Abstract
AIM
To determine if transient neurological abnormalities (TNA) at 9 months corrected age predict cognitive, behavioral, and motor outcomes at 6 years of age in extremely preterm infants.
METHOD
A cohort of 124 extremely preterm infants (mean gestational age 25.5wk; 55 males, 69 females), admitted to our unit between 2001 and 2003, were classified based on the Amiel-Tison Neurological Assessment at 9 months and 20 months corrected age as having TNA (n=17), normal neurological assessment (n=89), or neurologically abnormal assessment (n=18). The children were assessed at a mean age of 5 years 11 months (SD 4mo) on cognition, academic achievement, motor ability, and behavior.
RESULTS
Compared with children with a normal neurological assessment, children with TNA had higher postnatal exposure to steroids (35% vs 9%) and lower adjusted mean scores on spatial relations (84 [standard error {SE} 5] vs 98 [SE 2]), visual matching (79 [SE 5] vs 91 [SE 2]), letter–word identification (97 [SE 4] vs 108 [SE 1]), and spelling (76 [SE 4] vs 96 [SE 2]) (all p<0.05).
INTERPRETATION
Despite a normalized neurological assessment, extremely preterm children with a history TNA are at higher risk for lower cognitive and academic skills than those with normal neurological findings during their first year of school.
Extremely preterm infants, defined as those born before 28 weeks gestational age, are at an elevated risk both for developmental delays and for neurological injury. Although the survival of extremely preterm infants without substantial neurosensory impairment has increased, the burden of cognitive, learning, and behavior problems still affects more than 50% of this population.1,2 Unfortunately, developmental assessments in early childhood generally have poor validity in predicting which children are most likely to exhibit these problems at school age.3 With the tightening of governmental budgets, it is important to direct resources to those most likely to benefit. Further research is needed on early childhood characteristics associated with school-age outcomes, so that the children in greatest need can receive more focused interventions before school entry.
For some extremely preterm infants, signs of cerebral palsy (CP) will appear during the first year of their life with abnormal muscle tone and/or delayed motor milestones. There is also an inadequately studied subgroup of preterm infants who exhibit similar tone abnormalities suggestive of developing CP during infancy, but this subgroup appears to outgrow these impairments. This subgroup exhibits a syndrome that has previously been referred to as transient neurological abnormalities (TNA).4 TNA affects an estimated 11% to 60% of very low birthweight infants and an unknown number of term and larger preterm infants.5,6 The first to recognize this pattern of transient abnormal neurological signs was Drillien in 1972.5 She described a syndrome of abnormalities in muscle tone, posture, and primitive reflexes which resolved by 12 months corrected age. After following a cohort of very low birthweight infants until 6 to 7 years of age, Drillien et al.7 found that those children with TNA in infancy went on to have normal IQ but increased learning difficulties. Subsequent studies on TNA assessing long-term developmental and behavioral outcomes have had conflicting results. While some studies have found no association of TNA with later outcomes,8–10 others report increased difficulties with language, motor skills, and hyperactivity.11–13 Interpretation of these studies is difficult because they often included heterogeneous cohorts of preterm and term infants born before the 1990s. These studies’ results may not reflect the outcomes for extremely preterm newborn infants managed with current neonatology practice.
The primary aim of this study was to determine if the presence of TNA in extremely preterm infants during their first year of life predicts an increased risk of cognitive, behavioral, and motor problems at age 6 years. We hypothesized an increased risk of a broad spectrum of difficulties in these three domains at school entry. Our secondary aim was to determine the perinatal predictors of TNA for preterm children born since the year 2000.
METHOD
Study population
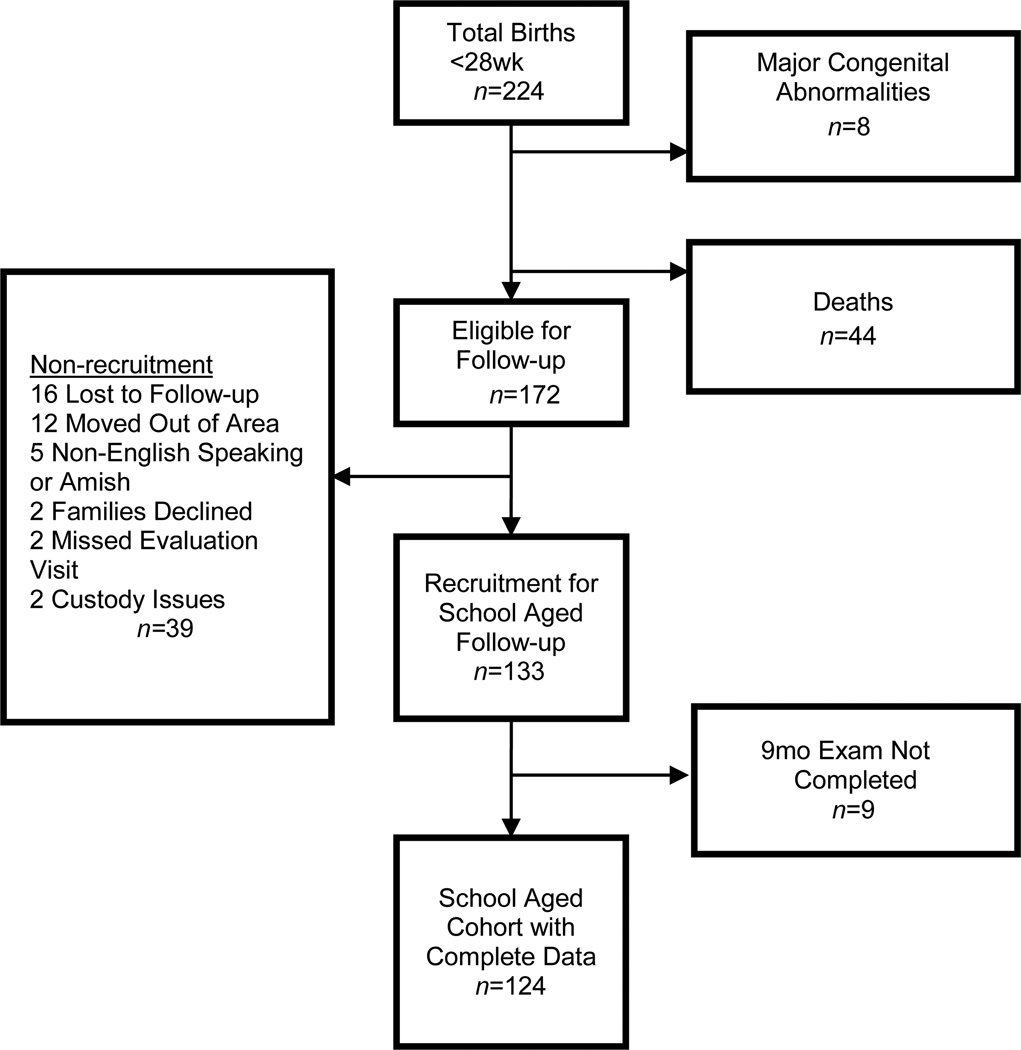
This was a prospective cohort study of 224 extremely preterm infants (<28wk gestation) admitted to the neonatal intensive care unit of Rainbow Babies & Children’s Hospital in Cleveland, Ohio, USA, between 1 January 2001 and 31 December 2003. Forty-four infants (20%) died before discharge and eight children with major malformations were excluded. Of the remaining 172 infants, 133 were recruited (77%). Of these 133 infants, 124 (93%) had complete neurological assessments in early childhood to allow for neurological classification (Fig. 1).
Figure 1.
Flow chart of participants.
Neonatal and maternal data were collected as part of our neonatal follow-up program at the time of infant discharge. As part of routine high-risk follow-up at 9 and 20 months corrected age, infants underwent a neurological examination based on the Amiel-Tison Neurological Assessment14 and completed the Bayley Scales of Infant Development (2nd edition) (BSID-2).15 The Amiel-Tison assessment evaluates active and passive muscle tone based on age-specific norms that change as the myelination progresses and the balance between the corticospinal and subcortical systems shifts. Children were classified at each visit as either having a normal or abnormal neurological assessment based on their muscle tone. Children who had a normal tone at both 9 and 20 months (n=89) were classified for this study as having a neurologically normal assessment (NNA). Children were classified as having TNA (n=17) if the assessment was abnormal at 9 months and then was followed by a normal assessment at 20 months. Children with an abnormal assessment (CP, hypotonia, or hypertonia) at 20 months (n=18) were classified as abnormal regardless of the outcome of the 9 month assessment. Scores from the BSID-2 included both the Mental Development Index (MDI) and the Psychomotor Developmental Index (PDI). Comparison of participants and non-participants based on demographic factors, neurological risk factors, and maternal social risk factors showed no statistically significant differences except for a higher recruitment among black survivors. Black participants were 61% (76/124) of the study sample but only 55% (95/172) of the surviving cohort (p=0.01).
Data collection
This is a secondary analysis of a subset of patients followed as part of a larger study of school-age outcomes in extremely low birthweight infants. Families were recruited for the school-age follow-up before their first year of kindergarten (the first year of formal education within the USA, at 5 to 6 years of age). During their first year of kindergarten, children were tested on areas of cognition, academic achievement, and motor function during a half-day session. Families completed questionnaires on the child’s health, family demographics, and behavior. Cognitive skills were assessed through an age-standardized brief intelligence assessment from the Woodcock–Johnson Tests of Cognitive Abilities (3rd edition).16 School achievement was assessed with select subtests of the Woodcock–Johnson Tests of Achievement (3rd edition), including letter word identification, spelling, and applied math problems.17 Achievement test results were standardized based on duration of time in school. Motor ability was assessed with the Bruininks–Oseretsky Test of Motor Proficiency (2nd edition),18 and visual–motor ability with the Beery Developmental Test of Visual-Motor Integration (5th edition).19 Parent ratings of behavior problems were obtained using the Child Behavior Checklist,20 specifically T scores from the externalizing, internalizing, attention, and total behavior problem scales. Socio-economic status was defined as the mean of the sample z-scores for maternal education, caregiver occupation,21 and US census-based median family income.22 The University Hospitals Case Medical Center institutional review board reviewed and approved this study. Informed consent was obtained from parents and teachers.
Statistical analysis
Group comparisons of clinical and demographic characteristics were completed using the analysis of variance for continuous variables, Kruskal–Wallis tests for severely skewed continuous variables followed by Kruskal–Wallis pairwise comparison follow-up tests for exact p values, and the χ2 or Fisher’s exact test for categorical variables. Owing to the non-parametric distribution of the BSID-2 scores, median scores were calculated and Hodges–Lehman median differences were estimated. Kindergarten testing scores were compared between the three groups using analysis of covariance with included covariates of ethnicity, sex, and socio-economic status. Relative risk for low IQ was adjusted for covariates and calculated by Poisson regression. To include the full cohort of children for cognitive and academic testing, those who were too low functioning cognitively to complete testing were assigned the lowest possible score of 50 for the BSID-2 and 40 for the Woodcock–Johnson tests, which is 4SD below the standardized mean score. Statistical testing was conducted using two-sided alternatives with a type I error level of 0.05.
RESULTS
Group characteristics
The TNA group and the NNA group did not differ significantly in gestational age, birthweight, sex, or perinatal morbidities including infection, necrotizing enterocolitis, patent ductus arteriosus, bronchopulmonary dysplasia, and abnormal cerebral ultrasound findings (Table I). However, the TNA group, compared with the NNA group, had a higher proportion of children who were white, had married mothers, and who received postnatal steroid therapy. Compared with the group with persistent neurological abnormalities, the TNA group had a lower proportion of severely abnormal head ultrasounds, defined as grade III to IV intraventricular hemorrhage and/or periventricular leukomalacia (p=0.014).
Table I.
Social demographic and clinical characteristics of study participants
| TNA (n=17) | NNA (n=89) | Persistent neurological abnormalities (n=18) |
TNA vs NNA (p) |
TNA vs persistent neurological abnormalities group (p) |
|
|---|---|---|---|---|---|
| Gestational age (wk), mean (SD) | 25.6 (1.0) | 25.6 (1.2) | 24.8 (1.5) | 0.963 | 0.075 |
| Birthweight (g), mean (SD) | 830 (162) | 808 (181) | 767 (200) | 0.647 | 0.301 |
| Small for gestational age, n (%)a | 3 (18) | 17 (19) | 3 (18) | 0.888 | >0.999 |
| Males, n (%) | 10 (59) | 36 (40) | 9 (50) | 0.167 | 0.601 |
| Black, n (%) | 6 (35) | 60 (67) | 12 (67) | 0.016 | 0.068 |
| Maternal antenatal steroids, n (%) | 15 (88) | 78 (88) | 14 (78) | 0.945 | 0.419 |
| Chorioaminitis, n (%) | 1 (6) | 12 (13) | 1 (6) | 0.396 | 0.967 |
| Cesarean delivery, n (%) | 6 (35) | 51 (57) | 8 (44) | 0.102 | 0.581 |
| Intubated at birth, n (%) | 15 (88) | 71 (80) | 18 (100) | 0.420 | 0.998 |
| 5 minute Apgar <6, n (%) | 7 (41) | 30 (34) | 8 (47) | 0.555 | 0.730 |
| Multiple birth, n (%) | 3 (18) | 18 (20) | 6 (33) | 0.807 | 0.295 |
| Oxygen dependent at 36wk PMA, n (%) | 12 (71) | 46 (52) | 11 (61) | 0.171 | 0.556 |
| Ventilator days, median (25th–75th centile) | 30 (14–57) | 20 (4–35) | 42 (12–82) | 0.094 | 0.409 |
| Postnatal steroids, n (%) | 6 (35) | 8 (9) | 5 (28) | 0.007 | 0.633 |
| Patent ductus arteriosis, n (%) | 11 (65) | 49 (55) | 13 (72) | 0.464 | 0.633 |
| Sepsis, meningitis, and/or NEC n (%)b | 9 (53) | 33 (37) | 10 (56) | 0.225 | 0.877 |
| Grade I/II IVH without PVL, n (%) | 4 (24) | 12 (13) | 1 (6) | 0.296 | 0.160 |
| Grade III/IV IVH and/or PVL, n (%) | 1 (6) | 5 (6) | 9 (50) | 0.966 | 0.014 |
| Maternal demographic characteristics at school-age testing | |||||
| Married, n (%) | 12 (71) | 38 (43) | 9 (50) | 0.042 | 0.218 |
| Educational level (y), mean (SD) | 13.2 (2.0) | 13.3 (2.4) | 13.3 (2.6) | 0.913 | 0.958 |
| Median family income, mean (SD)c | 61.6 (24.9) | 56.2 (35.2) | 56.8 (24.6) | 0.535 | 0.668 |
| Educational characteristics | |||||
| Age at testing (y), mean (SD) | 6.0 (.3) | 5.9 (.4) | 6.2 (.4) | 0.147 | 0.281 |
| Preschool attendance, n (%) | 16 (94) | 71 (80) | 16 (89) | 0.188 | 0.587 |
| Special-needs school/class, n (%) | 4 (24) | 3 (3) | 8 (44) | 0.008 | 0.198 |
| IEP or related service, n (%) | 7 (41) | 29 (33) | 15 (83) | 0.515 | 0.014 |
Birthweight greater than 2SD below expectation for gestational age based on Yudkin standards. Persistent neurological abnormalities group had one child who could not be classified at <24wk.
One case of meningitis in TNA group and one case in the persistent neurological abnormalities group.
Family income in thousands of dollars based on neighborhood tract of family residence from the 2000 US Census.22
TNA, transient neurological abnormalities; NNA, neurologically normal assessment; PMA, postmenstrual age; NEC, necrotizing enterocolitis; IVH, intraventricular hemorrhage; PVL, periventricular leukomalacia; IEP, individualized educational program.
Early childhood assessments
Based on the Amiel-Tison assessment, the presence of hypotonia at the 9-month assessment was more likely to be associated with a normal assessment at 20 months of age compared with any form of hypertonia (suspected CP, hypertonia, or mixed hypertonia/hypotonia) (relative risk=3.9, 95% confidence interval [CI] 1.6–9.5, p=0.001) (Tables II and III). At 9 months corrected age, the children with TNA had significantly lower scores on both the MDI and PDI of the BSID-2 compared with the NNA group. At 20 months corrected age compared with the NNA group, the TNA group continued to have a significantly lower PDI, whereas the groups did not differ significantly on the MDI. Comparison of the TNA group and the persistently abnormal neurological assessment group at 9 months showed higher MDI scores for the TNA group but similar PDI scores, whereas at 20 months corrected age the groups had similar MDI and PDI scores.
Table II.
Infant and toddler neurological assessment
| Transient neurological abnormalities (n=17) |
Persistent neurological abnormalities (n=18) |
|||
|---|---|---|---|---|
| Neurological assessment resultsa | 9mo | 20mo | 9mo | 20mo |
| Hypertonia, n | 2 | 0 | 5 | 3 |
| Hypotonia, n | 13 | 0 | 3 | 3 |
| Hypertonia and hypotonia, n | 1 | 0 | 2 | 0 |
| Cerebral palsy, n | 1 | 0 | 6 | 12 |
| Normal assessment, n | 0 | 17 | 2 | 0 |
Results are based on the Amiel-Tison assessment.
Table III.
Infant and toddler developmental testing
| TNA | NNA | Persistent neurological abnormalities |
TNA vs NNA | TNA vs persistent neurological abnormalities |
|
|---|---|---|---|---|---|
| (n=17) | (n=89) | (n=18) | Median difference (95% CI)a |
Median difference (95% CI)a |
|
| 9mo Bayley II | |||||
| PDI median (25th–75th centile) | 59 (56–71) | 89 (79–97) | 54 (50–70) | −24 (−31 to −17)b | 6 (0 to 14) |
| MDI median (25th–75th centile) | 92 (81–94) | 98 (94–101) | 83 (55–89) | −6 (−11 to −2)b | 8 (2 to 21)b |
| 20mo Bayley II | |||||
| PDI median (25th–75th centile) | 57 (50–81) | 86 (75–93) | 50 (50–55) | −20 (−31 to −8)b | 5 (0 to 24) |
| MDI median (25th–75th centile) | 71 (54–92) | 79 (69–87) | 57 (50–74) | −8 (−18 to 4) | 6 (−1 to 26) |
Standardized mean score is 100 and SD 15 for a normative sample.15
Estimated Hodges–Lehman median difference. Owing to the asymmetrical distribution for the transient neurological abnormalities (TNA) and persistent neurological abnormalities groups, the estimated medians will not be identical to calculated medians.
95% confidence intervals (95% CI) not including zero are significant.
NNA, neurologically normal assessment; PDI, Psychomotor Development Index; MDI, Mental Development Index.
Kindergarten outcomes
The children in all three groups were approximately 6 years of age at the time of evaluation and did not differ significantly in preschool attendance (Table IV). Children with TNA had higher rates of participation in a special-needs school classroom than the NNA group but did not differ on overall rates of individualized educational programs. Most children (83%) with persistent neurological abnormities had an individualized educational program and 44% required a special-needs classroom setting.
Table IV.
Kindergarten outcomes
| TNA | NNA | Persistent neurological abnormalities |
TNA vs NNA | TNA vs persistent neurological abnormalities |
||||
|---|---|---|---|---|---|---|---|---|
| n | Mean (SE) |
n | Mean (SE) |
n | Mean (SE) |
Adjusted mean difference (95% CI) |
Adjusted mean difference (95% CI) |
|
| Cognitive testing: Woodcock–Johnson Tests of Cognitive Abilitiesa | ||||||||
| Total IQ: brief intellectual ability | 15 | 81 (5) | 86 | 91 (2) | 17 | 65 (4) | −10 (−21 to 0) | 15 (2 to 28)e |
| Verbal comprehension | 15 | 83 (5) | 87 | 91 (2) | 18 | 72 (4) | −8 (−18 to 2) | 11(−1 to 23) |
| Spatial relations | 15 | 84 (5) | 87 | 98 (2) | 17 | 75 (4) | −14 (−24 to −4)f | 9 (−3 to 22) |
| Visual matching | 16 | 79 (5) | 86 | 91 (2) | 17 | 65 (5) | −12 (−22 to −1)e | 14 (1 to 28)e |
| Concept formation | 16 | 90 (4) | 87 | 94 (2) | 17 | 77 (4) | −4 (−14 to 5) | 13 (1 to 25)e |
| Academic testing: Woodcock–Johnson Tests of Achievementa | ||||||||
| Reading: letter–word identification | 16 | 97 (4) | 88 | 108 (1) | 14 | 101 (4) | −11 (−18 to −3)f | −4 (−14 to 7) |
| Spelling | 16 | 76 (4) | 88 | 96 (2) | 12 | 83 (5) | −20 (−29 to −11)g | −7 (−19 to 6) |
| Mathematics: applied problems | 16 | 90 (5) | 85 | 98 (2) | 15 | 77 (5) | −8 (−18 to 2) | 13 (1 to 26)e |
| Motor | ||||||||
| Motor proficiency: BOT2b | 16 | 38 (2) | 78 | 43 (1) | 16 | 28 (2) | −5 (−10 to 0) | 9 (3 to 16)f |
| Visual motor integrationa | 17 | 84 (4) | 88 | 92 (2) | 18 | 67 (4) | −7 (−16 to 1) | 17 (6 to 28)f |
| Behavior: Child Behavior Checklistb | ||||||||
| Internalizing problems | 17 | 48 (2) | 86 | 45 (1) | 16 | 47 (2) | 3 (−3 to 8) | 1 (−5 to 8) |
| Externalizing problems | 17 | 50 (3) | 86 | 47 (1) | 16 | 49 (3) | 2 (−3 to 8) | 1 (−7 to 8) |
| Total problems | 17 | 50 (3) | 86 | 46 (1) | 16 | 50 (3) | 3 (−3 to 9) | −1 (−8 to 7) |
| Attention problems | 17 | 59 (2) | 86 | 54 (1) | 16 | 57 (2) | 5 (2 to 9)c,d | 3 (−2 to 7)c |
Data are presented as adjusted mean (standard error [SE]). Means scores were adjusted for sex, ethnicity, and z-SES with the exception of sex for BOT2 and Child Behavior Checklist owing to sex norming of tests. z-SES is the mean of the sample’s z-scores of maternal education in years, family occupation,21 and median income for the neighborhood of residence based on data from the 2000 US Census.22
Standard scores represent the mean and SD for a normative sample with an average of 100 (SD 15).
Standard T scores represent normative sample with a mean of 50 and standard deviation of 10.
Non-parametric distribution, log of adjusted mean score used to normalize data and determine significance of difference between mean scores.
Non-significant when comparison made between transformed log scores, p=0.066.
p<0.05.
p<0.01.
p<0.001.
TNA, transient neurological abnormalities; NNA, neurologically normal assessment; 95% CI, 95% confidence interval; BOT2, Bruininks–Oseretsky Test of Motor Proficiency (2nd edition).
All three groups of extremely preterm infants scored below age standards on many portions of the cognitive, motor, and achievement tests. Group comparisons revealed a consistent pattern of cognitive outcomes in which the TNA group scored better than the persistent neurological abnormality group but more poorly than the NNA group. The TNA group scored significantly higher than the persistent neurological abnormality group on all cognitive and motor tests except those assessing verbal comprehension, spatial relations, and mathematics, but scored lower than the NNA group on measures of spatial relations, visual matching, reading, and spelling. There was a trend with borderline significance (p=0.065) of lower motor scores in the TNA group compared with the NNA group. There was also a suggestion of lower mean IQ in the TNA group compared with the NNA group (p=0.059). More children in the TNA group had an adjusted IQ<70 compared with the NNA group (relative risk=3.8, CI 95% 1.6–9.1, p=0.002) but the risk did not vary significantly between the TNA group and the group with persistent neurological abnormalities (relative risk=0.8, 95% CI 0.3–1.7, p=0.5). The groups did not differ significantly on the internalizing, externalizing, or total behavior problem scales of the Child Behavior Checklist, with mean scores for all three groups falling well within normative standards.
DISCUSSION
Extremely preterm newborn infants with TNA in infancy continued to show deficits in multiple developmental domains at school age compared with those without neurological abnormalities. This is the first study, to our knowledge, that examines TNA in a preterm cohort born since the routine introduction of antenatal steroids and surfactant. This is also the first study of school-age outcomes of children with TNA that includes a comparison group with persistent neurological abnormalities. The inclusion of this comparison group places these results in the context of the full spectrum of neurological outcomes of extremely preterm children. The BSID-2 showed significant motor and cognitive delays for both the TNA and the persistent neurological abnormalities groups. The average MDI scores at 20 months for all three groups were greater than 1SD below the normative mean, as is consistent with BSID-2 scores reported in the USA for the extremely preterm population and confirming the severe early delays observed in children born extremely preterm.23 At 6 years of age, the mean scores for children with TNA in cognitive and motor testing fell 1–2SD below standards for age. By comparison, the children in the NNA group had mean scores on cognitive and motor tests that were all within 1SD of normative standards. The TNA group also scored significantly below the NNA group in reading and spelling skills. Children with persistent neurological abnormalities scored consistently 2–3SD below the standard on cognitive and motor testing. This study failed to reveal group differences in parent ratings of child behavior problems.
Previous research on the school-age outcomes of TNA has been less conclusive, with some studies reporting continued developmental delays at school age but others failing to find lasting effects.7–13 This may be partly explained by the age the neurological deficits were detected and the length of time until resolution of these neurological deficits. The peak prevalence of TNA is 4 to 7 months.6,8,24 There are some hints that the longer the neurological deficit continues before resolving, the greater risk for longer-term developmental consequences. In a study of preterm infants born in 1978, scores on cognitive testing were lower at 3 and 5 years if TNA lasted greater than 6 months.25 Hack also reported in an abstract that mean IQ at 31 months in a group of very low birthweight infants with TNA born in 1977 was lower for children whose assessments normalized between 8 and 31 months than for those with a normal assessment before 8 months (Maureen Hack, personal communication). The identification in our study of TNA at 9 months corrected age may have reduced the number of children with TNA but it allowed us to exclude less severely affected children. In comparison, several previous studies of preterm infants identified with TNA at 4 to 7 months of age have failed to reveal residual effects at school age.8,9,26 Another possible reason for the discrepancy between our results and previous long-term follow-up studies is our extremely preterm population. The children from this cohort were born at a much earlier stage in neurological development than those in most of the previous studies. Risks for school-age sequelae may also have been increased by our use of a gestational-age-based cohort rather than a weight-based cohort. Our cohort excluded higher gestational age infants born small for gestational age who may have a different neurological risk pattern.
The infants with TNA were similar in demographic and perinatal morbidities to the NNA group, with no significant differences in several neonatal factors previously associated with increased risk of motor problems, including absence of maternal antenatal steroids,27 chorioaminitis,28 infection/necrotizing enterocolitis,29 and severely abnormal head ultrasound.30 The most notable difference between the groups was the increased postnatal steroid exposure in the children with TNA compared with the neurological normal group. The use of postnatal steroids has been a well-established risk factor for CP and developmental delay since the early 2000s31 but has not, to our knowledge, been associated with TNA. We did not replicate results of previous studies demonstrating associations of TNA with mechanical ventilation6,9, low Apgar scores,6,8 gestational age,32 and birthweight.9,32 These disparities may reflect sample differences or improved delivery-room management. The higher rates of TNA in married versus unmarried caregivers and in children of white versus black ethnicity were unexpected. Speculation about possible reasons for these associations may thus be unwarranted pending replication of the findings in other samples.
The etiology of TNA is unclear. Maturational delay or dysfunction of myelination has been suggested by one previous study that evaluated brainstem auditory evoked potentials in preterm children with TNA at 2 and 5 months.33 This is consistent with the well-established association between white matter damage detected at term-corrected age with cognitive and motor impairment at school age in preterm children.34,35 Residual cognitive impairment in the TNA group, despite resolution of early neuromotor deficits, may reflect limitations of compensatory neural plasticity.36 In our study, hypotonia was more frequently transient than hypertonia. Delayed myelination37 and cerebellar injury38 have both been associated with hypotonia and could either alone or together explain the high level of residual cognitive issues, especially the visual spatial difficulties and language problems.37,38 Hypertonia may represent more profound dysmyelination or a different mechanism of injury.
There are many limitations to our study. These include the relatively small number of children in the TNA and persistent neurological abnormalities groups, and the preponderance of white infants in the TNA group. Group sizes were sufficient to demonstrate residual effects of both forms of early neurological abnormality on cognition and motor performance, and ethnicity was controlled in the analysis. However, replication with a larger sample size would be useful, particularly in characterizing children with TNA and their outcomes. Another limitation is that our sample comprised only 74% of the survivors of extremely preterm birth. With the exception of ethnicity, the participants did not differ significantly from non-participants from our larger birth cohort in sociodemographic or perinatal factors, suggesting that our sample was largely representative of our total population. Our cohort also may not be representative of children born in other regions of the USA or outside academic medical centers.
By definition, children with TNA do not have neurological abnormalities after 1 to 2 years of age and thus may not be identified for special services before school entry. Unfortunately, once children’s motor assessments normalize they may no longer qualify for therapy services and families may be falsely reassured that their child is no longer at an increased risk for developmental issues. All preterm infants are at risk of having undiagnosed developmental problems but the children with TNA appear to be at greater risk. Future research should focus on developing effective interventions to target this population before school entry and to assess longitudinally whether the deficits of the children with TNA will become more evident as schoolwork becomes more challenging or if they will catch up to peers with advancing age.
In conclusion, extremely preterm children with TNA continue to have cognitive deficits at school entry relative to those without histories of abnormality on neurological examination in infancy. Despite a normalized neurological examination, children with TNA represent a high-risk group deserving early childhood surveillance. As children with TNA transition to school, they may also require additional developmental testing to ensure their educational needs are met and to increase their chance of school success.
What this paper adds.
Extremely preterm infants with transient neurological abnormalities in infancy are at greater risk of cognitive problems at 6 years.
Hypotonia in infancy is more likely to be transient than hypertonia in extremely preterm infants.
Postnatal steroid exposure increases the risk of developing transient neurological abnormalities.
ACKNOWLEDGEMENTS
This work was funded by grant HD050309 from the National Institutes of Health, which provided financial support for the design and conduct of the study. Research analysis was also financially supported by a Rainbow Fellowship Research Award Program (FRAP) grant and Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) grant 5T32HD060537. We also acknowledge the contributions of Anne Birnbaum, Elizabeth Roth, Dan Maier, Andrea Barkoukis Gefteas, Michelle R Jacobs, Alice Costiuc, and Ketrin Lengu in recruitment and data collection and coding.
ABBREVIATIONS
- BSID-2
Bayley Scales of Infant Development (2nd edition)
- MDI
Mental Development Index
- NNA
Neurologically normal assessment
- PDI
Psychomotor Developmental Index
- TNA
Transient neurological abnormalities
Footnotes
The authors have stated that they had no interests that might be perceived as posing a conflict or bias.
REFERENCES
- 1.Wilson-Costello D, Friedman H, Minich N, et al. Improved neurodevelopmental outcomes for extremely low birthweight infants in 2000–2002. Pediatrics. 2007;119:37–45. doi: 10.1542/peds.2006-1416. [DOI] [PubMed] [Google Scholar]
- 2.Hutchinson EA, De Luca CR, Doyle LW, Roberts G, Anderson PJ Victorian Infant Collaborative Study Group. School-age outcomes of extremely preterm or extremely low birthweight children. Pediatrics. 2013;31:e1053–e1061. doi: 10.1542/peds.2012-2311. [DOI] [PubMed] [Google Scholar]
- 3.Hack M, Taylor HG, Drotar D, et al. Poor predictive validity of the Bayley Scales of Infant Development for cognitive function of extremely low birthweight children at school age. Pediatrics. 2005;116:333–341. doi: 10.1542/peds.2005-0173. [DOI] [PubMed] [Google Scholar]
- 4.Amiel-Tison C. A method for neurologic evaluation within the first year of life. Curr Probl Pediatr. 1976;6:1–50. [PubMed] [Google Scholar]
- 5.Drillien C. Abnormal neurological signs in the first year of life in low-birthweight infants: possible prognostic significance. Dev Med Child Neurol. 1972;14:575–584. doi: 10.1111/j.1469-8749.1972.tb02639.x. [DOI] [PubMed] [Google Scholar]
- 6.Tudehope DI, Burns YR, O’Callaghan M, Mohay H. Minor neurological abnormalities during the first year of life in infants of birthweight <1500g. J Paediatr Child Health. 1981;17:265–268. doi: 10.1111/j.1440-1754.1981.tb01955.x. [DOI] [PubMed] [Google Scholar]
- 7.Drillien C, Thompson A, Burgoyne K. Low-birthweight children at early school-age: a longitudinal study. Dev Med Child Neurol. 1980;22:26–47. doi: 10.1111/j.1469-8749.1980.tb04303.x. [DOI] [PubMed] [Google Scholar]
- 8.Sommerfelt K, Pedersen S, Ellertsen B, Markestad T. Transient dystonia in non-handicapped low-birthweight infants and later neurodevelopment. Acta Paediatr. 1996;85:1445–1449. doi: 10.1111/j.1651-2227.1996.tb13950.x. [DOI] [PubMed] [Google Scholar]
- 9.d’Eugenio D, Slagle T, Mettelman B, Gross S. Developmental outcome of preterm infants with transient neuromotor abnormalities. Am J Dis Child. 1993;147:570–574. [PubMed] [Google Scholar]
- 10.Li A, Sauve R, Cerighton D. Early indicators of learning problems in high-risk children. J Dev Behav Pediatr. 1990;11:1–6. doi: 10.1097/00004703-199002000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Pe Benito F, Santello M, Faxas T, Ferretti C, Fisch C. Residual developmental disabilities in children with transient hypertonicity in infancy. Pediatr Neurol. 1989;5:154–160. doi: 10.1016/0887-8994(89)90064-7. [DOI] [PubMed] [Google Scholar]
- 12.Brandt I, Sticker EJ, Hocky M, Lentze MJ. Transient abnormal neurologic signs (TANS) in longitudinal study of very low birthweight preterm infants. Early Hum Dev. 2000;59:107–126. doi: 10.1016/s0378-3782(00)00090-6. [DOI] [PubMed] [Google Scholar]
- 13.Chaudhari S, Bhalerao M, Chitale A, Patil B, Pandit A, Hoge M. Transient tone abnormalities in high risk infants and cognitive outcome at five years. Indian Pediatr. 2010;47:931–935. doi: 10.1007/s13312-010-0157-4. [DOI] [PubMed] [Google Scholar]
- 14.Amiel-Tison C, Gosselin J. Guide for Examination and Evaluation. Baltimore, MD: Johns Hopkins University Press; 2001. Neurological Development from Birth to Six Years. [Google Scholar]
- 15.Bayley N. Manual for the Bayley Scales of Infant Development (2nd edition) San Antonio, TX: The Psychological Corporation; 1993. [Google Scholar]
- 16.Woodcock RC, Mc Grew KS, Mather S. Woodcock-Johnson III Tests of Cognitive Abilities. Itasca, IL: Riverside Publishing; 2001. [Google Scholar]
- 17.Woodcock RC, Mc Grew KS, Mather S. Woodcock-Johnson III Tests of Achievement. Itasca, IL: Riverside Publishing; 2001. [Google Scholar]
- 18.Bruininks RH, Bruininks BD. Bruininks-Oseretsky Test of Motor Proficiency: Examiners Manual. 2nd edition. Circle Pines, MN: AGS Publishing; 2005. [Google Scholar]
- 19.Beery KE, Beery NA. The Beery-Buktenica Developmental Test of Visual-motor Integration: Beery VMI, administration, scoring, and teaching manual. 5th edition. Minneapolis, MN: NCS Pearson; 2004. [Google Scholar]
- 20.Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age Forms and Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families; 2001. [Google Scholar]
- 21.Hauser RM, Warren JR. Socioeconomic indexes for occupations: a review, update, and critique. Soc Methodol. 1997;27:177–298. [Google Scholar]
- 22.Federal Financial Institutions Examination Council. [accessed 8 June 2011];Geocoding system. Available from: http://www.ffiec.gov/census/default.aspx.
- 23.Vohr BR, Stephens BE, Higgins RD, Bann CM, Hintz SR, Das A, et al. Are outcomes of extremely preterm infants improving? Impact of Bayley assessment on outcomes. J Pediatr. 2012;161:222–228. doi: 10.1016/j.jpeds.2012.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hack M, Caron B, Rivers A, Fararoff AA. The very low birthweight infant: the broader spectrum of morbidity during infancy and early childhood. J Dev Behav Pediatr. 1983;4:243–249. [PubMed] [Google Scholar]
- 25.Fedrizzi E, Zuccarino ML, Vizziello P. Clinical problems in neurodevelopmental diagnosis: a 7-year neurological and psychological follow-up study of low risk preterm infants. Ital J Neurol Sci. 1986;5(Suppl.):117–126. [PubMed] [Google Scholar]
- 26.Steward K, Deitz J, Crowe T, Robinson N, Bennett F. Transient neurologic signs in infancy and motor outcomes at 4½ years in children born biologically at risk. Top Early Child Spec. 1988;7:71–83. [Google Scholar]
- 27.Wong D, Abdel-Latif M, Kent A NICUS Network. Antenatal steroid exposure and outcomes of very premature infants: a regional cohort study. Arch Dis Child Fetal Neonatal Ed. 2014;99:F12–F20. doi: 10.1136/archdischild-2013-304705. [DOI] [PubMed] [Google Scholar]
- 28.Wu YW, Colford JM. Chorioamnionitis as a risk factor for cerebral palsy: a meta-analysis. JAMA. 2000;284:1417–1424. doi: 10.1001/jama.284.11.1417. [DOI] [PubMed] [Google Scholar]
- 29.Shah DK, Doyle LW, Anderson PJ, et al. Adverse neurodevelopment in preterm infants with postnatal sepsis or necrotizing enterocolitis is mediated by white matter abnormalities on magnetic resonance imaging at term. J Pediatr. 2008;153:170–175. doi: 10.1016/j.jpeds.2008.02.033. [DOI] [PubMed] [Google Scholar]
- 30.Pinto-Martin JA, Riolo S, Cnaan A, Holzman C, Susser MW, Paneth N. Cranial ultrasound prediction of disabling and non-disabling cerebral palsy at age two in a low birthweight population. Pediatrics. 1995;95:249–254. [PubMed] [Google Scholar]
- 31.Wilson-Costello D, Walsh MC, Langer JC, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Impact of postnatal corticosteroid use on neurodevelopment at 18 to 22 months’ adjusted age: effects of dose, timing, and risk of bronchopulmonary dysplasia in extremely low birthweight infants. Pediatrics. 2009;123:e430–e437. doi: 10.1542/peds.2008-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piccionlini O, Gianni ML, Vegnai C, Fumagalli M, Mosca F. Usefulness of an early neurofunctional assessment in predicting neurodevelopmental outcome in very low birthweight infants. Arch Dis Child Fetal Neonatal Ed. 2006;91:F111–F117. doi: 10.1136/adc.2005.073262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kitamoto I, Kukita J, Kurokawa T, Chen Y, Minami T, Ueda K. Transient neurologic abnormalities and BAEPs in high-risk infants. Pediatr Neurol. 1990;6:319–325. doi: 10.1016/0887-8994(90)90024-u. [DOI] [PubMed] [Google Scholar]
- 34.Spittle AJ, Cheong J, Doyle LW, et al. Neonatal white matter abnormality predicts childhood motor impairment in very preterm children. Dev Med Child Neurol. 2011;53:1000–1006. doi: 10.1111/j.1469-8749.2011.04095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woodward LJ, Clark CA, Bora S, Inder TE. Neonatal white matter abnormalities an important predictor of neurocognitive outcome for very preterm children. PLoS ONE. 7:e51879–e51879. doi: 10.1371/journal.pone.0051879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luciana M. Cognitive development in children born preterm: implications for theories of brain plasticity following early injury. Dev Psychopathol. 2003;15:1017–1047. doi: 10.1017/s095457940300049x. [DOI] [PubMed] [Google Scholar]
- 37.Bodensteiner JB. The evaluation of the hypotonic infant. Semin Pediatr Neurol. 2008;15:10–20. doi: 10.1016/j.spen.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 38.Limperopoulos C, Bassan H, Gauvreau K, et al. Does cerebellar injury in premature infants contribute to the high prevalence of long-term cognitive, learning, and behavioral disability in survivors? Pediatrics. 2007;120:584–593. doi: 10.1542/peds.2007-1041. [DOI] [PubMed] [Google Scholar]