Abstract
Background
New technologies—often with limited evidence to support their effectiveness—frequently diffuse into clinical practice and increase the costs of cancer care. We studied whether physician peer exposure was associated with the subsequent adoption of a new approach to adjuvant radiation therapy (brachytherapy) for the treatment of women with early stage breast cancer.
Methods
We performed a retrospective cohort study using SEER-Medicare data. Data from 2003–2004 was used to classify surgeons as early brachytherapy adopters and, among non-early adopters, whether they shared patients with early adopters (peer exposure). Data from 2005–2006 was used to examine whether women were more likely to receive brachytherapy if their surgeons were exposed to early adopters.
Results
Overall, the proportion of women receiving brachytherapy increased from 3.2% in 2003–2004 to 4.7% in 2005–2006. In this latter period, 2,087 patients were assigned to 328 non-early adopting surgeons. In unadjusted analyses, patients whose surgeons were connected to early adopters during 2003–2004 were significantly more likely to receive brachytherapy in 2005–2006 compared to those whose surgeons were not connected to early adopters (8.0% versus 4.1%, p=0.003). In adjusted analyses, the predicted probability of receiving brachytherapy in patients whose surgeon did have an early adopting peer was 3.9% vs. 1.0% among those whose surgeons did not have an early adopting peer(p=0.03).
Conclusions
Exposure to peers who were early adopters of brachytherapy was associated with a surgeon’s subsequent uptake of brachytherapy. This study provides an example of a novel approach to examining the diffusion of innovation in cancer care.
Keywords: Diffusion of innovation, physician patient-sharing networks, breast cancer, brachytherapy
INTRODUCTION
The cost of cancer care is growing rapidly and is expected to exceed $150 billion annually by 2020.(1) New technologies such as targeted cancer therapies, surgical techniques such as robotic assisted surgery, and new radiotherapy modalities have been important contributors to the rise in cancer spending over the past decade.(2–5) Frequently, these interventions diffuse into clinical practice without clear evidence of their effectiveness. Understanding the factors that influence provider adoption of new and unproven technologies is critical to develop strategies to better align clinical practice with available evidence, and to control health care costs.
The adoption of new technologies into clinical practice is influenced by several factors, including patient demand, clinical evidence, and payer policies.(6) Yet these factors are unable to explain the wide variation in the use of new cancer treatment modalities across geographic areas and between providers.(7) One of the more profound factors that influences provider behavior has largely been overlooked: the behavior of other providers.
Building on prior work on the diffusion of innovation,(8) we sought to apply the nascent methods of physician patient-sharing analysis to study peer influence in the adoption of one cancer care intervention. Physicians who share more patients with one another in insurance claims data are more likely to refer to and seek advice from one another.(9) Such patient sharing may reflect peer relationships which enable innovation to diffuse between providers. Previous studies have found that physicians who share patients with one another are more likely to practice in a similar manner in terms of costs, intensity of care, treatment rates and outcomes.(10–14) But what is not known is whether patient sharing is linked with the uptake of new technology over time.
To address this knowledge gap, we assessed the diffusion of brachytherapy, a new approach to adjuvant radiation therapy following breast conserving surgery (BCS) for early stage breast cancer. Brachytherapy temporarily implants radiation seeds using balloon catheters within and/or adjacent to the resection cavity. It is an excellent example to study for several reasons. First, despite a paucity of large randomized trials demonstrating their efficacy, new radiation therapy modalities such as brachytherapy have disseminated widely into clinical practice.(15–17) This rapid adoption has occurred despite concerns about effectiveness: recent observational studies suggest that brachytherapy may be associated with higher rates of complications and inferior cancer control compared to whole breast irradiation.(15, 18) Further, the costs associated with brachytherapy are significantly higher than those associated with whole breast irradiation, contributing to the high rate of health care spending.(4) Finally, recent work has determined that surgeons are an important influence on the use of brachytherapy: while rates of brachytherapy vary widely between geographic regions, the specific surgeon that a patient sees explains a much larger proportion of the variation than the region in which she lives.(4) Brachytherapy therefore offers an important case study into the ways in which expensive cancer therapies without overwhelming evidence to suggest their effectiveness diffuse into clinical practice.
To examine how physician peer exposure impacts the use of brachytherapy, we first identified surgeons who early adopters of brachytherapy using Medicare data. We then determined whether surgeons who shared patients with these early adopters were more likely to subsequently use brachytherapy. Surgeons may share patients with one another in claims data for a variety reasons including assisting one another with surgeries, seeing each other’s patient’s during evaluation and follow-up, receiving referrals from one another, and/or providing second opinions about cancer care. To the extent that patient sharing reflects exchanging clinical information, it may be linked with the adoption of new technology among one’s surgical peers.
METHODS
Overview
Our approach involved examining the use of brachytherapy for early stage breast cancer over two time periods. The initial time period (2003–2004), which was early during the adoption of brachytherapy into clinical practice, was used to classify surgeons according to whether they were early adopters of brachytherapy. Only surgeons who were not early adopters during the early time period were included in the final study sample. Among these non-early-adopting-surgeons, we determined whether they were connected to early adopting surgeons via shared patients (peer exposure) in the earlier time period. In the later time period (2005–2006), we then examined the practice patterns of these ‘non-early-adopting’ surgeons, to determine whether their peer exposure during the earlier time period affected the likelihood of their patients receiving brachytherapy. The two year time periods were chosen to allow for increased uptake of brachytherapy, adequate definition of peer exposure, and sufficient sample size for outcome analysis. Figure 1 provides an overview of the study design.
Figure 1.
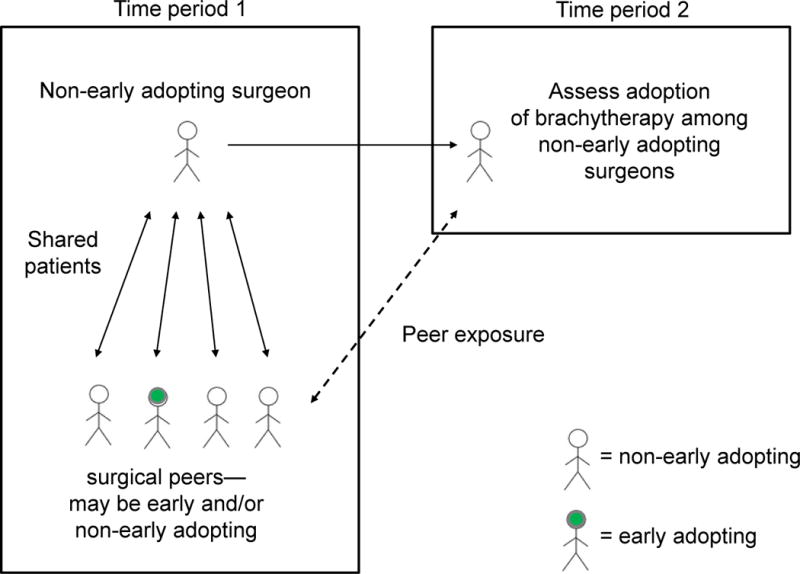
Overview of the study design. The first time period (2003–2004) was used to classify surgeons as early adopters vs. non-early adopters of brachytherapy. Among non-early adopters, we determined whether they were connected to early adopting surgeons via shared patients (peer exposure). In the later time period (2005–2006), we then examined the practice patterns of these non-early-adopting surgeons. Specifically, we assessed whether their peer exposure during the earlier time period was associated with the receipt of receiving brachytherapy during the later time period.
Data Source and Construction of Study Samples
The Surveillance, Epidemiology and End Results (SEER)-Medicare database links patient demographic and tumor-specific data collected by the SEER cancer registries to longitudinal health claims for Medicare enrollees. The population-based SEER registries operate and maintain high quality population-based cancer reporting systems and include a population comparable to the broader U.S. population.(19) Medicare is a US government program that provides insurance primarily for adults age 65 and older.
The 2003–2004 sample (Figure 2) was used to define (a) the number of shared patients between surgeons and (b) whether surgeons were early adopters. To determine the patient sharing, we included all women with non-metastatic, invasive stage I–III breast cancer diagnosed in 2003–2004. Additional patient inclusion criteria included: age 67 to 94 years, first or only tumor diagnosis, histologic characteristics consistent with epithelial origin, known month of diagnosis, diagnosis not reported from autopsy or death certificate, and did not receive a second non-breast cancer diagnosis in the year following diagnosis. To ensure that patients were likely to have complete Medicare claims, we only included patients with continuous enrollment in fee-for-service Medicare Parts A and B from 24 months before diagnosis through 12 months after surgery or diagnosis if patient did not receive surgery (or until death). This yielded a sample of 15,073 women. Patients were assigned to all of the surgeons that they saw from the 2 months before diagnosis to the 12 months following diagnosis. Surgeons who billed for care for the same patient were said to have a shared patient. Because surgeons who share a higher number of patients with one another are more likely to report knowing one another, we required physicians to share at least two patients to be considered connected.(9) There were a total of 1,282 pairs of surgeons who shared between 2 and 54 patients.
Figure 2.
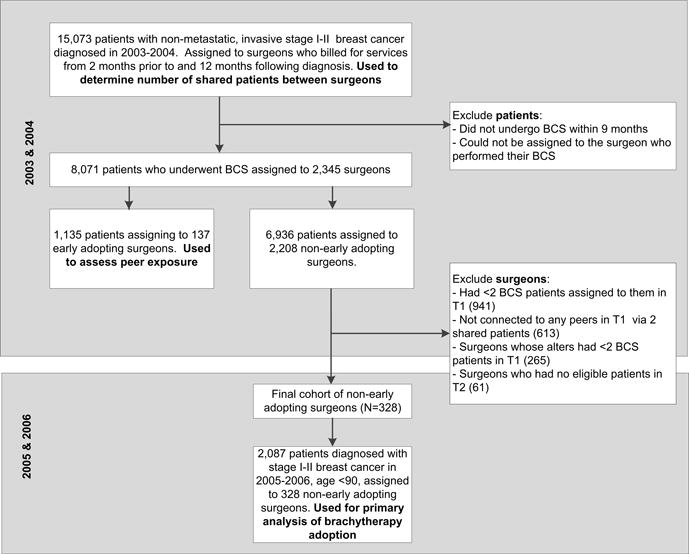
Patient and surgeon cohorts
To define whether surgeons were early adopters, we further limited the above sample to women who received BCS in the 9 months following their diagnosis and the surgeons who performed their BCS. Surgeons were defined as early adopters if any of their assigned patients underwent brachytherapy in the 9 months following BCS (see eTable 1 for codes for BCS and brachytherapy). To increase the potential accuracy of our classification of peer exposure, we required that non-early adopters had at least 2 BCS patients assigned to them, had no patients who received brachytherapy, be connected to at least 1 other surgeon (termed ‘alter’) via 2 or more shared patients, and that the sum of their alters’ patients be at least 2.
Our outcomes analysis focused on women with stage I and II breast cancer diagnosed during 2005–2006 who underwent BCS and were assigned to a non-early adopting surgeon. We employed the same patient inclusion criteria as above, further excluding women with stage III disease and women age 90 and over due to the small numbers in this sample.
Patient characteristics
For women diagnosed in 2005–2006, the primary outcome was whether the patient received brachytherapy within 9 months following BCS. Patient-level covariates included: age, race, marital status, median household income at the census tract or zip code level, cancer stage, tumor laterality, hormone receptor status, and comorbidity in the 2 years prior to diagnosis. Comorbidity was measured using a modified list of the conditions suggested by Elixhauser et al. that we had previously found were associated with survival in a non-cancer sample.(20) We categorized each patient’s number of comorbid conditions as 0, 1–2, or ≥3.
Peer exposures
Non-early adopting surgeons were classified according to whether they had alters who were early adopters of brachytherapy during the 2003–2004 time period. We further specified the total number of surgical peers that each surgeon was connected to (via ≥2 shared patients) and total number of surgical peers’ patients who underwent BCS in 2003–2004.
Surgeon, hospital, and regional characteristics
Surgical volume for each patient’s assigned surgeon was defined as the number of patients diagnosed in 2005–2006 for whom they performed BCS. Patients were assigned to the hospital where their surgery was performed. Because the use of brachytherapy may be driven by the availability of resources, we defined whether the hospital had at least one assigned patient who underwent brachytherapy (in 2003–2004). Additional hospital characteristics included teaching status and total bed size divided into quartiles. We included hospital referral region-level two year rate of mammogram among female Medicare enrollees age 67–69, divided in quartiles, as this has been shown previously to be associated with receipt of brachytherapy.(21)
Statistical analysis
After presenting descriptive statistics, we examined whether patient, surgeon, hospital and regional characteristics were associated with the receipt of brachytherapy among women diagnosed in 2005–2006 using chi-squared tests. We then performed unadjusted logistic regression analyses. Covariates that were significantly associated with brachytherapy in these models (p<0.05) were retained in the subsequent adjusted model, using patient as the unit of analysis in order to account for patient-level factors. We adjusted for clustering by surgeon using the GLIMMIX procedure in SAS, in which the surgeon was specified as a random effect, to account for the likelihood that patients who are treated by the same surgeon are more likely to share similar characteristics. We estimated the predicted probability of receiving brachytherapy among patients who did and did not have a surgeon who was connected to an early-adopting alter using the LS Means option in GLIMMIX.
In sensitivity analyses, we first altered our definition of non-early adopting surgeons to those who were assigned at least 4 patients who underwent BCS in 2003–2004 and whose alters had at least 4 or more patients in total. This created more stringent criteria with which to identify surgeons who were not early adopters and for peer exposure. Second, we adjusted for clustering of patients by hospital assignment. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary NC). The Yale Human Investigations Committee determined that this study did not constitute human subjects research.
RESULTS
During the 2003–2004 period, we identified 8,071 patients who underwent BCS from a total of 2,345 surgeons. Of these, 3.2% of women received brachytherapy, and 137 (5.8%) surgeons were defined as early adopters.
The 2,087 patients diagnosed in 2005–2006 who underwent BCS were assigned to one of 328 non-early adopting surgeons (Table 1). The majority of these women (92.1%) were white and nearly half were married (48.1%). Seventy-two percent had stage I disease and 84.8% were hormone receptor positive.
Table 1.
Characteristics of patients with stage I–II breast cancer in 2005–2006
| N | % | % received brachytherapy* | P-value** | |
|---|---|---|---|---|
| Overall | 2087 | 4.7 | ||
| Patient characteristics | ||||
| Age group | .23 | |||
| 67–69 | 403 | 19.3 | 4.7 | |
| 70–74 | 579 | 27.7 | 4.8 | |
| 75–79 | 527 | 25.3 | 5.9 | |
| 80–89 | 578 | 27.7 | 3.3 | |
| Race | .19 | |||
| White | 1923 | 92.1 | >3.9 | |
| Black | 103 | 4.9 | <10.7 | |
| Other | 61 | 2.9 | <18.0 | |
| Marital status | .10 | |||
| Married | 1004 | 48.1 | 5.5 | |
| Unmarried | 991 | 47.5 | >3.1 | |
| Unknown | 92 | 4.4 | <12.0 | |
| Income | .46 | |||
| <$33K | 272 | 13.0 | <4.0 | |
| $33 – <$40K | 253 | 12.1 | 4.7 | |
| $40K – <$50K | 416 | 19.9 | >4.8 | |
| $50K – <$63K | 469 | 22.5 | 3.6 | |
| ≥$63K | 677 | 32.4 | 5.5 | |
| Clinical characteristics | ||||
| Stage | <.001 | |||
| I | 1497 | 71.7 | >5.7 | |
| II | 590 | 28.3 | <1.9 | |
| Laterality | .85 | |||
| Right | 1035 | 49.6 | 4.7 | |
| Left | 1052 | 50.4 | 4.6 | |
| Hormone status | .02 | |||
| Negative | 235 | 11.3 | <4.7 | |
| Positive | 1769 | 84.8 | >4.2 | |
| Unknown | 83 | 4.0 | <13.3 | |
| Comorbid conditions | .42 | |||
| 0 | 980 | 47.0 | 5.0 | |
| 1–2 | 821 | 39.3 | >4.5 | |
| ≥3 | 286 | 13.7 | <3.8 | |
| Hospital characteristics | ||||
| Hospital brachytherapy use† | .60 | |||
| No | 1404 | 67.3 | 4.3 | |
| Yes | 379 | 18.2 | 5.5 | |
| Unknown | 304 | 14.6 | 4.9 | |
| Teaching hospital | .02 | |||
| No | 761 | 36.5 | 6.2 | |
| Yes | 1021 | 48.9 | 3.4 | |
| Unknown | 305 | 14.6 | 4.9 | |
| Total hospital beds | <.001 | |||
| Q1 (<216) | 441 | 21.1 | 2.7 | |
| Q2 (216 – 334) | 459 | 22.0 | 5.2 | |
| Q3 (335 – 442) | 433 | 20.8 | 8.8 | |
| Q4 (443 – 1161) | 449 | 21.5 | <2.4 | |
| Unknown | 305 | 14.6 | >3.9 | |
| Regional characteristics | ||||
| HRR-level two-year mammography rate | .047 | |||
| Q1 (52.4–58.1) | 502 | 24.1 | 5.4 | |
| Q2 (58.5–62.2) | 554 | 26.6 | 2.9 | |
| Q3 (62.2–65.4) | 506 | 24.3 | 6.3 | |
| Q4 (65.6–74.8) | 525 | 25.2 | 4.2 | |
| Surgeon characteristics | ||||
| Had an early-adopting alter | .003 | |||
| No | 1786 | 85.6 | 4.1 | |
| Yes | 301 | 14.4 | 8.0 | |
| Patient volume (in 2005–2006) (Mean, SD) | 13.5 | (11.9) | ||
| Number of peers in 2003–2004 (Mean, SD) | 2.4 | (1.6) | ||
| Number of peers’ BCS patients in 2003–2004 (Mean, SD) | 10.8 | (11.9) |
Exact percentages are not presented in some cells due to the Centers for Medicare and Medicaid Services’ prohibition against presenting cells sizes <11
Chi-square test of the association between each covariate and receipt of brachytherapy
Defined as whether the hospital had at least one assigned patient who underwent brachytherapy in 2003–2004
HRR: Hospital referral region
Among women who received BCS in 2005–2006, 5.9 % received brachytherapy. After limiting our sample to patients and surgeons who met final inclusion criteria in 2005–2006, 4.7% received brachytherapy (Table 1). Among these patients, 4.1% whose surgeon did not have an early adopting alter received brachytherapy, compared to 8.0% for patients whose surgeon did have an early adopting alter (p=.003). Brachytherapy use was higher among patients with stage I compared to stage II disease, and those treated at non-teaching versus teaching hospitals (6.2% versus 3.4%, p=0.02). Brachytherapy varied across hospital size, though a clear gradient was not observed.
In unadjusted logistic regression (Table 2), patients whose surgeon had an early adopting alter were significantly more likely to receive brachytherapy compared to patients whose surgeons did not have an early adopting alter (odds ratio [OR] 3.19, 95% Confidence Interval [CI] 1.05–9.70). Cancer stage and hospital size were also significantly associated with receipt of brachytherapy in unadjusted analyses. In adjusted analyses, having a surgeon who was connected to an early-adopting alter remained significantly associated with brachytherapy (OR 3.40, 95%CI 1.11–10.38). The predicted probability of receiving brachytherapy in patients whose surgeon did not have an early adopting alter was 1.0%, while the probability in patients whose surgeon did have an early adopting alter was 3.9%. The difference (2.9%) was statistically significant (p=.03).
Table 2.
Unadjusted and adjusted logistic regression models showing the association with receipt of brachytherapy among patients with stage I–II breast cancer in 2005–2006*
| UNADJUSTED | ADJUSTED | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Odds Ratio | 95% CI | P-value | Odds Ratio | 95% CI | P-value | |||
| Patient characteristics | ||||||||
| Age group | .07 | |||||||
| 67–69 | 1.00 | – | – | |||||
| 70–74 | 0.88 | 0.42 | 1.83 | |||||
| 75–79 | 1.55 | 0.75 | 3.20 | |||||
| 80–89 | 0.60 | 0.27 | 1.30 | |||||
| Race | .28 | |||||||
| White | 1.00 | – | – | |||||
| Black | 0.15 | 0.02 | 1.53 | |||||
| Other | 1.08 | 0.27 | 4.33 | |||||
| Marital status | .35 | |||||||
| Married | 1.00 | – | – | |||||
| Unmarried | 0.69 | 0.41 | 1.15 | |||||
| Unknown | 1.04 | 0.31 | 3.45 | |||||
| Income | .92 | |||||||
| <$33K | 1.00 | – | – | |||||
| $33 – <$40K | 1.02 | 0.36 | 2.91 | |||||
| $40K – <$50K | 1.12 | 0.43 | 2.93 | |||||
| $50K – <$63K | 0.99 | 0.36 | 2.72 | |||||
| ≥$63K | 1.35 | 0.52 | 3.53 | |||||
| Clinical characteristics | ||||||||
| Stage | <.001 | <.001 | ||||||
| I | 1.00 | – | – | 1.00 | – | – | ||
| II | 0.17 | 0.08 | 0.39 | 0.17 | 0.08 | 0.39 | ||
| Laterality | .81 | |||||||
| Right | 1.00 | – | – | |||||
| Left | 0.94 | 0.58 | 1.53 | |||||
| Hormone status | .54 | |||||||
| Negative | 1.00 | – | – | |||||
| Positive | 1.06 | 0.48 | 2.36 | |||||
| Unknown | 1.88 | 0.54 | 6.53 | |||||
| Comorbid conditions | .29 | |||||||
| 0 | 1.00 | – | – | |||||
| 1–2 | 1.03 | 0.61 | 1.73 | |||||
| ≥3 | 0.52 | 0.22 | 1.24 | |||||
| Hospital characteristics | ||||||||
| Hospital brachytherapy use† | .47 | |||||||
| No | 1.00 | – | – | |||||
| Yes | 1.80 | 0.70 | 4.61 | |||||
| Unknown | 1.15 | 0.49 | 2.72 | |||||
| Teaching hospital | .32 | |||||||
| No | 1.00 | – | – | |||||
| Yes | 0.54 | 0.25 | 1.20 | |||||
| Unknown | 0.73 | 0.29 | 1.83 | |||||
| Total hospital beds | .03 | .02 | ||||||
| Q1 (<216) | 1.00 | – | – | 1.00 | – | – | ||
| Q2 (216 – 334) | 1.56 | 0.52 | 4.68 | 1.61 | 0.52 | 5.03 | ||
| Q3 (335 – 442) | 3.48 | 1.23 | 9.88 | 3.74 | 1.27 | 11.02 | ||
| Q4 (443 – 1161) | 0.65 | 0.19 | 2.18 | 0.61 | 0.17 | 2.19 | ||
| Unknown | 1.50 | 0.50 | 4.49 | 1.46 | 0.47 | 4.53 | ||
| Regional characteristics | ||||||||
| HRR-level two-year mammography rate | .73 | |||||||
| Q1 (52.4–58.1) | 1.00 | – | – | |||||
| Q2 (58.5–62.2) | 0.64 | 0.22 | 1.91 | |||||
| Q3 (62.2–65.4) | 1.16 | 0.43 | 3.13 | |||||
| Q4 (65.6–74.8) | 1.13 | 0.39 | 3.28 | |||||
| Surgeon characteristics | ||||||||
| Had an early-adopting peer in 2003–2004 | .04 | .03 | ||||||
| No | 1.00 | – | – | 1.00 | – | – | ||
| Yes | 3.19 | 1.05 | 9.70 | 3.40 | 1.11 | 10.38 | ||
N=2,087 patients assigned to 328 surgeons; analyses adjusted for clustering by surgeon
Additional surgeon characteristics—patient volume in 2005–2006, number of peers in 2003–2004, and number of peers’ BCS patients in 2004–2004—were tested in unadjusted analyses and found not to be significantly associated with the receipt of brachytherapy. They were not included in the table for the sake of brevity.
Defined as whether the hospital had at least one assigned patient who underwent brachytherapy in 2003–2004
HRR: Hospital referral region
In sensitivity analyses, in which we changed the inclusion criteria for non-early adopting alters, we continued to find a significant association with brachytherapy (OR for having an early-adopting alter versus not, 7.50, 95%CI 1.84–30.63). Adjusting for clustering by hospital produced qualitatively similar results (OR 2.21, 95%CI 0.77–6.37 for initial alter criteria and OR 4.01, 95%CI 1.36–11.87 for revised alter criteria)
DISCUSSION
While the dissemination of cancer technology is a major driver of the high costs of cancer care, factors that influence adoption remain poorly understood. We found that peer exposure was significantly associated with the likelihood that surgeons would adopt a new approach to breast cancer care. The substantial difference in the predicted probability of brachytherapy use between surgeons with vs. without early-adopting peers suggest that peer exposure could be a major driver of the diffusion of innovation.
Rogers’ diffusion of innovation framework describes a process through which adopters come to learn about an innovation, develop a positive or negative attitude towards it, and decide to try and ultimately adopt it.(8) Social networks have been shown to shape perceptions of the innovation(22) and have been leveraged to promote the uptake of health interventions.(23–25) This study builds on prior work on the diffusion of innovation in important ways. Relatively few studies have specifically examined the role of social networks and peer influence in the diffusion of new cancer technologies. Demographic shifts, new technologies, and rising costs make this a critical area for investigation. A prior study in the UK found that a consultant’s team influenced the diffusion of breast conserving surgery, though this analysis did not fully account for individual patient characteristics.(26) More generally, prior studies which collect data on physician relationships have largely been unable to include patient and clinical factors that may impact adoption. We observed significant differences between patients whose surgeons did and did not have an early adopting peer (eTable 2) which underscores the need to adjust for these factors to isolate the impact of peer influence. Studies of physician social networks have typically relied on surveys to collect information on social ties between individuals. Such surveys can be costly to implement, challenging to perform, and prone to non-response, recall, and social desirability biases. Logistical burdens typically prevent researchers from surveying providers at multiple points in time, thus preventing a dynamic picture of peer social networks. Using insurance claims data to map potential peer influence expands the ease with which physician networks can be mapped across areas and over time.
Using claims data, however, we are unable to determine the mechanisms that may underpin the association between peer practice and subsequent physician adoption of brachytherapy. An average of 30% of patients in our sample saw more than 1 surgeon with nearly half of these seeing multiple surgeons on the same day. This suggests the possibility that these surgeons work closely with one another, potentially by assisting during surgeries and providing clinical advice. Being exposed to an early adopter may increase physician comfort and familiarity with the innovation, thus increasing the likelihood of adoption. Because brachytherapy involves the purchase of capital equipment, it is feasible that peers practicing at the same institution may be responsible for the observed associations. However, in sensitivity analyses in which we adjusted for clustering at the hospital-level, we continued to observe qualitatively similar associations between peer influence and brachytherapy adoption. An overlapping explanation is that competition between providers for referrals may help drive uptake. Fear of being ‘behind the curve’ may lead clinicians to invest in new technologies.(27)
This analysis has important limitations. First, though claims data offers multiple advantages in examining peer influence, it is also associated with well-known shortcomings including limited ability to risk adjust. However, the longitudinal study design and focus on differences among providers who were all non-users at baseline makes it less likely that confounders would bias findings. Second, we used all women with loco-regional breast cancer in calculating the number of shared patients between surgeons. Because we were unable to include women without breast cancer and women with different types of insurance, the shared number of patients between surgeons likely represents a lower bound. Using a higher threshold for shared patients—indicating a higher likelihood of physicians reporting advice and referral relationships with one another—continued to produce significant associations. Third, our analysis focused on surgeons, who typically implant the catheter for brachytherapy, and the strong relationship between peer exposure and subsequent brachytherapy use, suggesting their potential importance in the process. We did not account for radiation oncologists who deliver brachytherapy in our analyses,(4) and though we present secondary analyses in which we cluster patients within hospitals, we recognize the importance of examining the relative influence of peer influence vs structural features on the diffusion of innovation. Additionally, we did not examine potential indirect effects (i.e. peers of one’s peers) which may influence adoption. Finally, we examined diffusion over a limited timeframe when initial uptake of brachytherapy was low. It is likely that the impact of peer diffusion changes over time.
In summary, the results demonstrate that patients treated by surgeons with an early adopting peer were over twice as likely to receive brachytherapy following their breast conserving surgery. Peer influence is likely an important and understudied factor in the adoption of cancer technology, and using insurance claims data may present an important means to study this influence. If replicated in future studies, it is possible that this approach to examining peer influence may be useful in building interventions that seek to modify physician behavior. Prior work has used key opinion leaders to enhance the diffusion of innovation.(25) The current work may suggest that targeting physicians who are connected to early adopters may be important for increasing diffusion or attempting to reduce its spread.
Supplementary Material
Acknowledgments
Acknowledgements and funding: Dr. Pollack’s salary is supported by the National Cancer Institute (NCI) and Office of Behavioral and Social Sciences (K07 CA151910). Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number R01CA149045. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
The collection of the California cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract N01-PC-35136 awarded to the Northern California Cancer Center, contract N01-PC-35139 awarded to the University of Southern California, and contract N02-PC-15105 awarded to the Public Health Institute; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement #U55/CCR921930-02 awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California, Department of Public Health the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database. The interpretation and reporting of the SEER-Medicare data are the sole responsibility of the authors.
Disclosures: Dr. Gross and Ms. Soulos receive research funding from 21st Century Oncology LLC. Dr. Gross has also received funding from Medtronic and Johnson & Johnson; the authors report no other relationships or activities that could appear to have influenced the submitted work.
We thank Feng Dai, PhD at the Yale Center for Analytical Sciences for his statistical advice.
References
- 1.Mariotto AB, Robin Yabroff K, Shao Y, Feuer EJ, Brown ML. Projections of the Cost of Cancer Care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103:117–28. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dinan MA, Curtis LH, Hammill BG, Patz EF, Abernethy AP, Shea AM, et al. Changes in the use and costs of diagnostic imaging among Medicare beneficiaries with cancer, 1999–2006. JAMA. 2010;303(16):1625–31. doi: 10.1001/jama.2010.460. [DOI] [PubMed] [Google Scholar]
- 3.Yu JB, Soulos PR, Herrin J, Cramer LD, Potosky AL, Roberts KB, et al. Proton Versus Intensity-Modulated Radiotherapy for Prostate Cancer: Patterns of Care and Early Toxicity. J Natl Cancer Inst. 2013;105(1):25–32. doi: 10.1093/jnci/djs463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roberts KB, Soulos PR, Herrin J, Yu JB, Long JB, Dostaler E, et al. The Adoption of New Adjuvant Radiation Therapy Modalities Among Medicare Beneficiaries With Breast Cancer: Clinical Correlates and Cost Implications. Int J Radiat Oncol. 2013;85(5):1186–92. doi: 10.1016/j.ijrobp.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbash GI, Glied SA. New Technology and Health Care Costs — The Case of Robot-Assisted Surgery. N Engl J Med. 2010;363(8):701–4. doi: 10.1056/NEJMp1006602. [DOI] [PubMed] [Google Scholar]
- 6.Greer AL. Advances in the study of diffusion of innovation in health care organizations. Milbank Q. 1977:505–32. [PubMed] [Google Scholar]
- 7.Newhouse JP, Garber AM, Graham RP, McCoy MA, Mancher M, Kibria A. Variation in health care spending: target decision making, not geography. National Academies Press; 2013. [PubMed] [Google Scholar]
- 8.Rogers EM. Diffusion of Innovation. New York: The Free Press; 1995. [Google Scholar]
- 9.Barnett ML, Landon BE, O’Malley AJ, Keating NL, Christakis NA. Mapping Physician Networks with Self-Reported and Administrative Data. Health Serv Res. 2011;46:1592–609. doi: 10.1111/j.1475-6773.2011.01262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Landon BE, Keating NL, Barnett ML, Onnela JP, Paul S, O’Malley AJ, et al. Variation in patient-sharing networks of physicians across the United States. JAMA. 2012;308(3):265–73. doi: 10.1001/jama.2012.7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pollack CE, Weissman G, Bekelman JE, Liao KJ, Armstrong K. Physician social networks and variation in prostate cancer treatment in three cities. Health Serv Res. 2012;47:380–403. doi: 10.1111/j.1475-6773.2011.01331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pollack CE, Wang H, Bekelman JE, Weissman G, Epstein AJ, Liao KJ, et al. Physician social networks and variation in rates of complications following radical prostatectomy. Value Health. 2014;17(5):611–8. doi: 10.1016/j.jval.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pollack CE, Weissman G, Lemke KW, Hussey PS, Weiner JP. Patient sharing among physicians and costs of care: a netework analytic approach to care coordination using claims data. J Gen Intern Med. 2013;28:459–65. doi: 10.1007/s11606-012-2104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barnett ML, Christakis NA, O’Malley J, Onnela JP, Keating NL, Landen BE. Physician Patient-sharing Networks and the Cost and Intensity of Care in US Hospitals. Med Care. 2012;50:152–60. doi: 10.1097/MLR.0b013e31822dcef7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Presley CJ, Soulos PR, Herrin J, Roberts KB, James BY, Killelea B, et al. Patterns of use and short-term complications of breast brachytherapy in the national Medicare population from 2008–2009. J Clin Oncol. 2012;30(35):4302–7. doi: 10.1200/JCO.2012.43.5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abbott AM, Habermann EB, Tuttle TM. Trends in the use of implantable accelerated partial breast irradiation therapy for early stage breast cancer in the United States. Cancer. 2011;117(15):3305–10. doi: 10.1002/cncr.25927. [DOI] [PubMed] [Google Scholar]
- 17.Hattangadi JA, Taback N, Neville BA, Harris JR, Punglia RS. Accelerated Partial Breast Irradiation Using Brachytherapy for Breast Cancer: Patterns in Utilization and Guideline Concordance. J Natl Cancer Instit. 2012;104(1):29–41. doi: 10.1093/jnci/djr495. [DOI] [PubMed] [Google Scholar]
- 18.Smith GL, Xu Y, Buchholz TA, Giordano SH, Jiang J, Shih Y-CT, et al. Association between treatment with brachytherapy vs whole-breast irradiation and subsequent mastectomy, complications, and survival among older women with invasive breast cancer. JAMA. 2012;307(17):1827–37. doi: 10.1001/jama.2012.3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Warren J, Klabunde C, Schrag D, Bach P, Riley G. Overview of SEER-Medicare data: content, research applictions, and generalizability to the United States elderly population. Med Care. 2002;40(supp):IV-3–IV-18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 20.Elixhauser A, Steiner C, Harris D, Coffey R. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Sen S, Soulos PR, Herrin J, Roberts KB, Yu JB, Lesnikoski B-A, et al. For-profit hospital ownership status and use of brachytherapy after breast-conserving surgery. Surgery. 2014;155(5):776–88. doi: 10.1016/j.surg.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valente TW. Social Network Influences on Health Behavior: Models, Methods, and Applications. New York: Oxford University Press; 2010. [Google Scholar]
- 23.Valente TW. Network Interventions. Science. 2012;337:49–53. doi: 10.1126/science.1217330. [DOI] [PubMed] [Google Scholar]
- 24.Soumerai SB, McLaughlin TJ, Gurwitz JH, et al. Effect of Local Opinion Leaders on Quality of Care for Acute Myocardial Infarction: A Randomized Controlled Trial. JAMA. 1998;279:1358–63. doi: 10.1001/jama.279.17.1358. [DOI] [PubMed] [Google Scholar]
- 25.Flodgren G, Parmelli E, Doumit G, Gattellari M, O’Brien MA, Grimshaw J, et al. Local opinion leaders: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2011;8(8) doi: 10.1002/14651858.CD000125.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoy AR, Patrick H, Campbell B, Lyratzopoulos G. Measuring the influence of colleagues on a consultant team’s use of breast conserving surgery. Int J Technol Assess. 2010;26(02):156–62. doi: 10.1017/S0266462310000061. [DOI] [PubMed] [Google Scholar]
- 27.Escarce JJ, Bloom BS, Hillman AL, Shea JA, Schwartz JS. Diffusion of Laparoscopic Cholecystectomy among General Surgeons in the United States. Med Care. 1995;33(3):256–71. doi: 10.1097/00005650-199503000-00005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


