principles that are currently thought to underlie signaling from endosomes. Scaffolding: Growth factor receptors can engage signaling adaptors such as Grb2 in the endosome limiting membrane, driving downstream activation of MAP kinase (MAPK) modules. GPCRs likely signal from endosomes in an analogous manner, except using arrestins or beta-arrestins (Arr) as alternate signaling scaffolds. Sequestration: Wnt / Wingless signaling is proposed to occur from endosomes through physical sequestration of GSK3 into the endosome lumen. This reduces cytoplasmic GSK3 activity, stabilizing and promoting cytoplasmic accumulation of beta-catenin (β-cat) that functions as a downstream mediator of transcriptional signaling through binding to the transcription factor LEF. Catalysis: GPCRs can activate heterotrimeric G proteins (G) from the endosome limiting membrane, which promotes downstream signaling through production of cytoplasmic second messenger molecules such as cyclic AMP (cAMP).
A second connection in the signaling-endocytosis nexus, which has been proposed for many years but largely through indirect or correlative evidence, is that endosomes serve as sites of active signal initiation. There is now direct evidence that growth factor receptors engage key signaling adaptors in endosomes under physiologically relevant conditions, and that endosomes are major contributors to the net cellular response. It is likely that GPCRs signal analogously, using arrestins as alternate signaling scaffolds [48]. Moreover, as summarized above, there is now arguably direct evidence that canonical GPCR - G protein activation occurs in endosomes.
A natural next question is how endosome-based signaling is controlled and terminated. Proteolytic destruction of receptors in the endolysosome system is one obvious mechanism based on first principles, and there is considerable evidence that this underlies long-term down-regulation of many cellular signaling responses [47]. Another well-established mechanism is through ubiquitin-directed transfer of receptors from the limiting endosome membrane to intralumenal vesicles. This is opposite to endosome sequestration of GSK3 that is proposed to activate Wnt signaling, as discussed above. However, physical sequestration of receptors into intralumenal vesicles has long been recognized to terminate growth factor signals prior to receptor proteolysis [16]. A third possible mechanism is through endosome acidification, a process that has long been recognized to promote ligand-receptor dissociation and modulate growth factor responses [4,49]. It is presently thought that endosome acidification terminates some receptor-mediated signals (e.g., [50]) but not others (e.g., [42]). Arrestins likely also function in endosome signal termination, particularly of signals initiated by GPCRs to which arrestins directly bind. However, arrestins and arrestin-like proteins likely also have distinct trafficking and signaling functions that include promoting some cellular signals (as noted above and reviewed authoritatively in [48]).
Finally, we note that much of our current understanding is derived from study of simplified experimental models. Thus an important direction for future research is to delineate relationships between signaling and endocytosis as they exist in native systems. The physiological and therapeutic implications of such relationships are only beginning to be explored and likely significant. For example, the phenomenon of functional selectivity or agonist bias, now a major focus in GPCR-targeted therapeutics (see [51]), was recognized in large part through ligand-specific effects on GPCR endocytosis (reviewed in [52]). We anticipate that further elucidation of the role of endosomes as sites of both molecular sorting and signaling, and elucidating how chemically diverse ligands affect these functions, will provide fundamental biological insight useful for developing improved therapeutics and directing the actions of existing drugs more precisely.
Acknowledgments
The authors thank present and former members of the von Zastrow lab, as well as many other colleagues and collaborators at UCSF and elsewhere, for contributions of data, reagents, ideas and critical discussion. We regret being able to cite only a subset of important papers in this area. Work in the authors’ laboratory is supported by the U.S. National Institutes of Health. R.I. is supported by a K99/R00 Pathway to Independence Award from the NIH / NHLBI. N.T. is supported by a postdoctoral fellowship from the American Heart Association. B.L. is supported by a postdoctoral fellowship from the NIH / NIDA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Roshanak Irannejad, Email: roshanak.irannejad@ucsf.edu.
Mark von Zastrow, Email: mark.vonzastrow@ucsf.edu.
References
- 1•.Posner BI, Bergeron JJM. Assessment of internalization and endosomal signaling: studies with insulin and EGF. Methods in enzymology. 2014;535:293–307. doi: 10.1016/B978-0-12-397925-4.00017-1. A review of methods and early studies motivating the hypotheses of receptor down-regulation by endocytosis and active signaling from endosomes. [DOI] [PubMed] [Google Scholar]
- 2•.Haigler HT, McKanna JA, Cohen S. Direct visualization of the binding and internalization of a ferritin conjugate of epidermal growth factor in human carcinoma cells A-431. The Journal of Cell Biology. 1979;81:382–395. doi: 10.1083/jcb.81.2.382. Early primary data reporting endocytosis of growth factors and growth factor receptors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marsh M, Bolzau E, Helenius A. Penetration of Semliki Forest virus from acidic prelysosomal vacuoles. Cell. 1983;32:931–940. doi: 10.1016/0092-8674(83)90078-8. [DOI] [PubMed] [Google Scholar]
- 4•.Dunn WA, Hubbard AL. Receptor-mediated endocytosis of epidermal growth factor by hepatocytes in the perfused rat liver: ligand and receptor dynamics. The Journal of Cell Biology. 1984;98:2148–2159. doi: 10.1083/jcb.98.6.2148. The above two papers pioneer the general concept that endosomes represent a distinct membrane compartment and provide a thorough, early characterization of epidermal growth factor trafficking through endosomes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5•.Cohen S, Fava RA. Internalization of functional epidermal growth factor:receptor/kinase complexes in A-431 cells. The Journal of Biological Chemistry. 1985;260:12351–12358. Early primary data suggesting functional activity of growth factor receptors in endosomes. [PubMed] [Google Scholar]
- 6.Goh LK, Sorkin A. Endocytosis of receptor tyrosine kinases. Cold Spring Harbor Perspectives in Biology. 2013:5. doi: 10.1101/cshperspect.a017459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cosker KE, Segal RA. Neuronal Signaling through Endocytosis. Cold Spring Harbor Perspectives in Biology. 2014:6. doi: 10.1101/cshperspect.a020669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Fiore PP, von Zastrow M. Endocytosis, signaling, and beyond. Cold Spring Harbor perspectives in biology. 2014:6. doi: 10.1101/cshperspect.a016865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Irannejad R, von Zastrow M. GPCR signaling along the endocytic pathway. Current Opinion in Cell Biology. 2014;27:109–116. doi: 10.1016/j.ceb.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Traub LM, Bonifacino JS. Cargo recognition in clathrin-mediated endocytosis. Cold Spring Harbor Perspectives in Biology. 2013:5. doi: 10.1101/cshperspect.a016790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mayor S, Parton RG, Donaldson JG. Clathrin-Independent Pathways of Endocytosis. Cold Spring Harbor Perspectives in Biology. 2014:6. doi: 10.1101/cshperspect.a016758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13••.Boucrot E, Ferreira APA, Almeida-Souza L, Debard S, Vallis Y, Howard G, Bertot L, Sauvonnet N, McMahon HT. Endophilin marks and controls a clathrin-independent endocytic pathway. Nature. 2015;517:460–465. doi: 10.1038/nature14067. Description of fast endophilin-mediated endocytosis (FEME) of signaling receptors. [DOI] [PubMed] [Google Scholar]
- 14••.Boucrot E, Pick A, Çamdere G, Liska N, Evergren E, McMahon HT, Kozlov MM. Membrane fission is promoted by insertion of amphipathic helices and is restricted by crescent BAR domains. Cell. 2012;149:124–136. doi: 10.1016/j.cell.2012.01.047. Further elaboration of FEME, focusing on mechanisms of membrane deformation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oldenhof J, Vickery R, Anafi M, Oak J, Ray A, Schoots O, Pawson T, von Zastrow M, Van Tol HH. SH3 binding domains in the dopamine D4 receptor. Biochemistry. 1998;37:15726–15736. doi: 10.1021/bi981634+. [DOI] [PubMed] [Google Scholar]
- 16.Henne WM, Buchkovich NJ, Emr SD. The ESCRT pathway. Developmental cell. 2011;21:77–91. doi: 10.1016/j.devcel.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 17•.Whistler JL, Enquist J, Marley A, Fong J, Gladher F, Tsuruda P, Murray SR, Von Zastrow M. Modulation of postendocytic sorting of G protein-coupled receptors. Science (New York, NY) 2002;297:615–620. doi: 10.1126/science.1073308. Identification of a putative GPCR-associated sorting protein (GASP) that is capable of recognizing mammalian GPCRs without requiring ubiquitination. [DOI] [PubMed] [Google Scholar]
- 18.Simonin F, Karcher P, Boeuf JJM, Matifas A, Kieffer BL. Identification of a novel family of G protein-coupled receptor associated sorting proteins. Journal of Neurochemistry. 2004;89:766–775. doi: 10.1111/j.1471-4159.2004.02411.x. [DOI] [PubMed] [Google Scholar]
- 19••.He C, Wei Y, Sun K, Li B, Dong X, Zou Z, Liu Y, Kinch LN, Khan S, Sinha S, et al. Beclin 2 Functions in Autophagy, Degradation of G Protein-Coupled Receptors, and Metabolism. Cell. 2013;154:1085–1099. doi: 10.1016/j.cell.2013.07.035. Discovery of Beclin 2 as a GASP-interacting protein with dual functions in GPCR sorting and autophagy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diao J, Liu R, Rong Y, Zhao M, Zhang J, Lai Y, Zhou Q, Wilz LM, Li J, Vivona S, et al. ATG14 promotes membrane tethering and fusion of autophagosomes to endolysosomes. Nature. 2015 doi: 10.1038/nature14147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marley A, von Zastrow M. Dysbindin promotes the post-endocytic sorting of G protein-coupled receptors to lysosomes. PloS one. 2010:5. doi: 10.1371/journal.pone.0009325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22•.Rosciglione S, Thériault C, Boily M-O, Paquette M, Lavoie C. Gαs regulates the post-endocytic sorting of G protein-coupled receptors. Nature Communications. 2014;5 doi: 10.1038/ncomms5556. The above two papers propose a distinct sorting function of GASP through mediating alternate, ubiquitin-independent connectivity to the ESCRT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lauffer BEL, Melero C, Temkin P, Lei C, Hong W, Kortemme T, von Zastrow M. SNX27 mediates PDZ-directed sorting from endosomes to the plasma membrane. The Journal of Cell Biology. 2010;190:565–574. doi: 10.1083/jcb.201004060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Temkin P, Lauffer B, Jäger S, Cimermancic P, Krogan NJ, von Zastrow M. SNX27 mediates retromer tubule entry and endosome-to-plasma membrane trafficking of signalling receptors. Nature cell biology. 2011;13:715–721. doi: 10.1038/ncb2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steinberg F, Gallon M, Winfield M, Thomas EC, Bell AJ, Heesom KJ, Tavaré JM, Cullen PJ. A global analysis of SNX27-retromer assembly and cargo specificity reveals a function in glucose and metal ion transport. Nature cell biology. 2013;15:461–471. doi: 10.1038/ncb2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26•.Choy RW-Y, Park M, Temkin P, Herring BE, Marley A, Nicoll RA, von Zastrow M. Retromer mediates a discrete route of local membrane delivery to dendrites. Neuron. 2014;82:55–62. doi: 10.1016/j.neuron.2014.02.018. The above four papers describe the ASRT machinery, its wide range of cargoes, and function in surface delivery of neural signaling receptors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Small SA, Petsko GA. Retromer in Alzheimer disease, Parkinson disease and other neurological disorders. Nat Rev Neurosci. 2015;16:126–132. doi: 10.1038/nrn3896. [DOI] [PubMed] [Google Scholar]
- 28.Harrington AW, Hillaire CS, Zweifel LS, Glebova NO, Philippidou P, Halegoua S, Ginty DD. Recruitment of actin modifiers to TrkA endosomes governs retrograde NGF signaling and survival. Cell. 2011;146:421–434. doi: 10.1016/j.cell.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mok SA, Campenot RB. A nerve growth factor-induced retrograde survival signal mediated by mechanisms downstream of TrkA. Neuropharmacology. 2007;52:270–278. doi: 10.1016/j.neuropharm.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 30.Vieira AV, Lamaze C, Schmid SL. Control of EGF receptor signaling by clathrin-mediated endocytosis. Science (New York, NY) 1996;274:2086–2089. doi: 10.1126/science.274.5295.2086. [DOI] [PubMed] [Google Scholar]
- 31.Daaka Y, Luttrell LM, Ahn S, Della Rocca GJ, Ferguson SS, Caron MG, Lefkowitz RJ. Essential role for G protein-coupled receptor endocytosis in the activation of mitogen-activated protein kinase. The Journal of Biological Chemistry. 1998;273:685–688. doi: 10.1074/jbc.273.2.685. [DOI] [PubMed] [Google Scholar]
- 32.Whistler JL, Zastrow Mv. Dissociation of Functional Roles of Dynamin in Receptor-mediated Endocytosis and Mitogenic Signal Transduction. Journal of Biological Chemistry. 1999;274:24575–24578. doi: 10.1074/jbc.274.35.24575. [DOI] [PubMed] [Google Scholar]
- 33.Kranenburg O, Verlaan I, Moolenaar WH. Dynamin Is Required for the Activation of Mitogen-activated Protein (MAP) Kinase by MAP Kinase Kinase. Journal of Biological Chemistry. 1999;274:35301–35304. doi: 10.1074/jbc.274.50.35301. [DOI] [PubMed] [Google Scholar]
- 34.Seto ES, Bellen HJ. Internalization is required for proper Wingless signaling in Drosophila melanogaster. The Journal of Cell Biology. 2006;173:95–106. doi: 10.1083/jcb.200510123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35•.Taelman VF, Dobrowolski R, Plouhinec JL, Fuentealba LC, Vorwald PP, Gumper I, Sabatini DD, De Robertis EM. Wnt signaling requires sequestration of glycogen synthase kinase 3 inside multivesicular endosomes. Cell. 2010;143:1136–1148. doi: 10.1016/j.cell.2010.11.034. Evidence suggesting that endosomes can mediate signal transduction by sequestering a pathway-inhibitory kinase into intralumenal vesicles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36•.Mi K, Dolan PJ, Johnson GV. The low density lipoprotein receptor-related protein 6 interacts with glycogen synthase kinase 3 and attenuates activity. J Biol Chem. 2006;281:4787–4794. doi: 10.1074/jbc.M508657200. Evidence supporting pseudosubstrate inhibition mechanism of Wnt pathway activation. [DOI] [PubMed] [Google Scholar]
- 37•.Vinyoles M, Del Valle-Pérez B, Curto J, Viñas-Castells R, Alba-Castellón L, García de Herreros A, Duñach M. Multivesicular GSK3 Sequestration upon Wnt Signaling Is Controlled by p120-Catenin/Cadherin Interaction with LRP5/6. Molecular Cell. 2014;53:444–457. doi: 10.1016/j.molcel.2013.12.010. A recent study suggesting that distinct mechanisms of Wnt signaling are initiated from the plasma membrane relative to endosomes, and make different contributions to the functional Wnt response. [DOI] [PubMed] [Google Scholar]
- 38.Sigismund S, Algisi V, Nappo G, Conte A, Pascolutti R, Cuomo A, Bonaldi T, Argenzio E, Verhoef LGGC, Maspero E, et al. Threshold-controlled ubiquitination of the EGFR directs receptor fate. The EMBO journal. 2013;32:2140–2157. doi: 10.1038/emboj.2013.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Villaseñor R, Nonaka H, Conte-Zerial PD, Kalaidzidis Y, Zerial M. Regulation of EGFR signal transduction by analogue-to-digital conversion in endosomes. eLife. 2015 doi: 10.7554/eLife.06156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40•.Jean-Alphonse F, Bowersox S, Chen S, Beard G, Puthenveedu MA, Hanyaloglu AC. Spatially restricted G protein-coupled receptor activity via divergent endocytic compartments. The Journal of Biological Chemistry. 2014;289:3960–3977. doi: 10.1074/jbc.M113.526350. An interesting study supporting the hypothesis that distinct subpopulations of endosomes, and receptor-specific sorting between them, contributes to the generation of diversity in GPCR signaling via MAP kinase activation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sorkin A, McClure M, Huang F, Carter R. Interaction of EGF receptor and grb2 in living cells visualized by fluorescence resonance energy transfer (FRET) microscopy. Curr Biol. 2000;10:1395–1398. doi: 10.1016/s0960-9822(00)00785-5. [DOI] [PubMed] [Google Scholar]
- 42.Fortian A, Sorkin A. Live-cell fluorescence imaging reveals high stoichiometry of Grb2 binding to the EGF receptor sustained during endocytosis. Journal of Cell Science. 2014;127:432–444. doi: 10.1242/jcs.137786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosenbaum DM, Rasmussen SGF, Kobilka BK. The structure and function of G-protein-coupled receptors. Nature. 2009;459:356–363. doi: 10.1038/nature08144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steyaert J, Kobilka BK. Nanobody stabilization of G protein-coupled receptor conformational states. Current opinion in structural biology. 2011;21:567–572. doi: 10.1016/j.sbi.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45•.Irannejad R, Tomshine JC, Tomshine JR, Chevalier M, Mahoney JP, Steyaert J, Rasmussen GF, Sr, Sunahara RK, El-Samad H, Huang B, et al. Conformational biosensors reveal GPCR signalling from endosomes. Nature. 2013;495:534–538. doi: 10.1038/nature12000. Development of conformational biosensors, and direct evidence that GPCR and G protein activation occur in endosomes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46•.Tsvetanova NG, von Zastrow M. Spatial encoding of cyclic AMP signaling specificity by GPCR endocytosis. Nature chemical biology. 2014;10:1061–1065. doi: 10.1038/nchembio.1665. Development of subcellular optogenetics, and direct evidence that second messenger signal initiation from endosomes can produce different functional consequences than signal initiation from the plasma membrane. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Henry AG, White IJ, Marsh M, von Zastrow M, Hislop JN. The role of ubiquitination in lysosomal trafficking of δ-opioid receptors. Traffic (Copenhagen, Denmark) 2011;12:170–184. doi: 10.1111/j.1600-0854.2010.01145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shenoy SK, Lefkowitz RJ. β-Arrestin-mediated receptor trafficking and signal transduction. Trends in pharmacological sciences. 2011;32:521–533. doi: 10.1016/j.tips.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ebner R, Derynck R. Epidermal growth factor and transforming growth factor-alpha: differential intracellular routing and processing of ligand-receptor complexes. Cell Regul. 1991;2:599–612. doi: 10.1091/mbc.2.8.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gidon A, Al-Bataineh MM, Jean-Alphonse FG, Stevenson HP, Watanabe T, Louet C, Khatri A, Calero G, Pastor-Soler NM, Gardella TJ, et al. Endosomal GPCR signaling turned off by negative feedback actions of PKA and v-ATPase. Nat Chem Biol. 2014;10:707–709. doi: 10.1038/nchembio.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Strachan RT, Sun J-p, Rominger DH, Violin JD, Ahn S, Rojas Bie Thomsen A, Zhu X, Kleist A, Costa T, Lefkowitz RJ. Divergent transducer-specific molecular efficacies generate biased agonism at a G protein-coupled receptor (GPCR) The Journal of Biological Chemistry. 2014;289:14211–14224. doi: 10.1074/jbc.M114.548131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, et al. Functional selectivity and classical concepts of quantitative pharmacology. The Journal of Pharmacology and Experimental Therapeutics. 2007;320:1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]