Abstract
The aim of this study is to investigate the role of the purinergic receptor P2X3 in the peripheral and central nervous systems during acupuncture treatment for the visceral pain of irritable bowel syndrome (IBS). A total of 24 8-day-old Sprague–Dawley (SD) neonatal male rats (SPF grade) were stimulated using colorectal distention (CRD) when the rats were awake. The modeling lasted for 2 weeks with one stimulation per day. After 6 weeks, the rats were randomly divided into three groups of eight each: (1) the normal group (NG, n = 8); (2) the model group (MG, n = 8); and (3) the model + electroacupuncture group (EA, n = 8) that received electroacupuncture at a needling depth of 5 mm at the Shangjuxu (ST37, bilateral) and Tianshu (ST25, bilateral) acupoints. The parameters of the Han’s acupoint nerve stimulator (HANS) were as follows: sparse-dense wave with a frequency of 2/100 Hz, current of 2 mA, 20 min/stimulation, and one stimulation per day; the treatment was provided for seven consecutive days. At the sixth week after the treatment, the abdominal withdrawal reflex (AWR) score was determined; immunofluorescence and immunohistochemistry were used to measure the expression of the P2X3 receptor in myenteric plexus neurons, prefrontal cortex, and anterior cingulate cortex; and, a real-time PCR assay was performed to measure the expression of P2X3 messenger RNA (mRNA) in the dorsal root ganglion (DRG) and spinal cord. After stimulation with CRD, the expression levels of the P2X3 receptor in the inter-colonic myenteric plexus, DRG, spinal cord, prefrontal cortex, and anterior cingulate cortex were upregulated, and the sensitivity of the rats to IBS visceral pain was increased. Electroacupuncture (EA) could downregulate the expression of the P2X3 receptor and ease the sensitivity to visceral pain. The P2X3 receptor plays an important role in IBS visceral pain. The different levels of P2X3 in the peripheral enteric nervous system and central nervous system mediate the effects of the EA treatment of the visceral hyperalgesia of IBS.
Keywords: P2X3, Electroacupuncture, Irritable bowel syndrome, Visceral pain, Brain–gut axis
Introduction
Irritable bowel syndrome (IBS) is a gastrointestinal disorder involving different levels of the brain–gut axis, and visceral pain is one of the most common symptoms. The brain–gut axis can affect the outer periphery, the spinal cord, and the central nervous system, and various neurological neurotransmitters are involved. Because the enteric nervous system is associated with the central nervous system, gastrointestinal activity is regulated by the central nervous system, and the reciprocal relationships are called brain–gut interactions [1, 2]. In another study, patients with the visceral hypersensitivity of IBS not only exhibited high central reactivity to stimuli from the outer periphery but also showed increased visceral sensitivity to central stress events, which was considered to be the result of a change in the brain–gut axis [3].
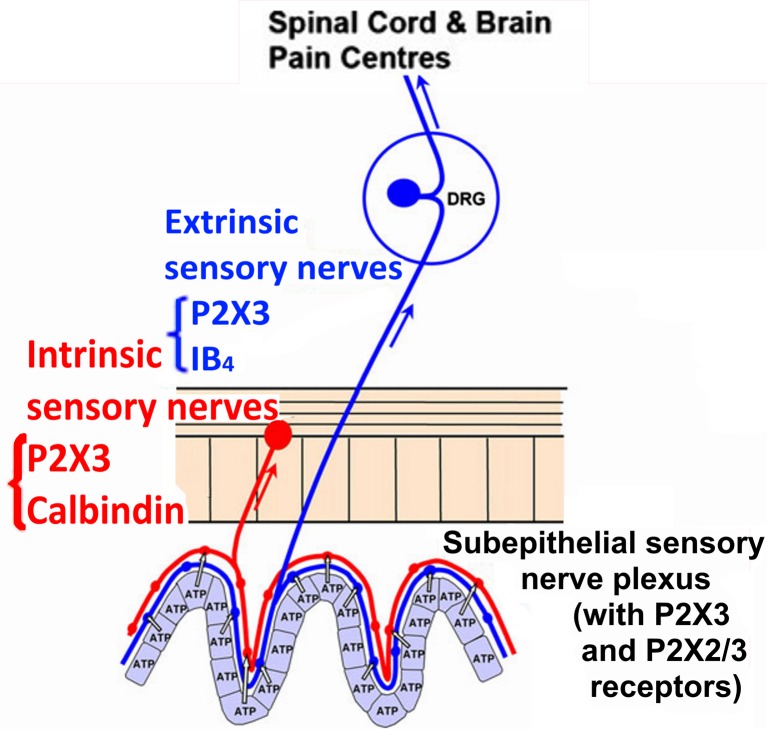
Burnstock, who discovered purinergic receptors, experimentally demonstrated that adenosine triphosphate (ATP) is released when the epithelial cells in tubular organs and saclike organs receive an expansionary stimulus; the stimulus acts on the nerve plexus of the purinergic receptor P2X2/3 under the epithelial mucosa, thus causing the transmission of pain signals to the pain center [4, 5, Fig. 1]. This assertion has been confirmed in many diseases, such as IBS and interstitial cystitis [6]. Clinical and fundamental research had found that a variety of analgesic drugs have good efficacy during the treatment of visceral pain, thus showing a broad therapeutic potential [7, 8]. The application of purinergic receptor antagonists has also expanded the treatment of visceral pain. As an important part of traditional Chinese medicine, acupuncture has 5000-year history in the treatment of IBS visceral pain and diarrhea and has been used extensively in clinics. The long-term clinical practice and fundamental research of our study group has also confirmed the effectiveness of acupuncture treatment for IBS [9–13] and has led to a preliminary explanation for a portion of the mechanism underlying the acupuncture treatment of IBS visceral pain [14]. As a neurotransmitter, ATP plays an important role in regulating the transduction of the visceral pain signal by binding to P2X receptors [15, 16], and ATP participates in the regulation of intestinal movement and gastrointestinal secretion. P2X receptors are widely distributed in the body, and they play an important role in the formation, transduction, and regulation of neuropathic pain, inflammatory pain, and visceral pain [17–20]. Many studies have shown that purinergic (P2X) receptors are involved in pain signal transmission, in which the P2X3 and P2X2/3 receptors are the main subtypes [21, 22]. Therefore, when studying the pathogenesis of IBS, it is necessary to investigate the relationship between the P2X receptor and IBS visceral hypersensitivity at various levels of the brain–gut axis.
Fig. 1.
Diagram from Geoffrey Burnstock. Purinergic signaling in the gastrointestinal tract and related organs in health and disease. Purinergic Signalling. 2014 Mar; 10(1):21
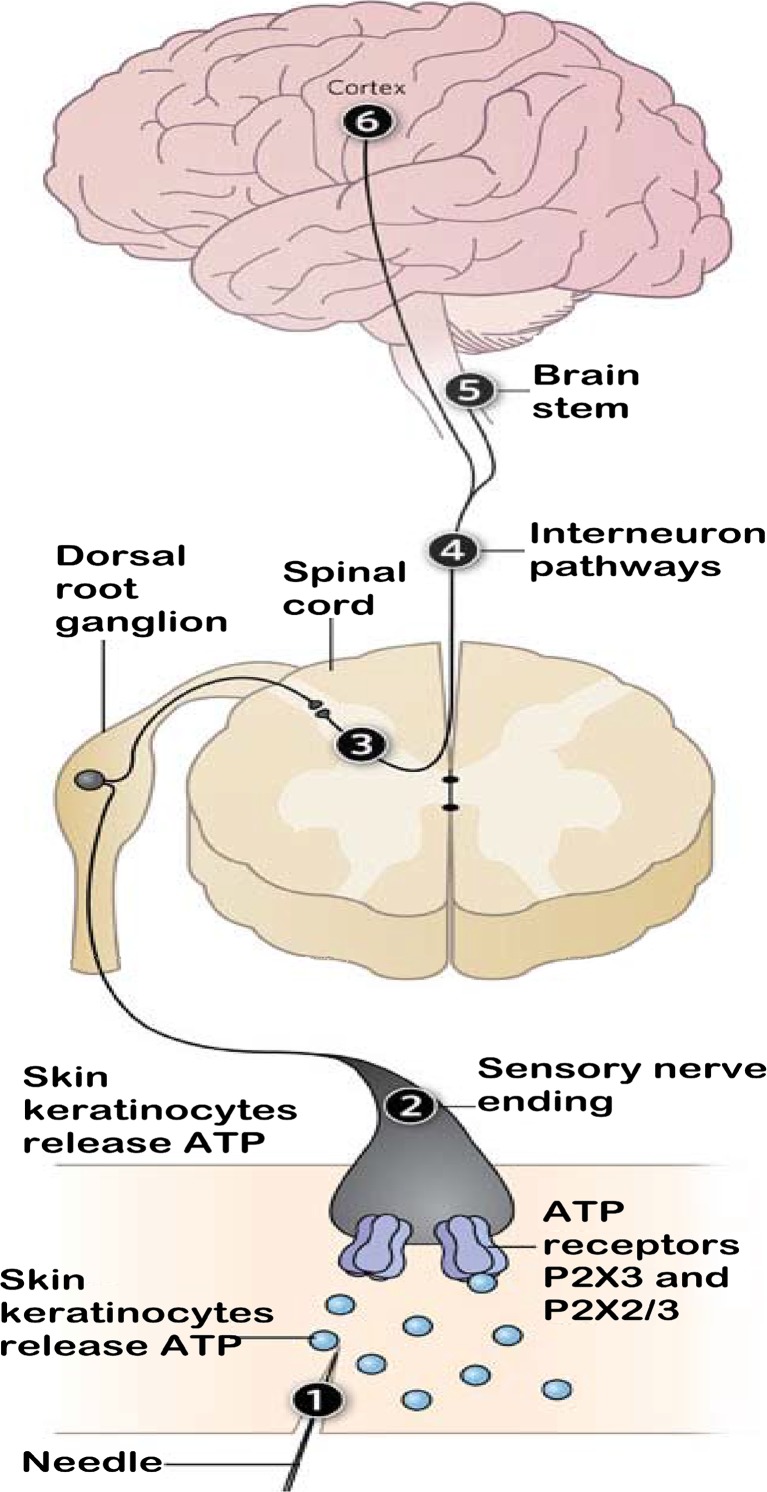
In a The Scientist article [23], Burnstock suggested that traditional Chinese acupuncture mainly acts through purinergic signals (Fig. 2). This assertion not only provided new ideas for the study of the mechanism underlying acupuncture but also presented new challenges. The experiments of this study were designed based on the above two viewpoints of Burnstock. The internationally recognized approach of colorectal distension (CRD) in rats was used to prepare a disease model of IBS visceral pain [24]; this model simulated the non-noxious distension stimulation of the epithelial cells of tubular organs and saclike organs, and the traditional Chinese acupuncture treatment for abdominal pain was applied. The above two viewpoints were also preliminarily confirmed by our experiments. Our animal study demonstrated that electroacupuncture (EA) could regulate the expression of the purinergic receptors in the peripheral and central pathways of visceral pain transmission to achieve remission of the IBS visceral hypersensitivity in rats. This regulation included regulation of the expression of purinergic receptors in the enteric nervous system of the outer periphery, the spinal cord of the central nervous system, and the anterior cingulate cortex and prefrontal cortex of the cerebral cortex. In this study, molecular biology and immunohistochemistry methods were used to explore the important role of the P2X3 receptor as a P2X receptor subtype in IBS visceral pain, and the impact of EA on the brain–gut neural signal transmission was observed at different levels, including the enteric nervous system, the dorsal root ganglion (DRG), the spinal cord, and the cerebral cortex. The scientific basis for the EA treatment of IBS visceral pain was revealed from the perspective of the regulation of P2X3, thus providing experimental evidence to elucidate the functional mechanism of acupuncture.
Fig. 2.
Diagram from Geoffrey Burnstock. Puncturing the Myth-purinergic signaling, not mystical energy, may explain how acupuncture works. The Scientist, 2011 Sep
Materials and methods
Animals
A total of 24 SPF-grade newborn Sprague–Dawley (SD) male rats (5 days old) were provided by the Experimental Animal Center of Fudan University. All treatments for the experimental animals were in line with the guidelines by International Association for the Study of Pain (IASP). On the eighth day after birth, the neonatal rats were stimulated using CRD when awake. The balloon was distended with 0.3 mL of air, keeping a pressure of 60 mmHg for 1 min. Pressure was measured with a sphygmomanometer and then slowly deflated and withdrawn the balloon from the anus. The distention was repeated two times (separated by 30 min) within an hour. The procedure for generating the rat model of chronic visceral hypersensitivity was described by Al-Chaer et al. [24]. The process of modeling lasted for 2 weeks. After 6 weeks, the rats were randomly divided into three groups of eight: (1) the normal group (NG, n = 8); (2) the model group (MG, n = 8); and (3) the electroacupuncture group (EA, n = 8) that received electroacupuncture at a needling depth of 5 mm at the Shangjuxu (ST37, bilateral) and Tianshu (ST25, bilateral) acupoints. The parameters of the Han’s acupoint nerve stimulator (HANS) were as follows: sparse-dense wave with a frequency of 2/100 Hz, current of 2 mA, 20 min/stimulation, and one stimulation per day. The treatment was provided for seven consecutive days. Tianshu acupoint (ST25) is level with the navel, 5 mm lateral to anterior median line; Shangjuxu acupoint (ST37) is 5 mm lateral to the anterior tubercle of the tibia and 15 mm below the knee joint. At the sixth week after the treatment, the abdominal withdrawal reflex (AWR) score was determined, and the rats were sacrificed by cervical dislocation under intraperitoneal anesthesia. The following tissues were collected and stored in liquid nitrogen: (1) the descending colon, (2) the DRG at the L6-S2 segments, (3) the spinal cord at the L6-S2 segments, and (4) the prefrontal cortex and the anterior cingulate cortex.
Indicators and methods of detection
-
The abdominal withdrawal reflex (AWR) score
The CRD stimulation was performed using a self-made balloon stimulator connected to a desktop sphygmomanometer and a syringe through a three-way valve to provide the distension stimulation at a constant pressure. When the rat was awake, the balloon was inserted from the anus along the rectum to reach the descending colon of the rat. CRD pain stimulation was applied at four different levels of pressure: 20, 40, 60, and 80 mmHg. Three AWR scores were measured for each rat, with each measurement lasting approximately 20 s and with 3-min interval between measurements. The mean value was calculated as the final score. The AWR scoring criteria are shown in Table 1.
-
Detection of the P2X3 receptor expression in the neurons of the colonic myenteric plexus by immunofluorescence
The experimental procedure was as follows: after blocking with 10 % normal goat serum for 20 min, rabbit anti-mouse P2X3 receptor antibody (Abcam, UK) at a 1:500 dilution was added in a dropwise manner, and the mixture was incubated at 37 °C for 2 h, followed by three 5-min washes with 0.01 M phosphate-buffered saline (PBS); goat anti-rabbit IgG-FITC (Santa Cruz, USA) at a 1:100 dilution was added in a dropwise manner and then incubated at room temperature in the dark for 1 h, followed by three 5-min washes using 0.01 M PBS. Images were acquired after the sample was mounted on a laser scanning confocal microscope (Leica TCS SP5 II, Germany).
-
Real-time PCR detection of the expression of P2X3 messenger RNA (mRNA) in DRG and spinal cord neurons
The total RNA was extracted from DRG and spinal cord tissue (L6-S2 segments), and the corresponding cDNA was synthesized using a reverse transcriptase reaction system (Shanghai Jrdun Biotechnology Co. Ltd.); the relative gene expression was determined using the ΔΔCT method. The following sequences were used for the primers for the P2X3 mRNA: NM_031075.2, primer F: 5′ TTG CCT TGT GCC ATT GTG 3′, primer R: 5′ CCT CTC AAC CTG CTT CTT TC 3′, Pos: 2631–2838, amplified product: size: 208 bps, product GC 45 %. The sequences of the primers for GAPDH mRNA were as follows: NM_017008.3, primer F: 5′ GTC GGT GTG AAC GGA TTT G 3′, primer R: 5′ TCC CAT TCT CAG CCT TGA C 3′, Pos: 86–266, amplified product: size: 181 bps, product GC 51 %.
-
Detection of the P2X3 receptor expression in the prefrontal cortex and anterior cingulate cortex in rats through an immunohistochemistry assay
Antigen retrieval was performed using the paraffin sections after microwave heating (0.01 M citrate buffer, pH 6.0), followed by three 5-min 0.01 M PBS (pH 7.4) washes. The endogenous peroxidase was inactivated using 0.3 % H2O2, and then, the sample was washed using 0.01 M PBS for 5 min × 3 times. Then, rabbit anti-mouse P2X3 receptor antibody (Abcam, ab10269) at a 1:300 dilution was added in a dropwise manner and incubated at 37 °C for 2 h, followed by three 5-min washes using 0.01 M PBS. The secondary antibody (EnVision kit, Shanghai Genentech Co. Ltd.) at a 1:500 dilution was added in a dropwise manner and incubated at room temperature for 30 min and then washed three times for 5 min each using 0.01 M PBS. DAB Chromogen Solution (EnVision kit, Shanghai Genentech Co. Ltd.) was added in a dropwise manner, and the developing time was controlled by monitoring the sample under a microscope. Image and data acquisition was conducted for the positive expression of the P2X3 receptor in the prefrontal cortex and anterior cingulate cortex, and the average integrated optical density (AOD) of the positive target was analyzed using the Motic image analysis system.
Table 1.
Abdominal withdrawal reflex (AWR) scoring criteria
| Score 0 | No behavioral response to colorectal distension (CRD) |
|---|---|
| Score 1 | Immobile during distension of colorectum (CR) and occasional appearance of brief head motion after a pause at the onset of the stimulation |
| Score 2 | A mild contraction of abdominal muscles, but no lifting of abdomen off the plattorm |
| Score 3 | A strong contraction of abdominal muscles and lifting of abdomen off the platform, no lifting of pelvic structure off the platform |
| Score 4 | Arching body and lifting of pelvic structure and scrotum |
Statistical methods
The experimental data were processed using the statistical software SPSS 18.0. Comparisons of multiple factors were performed using univariate analysis of variance (one-way ANOVA) and the LSD method. The results are presented as the mean ± SEM. P < 0.05 was considered statistically significant.
Results
In addition to downregulating the P2X3 expression in the neurons of the colon myenteric plexus that was abnormally increased in the rats with IBS visceral pain, EA could also adjust the expression of P2X3 and its mRNA to different levels in the central nervous system (DRG, spinal cord, cerebral prefrontal cortex, and anterior cingulate cortex) to regulate the brain–gut neural signal transmission and improve the conditions of visceral hyperalgesia in the rats with IBS.
Electroacupuncture could reduce the visceral hypersensitivity in rats with IBS
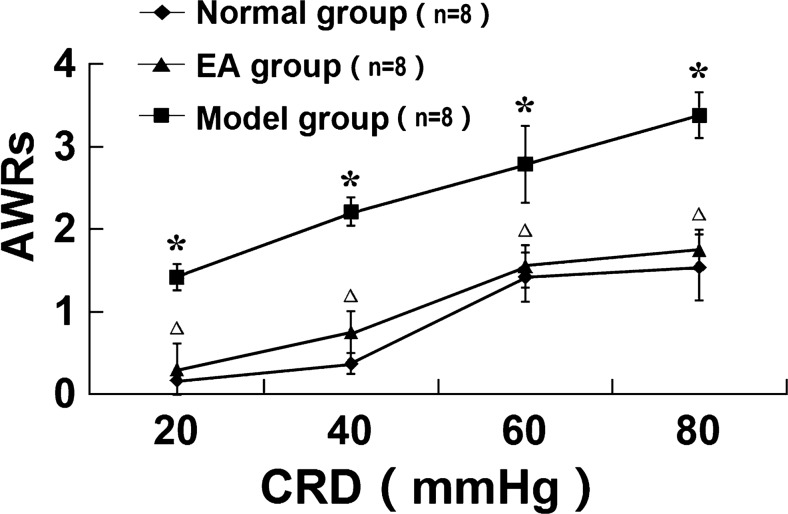
In this study, internationally accepted AWR scoring [17] was used to determine the change in the sensitivity to visceral pain. The IBS model in rats was prepared using the method of colorectal balloon distention. The AWR scores for the sensitivity to visceral pain were elevated at different pressures, indicating the development of visceral hypersensitivity after the CRD stimulation from the perspective of behavior. The experimental results showed that the CRD could lead to an increase in the sensitivity to visceral pain, and EA could reduce this visceral hypersensitivity, with a statistically significant difference (Fig. 3).
Fig. 3.
The AWR scores of the rats under different pressures. Compared with that in the normal group, the AWR score of the rats in the model group was significantly increased, *P < 0.01; compared with the score in the model group, the AWR score of the rats in the electroacupuncture group was significantly decreased, Δ P < 0.01
Electroacupuncture could regulate the P2X3 receptor in the neurons of the colon myenteric plexus in the rats with IBS visceral pain
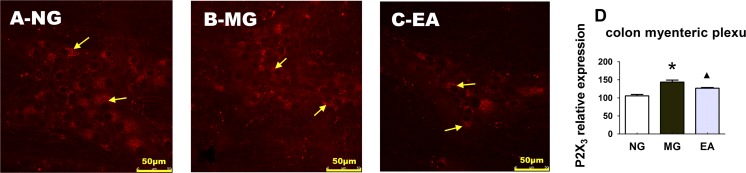
The CRD stimulation induced the visceral hyperalgesia of IBS. Meanwhile, the expression of the P2X3 receptor increased in the neurons of the colon myenteric plexus in the enteric nervous system of the rats, and further observation showed that EA could downregulate the expression of the P2X3 receptor in the colon myenteric plexus, with a statistically significant difference, P < 0.01 (Fig. 4).
Fig. 4.
Electroacupuncture regulated the P2X3 receptor in the myenteric plexus of the rats with the visceral hypersensitivity of IBS. a The expression of the P2X3 receptor in the neurons of the colon myenteric plexus in the normal rats. b The expression of the P2X3 receptor in the neurons of the colon myenteric plexus in the rats of the model group. c EA (at Shangjuxu and Tianshu) regulated the expression of the P2X3 receptor in the neurons of the colon myenteric plexus in the rats with IBS visceral pain. d Histogram of the differences in the P2X3 expression in the neurons of the colonic myenteric plexus in the different groups of rats, P < 0.01; *P < 0.01 MG vs. NG; ▲ P < 0.01 EA vs. MG
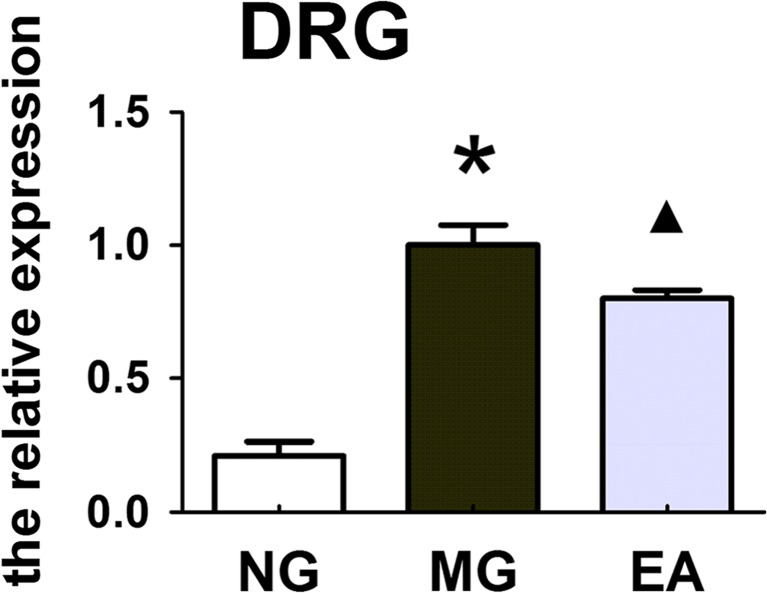
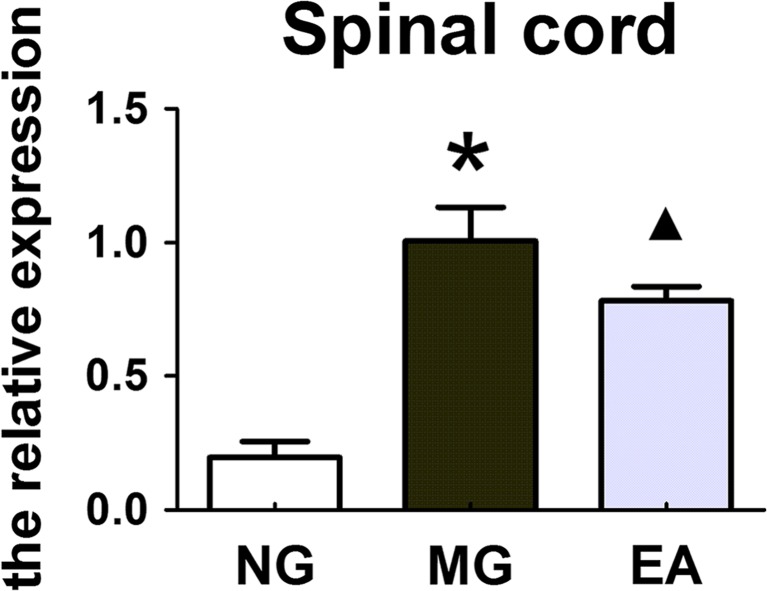
Electroacupuncture could reduce the expression of the P2X3 mRNA in the neurons of the DRG and spinal cord in the rats with IBS visceral pain
At the CNS portion of the nociceptive afferent nerve, EA altered the expression of P2X3 mRNA in the DRG and spinal cord of the rats with IBS visceral pain. The experimental results showed that compared with the expression in the normal rats, the expression of the P2X3 mRNA in the DRG and spinal cord of the rats with IBS visceral pain was upregulated, and these increased expression levels were reduced after the EA treatment at the Tianshu and Shangjuxu acupoints. The expression levels of P2X3 mRNA in the neurons of the DRG and spinal cord of the rats in different groups were different, P < 0.01 (Figs. 5 and 6).
Fig. 5.
The expression levels of P2X3 mRNA (2−△△Ct) in the neurons of the DRG of the rats in each group (NG, MG, and EA). The real-time PCR results showed that the expression levels of P2X3 mRNA in the DRG neurons of the rats in the different groups were different, P < 0.01; *P < 0.01 MG vs. NG; ▲ P < 0.01 EA vs. MG
Fig. 6.
The expression levels of P2X3 mRNA (2−△△Ct) in the neurons of the spinal cord of the rats in each group (NG, MG, and EA). The real-time PCR results showed that the expression levels of P2X3 mRNA in the spinal cord neurons of the rats in the different groups were different, P < 0.01; *P < 0.01 MG vs. NG; ▲ P < 0.01 EA vs. MG
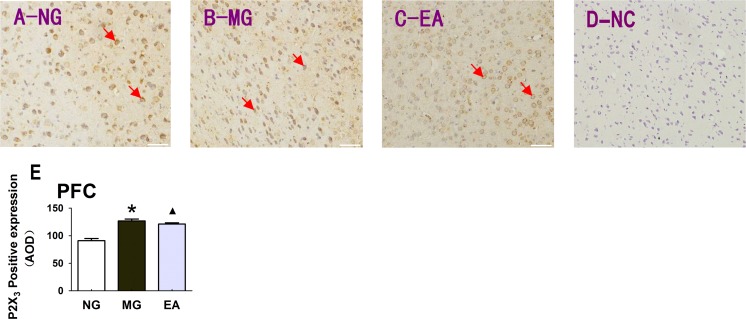
Electroacupuncture could downregulate the expression of the P2X3 receptor in the prefrontal cortex of the rats with IBS visceral pain
In the enteric nervous system of the outer periphery and in the primary afferent neurons, changes in the expression of the P2X3 receptor protein and gene were observed. Is there a similar change in the prefrontal cortex portion of the cerebral cortex in the higher level center? The results of an immunohistochemical assay showed that the colorectal balloon distention stimulation also changed the protein expression of P2X3 receptor in the prefrontal cortex, and EA also had an inhibitory regulatory effect on the expression of the P2X3 receptor in the prefrontal cortex (Fig. 7), magnification × 200.
Fig. 7.
The expression of the P2X3 receptor in the prefrontal cortex of the rats in each group. a The expression of the P2X3 receptor in the prefrontal cortex of the rats in the normal group. b The expression of the P2X3 receptor in the prefrontal cortex of the rats in the model group. c EA (at the Shangjuxu and Tianshu acupoints) regulates the expression of the P2X3 receptor in the prefrontal cortex of the rats with IBS visceral pain. d Negative control group (NC). e Histogram of the differences in the P2X3 expression in the prefrontal cortex of the rats in different groups, P < 0.01; *P < 0.01 MG vs. NG; ▲ P < 0.01 EA vs. MG
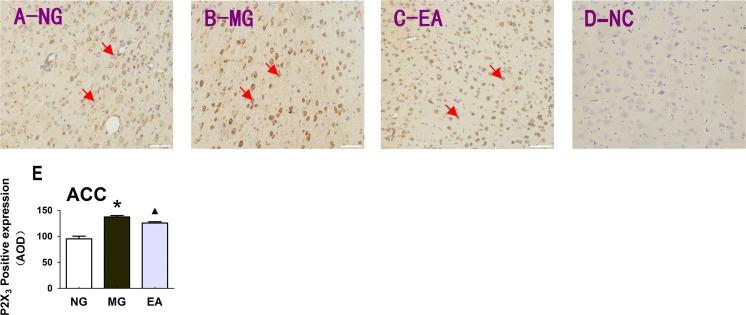
Electroacupuncture could inhibit the expression of the P2X3 receptor in the anterior cingulate cortex of the rats with IBS visceral pain
The anterior cingulate cortex is involved in complex visceral motor function and the pain response. The experiments in the CNS portion of the anterior cingulate cortex showed that the colorectal balloon distention stimulation could also alter the protein expression of P2X3 receptor in the anterior cingulate cortex, and EA also had an inhibitory regulatory effect on the expression of the P2X3 receptor in the anterior cingulate cortex (Fig. 8), magnification × 200.
Fig. 8.
The expression of the P2X3 receptor in the anterior cingulate cortex of the rats in each group. a The expression of the P2X3 receptor in the anterior cingulate cortex of the rats in the normal group. b The expression of the P2X3 receptor in the anterior cingulate cortex of the rats in the model group. c EA (at the Shangjuxu and Tianshu acupoints) regulates the expression of the P2X3 receptor in the anterior cingulate cortex of the rats with IBS visceral pain. d Negative control group (NC). e Histogram of the differences in the P2X3 expression in the anterior cingulate cortex of the rats in the different groups, P < 0.01; *P < 0.01 MG vs. NG; ▲ P < 0.01 EA vs. MG
Discussion
Using experiments, Burnstock demonstrated that when a hollow viscus (such as the intestine, ureter, and bladder) was extensively distended and overfilled, the epithelial cells in the cavity would be stimulated to release ATP, which would subsequently activate the P2X3 and P2X2/3 receptors in the subepithelial nerve endings to generate action potentials [25]; this change corresponds to hyperexcitability of the sensory neurons, and the action potentials would be transmitted to the central nervous system, resulting in a colicky sensation [26, 27]. In this study, the method of colorectal balloon distention was used to prepare an animal model of IBS visceral hyperalgesia in rats, and the expression of the P2X3 receptor in the colon myenteric plexus of the rats in the IBS model group was found to be higher than that of the rats in the normal group. The colon visceral hyperalgesia in the IBS rats might result from an increased level of ATP in the outer periphery, and the upregulated P2X receptors also led to hypersensitivity in nerves, thereby causing an abnormal pain response to the colorectal distension. The Shangjuxu point and the Tianshu point are an ancient and classical acupoint combination for the treatment of intestinal diseases using acupuncture. Our group has been engaged in clinical and basic research [9–13] on the treatment of IBS through acupuncture for years, and we have achieved desirable clinical efficacy. The preliminary mechanism of acupuncture in relieving visceral hypersensitivity has been elucidated in previous studies. Modern research has shown that treatment at the Shangjuxu point and the Tianshu point exerts its therapeutic effect by regulating the abnormally expressed serotonin (5-HT), SP, VIP, and c-Fos [12, 13] in the intestine, spinal cord, and brain of IBS rats with visceral hypersensitivity. Based on these findings, we selected the Shangjuxu point and the Tianshu point as the acupoints for stimulation by electroacupuncture. ATP, together with other sensory neurotransmitters, is stored in intestinal secretory cells, and these neurotransmitters transduce the signal from peripheral sensory neurons [28]. Antagonists of the P2X receptor, such as pyridoxal-phosphate-6-azophenyl-2′,4′-disulfonate (PPADS) and TNP-ATP, can significantly inhibit the afferent nerve activity caused by the increased ATP release due to the overfilling of the colon [29]. Our experiments also showed that compared with the rats in the model group, the rats that received EA had less CRD-induced visceral hypersensitivity. These findings demonstrated that ATP plays an important role via the P2X3 receptor in the mechanical signal transduction of visceral feelings and visceral hyperalgesia. The P2X3 receptor is involved in the mechanism by which EA regulates IBS visceral pain at the periphery, and EA most likely plays a role by directly or indirectly inhibiting the expression of the P2X3 receptor.
In the central mechanism, the P2X receptors at the CNS portion of primary afferent nerves can regulate the release of neurotransmitters and participate in the synaptic plasticity of the dorsal horn in the spinal cord. The P2X3 receptor in the DRG sensory neurons of rats with IBS visceral hypersensitivity plays an important role in ATP-mediated pain [30]. This receptor is specifically cloned and expressed in the afferent sensory neurons of the DRG, and it is highly selectively expressed in the small-diameter DRG cells that are involved in nociception. Our previous immunohistochemical experiments demonstrated that the P2X3 receptor in the DRG neurons of rats subjected to the CRD model of IBS was upregulated, and EA could reduce the expression of the P2X3 receptor in DRG cells [31]. The role of the P2X3 receptor in the activation of nociceptors in the rats with IBS was further explored at the gene level through real-time PCR. The results of our study showed that the expression of P2X3 mRNA in DRG neurons was also increased, and EA played a role in the downregulation. P2X3 may mediate the pathogenesis of IBS visceral pain at the DRG level of the brain–gut axis. This finding provides an experimental basis to further reveal the functional mechanism of the P2X receptors at each level of the brain–gut axis in the rats with visceral hypersensitivity.
The fibers of the neurons with positive immunoreactivity to the P2X3 receptor could project to the spinal dorsal horn II, and the P2X3 receptor could be expressed in these afferent nerve endings [32]. Acupuncture signals can be transmitted to the center of the marrow through the dorsal horn neurons in the spinal cord. After the downstream pain regulation system is activated, it exerts an analgesic effect by regulating the expression of the c-Fos, p38, and 5-HT receptors in the spinal dorsal horn [33, 34]. Thus, the regulation of IBS visceral pain by EA is achieved through the regulation of a variety of receptors at the spinal cord level. Previous studies demonstrated that EA can alleviate visceral hyperalgesia by downregulating the expression of the c-Fos, P2X2, and P2X3 receptors in the colon and the spinal cord [35], as well as the P2Y1 receptor expression in the DRG, in rats with visceral pain. This study investigated the mechanism by which EA regulates IBS visceral pain from the perspective of genes. In this study, the effect of EA on the P2X3 mRNA level was measured to develop a preliminary explanation for the mechanism by which EA and P2 receptors regulate IBS visceral pain. Compared with that in the normal rats, the expression of P2X3 mRNA in the spinal cord of the rats in the model group was increased. Compared with that in the rats in the model group, the expression of P2X3 mRNA in the spinal cord of the rats in the EA group was reduced, with similar results in the immunohistochemistry experiments [35]. This finding indicates that EA can play a role in reducing the development of IBS visceral hyperalgesia at the gene level, and the finding indicates that EA (at the Tianshu and Shangjuxu acupoints) can be used to treat IBS visceral pain.
The prefrontal cortex plays an important role in the perception of pain stimuli, such as the pain from internal organs [36, 37]. As a non-noxious stimuli, CRD is commonly used in studies of the treatment of visceral pain in the nervous system, and visceral hypersensitivity is one of the major pathological characteristics of IBS as a functional bowel disorder. In recent years, the neuroimaging of humans showed that in patients with IBS, the functional activity of the brain plays an important role in the response to visceral stimulation and reflects the severity of the IBS symptoms. As an important center of pain, the anterior cingulate cortex has extensive fiber links with the medial thalamus medulla, amygdala, periaqueductal gray matter, caudate nucleus, and other nuclei involved in the modulation of pain sensation [38–40]. Positron emission tomography (PET) showed that the cingulate cortex in patients with IBS was activated after CRD [41]. The results of an in situ hybridization and immunohistochemical study confirmed that the P2X3 receptor is expressed in the pain pathways of the brain in adult rats. P2X receptors can not only bind to ATP to mediate a rapid transient effect but also produce a chronic effect during long-term stimulation by ATP. Thus, various visceral stimuli (such as pain, pressure, and distension) can affect the central nervous system through the enteric nervous system [42], leading to changes in the activity of the corresponding region of the central nervous system, thus affecting the depression of the patients.
In this study, immunohistochemistry was used to detect the expression of the P2X3 receptor in the prefrontal cortex of the rats and in the model and EA groups. The results showed a difference in the expression of the P2X3 receptor in the prefrontal cortex of the rats in the EA group, indicating that EA could reduce the expression of the P2X3 receptor in the prefrontal cortex of the rats subjected to the model of IBS visceral pain. The AWR score analysis showed the following: The pain sensitivity of the rats in the model group was reduced, EA played a role by inhibiting the expression of the P2X3 receptor, and the P2X3 receptor also played a role in the central mechanism related to the regulation of IBS visceral pain by EA. The same detection method was used to detect the P2X3 receptor in the anterior cingulate cortex of the rats with IBS visceral pain, and the results showed that EA reduced the expression of the P2X3 receptor in the anterior cingulate cortex of the rats in the IBS model group; this finding suggested that EA could inhibit the expression of the P2X3 receptor and that the P2X3 receptor may play an important role in the central mechanism related to IBS visceral pain. The study revealed that EA is involved in the regulation of IBS visceral pain in the central mechanism and that the P2X3 receptor is part of one of the pathways of this function. The P2 receptor in the prefrontal cortex and cingulate cortex may be involved in IBS visceral pain from the perspective of synaptic facilitation [43], but this hypothesis still requires confirmation by further experiments. At the same time, in order to evaluate the effect of electroacupuncture objectively, we will add normal+electroacupuncture group into our future in-depth study in this direction. Additionally, the methods of Western blot can better measure the expression of the P2X3R protein.
The mechanism underlying the EA treatment of visceral pain involves an integrated process in the body at various levels from the outer periphery to the center; multiple factors, including nerves, immune and endocrine factors, also interact during this process, and this treatment is related to the acupuncture points, time, and other stimulation parameters. The EA treatment of visceral pain involves a variety of complex neural structures and neurotransmitters. The effects of this treatment may occur through the interactions between the spinal cord, thalamus, brain stem, and cortex in the high level center, as well as through the neuroendocrine-immune network. The mechanism underlying the EA treatment of visceral pain involves the interactions among different sensory afferents in the central nervous system; these interactions can occur at different levels for regions ranging from the outer periphery to the cortex. ATP is released from the nervous system to the extracellular space and mainly participates as a rapid neurotransmitter that mediates the neuronal depolarization current; ATP also coexists with some other neurotransmitters. The neurotransmitters interact with and regulate each other, and they work together to perform their functions. ATP and P2X receptors have received continuous attention in studies of the pathogenesis and EA treatment of IBS visceral pain, and elucidating the signal transduction mechanism requires further in-depth studies.
Acknowledgments
This research was funded by the Specialized Research Fund for the Doctoral Program of Higher Education, No. 20123107110008; the National Natural Sciences Foundation of China (No. 81403474); New Century Excellent Talents in University (No. NCET-13-0907); and the National Basic Research Program of China (973 program, No. 2009CB522900).
Conflict of interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Footnotes
Z. J. Weng, L. Y. Wu and C. L. Zhou contributed equally to this work.
Contributor Information
H. R. Liu, Email: lhr_tcm@139.com
H. G. Wu, Email: wuhuangan@126.com
References
- 1.O’Mahony L, McCarthy J, Kelly P, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128(3):541–551. doi: 10.1053/j.gastro.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 2.Kim DY, Camilleri M. Serotonin: a mediator of the brain-gut connection. Am J Gastroenterol. 2000;95(10):2698–2709. doi: 10.1111/j.1572-0241.2000.03177.x. [DOI] [PubMed] [Google Scholar]
- 3.Fukudo S, Nomura T, Muranaka M, et al. Brain-gut response to stress and cholinergic stimulation in irritable bowel syndrome. A preliminary study. J Clin Gastroenterol. 1993;17(2):133–141. doi: 10.1097/00004836-199309000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Burnstock G, Kennedy C. P2X receptors in health and disease. Adv Pharmacol. 2011;61:333–372. doi: 10.1016/B978-0-12-385526-8.00011-4. [DOI] [PubMed] [Google Scholar]
- 5.Burnstock G. Purinergic signalling in the gastrointestinal tract and related organs in health and disease. Purinergic Signal. 2014;10(1):3–50. doi: 10.1007/s11302-013-9397-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burnstock G. Purinergic signalling in the urinary tract in health and disease. Purinergic Signal. 2014;10(1):103–155. doi: 10.1007/s11302-013-9395-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burnstock G. Purinergic signalling: pathophysiology and therapeutic potential. Keio J Med. 2013;62(3):63–73. doi: 10.2302/kjm.2013-0003-RE. [DOI] [PubMed] [Google Scholar]
- 8.Ochoa-Cortes F, Liñán-Rico A, Jacobson KA, et al. Potential for developing purinergic drugs for gastrointestinal diseases. Inflamm Bowel Dis. 2014;20(7):1259–1287. doi: 10.1097/MIB.0000000000000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou EH, Ding GH, Wu HG, Qi L, Liu HR, Wang XM, Ma XP. Immediate effect of acupuncture on colon motility in patients with irritable bowel syndrome. Zhonghua Zhongyiyao Xuekan. 2011;29:293–296. [Google Scholar]
- 10.Ma XP. Acupuncture-moxibustion in treating irritable bowel syndrome: how does it work? World J Gastroenterol. 2014;20(20):6044–6054. doi: 10.3748/wjg.v20.i20.6044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ji J, Lu Y, Liu H, et al. Acupuncture and moxibustion for inflammatory bowel diseases: a systematic review and meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med. 2013;2013:158352. doi: 10.1155/2013/158352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu HG, Jiang B, Zhou EH, et al. Regulatory mechanism of electroacupuncture in irritable bowel syndrome: preventing MC activation and decreasing SP VIP secretion. Dig Dis Sci. 2008;53(6):1644–1651. doi: 10.1007/s10620-007-0062-4. [DOI] [PubMed] [Google Scholar]
- 13.Liu HR, Wang XM, Zhou EH, et al. Acupuncture at both ST25 and ST37 improves the pain threshold of chronic visceral hypersensitivity rats. Neurochem Res. 2009;34(11):1914–1918. doi: 10.1007/s11064-009-9972-1. [DOI] [PubMed] [Google Scholar]
- 14.Huang RJ, Zhao JM, Wu LY, et al. Mechanisms underlying the analgesic effect of moxibustion on visceral pain in irritable bowel syndrome: a review. Evid Based Complement Alternat Med. 2014;2014:895914. doi: 10.1155/2014/895914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burnstock G. The journey to establish purinergic signalling in the gut. Neurogastroenterol Motil. 2008;20(Suppl 1):8–19. doi: 10.1111/j.1365-2982.2008.01107.x. [DOI] [PubMed] [Google Scholar]
- 16.Giniatullin R, Nistri A. Desensitization properties of P2X3 receptors shaping pain signaling. Front Cell Neurosci. 2013;7:245. doi: 10.3389/fncel.2013.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burnstock G. Introduction and perspective, historical note. Front Cell Neurosci. 2013;7:227. doi: 10.3389/fncel.2013.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu GY, Shenoy M, Winston JH, et al. P2X receptor-mediated visceral hyperalgesia in a rat model of chronic visceral hypersensitivity. Gut. 2008;57(9):1230–1237. doi: 10.1136/gut.2007.134221. [DOI] [PubMed] [Google Scholar]
- 19.Soares-Bezerra RJ, Calheiros AS, da Silva Ferreira NC, et al. Natural products as a source for new anti-inflammatory and analgesic compounds through the inhibition of purinergic P2X receptors. Pharmaceuticals (Basel) 2013;6(5):650–658. doi: 10.3390/ph6050650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burnstock G, Nistri A, Khakh BS, et al. ATP-gated P2X receptors in health and disease. Front Cell Neurosci. 2014;8:204. doi: 10.3389/fncel.2014.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scalera A, Loguercio C. Focus on irritable bowel syndrome. Eur Rev Med Pharmacol Sci. 2012;16(9):1155–1171. [PubMed] [Google Scholar]
- 22.Wang WS, Tu WZ, Cheng RD, et al. Electroacupuncture and A-317491 depress the transmission of pain on primary afferent mediated by the P2X3 receptor in rats with chronic neuropathic pain states. J Neurosci Res. 2014;92(12):1703–1713. doi: 10.1002/jnr.23451. [DOI] [PubMed] [Google Scholar]
- 23.Burnstock G (2011) Puncturing the myth-purinergic signaling, not mystical energy, may explain how acupuncture works. The Scientist
- 24.Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology. 2000;119(5):1276–1285. doi: 10.1053/gast.2000.19576. [DOI] [PubMed] [Google Scholar]
- 25.Rong W, Spyer KM, Burnstock G. Activation and sensitisation of low and high threshold afferent fibers mediated by P2X receptors in the mouse urinary bladder. J Physiol. 2002;541(Pt 2):591–600. doi: 10.1113/jphysiol.2001.013469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burnstock G. Purine-mediated signalling in pain and visceral perception. Trends Pharmacol Sci. 2001;22(4):182–188. doi: 10.1016/S0165-6147(00)01643-6. [DOI] [PubMed] [Google Scholar]
- 27.Shinoda M, Feng B, Gebhart GF. Peripheral and central P2X receptor contributions to colon mechanosensitivity and hypersensitivity in the mouse. Gastroenterology. 2009;137(6):2096–2104. doi: 10.1053/j.gastro.2009.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tamir H, Gershon MD. Serotonin-storing secretory vesicles. Ann N Y Acad Sci. 1990;600:53–66. doi: 10.1111/j.1749-6632.1990.tb16872.x. [DOI] [PubMed] [Google Scholar]
- 29.Accarino AM, Azpiroz F, Malagelada JR. Attention and distraction: effects on gut perception. Gastroenterology. 1997;113(2):415–422. doi: 10.1053/gast.1997.v113.pm9247458. [DOI] [PubMed] [Google Scholar]
- 30.Shinoda M, La JH, Bielefeldt K, Gebhart GF. Altered purinergic signaling in colorectal dorsal root ganglion neurons contributes to colorectal hypersensitivity. J Neurophysiol. 2010;104(6):3113–3123. doi: 10.1152/jn.00560.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weng ZJ, Wu LY, Lu Y, et al. Electroacupuncture diminishes P2X2 and P2X3 purinergic receptor expression in dorsal root ganglia of rats with visceral hypersensitivity. Neural Regen Res. 2013;8(9):802–808. doi: 10.3969/j.issn.1673-5374.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82(4):1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 33.Xu KD, Liang T, Wang K, et al. Effect of pre-electroacupuncture on p38 and c-Fos expression in the spinal dorsal horn of rats suffering from visceral pain. Chin Med J (Engl) 2010;123(9):1176–1181. [PubMed] [Google Scholar]
- 34.Yu WC, Huang GY, Zhang MM, et al. Effect of connexin 43 knockout on acupuncture-induced down-regulation of c-fos expression in spinal dorsal horn in visceral pain mice. Acupunct Res. 2008;33(3):179–182. [PubMed] [Google Scholar]
- 35.Dong M. (2011) Regulatory effects of electroacupuncture on P2X2,3 receptor and c-fos of rats with irritable bowel syndrome visceral sensitivity. Shang Hai University of T.C.M. Master thesis
- 36.Gibney SM, Gosselin RD, Dinan TG, et al. Colorectal distension-induced prefrontal cortex activation in the Wistar-Kyoto rat: implications for irritable bowel syndrome. Neuroscience. 2010;165(3):675–683. doi: 10.1016/j.neuroscience.2009.08.076. [DOI] [PubMed] [Google Scholar]
- 37.Elsenbruch S. Abdominal pain in irritable bowel syndrome: a review of putative psychological, neural and neuro-immune mechanisms. Brain Behav Immun. 2011;25(3):386–394. doi: 10.1016/j.bbi.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 38.Shyu BC, Sikes RW, Vogt LJ, Vogt BA. Nociceptive processing by anterior cingulate pyramidal neurons. J Neurophysiol. 2010;103(6):3287–3301. doi: 10.1152/jn.00024.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yan N, Cao B, Xu J, et al. Glutamatergic activation of anterior cingulate cortex mediates the affective component of visceral pain memory in rats. Neurobiol Learn Mem. 2012;97(1):156–164. doi: 10.1016/j.nlm.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Sikes RW, Vogt LJ, Vogt BA. Distribution and properties of visceral nociceptive neurons in rabbit cingulate cortex. Pain. 2008;135(1–2):160–174. doi: 10.1016/j.pain.2007.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Naliboff BD, Berman S, Suyenobu B, et al. Longitudinal change in perceptual and brain activation response to visceral stimuli in irritable bowel syndrome patients. Gastroenterology. 2006;131(2):352–365. doi: 10.1053/j.gastro.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 42.Burnstock G. Physiopathological roles of P2X receptors in the central nervous system. Curr Med Chem. 2015;22(7):819–844. doi: 10.2174/0929867321666140706130415. [DOI] [PubMed] [Google Scholar]
- 43.Wang J, Zhang X, Cao B, et al. (2013) Facilitation of synaptic transmission in the anterior cingulate cortex in viscerally hypersensitive rats. Cereb Cortex. [Epub ahead of print] [DOI] [PMC free article] [PubMed]