Abstract
Extracellular purines and pyrimidines play major roles during embryogenesis, organogenesis, postnatal development and ageing in vertebrates, including humans. Pluripotent stem cells can differentiate into three primary germ layers of the embryo but may also be involved in plasticity and repair of the adult brain. These cells express the molecular components necessary for purinergic signalling, and their developmental fates can be manipulated via this signalling pathway. Functional P1, P2Y and P2X receptor subtypes and ectonucleotidases are involved in the development of different organ systems, including heart, blood vessels, skeletal muscle, urinary bladder, central and peripheral neurons, retina, inner ear, gut, lung and vas deferens. The importance of purinergic signalling in the ageing process is suggested by changes in expression of A1 and A2 receptors in old rat brains and reduction of P2X receptor expression in ageing mouse brain. By contrast, in the periphery, increases in expression of P2X3 and P2X4 receptors are seen in bladder and pancreas.
Keywords: Stem cells, Zebrafish, Heart, Bladder, Retina, Cochlea, Lung, Skeletal muscle, ATP, Adenosine
-
Synopsis
Introduction
Early embryos
Zebrafish
Frog embryos
Chick embryos
Mammalian embryos
Human embryos
Development in different systems
Cardiovascular system
Heart
Blood vessels
Skeletal muscle
Urinary bladder
Nervous system
Central nervous system
Peripheral nervous system
Retina
Inner ear
Gastrointestinal tract
Lung
Vas deferens
Other organs
Stem cells
Ageing
Summary
Introduction
Most early studies of the roles of nucleotide in development have been discussed in terms of their intracellular roles and as a source of energy. However, purines and pyrimidines are now generally accepted to have potent extracellular actions mediated by the activation of specific membrane receptors (see [1]); many of these previous studies can now be reinterpreted. ATP and adenosine play key roles from the very beginning of life, i.e. the moment of conception. Adenosine 5′-triphosphate (ATP) is obligatory for sperm movement [2] and is a trigger for capacitation, the acrosome reaction necessary to fertilize the egg [3]. Extracellular ATP also promotes a rapid increase in Na+ permeability of the fertilized egg membrane through the activation of a specific ATP receptor [4]. Mg2+–ATPase activity has been localized on the entire surface of unfertilized eggs and in preimplantation and postimplantation embryos [5, 6]. Together with the demonstration that ATP-activated spermatozoa show very high success rates in fertilization tests [3], this strongly suggests that ATP is a key sperm-to-egg signal in the process of fertilization.
In this review, the extracellular roles of purines and pyrimidines will be considered as signalling molecules in both embryological and postnatal development in a wide variety of systems in amphibians, birds and mammals, including humans. Readers are referred to reviews of the earlier literature about the involvement of purinergic signalling in both embryonic and postnatal development [7, 8] and very good reviews specifically about the development of the nervous system [9–11]. There have been relatively few reports about changes in purinergic transmission during ageing, and these are mostly concerned with the brain and cardiovascular system.
Together with muscarinic cholinergic receptors, extracellular receptors to ATP were shown to be the first functionally active membrane receptors in chick embryo cells at the time of germ layer formation. In gastrulating chick embryo, ATP caused rapid accumulation of inositol 1,4,5-trisphosphate (IP3) and Ca2+ mobilization in a similar way and to the same extent as acetylcholine (ACh), whereas other neuroendocrine substances such as insulin and noradrenaline (NA) had much weaker effects. This suggests that, alongside ACh, other phylogenetically old and universal regulators of cell metabolism such as ATP (and perhaps nitric oxide (NO)) might play leading roles in the functional regulation of gastrulation via the activation of specific receptors triggering Ca2+ mobilization.
Early embryos
The expression patterns of purinergic receptors and the ectonucleotidase enzymes that convert ATP to downstream nucleotides and nucleosides vary during development. This suggests, in turn, that these molecules may have important developmental roles, either to mediate particular physiological functions at different stages of development or to control the developmental processes themselves [12].
Zebrafish
Trigeminal ganglia of zebrafish express P2X3 receptors from a very early stage of development, most likely in neural crest-derived trigeminal cells rather than placode-derived cells [13]. Spinal sensory Rohon–Beard cells also expressed P2X3 receptors, also in the putative lateral line ganglion and in the early developing zebrafish. Ectodermal p2rx3.1 receptor, a paralog of the mammalian P2X3 receptor, is expressed in subpopulations of both neural and ectodermal cells in the embryonic head of zebrafish [14]. Knockdown of expression of this receptor disrupted craniofacial development, suggesting a critical role in this process.
Frog embryos
A novel P2Y receptor (p2y8) was cloned and sequenced and was expressed (as seen by Northern blots and in situ hybridization) in the neural plate of Xenopus embryos from stages 13 to 18 and again at stage 28 when secondary neurulation occurs in the tail bud [15]. It differs from other members of the P2Y receptor family having an intracellular C terminus with 216 amino acid residues (compared to 16 to 67 in the known P2Y receptors). It shows equipotent responses to the triphosphates ATP, uridine 5′-triphosphate (UTP), inosine triphosphate, cytidine triphosphate and guanosine triphosphate (GTP) and smaller responses to diphosphates and tetraphosphates when expressed as a recombinant receptor in Xenopus oocytes but is not responsive to inorganic phosphates. Activation of the p2y8 receptor evoked long duration responses (40–60 min). It was suggested that this novel P2Y receptor may be involved in the early formation of the nervous system.
Suramin and trypan blue, both substances that are known to be antagonists at P2 receptors (see [16]) as well as having other actions, have been shown to interfere with gastrulation [17]. If injected early when the dorsal lip is first invaginating, the Xenopus embryo develops no head or trunk and sometimes no tail; somites and notochord are also missing. If they are injected midway in gastrulation, embryos develop without heads, but with trunks and tails, while if injected at the end of gastrulation, the embryo is completely unaffected.
The nicotinic channels in myotomal muscle cells cultured from Xenopus embryos at stages 19–22 were shown to be opened by micromolar concentrations of exogenous ATP [18], following the earlier demonstration that ATP increases the sensitivity of receptors in adult frog skeletal muscles without increasing the affinity of ACh for the receptor or inhibitory acetylcholinesterase [19]. Since then, there have been a number of studies of the actions of ATP in developing Xenopus neuromuscular synapses (see [20]). Extracellular applications of ATP to developing Xenopus neuromuscular synapses in culture potentiate ACh responses of developing muscle cells during the early phase of synaptogenesis [21–23]. The possibility that extracellular ATP coreleased with ACh may serve as a positive trophic factor at developing neuromuscular synapses has also been raised [20, 21]. It is further suggested that calcitonin gene-related peptide (CGRP) and ATP coreleased with ACh from the nerve terminal may act together to potentiate postsynaptic ACh channel activity during the early phase of synaptogenesis [24]; it is claimed that CGRP actions are mediated by cyclic adenosine monophosphate (cAMP)-dependent protein kinase (PK) A, while ATP exerts its effects via PKC. In a later report from this group [25], they present results that suggest that endogenously released ATP, acting in concert with various PKs, is involved in the maintenance and/or development of the quantum size of synaptic vesicles at embryonic neuromuscular synapses.
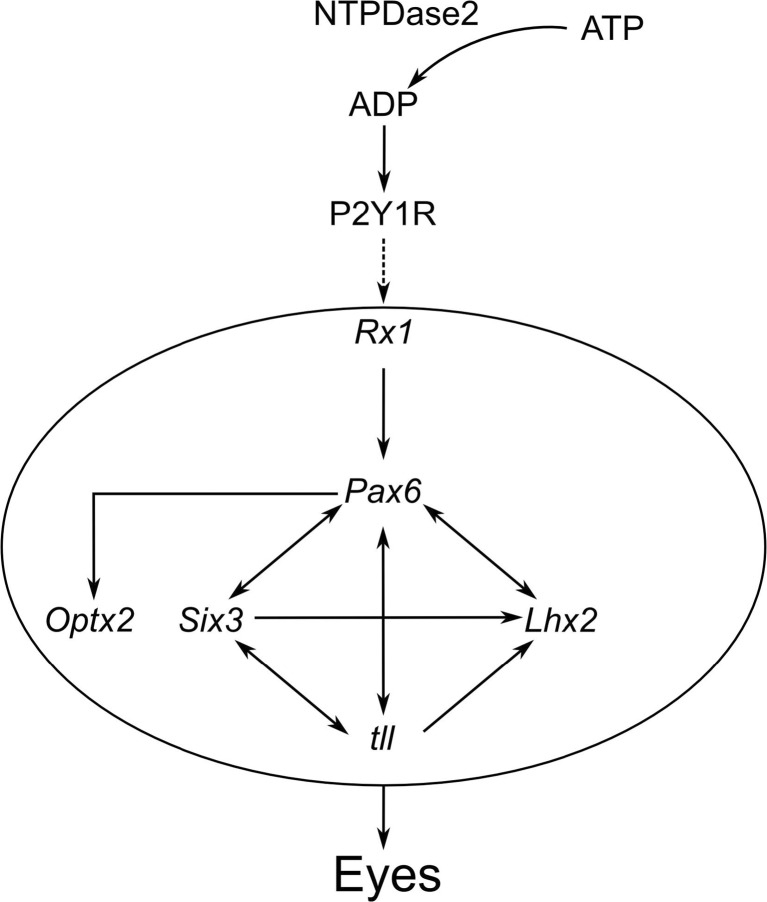
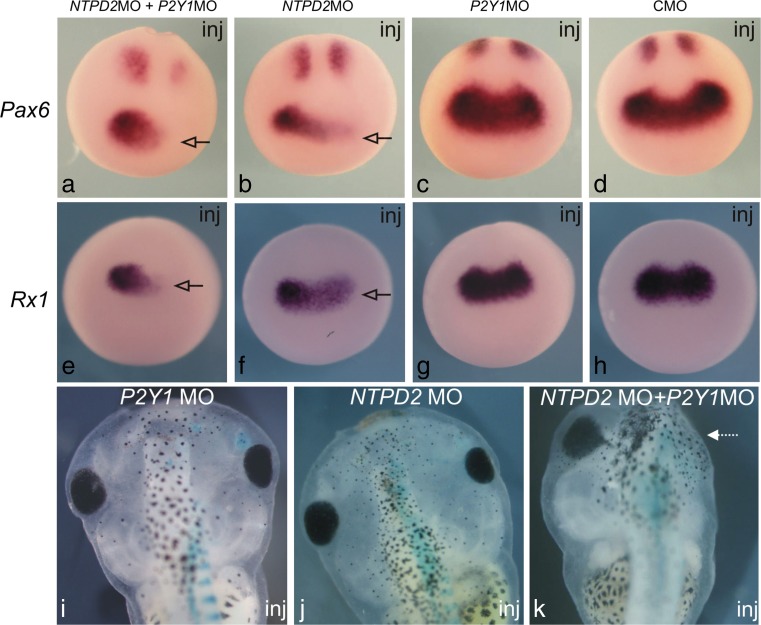
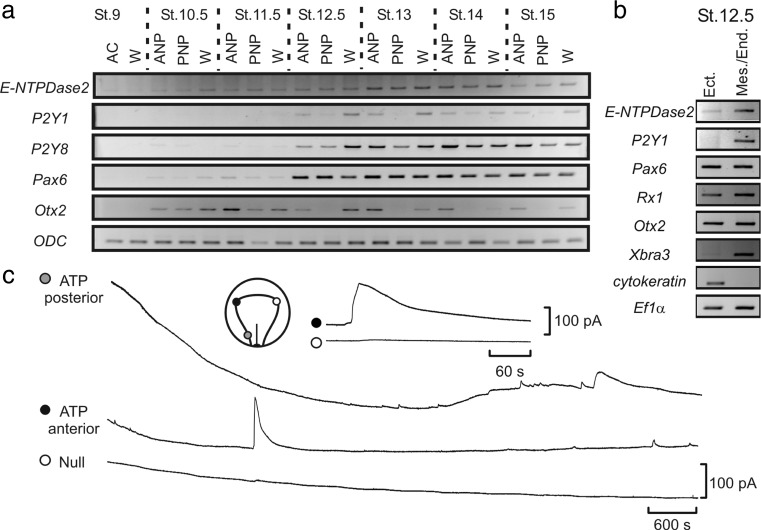
Purine-mediated signalling triggers eye development in Xenopus [26]. The authors have shown that overexpression of ecto-NTPDase2 (that converts ATP to adenosine 5′-diphosphate (ADP)) caused ectopic eye-like structures and occasionally complete duplication of the eye. Expression of ecto-NTPDase2 is essential for the expression of key genes required for eye development such as Rx1 and Pax6 (Figs. 1 and 2). It appears that the ecto-NTPDase2 produces ADP from ATP which is necessary for activation of the P2Y1 receptor. Although knockdown of P2Y1 expression had relatively mild effects, combined knockdown of ecto-NTPDase2 and the P2Y1 receptor could completely prevent eye development (Fig. 2). All the key components of purinergic signalling are present around stage 12 of embryogenesis when the key determination of eye development occurs (Fig. 3). In the eye field (the site of the future development of the eye and where key genes are activated to achieve this), a period of transient ATP release was observed (Fig. 3). However, this mechanism may be specific to lower vertebrate embryos, as knock out of NTPDase2, even in combination with knock out of the P2Y1 receptor, in mice does not prevent or alter eye development [27].
Fig. 1.
Purinergic signalling is upstream of the eye field transcription factors (EFTFs) that are expressed very early in development of the frog embryo and to control development of the eye (see [364]). ATP released in the presumptive eye field is converted to ADP via NTPDase2. The ADP can activate the P2Y1 receptor (P2Y1R), which then triggers expression of the transcription factor Rx1
Fig. 2.
Morpholino oligonucleotide knockdown of NTPDase2 (NTPD2MO) or the P2Y1 receptor (P2Y1MO) alters the expression of Pax6 and Rx1 (a–h). The morpholino constructs were injected on just one side of the early embryo to facilitate a comparison with the uninjected side as an internal control. NTPD2MO either on its own or in combination with P2Y1MO reduced Pax6 and Rx1 expression only on the injected side (arrows). However, injection of P2Y1MO on its own or a control morpholino (CMO) has no effect on expression of Pax6 and Rx1 (i–k). This reduced expression of Pax6 and Rx1 results in smaller eyes on the injected side (indicated by lacZ staining) or, in the case of the dual morpholino knockdown, no eyes (k, arrow) (reproduced with permission from [26])
Fig. 3.
The expression of the components of purinergic signalling and Pax6 and Otx2 during frog embryonic development. a NTPDase2 expression precedes induction of Pax6, and expression of the P2Y1R is coincident with the upregulation of Pax6 that occurs at St 12.5. b Both NTPDase2 and P2Y1 are expressed more strongly in the mesoderm/endoderm, suggesting that it is this tissue layer that initiates expression of the EFTFs in the ectoderm. c ATP is released from the neural plate during development. The inset diagram indicates the positioning of ATP biosensors in the very early frog embryo. A large transient signal is seen in the anterior region—the site of the eye field. ATP signalling is also seen in the posterior regions. The ATP release event is blown up in the inset traces (reproduced with permission from [26])
Chick embryos
In the chick, the thyroid gland most likely forms from the thyroid primordium via a process of evagination that appears to be induced by ATP [28]. The requirement for ATP was very precise, since it could not be replaced by pyrophosphate, AMP or ADP nor by GTP, suggesting a high degree of specificity of the ATP-induced effect. Together with muscarinic cholinergic receptors, extracellular receptors to ATP were shown to be the first functionally active membrane receptors in chick embryo cells at the time of germ layer formation [29]. In gastrulating chick embryo, ATP causes rapid accumulation of inositol phosphate and Ca2+ mobilization in a similar way and to the same extent as ACh, whereas other neuroendocrine substances such as insulin and NA have much weaker effects [29]. This suggests that, alongside ACh, other phylogenetically old and universal regulators of cell metabolism such as ATP (and perhaps NO) might play a leading role in the functional regulation of gastrulation via the activation of specific receptors triggering Ca2+ mobilization. Wide distribution of P2Y1 receptors in the 1-day-old chick brain was reported.
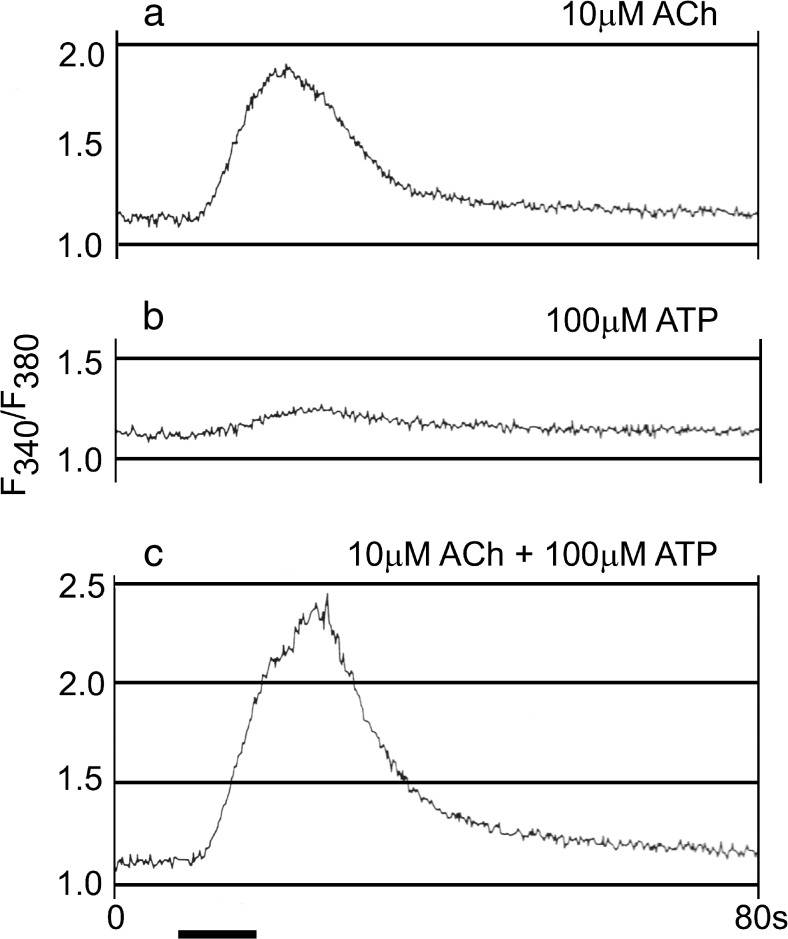
ATP acts on embryonic and developing cells of both nervous and non-nervous systems by increasing intracellular Ca2+ concentrations. Release of Ca2+ from intracellular stores is evoked in the otocyst epithelium of the early embryonic chick, incubated for 3 days (stage 18 to 19) [30] (Fig. 4), in developing chick myotubes [31] and in dissociated cells from whole early embryonic chicks [29, 32]. Programmed cell death was demonstrated in proliferative regions of chick optic tectum during early development, particularly in the ventricular zone between stages E7.5 and E8. This is of interest since P2X7 receptors mediate apoptosis. A P2X receptor subunit in embryonic chick brain has been cloned and characterized, which is highly homologous to the mammalian P2X4 receptor (human and rat) with approximately 75 % sequence identity [33].
Fig. 4.
Interaction between acetylcholine (ACh) and ATP recorded in an otocyst from chick embryo. a The response to 10 μM ACh. b The response to 100 μM ATP. c The response to the coapplication of 10 μM ACh and 100 μM ATP. The records in a–c were taken in this order at 5-min intervals. The bath solutions contained 25 mM Ca2+ (reproduced with permission from [30])
In a study, the expression of the G protein-coupled P2Y1 receptor during embryonic development of the chick was described [34]. During the first 10 days of embryonic development, the P2Y1 receptor is expressed in a developmentally regulated manner in the limb buds, mesonephros, brain, somites and facial primordia (Fig. 5), suggesting that there may be a role for ATP and P2Y1 receptors in the development of these systems.
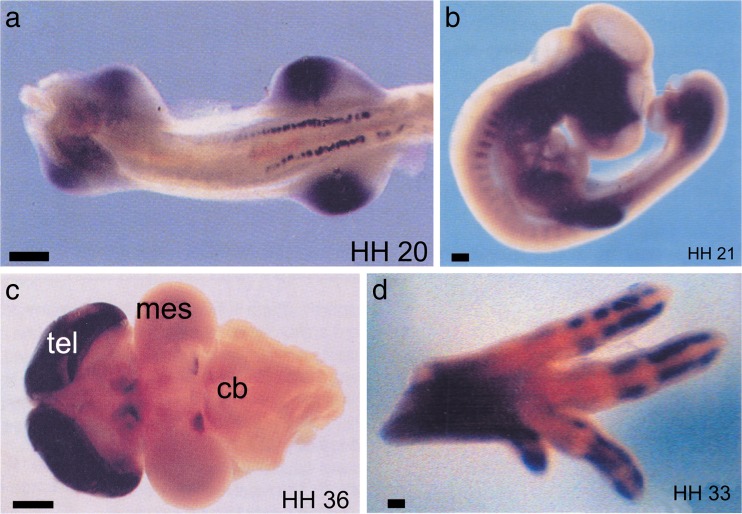
Fig. 5.
Expression of P2Y1 receptors during embryonic development of the chick as visualized by whole-mount in situ hybridization. Stages of development are shown in bottom right corner. a Ventral view of stage 20 embryo showing P2Y1 expression in mesonephros and limb buds (scale bar = 200 μm). b Lateral view of the chick somite at stage 21 showing P2Y1 expression in the anterior region. The dark area in the head region is due to an artefact of photography (scale bar = 200 μm). c Dorsal view of stage 36 brain (anterior to the left), showing increased levels of expression in telecephlon (tel), dorsal diencephlon and posterior midbrain. mes mesencephalon, cb cerebellum (scale bar = 1 mm). d An anterior-uppermost view of a leg at embryonic stage 33. Expression of P2Y1 is seen in the digits, but not in areas of joint formation. The same expression pattern is also seen in the wing (scale bar = 100 μm) (reproduced with permission from [34])
Adenosine has been implicated in growth regulation of the vascular system in the chick embryo [35], in common with a similar role claimed for experimental angiogenesis in the chorioallantoic membrane [36–38].
Mammalian embryos
Puff-applied ATP has been shown to have two main effects on a mouse mesodermal stem cell line: an increase in intracellular Ca2+ concentrations and a subsequent hyperpolarization due to Ca2+-activated K+ conductance [39] (Fig. 6). The author speculated that the transient increase in intracellular Ca2+ may influence mesodermal cell differentiation, particularly in relation to muscle differentiation. In a subsequent paper [40], two myoblastic cell lines, one from rat, the other from mouse, showed similar properties to those of the myogenic clonal cells derived from the mouse mesodermal stem cell line described above.
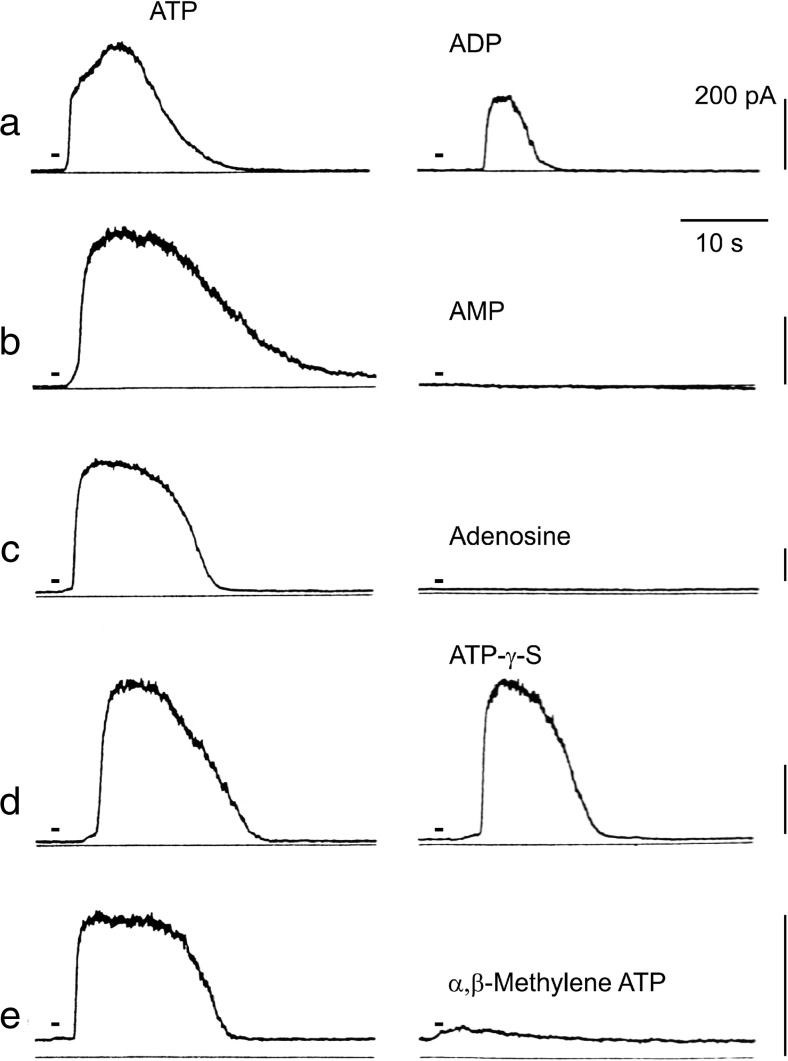
Fig. 6.
K+ responses to ATP analogues of a mouse mesodermal cell line. Each of the two traces was obtained from the same cell (a–e). The responses induced by ATP (left traces) and ATP analogues (right traces) are shown. The names of the analogues are shown near the traces. Each drug was applied at 20 μM, and the holding potentials were 0 mV (reproduced with permission from [39])
ATP and ADP have been shown to enhance, reduce or have no effect (depending on the dose used) on the incidence of trypan blue-induced teratogenic malformations in the rat foetus at day 20 [41]. Concomitant administration of ATP and cortisone in mice either decreases the teratogenic effect of cortisone (50 μg ATP) or enhances its teratogenic effect (>100 μg ATP) [42]. Mouse heads of embryos from 14 to 24 pairs of body somites exposed to an ATP-containing medium have been demonstrated to undergo rapid epithelial thickening and invagination, a process that appears to take part in the shaping of nasal pits and formation of the primary palate [43]. The trophoblast appears at the blastocyst stage of embryogenesis and contributes to placentation. ATP increases [Ca2+]i via a P2Y receptor in proliferating bovine trophoblast cells [44].
Besides ATP, a number of reports implicate adenosine as one of the endogenous effectors that can selectively modulate cell growth during embryonic development. For example, adenosine is shown to potentiate the delaying effect of dibutyryl cAMP (a membrane-permeable analogue of cAMP) on meiosis resumption in denuded mouse oocytes [45]. The role of adenosine has been particularly well characterized in the morphogenetic outgrowth of vertebrate limb buds [46]. Embryonic limb development in the mouse is driven by rapid mesenchymal cell proliferation induced by trophic substances secreted by the apical ectodermal ridge. This interaction can be restricted experimentally by pharmacological agents that elevate intracellular cAMP levels or physiologically by the onset of programmed cell death triggered by naturally occurring negative regulators of growth. Mutations that affect the pattern of limb/bud outgrowth provide invaluable experimental means to investigate these growth-regulatory processes. Knudsen and Elmer [46] studied the regulation of polydactylous outgrowth (an expression of the Hemimelia-extra toe (HmX/+) mutant phenotype) in hindlimb bud explants. Polydactylous growth is enhanced by exogenous adenosine deaminase, the enzyme which catalyzes the inactivation of endogenous adenosine. This effect was reversed by coexposure to hydrolysis-resistant adenosine analogues. Since the P1 receptor antagonist, caffeine, could completely prevent suppression of polydactylous outgrowth, the effects of an adenosine analogue were most likely mediated by activation of specific extracellular receptors.
Micromolar concentrations of adenosine, inosine and hypoxanthine, but not guanosine, block the second or third cleavage of mouse embryos developing in vitro [47]. Zygotes or early two-cell embryos, cultured in a purine-containing medium for 24 h, resume development following transfer to purine-free conditions. The precise mechanism of the purine-sensitive process is not known, but embryos conceived in vivo are sensitive until approximately 28–30 h after fertilization and are no longer sensitive by 34 h [48]. However, a later study by this group has shown that the purine-induced block can be reversed by compounds that elevate cAMP [49]. In mouse embryonic development, adenosine deaminase increased 74-fold between days 7 and 9; deoxyadenosine kinase increased 5.4-fold during the same period; adenosine kinase, deoxyguanosine kinase and purine nucleoside phosphorylase exhibited less than 2-fold changes in activity between days 7 and 13 [50]. The authors concluded that while phosphorylation of adenosine was the principal route of metabolism up to day 9, after which, there is a switch to deamination. The possible role of ecto-adenosine deaminase in the development of the nervous system and the neurological abnormalities that occur in adenosine deaminase-deficient patients is discussed in a review by Franco et al. [51].
Taken together, these results point to a role for purines in both physiological fertilization and normal development and also underline that alterations of the purinergic regulation of embryonal growth might be involved in the onset of morphological malformations. Depending upon the purine derivative, and probably upon the purinoceptor involved as well, ATP and adenosine can act as both positive and negative regulators of growth. This is also consistent with data obtained from in vitro cell lines which implicates purines in both cell proliferation and apoptosis. Further studies are needed to better characterize the receptor subtypes involved and also to identify more precisely the developmental events specifically controlled by purines.
Human embryos
A few studies have been made of receptors to purines and pyrimidines in human embryos. Human embryonic kidney cells (HEK293) endogenously express P2Y1 and P2Y2 receptors [52]. These embryonic cells have also been shown to express an endogenous A2B receptor [53]. ATP and adenosine-5′-O-3-thiotriphosphate were shown to stimulate DNA synthesis in human foetal astrocyte cultures [54]. In addition, ATP stimulated a mitogen-activated protein kinase (MAPK) termed extracellular signal-regulated protein (ERK), a key component of signal transduction pathways involved in cellular proliferation and differentiation. The activation of MAPK was mediated, at least in part, by P2 receptors since suramin produced 50 % block.
There has been a study of plasma ATP levels in the foetus at the time of obstetrical delivery, samples being collected immediately after clamping of the cord [55], and the results showed that the plasma ATP was significantly higher in arterial compared to venous or maternal venous blood. It was suggested, therefore, that the ATP in arterial blood was of foetal origin and that the levels decrease in response to stress during vaginal delivery and correlate with the oxygen supplied from the placenta.
In a study of human fibroblasts, differential sensitivity to adenosine was demonstrated in donors of different ages [56]. Foetal fibroblasts were the most sensitive to adenosine, which produced inhibition of growth and RNA synthesis; in contrast, fibroblasts taken from 4-year-old donors showed growth stimulation to adenosine. Activation of A2 receptors by adenosine stimulates l-arginine transport and NO synthase in human foetal endothelial cells [57]. Adenosine selectively inhibits tumour necrosis factor-α production in the human newborn [58]. Expression of purinergic P2X4, P2X7 and P2Y2 receptors and their activation led to increase in [Ca2+]i, which is involved in the changing demand for calcium with increased foetal growth over gestation in the first trimester and term human placenta [59].
Overall, our survey of purinergic signalling during early development in a range of species provides substantial evidence favouring its involvement in a variety of developmental processes. The current state of the literature therefore suggests that, in future, more precise molecular genetic manipulations of specific components of purinergic signalling to determine their roles in the regulation of development would be well worthwhile.
Development in different systems
Cardiovascular system
Heart
Studies of the development of pharmacological sensitivity to adenosine analogues in embryonic chick heart [60, 61] show that pharmacological sensitivity to A1 receptor agonists begins at embryonic day 7 and then increases continuously to day 12 when the atria became fully responsive. Ligand binding shows that A1 receptors are present at days 5 and 6, but are not responsive to adenosine, and the author concluded that the development of sensitivity to A1 receptor-mediated negative chronotropic responses was not paralleled by developmental changes in adenosine inhibition of adenylyl cyclase or by the development of sympathetic and parasympathetic innervation. Chronic exposure of the embryonic chick heart (15–17 days old) to R-N6-2-phenylisopropyladenosine (R-PIA) produces down-regulation of A1 receptors and desensitization of the negative inotropic response to adenosine [62]. A study of ventricular cells cultured from chick embryos 14 days in ovo showed that a functional A2A receptor is expressed and mediates augmentation of myocyte contractility [63]. The A2A receptor coexists with an A2B receptor, although it has 50-fold higher affinity, and the authors suggest that high affinity A2A receptors play an important modulatory role in the presence of low levels of adenosine, while the low affinity A2B receptor becomes functionally important when the adenosine level is high.
In foetal sheep, centrally administered adenosine influences cardiac function [64]. The ontogeny of A1 receptors was studied in rats with binding assays (using [3H]1,3-dipropyl-8-cyclopentylxanthine (DPCPX)), an A1 antagonist, and by in situ hybridization of messenger RNA (mRNA) [65]. In a later study of mouse embryo cardiac function [66], adenosine, via A1 receptors, was shown to potently regulate heart rate via multiple effector systems at very early stages of prenatal development (9–12 days postconception). At gestational days 8–11, mRNA expression for A1 receptors was detected in the atrium (one of the earliest G protein-coupled receptor genes to be expressed in the heart), but not in other foetal structures, while at gestational day 14, A1 mRNA was present in the CNS (thalamus, ventral horn of spinal cord) as well as the atrium; by gestational age 17, patterns of A1 receptor expression in the brain were similar to those observed in adults [67, 68]. Determination of A1 receptor density in developing rat heart using [3H]DPCPX showed that functional A1 receptors are present in greater numbers in the immature perinatal heart than in the adult heart [69].
Intravenous infusion of adenosine analogues into foetal lambs produced dose-dependent bradycardia and hypotension [70–72]. In contrast, in the newborn, 5′-N-ethylcarboxamidoadenosine (NECA) produced dose-dependent tachycardia, while R-PIA and cyclohexyladenosine produced dose-dependent bradycardia. Although adenosine causes cardiovascular changes in pregnant ewes, the effects are well tolerated and do not significantly affect the cardiorespiratory status of the foetus [73].
P2 receptors are expressed in human foetal heart [74]. P2X1, P2X3 and P2X4 receptor subtypes as well as P2Y2, P2Y4 and P2Y6 receptors were present. A subunit of the P2X receptor family was isolated from cardiomyocytes and brain from 14-day-old chick embryos that had 75 % identity with the rat and human P2X4 receptor [33].
The postnatal development of A1 receptors in the heart has been studied extensively (see [7]). In preinnervated immature rat myocytes, A1 receptors are present in greater numbers compared to the adult and they are functionally coupled at their effector sites [69].
Adenosine formation and release by neonatal rat ventricular myocytes in culture have been described [75, 76]. Adenosine and hypoxia effects on the atrioventricular node of neonatal rabbit hearts have been reported [77]. The adenosine agonist NECA increases cardiac output in developing Xenopus tadpoles through a combination of increased filling and accelerated growth of heart and vessels [78]. Adenosine was claimed not to be as effective as a vasodilator of internal carotid arteries in the newborn pig as it is in the adult. In foetal sheep, centrally administered adenosine influences cardiac function. Expression of A1 and A2A receptor genes in rat peripheral arterial chemoreceptors is differential during postnatal development [79, 80].
Expression of P2Y receptor subtypes in myocytes changes from neonate rat heart to the adult [81]. Expression of P2Y1 receptors is higher in comparison to P2Y2, P2Y4 and P2Y6 receptors in the neonatal myocyte, while P2Y4 receptors were not detected in adult myocytes. In neonatal heart fibroblasts, P2Y1 and P2Y6 receptors were expressed at higher levels than P2Y2 and P2Y4 receptors. RT-PCR studies of the foetal human heart showed that P2X1, P2X3 and P2X4 subtypes were expressed together with P2Y2, P2Y4 and P2Y6 receptors [74]. ATP and α,β-methylene ATP (α,β-meATP) intravenous injection increased heart rate via P2X receptors in rats aged 21, 56 and 100 days, with the most potent effect in 21-day-old animals [82]. It was claimed that P2Y receptor-mediated changes were more prominent in the later developmental stages [83]. ATP increased expression of the immediate-early genes c-fos and jun B in cultured neonatal cardiac myocytes by a different pathway from that produced by NA [84]. UTP caused hypertrophic growth in neonatal rat cardiomyocytes, while prolonged exposure to ATP had hypertrophic growth inhibitory effects [85]. P2Y4 receptors were shown to be involved in myocardial contractile activity in rats during postnatal development [86].
Blood vessels
Adenosine was claimed not to be as effective as a vasodilator of internal carotid arteries in the newborn as it was in the adult pig [87]. It was suggested that neonatal brain adenosine may play a role in regulating blood flow during hypoxia [88]. Adenosine appears to play an important role in the regulation of coronary blood flow in the newborn lamb [89]. Adenosine-induced vasodilatation of the guinea pig coronary vascular bed is greater in immature guinea pig heart (5 days) compared to mature heart (1–2 months) [90]. Responses of isolated mesenteric arteries of the beagle to both β-adrenoceptors and P1 receptors are less in old compared to young animals [91].
In the mesenteric artery of the rat, the sympathetic and sensory nerve fibre plexuses develop over the first three postnatal weeks, but functionally mature nerve-mediated contractile responses cannot be elicited before 14 days postnatal [92], correlating with the appearance of adult-like excitatory junction potentials (EJPs) [93]. Prior to this period, intracellular recordings from animals aged 4 to 9 days old showed slow depolarizing potentials which were mediated by α-adrenoceptors. From day 9 onwards, EJPs, which were resistant to α-adrenergic antagonists, were recorded [93] and are likely to be mediated by ATP (see [94]). Following denervation studies in the rat mesenteric vascular bed, electrical responses similar to those seen during the early stages of development were recorded [95], suggesting that a similar sequence of events occurs during regeneration as takes place during development. P2X receptor subtype mRNA expression in rat mesenteric artery showed high expressions for P2X1 and P2X4 at postnatal day 7, which remained during development until day 360 [96, 97]. A reduction in spontaneous and α1-adrenoceptor-induced release of ATP from endothelial cells of the rat tail artery occurs with advancing age [98]. The endothelium-dependent relaxant response of the aorta to ATP, mediated by NO, was greatest in 4-week-old rats but declined progressively at 45 and 105 weeks [99].
Oxygen-induced pulmonary vasodilatation is mediated by ATP in newborn lambs; it is attenuated by combined administration of P1 and P2Y receptor antagonists [100]. However, it appears to differ from the mechanism of oxygen-induced pulmonary vasodilatation in the late gestational foetal lamb [101–103]. Infusion of ATP caused a significant decrease in pulmonary vascular resistance in normoxic and hypoxic newborn lambs [104]. Adenosine is also a pulmonary vasodilator in newborn lambs [71].
Skeletal muscle
ATP actions on patched membranes of myoblasts and myotubes cultured from 12-day-old chicken embryos were first demonstrated by Kolb and Wakelam [105]. Using biochemical methods, ATP-induced cation influx was later demonstrated in myotubes prepared from 11-day-old chick embryos and shown to be additive to cholinergic agonist action [106]. Later papers from this group claimed that the myotube P2 receptor triggers phosphoinositide turnover [31, 107] and alters Ca2+ influx through dihydropyridine-sensitive channels [108]. ATP has a potent depolarizing action on myotubes derived from pectoral muscle cultured from 11-day chick embryos [109], and its physiological and pharmacological properties have been described in a series of papers [110–114]. The myotube P2 receptor is not activated by ADP, AMP, adenosine or the non-hydrolyzable ATP analogues α,β-meATP or β,γ-methylene ATP [109]. A single class of ATP-activated ion channel conducts both cations and anions in the myotube [111], and the P2 receptors involved showed marked desensitization [112]. The sensitivity of extracellular ATP has been tested at various stages of development of different muscles [115]. At embryonic day 6, ATP (50–100 μM) elicited vigorous contractions in all the muscles tested, but by embryonic day 17, none of the muscles contracted in response to ATP. The distribution of 5′-nucleotidase during the development of chick striated muscle showed that there is a more restricted distribution in the adult compared to the embryo [116]. A mammalian P2X1 receptor orthologue was identified in embryonic chicken skeletal muscle, perhaps forming heteromultimers with P2X4 and P2X5 receptor subunits [117]. Both P2X5 and P2X6 receptors were present in developing chick skeletal muscles [34, 118].
Purinoceptors were present in mouse C2C12 myotubes [119–123]. P1 receptors activating cAMP were identified as well as P2Y2 receptors, sensitive to ATP and UTP. Occupation of the receptor by ATP or UTP led to formation of IP3 and release of Ca2+ from internal stores as well as from the extracellular space. The responses to ATP of myotubes prepared from E18 mouse embryos from normal and mutant mdg/mdg mice with muscular dysgenesis were studied by Tassin et al. [124]. Using Fura-2 as a probe, they showed that many of the mdg/mdg myotube preparations showed little or no increase in cytoplasmic Ca2+ levels. The presence of functional P2X receptors in freshly isolated skeletal muscle cells from prenatal mice has been reported [125]. A punctate staining pattern for P2X7-like receptors was present at postnatal day 1 in mouse motor nerve terminals [126]. It was concluded that P2X7 receptors are expressed by both myelinating Schwann cells and motor neuron terminals, so an autoregulatory role for ATP released by nerve terminals during synaptic transmission was suggested.
P2 receptor expression and responses to ATP change during development of skeletal muscle [34, 115, 127, 128]. P2X5, P2X6 and P2X2 receptors were expressed sequentially with P2X5 and P2X6 receptors associated with the development of the myotube, while P2X2 and P2Y1 receptors appeared to be involved in the formation of the skeletal neuromuscular junction [128–131]. The possibility that extracellular ATP, coreleased with ACh, may serve as a trophic factor during the development of the Xenopus neuromuscular junction has been considered [20, 25]. P2Y13 receptors regulate phosphate metabolism and fibroblast growth factor-23 secretion during skeletal bone development [132].
Urinary bladder
Expression of P2X1 receptor transcripts was much lower in foetal human bladder than in adult bladder; P2X4 and P2X7 receptors were also expressed in the foetus [133]. The P2 receptor expression shifted from the dome to the body of the bladder with increasing gestation. Obstruction of the foetal male sheep bladder resulted in enlarged, hypocontractile and compliant bladder [134]. However, there was no conclusive evidence for changes in purinergic (or in cholinergic or nitrergic) neurotransmission.
Responses of the rabbit urinary bladder to both parasympathetic cotransmitters ACh and ATP were recognized in newborn animals, but adrenoceptors were poorly expressed until a later stage (see [135]). Newborn bladders generated much greater tension in response to ATP than adult tissue and then declined, while the response to cholinergic agonists did not decline. Rat urinary bladder responses to adenosine (inhibitory) and ATP (excitatory) mediated by P1 and P2X receptors, respectively, occurred at P2, the earliest day studied. In the neonate, adenosine was more potent than in the adult, while ATP potency initially increased with age but then declined; it was highest between P10 and P25. The main pathway for nerve activation of the urinary bladder of newborn mice was cholinergic, with only a small contribution of the purinergic component; this was in contrast to adult bladder, which was equally dependant on cholinergic and purinergic components [136]. These differences were due to changes in ATP release, rather than to changes in receptor function.
In neonates and adults, the rate and pattern of breakdown of ATP and adenosine by ectoenzymes in the rat urinary bladder were identical. This suggested that the marked differences in potency to ATP and adenosine during development were likely to be due to changes in receptor number and/or agonist affinity or efficacy. Small clusters of P2X receptors (about 0.4 μm in diameter) were present on smooth muscle cells at day P1 in rat urinary bladder, although few varicose nerve fibres were present at that time [137]. At P4, many varicose fibres were present and some small clusters of P2X receptors were seen in association with varicosities. Many of the P2X receptor clusters were found adjacent to varicosities of parasympathetic nerve fibres by P21. Increased purinoceptor-mediated contractions were elicited in newborn rat detrusor smooth muscle, which reached adult levels 1 month after birth [138].
Contractile responses of the rat bladder to ATP, released as a cotransmitter from parasympathetic nerves, increased with age [139].
Nervous system
Central nervous system
The emphasis in earlier studies of purines in the CNS was largely about adenosine [7, 140, 141], but since purinergic neurotransmission involving ATP as a transmitter was clearly demonstrated in the medial habenula of the rat brain [142], many more papers have been appearing concerning P2 receptors in the brain and spinal cord (see [8, 143, 144]).
Adenosine receptors appeared early and reached higher adult levels in the brains (most notably in the cerebellum) of mice pups chronically exposed in utero to caffeine [145]. A study of the distribution of adenosine-binding sites in the cat visual cortex showed changes in the laminar binding pattern during postnatal development [146]. Adenosine receptors were detected in the rat forebrain in young neonates, preceding N-protein coupling [147]. In a study of the developing guinea pig brain, A1 receptors were present from E19 and it was claimed that in cerebellum, but not in forebrain, postnatal coupling of adenosine A1 receptors to associated G proteins is much more extensive than in the prenatal period [148].The developmental properties of adenosine A2A receptors differ from those of A1 receptors during postnatal development of rat striatum. A2A receptor binding sites were low at birth (about 3 % of adult levels) and then increased mostly between birth and 5 days and then again from 15 days to adulthood [149]. In contrast, expression of A1 receptor mRNA in the brain was first described at gestation day 14. It was restricted to portions of neuroepithelium caudate putamen, piriform cortex, hypoglossal nucleus and ventral horn of spinal cord. By gestational age 17, A1 receptor expression pattern in the brain was similar to those observed in adults. The development of adenosine uptake sites in the guinea pig brain has been described. A1 receptors are widely distributed at birth (about 10 % of adult levels) and then increase gradually until adulthood, with a peak during the second week of postnatal life [150–152]. The ratio of adenosine (A2A) receptors to dopamine D2 receptors in the rat striatum increases with age, involving both presynaptic and postsynaptic mechanisms [153]. A decrease in striatal A2 receptor mRNA expression has been demonstrated with in situ hybridization histochemistry in rat striatum between 3 and 24 months, but it was suggested that this may be related to neuronal loss over the same period [154]. Postnatal changes in expression of A2A receptors have been described in various brain regions [155]. It was suggested that postnatal changes in adenosine receptors may explain age-dependent differences in stimulatory caffeine effects and protection against seizures throughout development. Further, since A2A receptors show a codistribution with D2 receptors throughout development, they also speculate that caffeine may partly exert its actions via dopamine receptors. Wide distribution of P2Y1 receptors was reported in the 1-day-old chick brain [156]. Age-dependent changes in presynaptic neuromodulation via A1 receptors have been described in hippocampus of mouse [157] and rat [158, 159]. There is a down-regulation and reduced responsiveness to presynaptic A1 receptors modulating ACh release during postnatal development and ageing. A1 receptor down-regulation occurred in foetal brain after caffeine or theophylline treatment of pregnant rats [160]. Down-regulation and reduced responsiveness to presynaptic A1 receptors modulating ACh release in the hippocampus during postnatal development and ageing were reported [161].
Caffeine decreased the incidence of neonatal respiratory disturbances, perhaps reflecting the early dominance of the adenosinergic system in the brainstem [162]. The developmental properties of adenosine A2A receptors differed from those of A1 receptors during postnatal development of rat striatum. A2A receptor binding sites were low at birth (about 3 % of adult levels) and increased mostly between birth and 5 days and then again from 15 days to adulthood. The ratio of adenosine A2A receptors to dopamine D2 receptors in the rat striatum increased with age, at both presynaptic and postsynaptic sites. A1 and A2A receptors played a major role during the development of the rat uncrossed retinotectal pathways [163].
Rhythmic movements were regulated by purinergic transmitters in frog embryos [164]. ATP was released during swimming that activated P2Y receptors to cause an increase in the excitability of the spinal motor circuits by inhibiting voltage-gated K+ currents via a P2Y1 receptor [164, 165]. Adenosine, resulting from the breakdown of ATP, acted on P1 receptors to reduce the voltage-gated Ca2+ currents lowering excitability of the motor circuits to oppose the actions of ATP [166]. It was suggested that a gradually changing balance between ATP and adenosine underlies the rundown of the motor pattern for swimming in Xenopus. In an earlier study, Dale [167] presented evidence to suggest that delayed production of adenosine underlies temporal modulation of swimming in the frog embryo and is likely to result from feed-forward inhibition of the 5′-ectonucleotidase in the spinal cord. A homologue of apyrase was identified in Xenopus during early development [168]. Subsequent comprehensive studies have shown that the NTPDase, NPPase gene families and CD73 are expressed at all stages of development in Xenopus [26, 169, 170].
ATP [171] and adenosine [172] modulate the activity of inspiratory neurons in the brain stem of neonatal rats (see [7]). The activity of neurons in the rostral ventrolateral medulla and the respiratory motor output were depressed by adenosine, with a more pronounced decrease in respiratory activity in younger animals. During the first 2 weeks of postnatal development, ATP excitation of glutamate inspiratory drive to mouse hypoglossal neurons remained constant. A secondary inhibitory response occurred, mediated by adenosine acting via A1 receptors after enzymatic breakdown of extracellular ATP [173]. Adenosine, acting on A1 receptors after breakdown of ATP, evoked a secondary inhibitory response. It has been claimed that adenosine plays a central role in modulating ventilation in the newborn piglet and is involved in the diphasic ventilatory responses to hypoxia. R-PIA, an adenosine receptor agonist, caused a decrease in ventilation which was blocked by theophylline indicating mediation by P1 receptors [174]. R-PIA caused a considerably more pronounced effect in 1- to 3-day-old animals than in 8-day-old animals and was shown to bind with higher affinity in the brains from newborn animals compared to older animals. The authors suggested that this might explain the potent therapeutic effect of the adenosine antagonist, theophylline, on recurrent apnea in preterm infants. In studies of anesthetized newborn piglets, it was concluded that adenosine contributes to ventilatory depression caused by hypoxia [175, 176]. In another investigation of the role of adenosine in the hypoxic ventilatory response of the newborn piglet, the authors concluded that adenosine plays a central role in modulating ventilation in the newborn piglet and is involved in the biphasic ventilatory responses to hypoxia [177]. It has been claimed that the inspiratory output of XII motor neurons that control the airways in mammals is potentiated by P2Y1 receptors in neonatal rat in vitro [178].
Responses of sympathetic preganglionic neurons in a neonatal rat brain stem–spinal cord preparation were evoked by ATP and adenosine. In rat locus coeruleus neurons, P2 receptors first appear to be functional soon after birth, increasing thereafter to reach maturity in animals older than 18 days [179]. α,β-MeATP was strongly active on glycinergic presynaptic nerve terminals projecting to rat substantia gelatinosa neurons at P28–30, perhaps contributing to the fine control of the pain signal in spinal cord dorsal horn neurons [180]. Excitatory synapses, mediated by both glutamate and ATP in rat superficial dorsal horn, were functional from the first postnatal days [181]. P2X3 receptors on motoneurons of the nucleus ambiguus were down-regulated during the first two postnatal weeks, indicating their involvement in the control of oesophageal motor activity in early development [182].
Extracellular ATP facilitated the release of dopamine via P2 receptor activation in parts of the mesolimbic system, including organotypic slice cocultures of the ventral tegmentum, substantia nigra and prefrontal cortex [183]. These authors showed a time-, region- and cell type-dependent in vitro and in vivo expression pattern of different P2 receptor types in the dopaminergic systems, suggesting an important role of purinergic signalling in development and growth. Both ATP and adenosine have been shown to modulate the activity of inspiratory neurons in the brain stem of neonatal rats (see [7]). Adenosine depressed both the activity of neurons in the rostral division of the ventrolateral medulla and the respiratory motor output, with a more pronounced decrease in respiratory activity in younger animals. ATP excitation of glutamate inspiratory drive to mouse hypoglossal neurons remained constant during the first 2 weeks of postnatal development. A secondary inhibitory response was due to adenosine acting on A1 receptors after breakdown of ATP. ATP and adenosine mediate responses of sympathetic preganglionic neurons in a neonatal rat brain stem–spinal cord preparation. Activation of P2Y1 receptors in the glomerulus stimulated neuronal network activity in the developing olfactory bulb [184].
Intense immunolabelling of P2X3 receptors in the postnatal (P7 and P14), but not adult, rat brain was reported [185]. Staining was restricted to the hindbrain, in particular, the mesencephalic trigeminal nucleus, the superior and inferior olive, the intermediate reticular zone, the spinal trigeminal tract and the prepositus hypoglossal nucleus at E16. P2X7 receptor mRNA was detected in the E19 rat by in situ hybridization in brain ependymal, but not neurons [186]. Human foetal astrocytes expressed low levels of P2X7 receptor mRNA and protein in primary cultures [187]. P2X7 receptors mediated caspase-8/9/3-dependent apoptosis in developing rat primary cortical neurons [188].
Green fluorescent protein-tagged P2X2 receptor studies of embryonic hippocampal neurons led to the claim that ATP application can lead to changes in dendritic morphology and receptor distribution [189]. P2X2 receptors were identified on Purkinje neurons in the neonatal cerebellum, mRNAs for P2X1-4 and P2X6 subunits were identified in the cerebellum during the first postnatal week, and coexpression of two units in Purkinje cells demonstrated with patch clamping [190].
Purinergic signalling in precursor cells, neuroglial progenitors and differentiating neurons during neurogenesis of embryonic rat neocortex was reported [191]. Neuroglial progenitors from the ventricular and subventricular zones showed increase in [Ca2+]i in response to ATP. A detailed study of the expression pattern for P2X3 receptors in embryonic neurogenesis was published [192]. P2X3 receptors first appeared in the hindbrain neural tube and sensory ganglia at E11–11.5. At E14.5, P2X3 receptors appeared in the optic tract, nucleus tractus solitarius and mesencephalic trigeminal nucleus. However, P2X3 immunoreactivity was down-regulated in early postnatal brain stem.
P2X receptor expression changed during postnatal development of the rat cerebellum [193]. All P2X receptor subtypes were expressed (except P2X3) on Purkinje cells and deep cerebellar nuclei at P3. At P7, there was upregulation of P2X5 and P2X6 receptors, and microglial cells expressed P2X1 and P2X7 receptor immunoreactivity. Dendritic trees of Purkinje cells were intensely labelled for P2X1-7 receptors (except for P2X3) at P14. P2X4 receptors were expressed on microglia and P2X5 receptors on granular cells. At P21 and P66, the P2X receptors were down-regulated on Purkinje cells and deep cerebellar nuclei, but P2X5 receptors on granular cells were upregulated. Endogenous release of ATP started to enhance synaptic activity in rat Purkinje neurons by the end of the second postnatal week [194].
The sequential expression of P2X receptor subtypes during embryonic rat brain development revealed that P2X3 receptors appeared first at E11, P2X2 and P2X7 receptors at E14, while P2X4, P2X5 and P2X6 receptors did not appear until birth and P2X1 receptors even later [195]. ATP inhibited motor axon outgrowth during early embryonic neurogenesis, probably via the P2X3 receptor, and it was suggested that P2X7 receptors were involved in programmed cell death during embryogenesis. At E9.5, P2X3 receptors were shown to be present in the hindbrain, midbrain, diencephalon and forebrain neuroectoderm of mouse brain and, at E10.5, in the marginal layer of diencephalon, midbrain and hindbrain [196]. Spontaneous and evoked postsynaptic currents in embryonic chick hypothalamus appeared to arise from the concurrent activation of both γ-aminobutyric acid (GABA) and P2X receptors [197].
ATP acting via P2X and P2Y receptors contributed to modulate network-driven giant depolarizing potentials in the rat hippocampus during early stages of postnatal development [198]. ATP, via both P2X and P2Y receptors, appeared to shape hippocampal connectivity during postnatal development [199]. Activation of P2Y1 receptors increased the frequency of GABAA-mediated spontaneous postsynaptic current in CA3 principal neurons in early postnatal (P1–P6) rat hippocampus [200]. In vitro studies of sensorimotor cortical neurons from 14-day-old and 30-day-old rats have shown that Ca2+ release could be evoked by ATP indicating the presence of P2Y receptors [201]. Almost all P14 neurons appeared to possess such receptors, whereas only about one third of neurons from P30 rats responded to ATP, suggesting that substantial changes in signalling mechanisms occur in neocortical neurons in the third to fourth week of postnatal development.
Changes in the distribution of the ectoenzymes involved in the breakdown of ATP and adenosine in the brain during foetal and neonatal development have been reported [7, 202]. Ecto-5′-nucleotidase was redistributed during development of the cat visual cortex. Ecto-5′-nucleotidase levels were low at 30 and 35 days of gestation of foetal guinea pigs but increased rapidly during the 40- to 60-day period. In contrast, adenosine deaminase was present at 30 days of gestation and was maintained at the same level until 60 days. Adenosine deaminase-staining neurons were observed in the olfactory cortex of rat embryos as early as E15. Ca2+–ATPase in the rat spinal cord during embryonic development was intensely active in the roof and floor plates, but not in the basal and lateral plates at E12, suggesting a role for Ca2+–ATPase in early differentiation of neuroepithelial cells. ATP induced a rise in [Ca2+]i in embryonic spinal cord astrocytes. P1 and P2 receptor involvement in the proliferation of human foetal cortical astrocytes was reported. Postnatal development of ATPase, ADPase and ATP diphosphohydrolase activity in the cerebral cortex has also been studied [203, 204]. The activities increased steadily from birth, reaching maximum values at 21 days of age. A marked increase in activity of ecto-5′-nucleotidase was also seen in rat olfactory bulb during neonatal development [205]. During early postnatal life, the enzyme was found within synapses in the brain, but a glial pattern of expression dominated in the adult, with the exception of the olfactory bulb where both glial and synaptic staining was present in mature adults. Ecto-NTPDase2 was expressed transiently at E18 in astrocytic cells in the outer molecular layer of the dentate gyrus and in cerebellar white matter [206]. Postnatal development of ecto-nucleotidase activity in the cerebral cortex increased steadily from birth, reaching maximum values at 21 days of age. Increased activity of ecto-5′-nucleotidase was also seen in rat olfactory bulb during neonatal development. Ecto-5′-nucleotidase was present first in immature Purkinje cells at birth and increased throughout postnatal development. In contrast, alkaline phosphatase activity did not appear on the granular neurons in the developing cerebellum until day 7 postnatal. Ecto-ATPase, ecto-ADPase and ecto-5′-nucleotidase activities were shown to change in relation to age in synaptosomes of rat spinal cord [207]. In synaptic plasma membranes isolated from rat cerebral cortex, NTPDase1 activity increased from birth to day 30, after which it declined [208]. In general, ATP and ADP hydrolysis decreased in older animals.
It is known that neonatal hypothyroidism leads to abnormal development of the CNS. Hypothyroidism changes adenine nucleotide hydrolysis by ecto-5′-nucleotidase activities in synaptosomes from hippocampus and cerebral cortex in rats in different phases of postnatal development [209]. Neonatal hypothyroidism enhances the metabolism of adenine nucleotides in astrocyte cultures from rat brain [210]. Interactions between thyroid hormones and adenosine receptor-mediated responses during brain development are discussed in a review by Ahmed et al. [211]. Leukaemia inhibitory factor is required for normal development of hippocampal astrocytes, a process that is regulated by spontaneous release of ATP from neurons [212].
Peripheral nervous system
The neural ectoderm folds to form the neural tube during early embryological development. Cells in the neural crest migrate into the mesoderm and then differentiate and mature to become glial cells and neurons. Some form primary afferent neurons of the dorsal root ganglia (DRG), while others form postganglionic neurons of the sympathetic and parasympathetic ganglia. Other cells form the enteric nervous system and adrenomedullary chromaffin cells. The sensory neurons of nodose, petrosal and trigeminal ganglia are derived partly or entirely from the neural placodes. Adenosine inhibited neurite outgrowth of chick sympathetic neurons taken from 11-day chick embryos and killed by apoptosis about 80 % of sympathetic nerves supported by growth factor over the next 2 days in culture [213]. Specific A1 or A2 agonists were not neurotoxic. The toxic effects of adenosine were not antagonized by aminophylline but were prevented by an adenosine transporter or adenosine deaminase inhibitor, suggesting an intracellular site of action for the toxic effects of adenosine. It was concluded that adenosine and its breakdown enzymes play an important role in the regulation of growth and development of sympathetic neurons. In follow-up experiments, the authors suggested that adenosine induced apoptosis by inhibiting mRNA and protein synthesis [214].
Responses to ATP were described in ciliary neurons acutely dissociated from embryonic chick ciliary ganglia taken at day 14 [215]. The relative potency of agonists in producing transient inward currents with patch recording is ATP > 2-methylthioATP > ADP; neither adenosine, AMP nor α,β-meATP is effective, but suramin is an antagonist. The authors suggest that the P2 receptor subtype involved might be P2Y, but in view of subsequent knowledge about the functional properties of cloned subtypes of the P2 receptor family, it seems more likely to belong to the P2X receptor family. ATP-evoked currents in cultured DRG neurons from rat embryos modulate spontaneous glutamate release via P2X2 and P2X3 receptors [216]. Early expression of P2X3 receptors in prenatal human DRG neurons has been reported [217].
P2X receptors on cultured embryonic DRG neurons mediate the release of substance P [218]. P2X3 receptors were expressed on most neurons in embryonic mouse trigeminal ganglia and DRG. This was in contrast to adult ganglia, which do not express P2X3 receptors on large-diameter neurons [192, 196, 219]. Most sensory neurons in mouse DRG, trigeminal and nodose ganglia expressed P2X3 receptors at E14. However, after birth, there was a decline to about 50 % of neurons showing positive staining [219]. Isolectin B4 (IB4)-positive neurons in sensory ganglia appeared at birth. They increased to about 50 % by P14, when they mostly expressed P2X3 receptors. Peptide release from neurons in embryonic DRG is augmented by ATP via P2Y receptors [220].
Rat superior cervical ganglia (SCG) sympathetic neurons were responsive to ATP and α,β-meATP at E18, birth and during the early postnatal period, via P2X2/3 heteromultimer receptors; these responses were reduced in mature rats [221]. This change in P2X receptor expression occurs when synaptogenesis is taking place in the SCG, suggesting a role for purinergic signalling in this process. During early postnatal life, IB4-binding DRG neurons (that express P2X3 receptors) switch from nerve growth factor to glial cell-derived neurotrophic factor dependence [222]. P2 receptors modulated NA release from chick sympathetic neurons cultured from 12-day-old embryos [223, 224]. This suggested that both a facilitatory P2 receptor and an inhibitory receptor were involved. Cultured mice and rat paravertebral sympathetic neurons from the first few days after birth appeared to express different purinoceptors [225]. Neurons from both species responded via P2Y receptors activated by UTP to cause depolarization and NA release.
Ninety-three percent of the tyrosine hydroxylase-negative (parasympathetic) neurons in the rat pelvic ganglion expressed P2X2 receptors in both young and old rats [226]. P2X7 receptors in satellite glial cells were shown to mediate functional expression of P2X3 receptors in immature DRG neurons [227].
Retina
There are many studies of purinergic signalling in the retina of adult mammals, but relatively few reports about embryonic retina. Spontaneous waves of excitation in the developing rabbit retina were detected at E22, and involvement of purinergic receptor activation in these waves was investigated [228]. Adenosine has been implicated in chick retinal development. A1 receptors were suggested to have different functions in the embryonic retina [229].
ATP-induced rise in intracellular Ca2+ was mediated by P2Y2 or P2Y4 receptors in embryonic chick neural retina, and there was a decline of ATP-induced rise in intracellular Ca2+ just prior to synaptogenesis (see [7]). Large Ca2+ signals in chick retinal cells were triggered at E3 by ATP and UTP, acting via P2Y receptors [230]. Simultaneously, ATP, ADP and UTP stimulated proliferation of retinal cells via P2Y1 and P2Y4 receptors and multiple intracellular signalling cascades [231]. Autocrine or paracrine release of ATP has been claimed to be involved in the regulation of DNA synthesis in the neural retina at early embryonic stages [232]. Suramin and pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid inhibited the ATP-induced increase in [3H]thymidine incorporation in retinal cultures from E3. The change in Ca2+ signalling mediated by P2u (i.e. P2Y2 or P2Y4) receptors during development may be involved in the differentiation of neuroepithelial cells or progenitor cells into neurons. P2 receptors appear to mediate the regulation of retinal progenitor cell proliferation at early embryonic stages, perhaps in collaboration with growth factors [232]. Proliferation of both bipolar and Müller cells in early developing chick retina at E6–8 is probably mediated by P2Y1 receptors [233]. ATP induced proliferation of cultured retinal cells [233]. ATP evoked increase in [Ca2+]i in radial Müller cells in the postnatal rabbit retina [234].
ATP signalling associated with Ca2+ waves is present in both retinal pigment epithelium [235, 236] and in the developing neural retina [230, 237]. Activation of P2 receptors in the chick embryo retina evoked an increase in [Ca2+]i and controls the rate of division of progenitor cells [231, 236]. Pearson et al. [236] showed that the ATP release in the retinal pigment epithelium came about through the spontaneous gating of connexin43 hemichannels. Blockade of these hemichannels with a connexin mimetic peptide not only greatly reduced ATP release but also greatly reduced the proliferation of progenitor cells in the adjacent neural retina.
Guanine nucleotides block agonist-driven Ca2+ influx in chick embryo retinal explants [238]. RT-PCR studies revealed changes in the expression of P2X3 and P2X5 receptor mRNA at different postnatal stages, P23 to P210, of pigmented retinas [239]. Also, P2X7 mRNA showed positive identification in the retina of postnatal rats (P23–P210) [240]. Data has been presented that suggests that purinergic signalling via P2Y1 and P2Y4 receptors on Müller cells may contribute to differentiation in the postnatal rat retina [241].
Inner ear
P2X2 receptor mRNA expression was present in the precursors of the cells bordering the cochlear endolymphatic compartment at E12 and in spinal and vestibular ganglia during embryonic development of the rat inner ear [242]. P2X2 receptor mRNA was not expressed in inner and outer hair cells until after P10 through P12, concomitant with the onset of hearing. These data are in accordance with the roles of the P2X2 receptor both in the process of labyrinthine development and in the regulation of auditory and vestibular sensory transduction. P2X1 receptors provide the signal transduction pathway for development of afferent and efferent innervation of the sensory hair cells and cochlea morphogenesis [243]. Using confocal immunofluorescence, P2X3 receptor expression has been characterized in the mouse cochlea from E16 [244]. Spiral ganglion neuron cell bodies and peripheral neurites projecting to the inner and outer hair cells were labelled for P2X3 receptor protein from E18 to P6. They were diminished around P6 and were no longer present at the onset of hearing (around P11). This suggests a role for P2X3 receptor-mediated purinergic signalling in cochlea synaptic reorganization and establishment of neurotransmission that occurs just prior to the onset of hearing function [245]. Brain-derived neurotrophic factor-mediated development of spiral ganglion neurons was shown to be inhibited by P2X3 and P2X2/3 receptor-mediated signalling in neonatal rat cochlea [246].
P2X7 receptor immunolabelling was observed in the primary auditory neurons of the spiral ganglion from E18 to adult and in the fibres innervating the sensory inner and outer hair cells from birth to adult [247]. The authors speculated that P2X7 receptors may be involved in signal transduction and modulation as well as in regulating cell death during development and in pathological conditions. It was reported that P2Y4 and P2X2 receptors were coexpressed and contributed to the regulation of short circuit currents in rat cochlear neonatal outer sulcus cells [248]. They showed further that P2Y4 receptor expression rapidly declined postnatally and reached near adult levels on postnatal day 14. In a later paper, the expression of P2Y1, P2Y2, P2Y4, P2Y6 and P2Y12 receptors was identified in the rat cochlea during development [249]. Beginning in the late embryonic period, P2Y receptors were found in the cells lining the cochlea partition associated with the electrochemical environment that provides the driving force for sound transduction. Early postnatal P2Y2 and P2Y4 protein expression in the greater epithelial ridge supported the view that initiation and regulation of spontaneous activity in the hair cells prior to hearing onset are mediated by purinergic signalling.
Evidence was presented to suggest that P2X3 receptors mediate vestibular synaptic augmentation in the inner ear of chicken embryos [250]. They showed further that P2Y receptor (P2Y1, P2Y2 and P2Y6) mRNA was present during the early stage (E15) rather than the later stage (E21) of the development of the vestibular system. It was suggested that temporal changes in P2Y receptor expression during development might be involved in the establishment of the endolymphatic ion composition needed for vestibular and auditory transduction. A transient structure, Köllicker’s organ, consisting of epithelial cells that lie adjacent to the hair cells, appears during the development of the cochlea. Spontaneous electrical potentials in these cells were mediated by spontaneous release of ATP via connexin hemichannels to act via P2X and P2Y receptors [251, 252]. These cells exhibit spontaneous Ca2+ waves activated by ATP [253] and may allow the establishment of the tonotopic organization of the cochlea and spiral ganglion cell innervation, although this is disputed [254]. Changes in expression of the ectonucleotidases, NTPDase5 and NTPDase6, and uridine diphosphate-activated P2Y6 receptors were observed during rat cochlea development [255].
In the developed cochlea, hemichannel-mediated ATP release may be important in regulating the electromotility of the outer hair cells [256]. This electromotility is an active amplifier of mechanical movement of the basilar membrane and determines the sensitivity of the cochlea to sound stimulation. A review by Mammano [254] summarizes the current understanding of the roles of ATP in cochlear development and function and the possible roles of connexin hemichannels. Interestingly, mutations or deletions of connexins, in particular, Cx26 [257] and Cx30, cause sensorineural hearing loss, suggesting that ATP release via these hemichannels may have fundamentally important roles in the development and maintenance of a functional cochlea [254, 258].
Gastrointestinal tract
Non-adrenergic, non-cholinergic (NANC) nerve-mediated responses were observed before birth in mouse and rabbit small intestine [259]. Quinacrine fluorescence, which is a marker for high levels of vesicle-bound ATP, was observed before birth in enteric neurons of rabbit ileum and stomach, 3 days before catecholamine fluorescence was detected in enteric nerves [260]. In the guinea pig taenia coli, the purinergic NANC inhibitory system appeared at 8 weeks of gestation, while cholinergic excitatory transmission was not present until birth [261].
P2X3 receptor immunoreactivity in the myenteric plexus of the embryonic rat stomach was present on both extrinsic and intrinsic nerves [262]. The extrinsic sensory nerve fibres first expressed P2X3 receptors as early as E12, which extended rapidly on to the whole stomach by E14. However, the intrinsic enteric neuron cell bodies slowing positive P2X receptor immunoreactivity did not appear until birth (P1), peaked by P14 and then decreased in maturing animals. P2X3 and P2X5 receptors are present in large numbers of myenteric neurons in newborn guinea pigs compared to adults, whereas P2X5 receptor mRNA is found more frequently in adults [263]. Intraganglionic laminar nerve endings and intramuscular arrays were seen first postnatally at P1 and P7, respectively [262].
Several studies of postnatal developmental changes in purinergic signalling in the small intestine have been reported. ATP and ADP produced contractile responses in rat duodenal segments at P1 [264]. This response increased with age to day 7, followed by a decrease, and was non-existent by day 21. The relaxant responses to ATP and ADP, however, appeared at day 21 and continued to increase up to day 70 and probably later. Responses to adenosine or AMP were not elicited until day 14.
A2B receptors in the longitudinal muscle of the rat duodenum were present at day 15, while A1 receptors did not appear until after day 20; both receptor subtypes mediated relaxation. A2B receptors in the muscularis mucosa mediated contraction from day 10. P2Y receptors mediated relaxation of the longitudinal muscle at day 25, while in the muscularis mucosa, P2Y receptors mediated contraction. After day 20, contractions were mediated by both P2X and P2Y receptors. The longitudinal muscle of the rat colon relaxed via A2B and P2Y receptors, while the muscularis mucosa contracted via A1 and probably P2Y2 or P2Y4 receptors [264].
In the mouse gastrointestinal tract, combined pharmacological and immunohistochemical studies showed that contraction was mediated by P2Y1 receptors from P3 to P8 [265]. However, relaxation of longitudinal muscle throughout the gastrointestinal tract from day 12 onwards was via P2Y1 receptors located both on smooth muscle and on a subpopulation of myenteric neurons. P1, P2Y2 and/or P2Y4 receptors and were also present on intestinal smooth muscles. The relaxant response during postnatal development was mediated by P2Y1 receptors that gradually appeared along the length of the gastrointestinal tract, in the stomach from day 3, from day 6 in the duodenum, day 8 in the ileum and day 12 in the colon. The shift from contraction to relaxation occurred 1 week before weaning, suggesting that this may contribute to the changes that take place in the gut when the food compositions change from maternal milk to solid food.
Adenosine deaminase was localized predominantly on the keratinized squamous epithelium that lines the mucosal layer of the oesophagus, forestomach and the simple columnar epithelium of proximal small intestine of the mouse gastrointestinal tract. The levels of adenosine deaminase in these tissues were low at birth but reached very high levels within the first 2 weeks of postnatal life [266]. There was strong immunostaining of P2X4 receptors on the epithelial cells of proximal and distal newborn rat gut, P2X6 receptor immunostaining in capillary vessels in proximal newborn gut and differences in the amounts of P2X4 and P2X6 receptor expression in newborn compared to adult proximal and distal intestine [267].
Lung
The development of purinergic signalling in the lung described in early papers has been reviewed [7]. Increase in [Ca2+]i signals in rat foetal lung epithelial cells was evoked by ATP and UTP. In epithelia explanted from foetal rat lung, receptors to adenosine, ATP and UTP were located on apical membranes throughout the lung, while basolateral receptors for these agonists were functional later in gestation in distal lung and in trachea. P2X7 mRNA was detected in E19 rat embryos by in situ hybridization in bronchial epithelium. ATP increased surfactant secretion as early as day 1 in newborn rats, but the effect of UTP did not appear until 4 days after birth. Adenosine analogues interrupted foetal breathing movements, but apnea was not produced in newborn lambs. Adenosine, ATP and ADP evoked oxygen-induced pulmonary vasodilatation in foetal lambs, probably via both A2A and P2Y receptors [268]. P2X3 receptors are expressed on vagal sensory nerve terminals in rat lung where they make contact with neuroepithelial bodies (NEBs) a few days before birth [269]. This is consistent with the function of NEBs as oxygen sensors before the carotid body O2 sensory system is fully developed at about 2 weeks after birth. Adenosine played a central role in modulating ventilation in the newborn piglet and was involved in the biphasic ventilatory responses to hypoxia.
Adenosine has a regulatory role in lung surfactant secretion in the newborn rabbit [270]. Ventilation-induced increase in secretion of lung surfactant was inhibited by P1 receptor antagonists. ATP stimulation of surfactant in type II cells in adult rats is mediated by both a P2Y2 receptor coupled to phospholipase C and a receptor coupled to adenylate cyclase; UTP also activated the P2Y2 receptor but did not stimulate cAMP formation. ATP increased cAMP formation in newborns but did not promote phospholipase D activation until day 4. Thus, the adenylate cyclase-coupled ATP signalling mechanism is functional early in development, but the P2Y2 receptor pathway is not.
Vas deferens
Rats are not sexually active until about 10 weeks, so that changes in purinergic signalling in the developing vas deferens might occur later than in the gut. ATP and NA are cotransmitters in the sympathetic nerves supplying the vas deferens (see [271]). EJPs produced by ATP in response to sympathetic nerve stimulation of the vas deferens were not observed in mice less than 18 days postnatal [272]. At 3 weeks postnatal (the earliest time studied), the responses of the rat vas deferens to field stimulation with single or trains of pulses lacked the adrenergic component, but the non-adrenergic component was present [273]. Responses to ATP first appeared at day 15 and then increased with age [264].
Adenosine, acting via prejunctional A1 receptors, inhibited sympathetic neurotransmission in the rat vas deferens when nerve-mediated contractions were first seen at day 15, but its potency decreased with age. Inhibitory postjunctional A2-like receptors and prejunctional A1 receptors were present from days 10 to 15, respectively. Postjunctional excitatory A1 receptors did not appear until after day 20. Stimulation of the hypogastric nerve produced monophasic contractions of the vas deferens in 2-week-old guinea pigs. Nerve-evoked contractions of the circular muscle layer of the guinea pig vas deferens decreased with increasing age, apparently due to purinergic postjunctional rather than prejunctional mechanisms. Postnatal androgen exposure of hypogonadal mice that are deprived of androgens after birth resulted in inhibition of purinergic EJPs in the vas deferens in adult mice, and P2X1 receptors could not be demonstrated with immunofluorescence [274].
Other organs
Chondrocytes, isolated from the cephalic region of day 19 chick embryo sterna, release nucleotides into the extracellular milieu, although they are rapidly degraded; it is claimed that they are involved in both chondrocyte maturation and matrix mineralization [275]. Extracellular ATP modulates [Ca2+]i in retinoic acid-treated chondrocytes isolated the cephalic portion of day 14 chick embryonic sterna [276]. They speculate that immature chondrocytes may generate adenine nucleotides that then act in a paracrinal manner on chondrocytes that are at a later stage of maturation. Rapid deamination of adenosine in cultures of foetal mouse calvarial bones was shown and taken to account, at least in part, for the failure to observe effects of adenosine in bone metabolism in culture [277]. Cells of osteogenic and chondrogenic lineage derived from foetal metatarsal bones were exposed to ATP4-; cells of haemopoietic origin were permeabilized and killed, while cells of non-haemopoietic origin (e.g. osteoblasts, chondrocytes) were insensitive to ATP4- and survived [278]. This system allows the study of the properties and functions of osteogenic or chondrogenic cells without interference by the presence of cells of haemopoietic origin. ATP pyrophosphohydrolase has been purified and partially characterized from foetal bovine epiphyseal cartilage of patients with chondrocalcinosis [279]. The most prominent location of P2X7 receptor mRNA in E19 rat embryos is the bone marrow, but bone marrow cells from mouse femur also showed strong immunoreactivity for P2X receptors [186].
In the liver, the hepatic ATP/ADP ratio showed significant decrease during the first day of postnatal life in starved newborn rats, correlating directly to the gluconeogenic flux [280].
Enzymes for the breakdown of extracellular purines were described in the developing rat testis, but full metabolic involvement in terms of Mg2+ ATPase and 5′-nucleotidase was not achieved until 45 days postnatal [281].
In rat kidney, intrarenal adenosine is a physiological regulator of glomerular filtration rate and renal blood flow and appears to play a key role in the hypoxaemia-induced renal insufficiency in newborn rats [282]. Adenosine is involved in the regulation of the action of insulin on rat fat cell metabolism during postnatal development and ageing [283].
ATP and ADP and, to a lesser extent, AMP and adenosine increased insulin secretion from the isolated perfused newborn dog pancreas [284].
P2Y2 receptors are strongly expressed on NA-containing adrenal chromaffin cells, but very little on adrenaline-containing cells in mature rats [285]. In contrast, in newborn rats, P2Y2 receptors are expressed equally on both NA and adrenaline-containing cells. A loss of P2Y2 receptor expression on both NA- and adrenaline-containing cells occurs in the adrenal gland of old (22 month) rats compared to newborn animals. It was suggested that ATP, acting via P2Y2 receptors, may influence the phenotypic expression of chromaffin cells into NA- or adrenaline-containing cells during early development.
Differential coupling of P2Y1 receptors to Gα14 and Gαq/11 proteins during the development of rat salivary (submandibular) gland has been described, with two bands (42 and 52 kDa) present in 1-week-old rats, but only the 42 kDa band was detected in the submandibular gland cells of 4- to 6-week-old rats [286, 287].
In the skin, Merkel cells appear in the epidermis of planum nasale of rat foetuses from the 16th day of intrauterine development and nerve fibres form close associations with them by day 20 [288]. Since it is known that Merkel cells contain high levels of peptide-bound ATP and are in close association with sensory fibres expressing P2X3 receptors (see [289]), this is of interest.
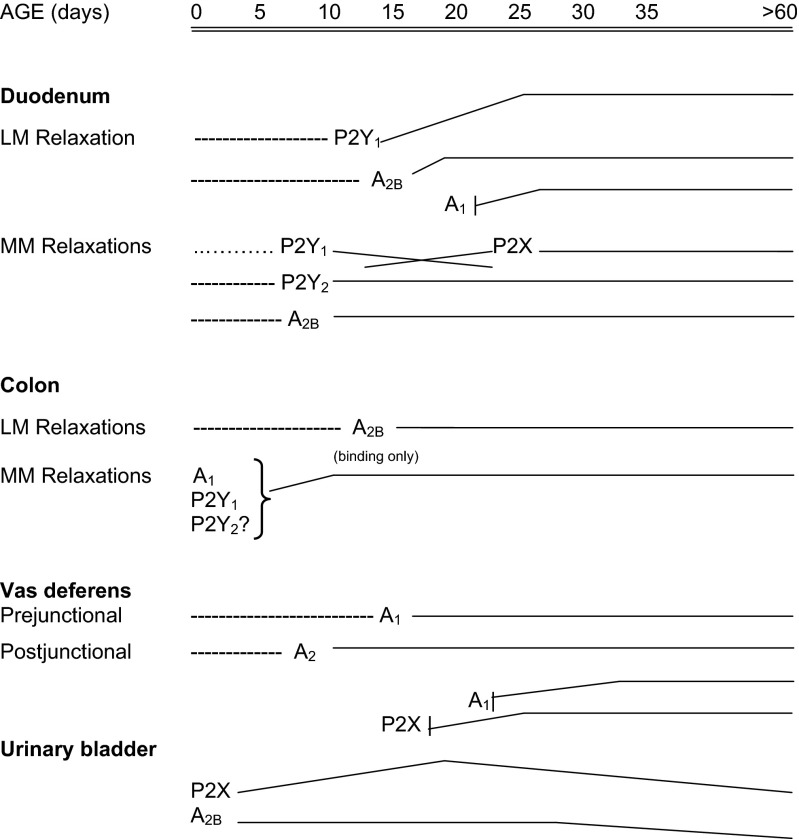
Figure 7 summarizes the expression of purinoceptors during postnatal development of duodenum, colon, vas deferens and urinary bladder.
Fig. 7.
Diagram summarizing the development of functional responses mediated by purine receptors in the rat duodenum, colon, urinary bladder and vas deferens. The dashed lines represent ages at which it was not possible to study functional responses, and the solid lines show when responses were observed, with the slope of the line indicating whether a response, in general, increased or decreased with age (reproduced with permission from [264])
Stem cells
Pluripotent stem cells (embryonic carcinoma and embryonic stem cells) have features of early embryonic cells [290, 291]. They are capable of differentiating into the three primary germ layers of the embryo. Embryonic stem cells are present in foetal as well as in adult tissue. The importance of purinergic signalling in stem cell biology, including regulation of proliferation, differentiation and cell death, has been revealed [9, 292–294].
The P19 murine embryonal carcinoma cell line has been used for studying mechanisms of early neurogenesis [295]. Cultured P19 cells treated with retinoic acid form tri-dimensional cell aggregates, which resemble the blastula stage of embryonic development [296–298]. Differentiation into neuronal cells occurs by day 8, while glial cells predominate in the culture on day 14. Purinergic receptor activity was shown to be essential for proliferation and the progress of neurogenesis [299]. Differentiating P19 cells at the neural progenitor stage exposed to blockers of P2Y and P2X receptor-mediated calcium signal transduction led to inhibition of proliferation.
P2Y2 receptors potentiate proliferation of embryonic stem cells [300]. Early in embryonic development, P2X receptors also appear. Neuronal progenitors, isolated from midbrain of E10.5 mice, possess all components of the purinergic signalling system. mRNA for adenosine, P2X and P2Y receptors and ectonucleotidases are expressed, and stimulation of purinoceptors elicited neuronal formation [301]. P2X3, P2X4, P2Y1 and P2Y4 receptor expression was high in embryonic P19 cells but then decreased following induction of differentiation [302]. P2X4/6 and P2X2/6 heteromultimer receptors may also be expressed on P19 cells. P2X6 receptor subtype expression increased during prenatal and postnatal mouse brain development [303]. P2Y1 receptors are the major subtype involved in regulating cell proliferation and differentiation, less so by the P2Y2 receptor subtype and with only a minor role for P2X4 receptors [299]. Chronic inhibition of P2Y1 and, possibly, P2X2 receptor activity induced differentiation of P19 cells, resulting in loss of NMDA receptor activity in neuronal-differentiated cells, whereas blockade of P2Y2 and, possibly, P2X2 receptors resulted in inhibition of cholinergic receptor responses.
Isolated neural stem cells from foetal brain or neurogenic areas of adult brain proliferate as free-floating spherical expansions, called neurospheres, expressing the neural progenitor marker nestin [304]. Removal of growth factors led cells of the outer layers of the neurosphere to migrate and differentiate into three major neural phenotypes, neurons, astrocytes and oligodendrocytes [305, 306]. Neurospheres from foetal rat brain expressed P2X2–7, as well as P2Y1, P2Y2, P2Y4 and P2Y6 receptors [307]. Proliferation of human neural stem cells cultured from telecephalon tissues from a 15-week gestational age embryo was induced by ATP, and activation of P2 receptors released [Ca2+]i from thapsigargin-sensitive intracellular stores [308], indicating mediation via P2Y receptors.
Evidence was presented to identify a novel role for P2X7 receptors in control of mouse embryonic stem cells, showing that they can mediate a pro-survival or pro-death signal depending on the mode of activation [309]. P2X7 receptor expression and activity are upregulated in mouse embryonic stem cells and modulate proliferation and suppress differentiation [310]. Expression and regulation of the ATP-binding cassette transporter, ABCG2, in human embryonic stem cells have been reported [311].
P2Y receptors are involved in regulation of embryonic and postnatal neurogenesis. P2Y and muscarinic ACh receptors appear to be the first functionally active receptors, present after gastrulation [29]. P2Y receptors, especially the P2Y1 subtype, were widely expressed in the embryonic rat brain as early as day 11 [312]. A marked decrease in mRNA to P2Y1 receptors and upregulation of mRNA for P2Y2 receptors were observed on freshly isolated astrocytes during development of rat hippocampus [313]. P2Y receptor proteins were strongly expressed transiently in structures that were likely to be involved in functions specific to embryonic development. P2Y4 receptors disappeared from the brain stem and ventral spinal cord postnatally. Calcium waves propagate through radial glial cells in the proliferative central ventricular zone, requiring both P2Y1 receptors and connexin hemichannels [314].
Subventricular zone-derived neurospheres express two ecto-nucleotidases, NTPDase2 and alkaline phosphatase, which are markers for subsets of progenitor cells in the developing and adult mouse brain, supporting the notion that purinergic signalling contributes to embryonic, postnatal and adult neurogenesis [315]. There is widespread mRNA expression for ectonucleotide pyrophosphatase/phosphodiesterase 1-3 (E-NPP1-3) in different rat brain regions during development and ageing [316]. There was an increase in both ATP and ADP hydrolysis by ecto-NTPDases 1–3 in synaptic plasma membranes isolated from rats in the first and second months of life and then decreased in adult life [317].
Hypothalamic tanycytes comprise a third neurogenic cell population in the adult brain capable of generating new neurons in the hypothalamus [318–320]. They can also release ATP, particularly in response to glucose, which can act at P2Y1 receptors to trigger Ca2+ waves [321, 322]. Like other neural stem cells in the brain, tanycytes also express NTPDase2 [201]. The role of ATP in tanycyte proliferation and differentiation has not, so far, been examined. Given that tanycytes have several similarities to radial glial cells, which are regulated by ATP signalling [314], an investigation of the actions of ATP would seem warranted [322].
Ageing
Some studies of changes in purinergic signalling in ageing have been published. Two populations of binding sites for adenosine, corresponding to A1 and A2 receptors, were described in the brain of both young and old rats, but both the binding sites were greater in old rats [323]. Adenosinergic inhibition of synaptic potentials was significantly enhanced in hippocampal slices from aged rats contributing to age-related decline in synaptic efficacy [324]. Activation by endogenous adenosine via A1 receptors mediated synaptic plasticity (both long-term potentiation and long-term depression), and this was maintained in aged rats [325]. Caffeine has been used to counteract age-related cognitive decline. For example, acute treatment with caffeine was claimed to reverse the impairment of olfactory discrimination and short-term social memory in ageing rats [326]. In layer 5 of cortex, synapses exhibit age-dependent changes in their integrative properties. For young rats (<P14), the probability of transmitter release is high and the synapses exhibit depression with repeated high-frequency stimulation [327, 328]. However, these synapses in older rats (>P30) exhibit a much lower release probability and change from being depressed during high-frequency stimulation to being facilitated. Kerr et al. [329] have shown that this developmental change in the integrative properties of these synapses can be explained by a developmental change in the extracellular concentration of adenosine acting via the A1 receptor. At young ages, adenosine levels are low, but increase with age. The presynaptic action of adenosine on the A1 receptor causes inhibition of transmitter release and shifts the synapses to a low probability release mode, which is then capable of facilitation during high-frequency trains of stimuli.
Extracellular levels of adenosine in the striatum were not affected by age, although there were differences in the mechanisms of adenosine release and metabolism. For example, erythro-2-(hydroxy-3-nonyl) adenine, an adenosine deaminase inhibitor, increased adenosine levels in the striatum of young, but not old, rats [330]. Maintenance of a constant extracellular adenosine level in the ageing brain may be a homeostatic mechanism. In old striatum, levels of A2 receptor mRNA and A2 receptor binding sites were reduced by 32 and 20 %, respectively [154]. A1 and A2A receptors both played a role in controlling motor activity in rats [153]. 2-[4-(2-p-Carboxyethyl)phenylamino]-5′-N-ethyl-carboxamidoadenosine (CGS21680), an A2A receptor agonist, significantly increased spontaneous outflow of glutamate and aspartate in young, but not in old rats [331]. In contrast, there was increased spontaneous outflow of GABA in old, but not young rats exposed to CGS21680 [332]. A1 and A2A receptor binding was modified in aged striatum, hippocampus and cortex of the rat [333].
Modulation of cortical ACh release via P1 receptors was decreased in ageing rats [334], and a decrease in A1 receptor gene expression was described in mouse cerebral cortex of aged rats [335]. Reduced efficiency of A1 receptors to modulate synaptic transmission in the hippocampus of aged rats has been reported [336]. Down-regulation of adenosinergic function may underlie the enhanced excitability seen in hippocampal neurons of the CA1 subregion of aged animals [159]. Cross talk between A1 and A2A receptors in the hippocampus and cortex has been reported and A2A receptors shown to control A1 receptor function via PKC, but not PKA, in young adult, but not aged rats [337]. Adenosinergic activities seem to be unaffected by ageing in the cerebellum and substantia nigra. Neuron–glial synaptic currents were mediated by P2X as well as glutamate receptors. In the ageing mouse brain, the density of P2X receptors is reduced as is their role in glial synaptic current generation [338].
Ischaemic tolerance is impaired in aged heart. RT-PCR analysis showed age-related decline of A3 receptors and induction of A2B receptors in ageing mouse myocardium, changes that were mimicked by ischaemia in young, but not old, hearts [339]. They showed further that in aged hearts, ischaemia selectively reduced A1 receptor levels. An increase in density of A1 receptors in rabbit heart in old age in contrast to the diminished β-adrenergic responsiveness has been reported in the senescent heart [340]. There are conflicting reports about changes in purinergic signalling in the vascular system during old age. However, this may be explained by the variation in expression of P2 receptor subtypes in different vessels in different species and in different pathophysiological conditions (see [341]). Age-related changes in the relative importance of NA and ATP as mediators of the contractile responses of the rat tail artery to sympathetic nerve stimulation have been reported. The ATP component was dominant in young rats but declined with age [342]. ATP appeared to be the sole mediator of the sympathetic contractile response in the tail artery in some young rats. However, there was a shift from purinergic to adrenergic signalling in old age, which was reflected by the responses to ATP and α,β-meATP, as well as the expression of P2X receptors [343].
Expression of P2Y1 and P2Y2 receptors and contractile responses to 2-methythio ADP and UTP were also decreased with age. It was speculated that the dramatic reduction in expression of P2 receptors in the rat tail artery during development and ageing was related to the role of the tail artery in temperature regulation. Constriction of rat mesenteric arteries by ATP decreased with age [344]. ATP is a cotransmitter with NA in sympathetic nerves supplying blood vessels in young human skin; NA, however, becomes the dominant neurotransmitter in old age [345]. Cerebral microvessels in old rats showed reduced ATPase activity, perhaps contributing to the blood–brain barrier function changes found in old rats [346]. Ageing is associated with impaired ability to modulate sympathetic vasoconstrictor activity and reduced exercise hyperaemia. ATP-induced vasodilation was low in the sedentary elderly, and interstitial ATP content during exercise and P2Y2 receptor expression were higher in the active elderly compared to the sedentary elderly [347].
In the corpus cavernosum of rats, ageing was accompanied by a decrease in P2X1, but an increase in P2X7 receptors, and there was increased expression of P2Y4 receptors in the bladder [348]. It was suggested that these changes in purinergic signalling probably contribute to the development of erectile dysfunction and higher detrusor activation in ageing rats.
Contractile responses of the aged rat bladder to ATP were significantly greater than those of the young bladder, compared to little change in the responses to ACh or KCl [349]. The purinergic component of nerve-mediated contractions of the human bladder was also increased with age, due largely to increased release of ATP [350]. The response of the bladder to α,β-meATP increased with age [351], but P2X1 and P2X3 receptor mRNA did not change with age. In aged male mice, there was an increase in bladder voiding, augmented P2X3 receptor-mediated afferent nerve firing during bladder filling and an increase in urothelial activation by ATP [352]. The authors speculated that this may reflect the increase in overactive bladder in ageing humans. In old guinea pigs, the purinergic component of sympathetic cotransmission was dominant in seminal vesicles [353]. P2X4 receptor expression increased in pancreatic islets in old age, but P2Y1 receptor expression was lost [354].
An increase in density of A1 receptors in rabbit heart in old age has been claimed [340] in contrast to the diminished β-adrenergic responsiveness in the senescent heart [355]. In the immature (25 days old) rat heart, adenosine was found to have little role in the modulation of contractile responsiveness via activation of either A1 or A2 receptors, but enhanced antiadrenergic and stimulatory functions of adenosine via A1 and A2 receptor activation were present in the heart of mature (79 days old) rats [356]. Reduced adenosine A1 receptor and Gα protein coupling in rat ventricular myocardium during ageing has been reported [357]. Age-related decline in β-adrenergic and adenosine A1 receptor function in the rat heart is attenuated by dietary restriction [358].
Age-related changes in P2 receptor mRNA have been described in rat arteries [359]. P2X1 receptor mRNA was reduced in basilar artery in 19-month compared to 2-month-old rats, while P2Y1 and P2Y2 receptor mRNA increased. mRNA for P2X receptors was described in postnatal rat mesenteric arteries [360]. P2X1 and P2X4 receptors were most strongly expressed, P2X2 and P2X7 receptors less so, while P2X3 and P2X5 receptors were only weakly expressed and no expression of P2X6 receptors; no differences in expression were seen between 7 and 28 days postnatal.
Summary
Several interesting generalizations emerge from the analysis in this review.
Purinergic signalling is present from the earliest developmental stages (before classical adrenergic signalling) and usually declines with maturation; sometimes, this involves reduction in potency of purines and pyrimidines, and sometimes, particular purinoceptor subtypes are no longer expressed. In a few cases, purinergic signalling has been demonstrated to control major developmental processes.
There are examples where a similar sequence of events occurs during regeneration after trauma to adult tissues as occurs during development, e.g. skeletal muscle and mesenteric arteries.
During postnatal development, responses to nucleosides and nucleotides increase or decrease with age depending on the physiological demands of the particular system involved.
Both P1 and P2 receptors are present early in development of many systems, but there are a few examples where the earliest effects of ATP are mediated via adenylate cyclase, and only later in receptor activation does the IP3 second messenger system for P2Y receptor transduction come into operation (e.g. type III pneumocytes in lung). Fast P2X purinergic signalling may appear a little later in development compared to P1 and P2Y receptors, except perhaps in urinary bladder.
There are some compelling pioneering studies that implicate extracellular purines and pyrimidines in embryonic and stem cell functions, but the roles of purinergic signalling in development are still in its infancy. ATP is an ancient signalling molecule utilized early in evolution and has been retained as a very successful communicating molecule for the activities of most cells in the body (see [361–363]). This supports the view that it would have also been utilized in the complex mechanisms involved in embryonic development and regeneration, in keeping with the general principle that ‘ontogeny repeats phylogeny’.
Acknowledgments
The authors thank Dr. Gillian E. Knight for her excellent editorial assistance.
References
- 1.Burnstock G. Purine and pyrimidine receptors. Cell Mol Life Sci. 2007;64:1471–1483. doi: 10.1007/s00018-007-6497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yeung CH. Temporary inhibition of the initiation of motility of demembranated hamster sperm by high concentrations of ATP. Int J Androl. 1986;9:359–370. doi: 10.1111/j.1365-2605.1986.tb00898.x. [DOI] [PubMed] [Google Scholar]
- 3.Foresta C, Rossato M, Di Virgilio F. Extracellular ATP is a trigger for the acrosome reaction in human spermatozoa. J Biol Chem. 1992;267:1–5. [PubMed] [Google Scholar]
- 4.Kupitz Y, Atlas D. A putative ATP-activated Na+ channel involved in sperm-induced fertilization. Science. 1993;261:484–486. doi: 10.1126/science.8392753. [DOI] [PubMed] [Google Scholar]
- 5.Smith R, Koenig C, Pereda J. Adenosinetriphosphatase (Mg-ATPase) activity in the plasma membrane of preimplantation mouse embryo as revealed by electron microscopy. Anat Embryol (Berl) 1983;168:455–466. doi: 10.1007/BF00304281. [DOI] [PubMed] [Google Scholar]
- 6.Ishikawa T, Seguchi H. Localization of Mg++-dependent adenosine triphosphatase and alkaline phosphatase activities in the postimplantation mouse embryos in day 5 and 6. Anat Embryol (Berl) 1985;173:7–11. doi: 10.1007/BF00707299. [DOI] [PubMed] [Google Scholar]
- 7.Burnstock G. Purinergic signalling in development. In: Abbracchio MP, Williams M, editors. Handbook of experimental pharmacology, volume 151/I. Purinergic and pyrimidinergic signalling I—molecular, nervous and urinogenitary system function. Berlin: Springer-Verlag; 2001. pp. 89–127. [Google Scholar]
- 8.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- 9.Zimmermann H. Nucleotide signaling in nervous system development. Pflugers Arch. 2006;452:573–588. doi: 10.1007/s00424-006-0067-4. [DOI] [PubMed] [Google Scholar]
- 10.Zimmermann H. Purinergic signaling in neural development. Semin Cell Dev Biol. 2011;22:194–204. doi: 10.1016/j.semcdb.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 11.Dale N. Dynamic ATP signalling and neural development. J Physiol. 2008;586:2429–2436. doi: 10.1113/jphysiol.2008.152207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Massé K, Dale N. Purines as potential morphogens during embryonic development. Purinergic Signal. 2012;8:503–521. doi: 10.1007/s11302-012-9290-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Norton WHJ, Rohr KB, Burnstock G. Embryonic expression of a P2X3 receptor encoding gene in zebrafish. Mech Dev. 2000;99:149–152. doi: 10.1016/s0925-4773(00)00472-x. [DOI] [PubMed] [Google Scholar]
- 14.Kucenas S, Cox JA, Soto F, Lamora A, Voigt MM. Ectodermal P2X receptor function plays a pivotal role in craniofacial development of the zebrafish. Purinergic Signal. 2009;5:395–407. doi: 10.1007/s11302-009-9165-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bogdanov YD, Dale L, King BF, Whittock N, Burnstock G. Early expression of a novel nucleotide receptor in the neural plate of Xenopus embryos. J Biol Chem. 1997;272:12583–12590. doi: 10.1074/jbc.272.19.12583. [DOI] [PubMed] [Google Scholar]
- 16.Burnstock G. P2 Purinoceptors: historical perspective and classification. In: Chadwick DJ, Goode JA, editors. P2 purinoceptors: localization, function and transduction mechanisms. Chichester: Wiley; 1996. pp. 1–29. [DOI] [PubMed] [Google Scholar]
- 17.Gerhart J, Danilchik M, Doniach T, Roberts S, Rowning B, Stewart R. Cortical rotation of the Xenopus egg: consequences for the anteroposterior pattern of embryonic dorsal development. Development. 1989;107(Suppl):37–51. doi: 10.1242/dev.107.Supplement.37. [DOI] [PubMed] [Google Scholar]
- 18.Igusa Y. Adenosine 5′-triphosphate activates acetylcholine receptor channels in cultured Xenopus myotomal muscle cells. J Physiol. 1988;405:169–185. doi: 10.1113/jphysiol.1988.sp017327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akasu T, Hirai K, Koketsu K. Increase of acetylcholine-receptor sensitivity by adenosine triphosphate: a novel action of ATP on ACh-sensitivity. Br J Pharmacol. 1981;74:505–507. doi: 10.1111/j.1476-5381.1981.tb09997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fu WM. Regulatory role of ATP at developing neuromuscular junctions. Prog Neurobiol. 1995;47:31–44. doi: 10.1016/0301-0082(95)00019-r. [DOI] [PubMed] [Google Scholar]
- 21.Fu WM, Poo MM. ATP potentiates spontaneous transmitter release at developing neuromuscular synapses. Neuron. 1991;6:837–843. doi: 10.1016/0896-6273(91)90179-4. [DOI] [PubMed] [Google Scholar]
- 22.Fu WM. Potentiation by ATP of the postsynaptic acetylcholine response at developing neuromuscular synapses in Xenopus cell cultures. J Physiol. 1994;477(Pt 3):449–458. doi: 10.1113/jphysiol.1994.sp020206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu WM, Huang FL. Potentiation by endogenously released ATP of spontaneous transmitter secretion at developing neuromuscular synapses in Xenopus cell cultures. Br J Pharmacol. 1994;111:880–886. doi: 10.1111/j.1476-5381.1994.tb14820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu B, Fu WM. Regulation of postsynaptic responses by calcitonin gene related peptide and ATP at developing neuromuscular junctions. Can J Physiol Pharmacol. 1995;73:1050–1056. doi: 10.1139/y95-149. [DOI] [PubMed] [Google Scholar]
- 25.Fu WM, Chen YH, Lee KF, Liou JC. Regulation of quantal transmitter secretion by ATP and protein kinases at developing neuromuscular synapses. Eur J Neurosci. 1997;9:676–685. doi: 10.1111/j.1460-9568.1997.tb01416.x. [DOI] [PubMed] [Google Scholar]
- 26.Massé K, Bhamra S, Eason R, Dale N, Jones EA. Purine-mediated signalling triggers eye development. Nature. 2007;449:1058–1062. doi: 10.1038/nature06189. [DOI] [PubMed] [Google Scholar]
- 27.Gampe K, Haverkamp S, Robson SC, Gachet C, Hüser L, Acker-Palmer A, Zimmermann H. NTPDase2 and the P2Y1 receptor are not required for mammalian eye formation. Purinergic Signal. 2015;11:155–160. doi: 10.1007/s11302-014-9440-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hilfer SR, Palmatier BY, Fithian EM. Precocious evagination of the embryonic chick thyroid in ATP-containing medium. J Embryol Exp Morpholog. 1977;42:163–175. [Google Scholar]
- 29.Laasberg T. Ca2+-mobilizing receptors of gastrulating chick embryo. Comp Biochem Physiol C. 1990;97:9–12. doi: 10.1016/0742-8413(90)90164-5. [DOI] [PubMed] [Google Scholar]
- 30.Nakaoka Y, Yamashita M. Ca2+ responses to acetylcholine and adenosine triphosphate in the otocyst of chick embryo. J Neurobiol. 1995;28:23–34. doi: 10.1002/neu.480280104. [DOI] [PubMed] [Google Scholar]
- 31.Häggblad J, Heilbronn E. P2-purinoceptor-stimulated phosphoinositide turnover in chick myotubes. Calcium mobilization and the role of guanyl nucleotide-binding proteins. FEBS Lett. 1988;235:133–136. doi: 10.1016/0014-5793(88)81248-1. [DOI] [PubMed] [Google Scholar]
- 32.Lohmann F, Drews U, Donié F, Reiser G. Chick embryo muscarinic and purinergic receptors activate cytosolic Ca2+ via phosphatidylinositol metabolism. Exp Cell Res. 1991;197:326–329. doi: 10.1016/0014-4827(91)90441-v. [DOI] [PubMed] [Google Scholar]
- 33.Ruppelt A, Liang BT, Soto F. Cloning, functional characterization and developmental expression of a P2X receptor from chick embryo. Prog Brain Res. 1999;120:81–90. doi: 10.1016/s0079-6123(08)63547-5. [DOI] [PubMed] [Google Scholar]
- 34.Meyer MP, Clarke JDW, Patel K, Townsend-Nicholson A, Burnstock G. Selective expression of purinoceptor cP2Y1 suggests a role for nucleotide signalling in development of the chick embryo. Dev Dyn. 1999;214:152–158. doi: 10.1002/(SICI)1097-0177(199902)214:2<152::AID-AJA5>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 35.Adair TH, Montani JP, Strick DM, Guyton AC. Vascular development in chick embryos: a possible role for adenosine. Am J Physiol. 1989;256:H240–H246. doi: 10.1152/ajpheart.1989.256.1.H240. [DOI] [PubMed] [Google Scholar]
- 36.Fraser RA, Ellis EM, Stalker AL. Experimental angiogenesis in the chorio-allantoic membrane. Bibl Anat. 1979;18:25–27. [PubMed] [Google Scholar]
- 37.Teuscher E, Weidlich V. Adenosine nucleotides, adenosine and adenine as angiogenesis factors. Biomed Biochim Acta. 1985;44:493–495. [PubMed] [Google Scholar]
- 38.Dusseau JW, Hutchins PM, Malbasa DS. Stimulation of angiogenesis by adenosine on the chick chorioallantoic membrane. Circ Res. 1986;59:163–170. doi: 10.1161/01.res.59.2.163. [DOI] [PubMed] [Google Scholar]
- 39.Kubo Y. Properties of ionic currents induced by external ATP in a mouse mesodermal stem cell line. J Physiol. 1991;442:691–710. doi: 10.1113/jphysiol.1991.sp018815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kubo Y. Electrophysiological and immunohistochemical analysis of muscle differentiation in a mouse mesodermal stem cell line. J Physiol. 1991;442:711–741. doi: 10.1113/jphysiol.1991.sp018816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beaudoin AR. Effect of adenosine triphosphate and adenosine diphosphate on the teratogenic action of trypan blue in rats. Neonatology. 1976;28:133–139. [Google Scholar]
- 42.Gordon HW, Tkaczyk W, Peer LA, Bernhard WG. The effect of adenosine triphosphate and its decomposition products on cortisone induced teratology. J Embryol Exp Morpholog. 1963;11:475–482. [PubMed] [Google Scholar]
- 43.Smuts MS. Rapid nasal pit formation in mouse embryos stimulated by ATP-containing medium. J Exp Zool. 1981;216:409–414. doi: 10.1002/jez.1402160309. [DOI] [PubMed] [Google Scholar]
- 44.Nakano H, Shimada A, Imai K, Takahashi T, Hashizume K. ATP-evoked increase in intracellular calcium via the P2Y receptor in proliferating bovine trophoblast cells. Cell Tissue Res. 2003;313:227–236. doi: 10.1007/s00441-003-0754-9. [DOI] [PubMed] [Google Scholar]
- 45.Petrungaro S, Salustri A, Siracusa G. Adenosine potentiates the delaying effect of dbcAMP on meiosis resumption in denuded mouse oocytes. Cell Biol Int Rep. 1986;10:993. doi: 10.1016/0309-1651(86)90121-9. [DOI] [PubMed] [Google Scholar]
- 46.Knudsen TB, Elmer WA. Evidence for negative control of growth by adenosine in the mammalian embryo: induction of Hmx/+ mutant limb outgrowth by adenosine deaminase. Differentiation. 1987;33:270–279. doi: 10.1111/j.1432-0436.1987.tb01567.x. [DOI] [PubMed] [Google Scholar]
- 47.Nureddin A, Epsaro E, Kiessling AA. Purines inhibit the development of mouse embryos in vitro. J Reprod Fertil. 1990;90:455–464. doi: 10.1530/jrf.0.0900455. [DOI] [PubMed] [Google Scholar]
- 48.Loutradis D, John D, Kiessling AA. Hypoxanthine causes a 2-cell block in random-bred mouse embryos. Biol Reprod. 1987;37:311–316. doi: 10.1095/biolreprod37.2.311. [DOI] [PubMed] [Google Scholar]
- 49.Fissore R, O’Keefe S, Kiessling AA. Purine-induced block to mouse embryo cleavage is reversed by compounds that elevate cyclic adenosine monophosphate. Biol Reprod. 1992;47:1105–1112. doi: 10.1095/biolreprod47.6.1105. [DOI] [PubMed] [Google Scholar]
- 50.Jenuth JP, Mably ER, Snyder FF. Modelling of purine nucleoside metabolism during mouse embryonic development: relative routes of adenosine, deoxyadenosine, and deoxyguanosine metabolism. Biochem Cell Biol. 1996;74:219–225. doi: 10.1139/o96-022. [DOI] [PubMed] [Google Scholar]
- 51.Franco R, Casado V, Ciruela F, Saura C, Mallol J, Canela EI, Lluis C. Cell surface adenosine deaminase: much more than an ectoenzyme. Prog Neurobiol. 1997;52:283–294. doi: 10.1016/s0301-0082(97)00013-0. [DOI] [PubMed] [Google Scholar]
- 52.Schachter JB, Sromek SM, Nicholas RA, Harden TK. HEK293 human embryonic kidney cells endogenously express the P2Y1 and P2Y2 receptors. Neuropharmacology. 1997;36:1181–1187. doi: 10.1016/s0028-3908(97)00138-x. [DOI] [PubMed] [Google Scholar]
- 53.Cooper J, Hill SJ, Alexander SP. An endogenous A2B adenosine receptor coupled to cyclic AMP generation in human embryonic kidney (HEK 293) cells. Br J Pharmacol. 1997;122:546–550. doi: 10.1038/sj.bjp.0701401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Neary JT, McCarthy M, Kang Y, Zuniga S. Mitogenic signaling from P1 and P2 purinergic receptors to mitogen-activated protein kinase in human fetal astrocyte cultures. Neurosci Lett. 1998;242:159–162. doi: 10.1016/s0304-3940(98)00067-6. [DOI] [PubMed] [Google Scholar]
- 55.Fukuda S, Katoh S, Yamamoto K, Hashimoto M, Kitao M. Correlation between levels of plasma adenosine triphosphate and stress to the fetus at delivery. Biol Neonate. 1990;57:150–154. doi: 10.1159/000243185. [DOI] [PubMed] [Google Scholar]
- 56.Bynum JW. Differential adenosine sensitivity in fibroblasts from different age donors. Exp Gerontol. 1980;15:217–225. doi: 10.1016/0531-5565(80)90027-3. [DOI] [PubMed] [Google Scholar]
- 57.Sobrevia L, Yudilevich DL, Mann GE. Activation of A2-purinoceptors by adenosine stimulates L-arginine transport (system y+) and nitric oxide synthesis in human fetal endothelial cells. J Physiol. 1997;499:135–140. doi: 10.1113/jphysiol.1997.sp021916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Levy O, Coughlin M, Cronstein BN, Roy RM, Desai A, Wessels MR. The adenosine system selectively inhibits TLR-mediated TNF-α production in the human newborn. J Immunol. 2006;177:1956–1966. doi: 10.4049/jimmunol.177.3.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roberts VH, Waters LH, Powell T. Purinergic receptor expression and activation in first trimester and term human placenta. Placenta. 2007;28:339–347. doi: 10.1016/j.placenta.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 60.Blair TA, Parenti M, Murray TF. Development of pharmacological sensitivity to adenosine analogs in embryonic chick heart: role of A1 adenosine receptors and adenylyl cyclase inhibition. Mol Pharmacol. 1989;35:661–670. [PubMed] [Google Scholar]
- 61.Hatae J, Sperelakis N, Wahler GM. Development of the response to adenosine during organ culture of young embryonic chick hearts. J Dev Physiol. 1989;11:342–345. [PubMed] [Google Scholar]
- 62.Shryock J, Patel A, Belardinelli L, Linden J. Downregulation and desensitization of A1-adenosine receptors in embryonic chicken heart. Am J Physiol. 1989;256:H321–H327. doi: 10.1152/ajpheart.1989.256.2.H321. [DOI] [PubMed] [Google Scholar]
- 63.Liang BT, Haltiwanger B. Adenosine A2a and A2b receptors in cultured fetal chick heart cells. High- and low-affinity coupling to stimulation of myocyte contractility and cAMP accumulation. Circ Res. 1995;76:242–251. doi: 10.1161/01.res.76.2.242. [DOI] [PubMed] [Google Scholar]
- 64.Egerman RS, Bissonnette JM, Hohimer AR. The effects of centrally administered adenosine on fetal sheep heart rate accelerations. Am J Obstet Gynecol. 1993;169:866–869. doi: 10.1016/0002-9378(93)90017-d. [DOI] [PubMed] [Google Scholar]
- 65.Rivkees SA. The ontogeny of cardiac and neural A1 adenosine receptor expression in rats. Brain Res Dev Brain Res. 1995;89:202–213. doi: 10.1016/0165-3806(95)00120-3. [DOI] [PubMed] [Google Scholar]
- 66.Hofman PL, Hiatt K, Yoder MC, Rivkees SA. A1 adenosine receptors potently regulate heart rate in mammalian embryos. Am J Physiol. 1997;273:R1374–R1380. doi: 10.1152/ajpregu.1997.273.4.R1374. [DOI] [PubMed] [Google Scholar]
- 67.Weber RG, Jones CR, Lohse MJ, Palacios JM. Autoradiographic visualization of A1 adenosine receptors in rat brain with [3H]8-cyclopentyl-1,3-dipropylxanthine. J Neurochem. 1990;54:1344–1353. doi: 10.1111/j.1471-4159.1990.tb01968.x. [DOI] [PubMed] [Google Scholar]
- 68.Reppert SM, Weaver DR, Stehle JH, Rivkees SA. Molecular cloning and characterization of a rat A1-adenosine receptor that is widely expressed in brain and spinal cord. Mol Endocrinol. 1991;5:1037–1048. doi: 10.1210/mend-5-8-1037. [DOI] [PubMed] [Google Scholar]
- 69.Cothran DL, Lloyd TR, Taylor H, Linden J, Matherne GP. Ontogeny of rat myocardial A1 adenosine receptors. Biol Neonate. 1995;68:111–118. doi: 10.1159/000244226. [DOI] [PubMed] [Google Scholar]
- 70.Yoneyama Y, Power GG. Plasma adenosine and cardiovascular responses to dipyridamole in fetal sheep. J Dev Physiol. 1992;18:203–209. [PubMed] [Google Scholar]
- 71.Konduri GG, Woodard LL, Mukhopadhyay A, Deshmukh DR. Adenosine is a pulmonary vasodilator in newborn lambs. Am Rev Respir Dis. 1992;146:670–676. doi: 10.1164/ajrccm/146.3.670. [DOI] [PubMed] [Google Scholar]
- 72.Koos BJ, Mason BA, Ducsay CA. Cardiovascular responses to adenosine in fetal sheep: autonomic blockade. Am J Physiol. 1993;264:H526–H532. doi: 10.1152/ajpheart.1993.264.2.H526. [DOI] [PubMed] [Google Scholar]
- 73.Mason BA, Ogunyemi D, Punla O, Koos BJ. Maternal and fetal cardiorespiratory responses to adenosine in sheep. Am J Obstet Gynecol. 1993;168:1558–1561. doi: 10.1016/s0002-9378(11)90798-4. [DOI] [PubMed] [Google Scholar]
- 74.Bogdanov YD, Wildman SS, Clements MP, King BF, Burnstock G. Molecular cloning and characterization of rat P2Y4 nucleotide receptor. Special Report. Br J Pharmacol. 1998;124:428–430. doi: 10.1038/sj.bjp.0701880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Meghji P, Holmquist CA, Newby AC. Adenosine formation and release by neonatal-rat heart cells in culture. Biochem J. 1985;229:799–805. doi: 10.1042/bj2290799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Meghji P, Skladanowski AC, Newby AC, Slakey LL, Pearson JD. Effect of 5′-deoxy-5′-isobutylthioadenosine on formation and release of adenosine from neonatal and adult rat ventricular myocytes. Biochem J. 1993;291:833–839. doi: 10.1042/bj2910833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Young ML, Ramza BM, Tan RC, Joyner RW. Adenosine and hypoxia effects on atrioventricular node of adult and neonatal rabbit hearts. Am J Physiol. 1987;253:H1192–H1198. doi: 10.1152/ajpheart.1987.253.5.H1192. [DOI] [PubMed] [Google Scholar]
- 78.Tang YY, Rovainen CM. Cardiac output in Xenopus laevis tadpoles during development and in response to an adenosine agonist. Am J Physiol. 1996;270:R997–R1004. doi: 10.1152/ajpregu.1996.270.5.R997. [DOI] [PubMed] [Google Scholar]
- 79.Gauda EB, Northington FJ, Linden J, Rosin DL. Differential expression of A2a, A1-adenosine and D2-dopamine receptor genes in rat peripheral arterial chemoreceptors during postnatal development. Brain Res. 2000;872:1–10. doi: 10.1016/s0006-8993(00)02314-3. [DOI] [PubMed] [Google Scholar]
- 80.Gauda EB, Cooper RZ, Donnelly DF, Mason A, McLemore GL. The effect of development on the pattern of A1 and A2a-adenosine receptor gene and protein expression in rat peripheral arterial chemoreceptors. Adv Exp Med Biol. 2006;580:121–129. doi: 10.1007/0-387-31311-7_19. [DOI] [PubMed] [Google Scholar]
- 81.Webb TE, Boluyt MO, Barnard EA. Molecular biology of P2Y purinoceptors: expression in rat heart. J Auton Pharmacol. 1996;16:303–307. doi: 10.1111/j.1474-8673.1996.tb00040.x. [DOI] [PubMed] [Google Scholar]
- 82.Anikina TA, Sitdikov FG, Khamzina EY, Bilalova GA. Role of purinoceptors in cardiac function in rats during ontogeny. Bull Exp Biol Med. 2005;140:483–485. doi: 10.1007/s10517-006-0002-x. [DOI] [PubMed] [Google Scholar]
- 83.Anikina TA, Bilalova GA, Zverev AA, Sitdikov FG. Role of P2X and P2Y receptors in rat myocardial contractility during ontogeny. Bull Exp Biol Med. 2007;143:695–698. doi: 10.1007/s10517-007-0217-5. [DOI] [PubMed] [Google Scholar]
- 84.Zheng JS, Boluyt MO, O'Neill L, Crow MT, Lakatta EG. Extracellular ATP induces immediate-early gene expression but not cellular hypertrophy in neonatal cardiac myocytes. Circ Res. 1994;74:1034–1041. doi: 10.1161/01.res.74.6.1034. [DOI] [PubMed] [Google Scholar]
- 85.Pham TM, Morris JB, Arthur JF, Post GR, Brown JH, Woodcock EA. UTP but not ATP causes hypertrophic growth in neonatal rat cardiomyocytes. J Mol Cell Cardiol. 2003;35:287–292. doi: 10.1016/s0022-2828(03)00009-9. [DOI] [PubMed] [Google Scholar]
- 86.Anikina TA, Anisimova IN, Sitdikov FG. Involvement of P2Y receptors in myocardial contractile activity of rats during postnatal ontogeny. Bull Exp Biol Med. 2012;152:672–674. doi: 10.1007/s10517-012-1603-1. [DOI] [PubMed] [Google Scholar]
- 87.Laudignon N, Aranda JV, Varma DR. Effects of adenosine and its analogues on isolated internal carotid arteries from newborn and adult pigs. Biol Neonate. 1990;58:91–97. doi: 10.1159/000243238. [DOI] [PubMed] [Google Scholar]
- 88.Park TS, Van Wylen DG, Rubio R, Berne RM. Increased brain interstitial fluid adenosine concentration during hypoxia in newborn piglet. J Cereb Blood Flow Metab. 1987;7:178–183. doi: 10.1038/jcbfm.1987.41. [DOI] [PubMed] [Google Scholar]
- 89.Mainwaring RD, Mentzer RM, Jr, Ely SW, Rubio R, Berne RM. The role of adenosine in the regulation of coronary blood flow in newborn lambs. Surgery. 1985;98:540–546. [PubMed] [Google Scholar]
- 90.Matherne GP, Headrick JP, Berne RM. Ontogeny of adenosine response in guinea pig heart and aorta. Am J Physiol. 1990;259:H1637–H1642. doi: 10.1152/ajpheart.1990.259.6.H1637. [DOI] [PubMed] [Google Scholar]
- 91.Shimizu I, Toda N. Alterations with age of the response to vasodilator agents in isolated mesenteric arteries of the beagle. Br J Pharmacol. 1986;89:769–778. doi: 10.1111/j.1476-5381.1986.tb11181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Serio M, Montagnani M, Potenza MA, Mansi G, De Salvatore G, Mitolo-Chieppa D. Postnatal developmental changes of receptor responsiveness in rat mesenteric vascular bed. J Auton Pharmacol. 1996;16:63–68. doi: 10.1111/j.1474-8673.1996.tb00413.x. [DOI] [PubMed] [Google Scholar]
- 93.Hill CE, Hirst GD, Van Helden DF. Development of sympathetic innervation to proximal and distal arteries of the rat mesentery. J Physiol. 1983;338:129–147. doi: 10.1113/jphysiol.1983.sp014665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Burnstock G. Noradrenaline and ATP: cotransmitters and neuromodulators. J Physiol Pharmacol. 1995;46:365–384. [PubMed] [Google Scholar]
- 95.Hill CE, Hirst GD, Ngu MC, Van Helden DF. Sympathetic postganglionic reinnervation of mesenteric arteries and enteric neurones of the ileum of the rat. J Auton Nerv Syst. 1985;14:317–334. doi: 10.1016/0165-1838(85)90079-7. [DOI] [PubMed] [Google Scholar]
- 96.Phillips JK (1998) Neuroreceptor mediated vascular control mechanisms in the rat. PhD Thesis Canberra
- 97.Phillips JK, Hill CE. Neuroreceptor mRNA expression in the rat mesenteric artery develops independently of innervation. Int J Dev Neurosci. 1999;17:377–386. doi: 10.1016/s0736-5748(99)00032-5. [DOI] [PubMed] [Google Scholar]
- 98.Hashimoto M, Shinozuka K, Bjur RA, Westfall DP, Hattori K, Masumura S. The effects of age on the release of adenine nucleosides and nucleotides from rat caudal artery. J Physiol. 1995;489(Pt 3):841–848. doi: 10.1113/jphysiol.1995.sp021096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ueda H, Moritoki H. Possible association of decrease of ATP-induced vascular relaxation with reduction of cyclic GMP during aging. Arch Int Pharmacodyn Ther. 1991;310:35–45. [PubMed] [Google Scholar]
- 100.Crowley MR. Oxygen-induced pulmonary vasodilation is mediated by adenosine triphosphate in newborn lambs. J Cardiovasc Pharmacol. 1997;30:102–109. doi: 10.1097/00005344-199707000-00015. [DOI] [PubMed] [Google Scholar]
- 101.Morin FC, Egan EA. Pulmonary hemodynamics in fetal lambs during development at normal and increased oxygen tension. J Appl Physiol. 1992;73:213–218. doi: 10.1152/jappl.1992.73.1.213. [DOI] [PubMed] [Google Scholar]
- 102.Konduri GG, Theodorou AA, Mukhopadhyay A, Deshmukh DR. Adenosine triphosphate and adenosine increase the pulmonary blood flow to postnatal levels in fetal lambs. Pediatr Res. 1992;31:451–457. doi: 10.1203/00006450-199205000-00007. [DOI] [PubMed] [Google Scholar]
- 103.Konduri GG, Gervasio CT, Theodorou AA. Role of adenosine triphosphate and adenosine in oxygen-induced pulmonary vasodilation in fetal lambs. Pediatr Res. 1993;33:533–539. doi: 10.1203/00006450-199305000-00022. [DOI] [PubMed] [Google Scholar]
- 104.Konduri GG, Woodard LL. Selective pulmonary vasodilation by low-dose infusion of adenosine triphosphate in newborn lambs [see comments] J Pediatr. 1991;119:94–102. doi: 10.1016/s0022-3476(05)81047-9. [DOI] [PubMed] [Google Scholar]
- 105.Kolb HA, Wakelam MJ. Transmitter-like action of ATP on patched membranes of cultured myoblasts and myotubes. Nature. 1983;303:621–623. doi: 10.1038/303621a0. [DOI] [PubMed] [Google Scholar]
- 106.Häggblad J, Eriksson H, Heilbronn E. ATP-induced cation influx in myotubes is additive to cholinergic agonist action. Acta Physiol Scand. 1985;125:389–393. doi: 10.1111/j.1748-1716.1985.tb07734.x. [DOI] [PubMed] [Google Scholar]
- 107.Häggblad J, Heilbronn E. Externally applied adenosine-5′-triphosphate causes inositol triphosphate accumulation in cultured chick myotubes. Neurosci Lett. 1987;74:199–204. doi: 10.1016/0304-3940(87)90149-2. [DOI] [PubMed] [Google Scholar]
- 108.Eriksson H, Heilbronn E. Extracellularly applied ATP alters the calcium flux through dihydropyridine-sensitive channels in cultured chick myotubes. Biochem Biophys Res Commun. 1989;159:878–885. doi: 10.1016/0006-291x(89)92190-6. [DOI] [PubMed] [Google Scholar]
- 109.Hume RI, Hönig MG. Excitatory action of ATP on embryonic chick muscle. J Neurosci. 1986;6:681–690. doi: 10.1523/JNEUROSCI.06-03-00681.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hume RI, Thomas SA. Multiple actions of adenosine 5′-triphosphate on chick skeletal muscle. J Physiol. 1988;406:503–524. doi: 10.1113/jphysiol.1988.sp017393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Thomas SA, Hume RI. Permeation of both cations and anions through a single class of ATP-activated ion channels in developing chick skeletal muscle. J Gen Physiol. 1990;95:569–590. doi: 10.1085/jgp.95.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Thomas SA, Hume RI. Irreversible desensitization of ATP responses in developing chick skeletal muscle. J Physiol. 1990;430:373–388. doi: 10.1113/jphysiol.1990.sp018296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Thomas SA, Hume RI. Single potassium channel currents activated by extracellular ATP in developing chick skeletal muscle: a role for second messengers. J Neurophysiol. 1993;69:1556–1566. doi: 10.1152/jn.1993.69.5.1556. [DOI] [PubMed] [Google Scholar]
- 114.Thomas SA, Zawisa MJ, Lin X, Hume RI. A receptor that is highly specific for extracellular ATP in developing chick skeletal muscle in vitro. Br J Pharmacol. 1991;103:1963–1969. doi: 10.1111/j.1476-5381.1991.tb12360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wells DG, Zawisa MJ, Hume RI. Changes in responsiveness to extracellular ATP in chick skeletal muscle during development and upon denervation. Dev Biol. 1995;172:585–590. doi: 10.1006/dbio.1995.8062. [DOI] [PubMed] [Google Scholar]
- 116.Mehul B, Doyennette-Moyne MA, Aubery M, Codogno P, Mannherz HG. Enzymatic activity and in vivo distribution of 5′-nucleotidase, an extracellular matrix binding glycoprotein, during the development of chicken striated muscle. Exp Cell Res. 1992;203:62–71. doi: 10.1016/0014-4827(92)90040-f. [DOI] [PubMed] [Google Scholar]
- 117.Soto F, Krause U, Borchardt K, Ruppelt A. Cloning, tissue distribution and functional characterization of the chicken P2X1 receptor. FEBS Lett. 2003;533:54–58. doi: 10.1016/s0014-5793(02)03751-1. [DOI] [PubMed] [Google Scholar]
- 118.Ruppelt A, Ma W, Borchardt K, Silberberg SD, Soto F. Genomic structure, developmental distribution and functional properties of the chicken P2X5 receptor. J Neurochem. 2001;77:1256–1265. doi: 10.1046/j.1471-4159.2001.00348.x. [DOI] [PubMed] [Google Scholar]
- 119.Henning RH, Nelemans A, Van den Akker J, Den Hertog A. The nucleotide receptors on mouse C2C12 myotubes. Br J Pharmacol. 1992;106:853–858. doi: 10.1111/j.1476-5381.1992.tb14424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Henning RH, Duin M, Den Hertog A, Nelemans A. Characterization of P2-purinoceptor mediated cyclic AMP formation in mouse C2C12 myotubes. Br J Pharmacol. 1993;110:133–138. doi: 10.1111/j.1476-5381.1993.tb13782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Henning RH, Duin M, Den Hertog A, Nelemans A. Activation of the phospholipase C pathway by ATP is mediated exclusively through nucleotide type P2-purinoceptors in C2C12 myotubes. Br J Pharmacol. 1993;110:747–752. doi: 10.1111/j.1476-5381.1993.tb13875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Henning RH, Duin M, van Popta JP, Nelemans A, Den Hertog A. Different mechanisms of Ca2+-handling following nicotinic acetylcholine receptor stimulation, P2U-purinoceptor stimulation and K+-induced depolarization in C2C12 myotubes. Br J Pharmacol. 1996;117:1785–1791. doi: 10.1111/j.1476-5381.1996.tb15355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Henning RH. Purinoceptors in neuromuscular transmission. Pharmacol Ther. 1997;74:115–128. doi: 10.1016/s0163-7258(97)00015-6. [DOI] [PubMed] [Google Scholar]
- 124.Tassin AM, Haggblad J, Heilbronn E. Receptor-triggered polyphosphoinositide turnover produces less cytosolic free calcium in cultured dysgenic myotubes than in normal myotubes. Muscle Nerve. 1990;13:142–145. doi: 10.1002/mus.880130210. [DOI] [PubMed] [Google Scholar]
- 125.Collet C, Strube C, Csernoch L, Mallouk N, Ojeda C, Allard B, Jacquemond V. Effects of extracellular ATP on freshly isolated mouse skeletal muscle cells during pre-natal and post-natal development. Pflugers Arch. 2002;443:771–778. doi: 10.1007/s00424-001-0758-9. [DOI] [PubMed] [Google Scholar]
- 126.Moores TS, Hasdemir B, Vega-Riveroll L, Deuchars J, Parson SH. Properties of presynaptic P2X7-like receptors at the neuromuscular junction. Brain Res. 2005;1034:40–50. doi: 10.1016/j.brainres.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 127.Ryten M, Hoebertz A, Burnstock G. Sequential expression of three receptor subtypes for extracellular ATP in developing rat skeletal muscle. Dev Dyn. 2001;221:331–341. doi: 10.1002/dvdy.1147. [DOI] [PubMed] [Google Scholar]
- 128.Ryten M, Dunn PM, Neary JT, Burnstock G. ATP regulates the differentiation of mammalian skeletal muscle by activation of a P2X5 receptor on satellite cells. J Cell Biol. 2002;158:345–355. doi: 10.1083/jcb.200202025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ryten M, Koshi R, Knight GE, Turmaine M, Dunn PM, Cockayne DA, Ford APDW, Burnstock G. Abnormalities in neuromuscular junction structure and skeletal muscle function in mice lacking the P2X2 nucleotide receptor. Neuroscience. 2007;148:700–711. doi: 10.1016/j.neuroscience.2007.06.050. [DOI] [PubMed] [Google Scholar]
- 130.Choi HB, Hong SH, Ryu JK, Kim SU, McLarnon JG. Differential activation of subtype purinergic receptors modulates Ca2+ mobilization and COX-2 in human microglia. Glia. 2003;43:95–103. doi: 10.1002/glia.10239. [DOI] [PubMed] [Google Scholar]
- 131.Ling KK, Siow NL, Choi RC, Tsim KW. ATP potentiates the formation of AChR aggregate in the co-culture of NG108-15 cells with C2C12 myotubes. FEBS Lett. 2005;579:2469–2474. doi: 10.1016/j.febslet.2005.03.054. [DOI] [PubMed] [Google Scholar]
- 132.Wang N, Robaye B, Gossiel F, Boeynaems JM, Gartland A. The P2Y13 receptor regulates phosphate metabolism and FGF-23 secretion with effects on skeletal development. FASEB J. 2014;28:2249–2259. doi: 10.1096/fj.13-243626. [DOI] [PubMed] [Google Scholar]
- 133.O'Reilly BA, Kosaka AH, Chang TK, Ford AP, Popert R, Rymer JM, McMahon SB. A quantitative analysis of purinoceptor expression in human fetal and adult bladders. J Urol. 2001;165:1730–1734. [PubMed] [Google Scholar]
- 134.Thiruchelvam N, Wu C, David A, Woolf AS, Cuckow PM, Fry CH. Neurotransmission and viscoelasticity in the ovine fetal bladder after in utero bladder outflow obstruction. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1296–R1305. doi: 10.1152/ajpregu.00688.2002. [DOI] [PubMed] [Google Scholar]
- 135.Burnstock G. Purinergic signalling in lower urinary tract. In: Abbracchio MP, Williams M, editors. Handbook of experimental pharmacology, volume 151/I. Purinergic and pyrimidinergic signalling I—molecular, nervous and urinogenitary system function. Berlin: Springer-Verlag; 2001. pp. 423–515. [Google Scholar]
- 136.Ekman M, Andersson KE, Arner A. Developmental regulation of nerve and receptor mediated contractions of mammalian urinary bladder smooth muscle. Eur J Pharmacol. 2006;532:99–106. doi: 10.1016/j.ejphar.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 137.Dutton JL, Hansen MA, Balcar VJ, Barden JA, Bennett MR. Development of P2X receptor clusters on smooth muscle cells in relation to nerve varicosities in the rat urinary bladder. J Neurocytol. 1999;28:4–16. doi: 10.1023/a:1007043132537. [DOI] [PubMed] [Google Scholar]
- 138.Tugay M, Yildiz F, Utkan T, Gacar N, Ulak G, Erden F. Age-related smooth muscle reactivity changes in the rat bladder: an in vitro study. Pharmacol Res. 2003;48:329–334. doi: 10.1016/s1043-6618(03)00105-1. [DOI] [PubMed] [Google Scholar]
- 139.Kageyama S, Fujita K, Suzuki K, Shinbo H, Masuda N, Uchida W. Effect of age on the responses of rat bladder detrusor strips to adenosine triphosphate. BJU Int. 2000;85:899–904. doi: 10.1046/j.1464-410x.2000.00527.x. [DOI] [PubMed] [Google Scholar]
- 140.Dunwiddie TV. The physiological role of adenosine in the central nervous system. Int Rev Neurobiol. 1985;27:63–139. doi: 10.1016/s0074-7742(08)60556-5. [DOI] [PubMed] [Google Scholar]
- 141.Fredholm BB. Adenosine receptors in the central nervous system. News Physiol Sci. 1995;10:122–128. [Google Scholar]
- 142.Edwards FA, Gibb AJ, Colquhoun D. ATP receptor-mediated synaptic currents in the central nervous system. Nature. 1992;359:144–147. doi: 10.1038/359144a0. [DOI] [PubMed] [Google Scholar]
- 143.Gibb AJ, Halliday FC. Fast purinergic transmission in the central nervous system. Semin Neurosci. 1996;8:225–232. [Google Scholar]
- 144.Abbracchio MP. ATP in brain function. In: Jacobson KA, Jarvis MF, editors. Purinergic approaches in experimental therapeutics. New York: Wiley-Liss; 1997. pp. 383–404. [Google Scholar]
- 145.Marangos PJ, Boulenger JP, Patel J. Effects of chronic caffeine on brain adenosine receptors: regional and ontogenetic studies. Life Sci. 1984;34:899–907. doi: 10.1016/0024-3205(84)90207-8. [DOI] [PubMed] [Google Scholar]
- 146.Shaw C, Hall SE, Cynader M. Characterization, distribution, and ontogenesis of adenosine binding sites in cat visual cortex. J Neurosci. 1986;6:3218–3228. doi: 10.1523/JNEUROSCI.06-11-03218.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Morgan PF, Marangos PJ. Ontogenetic appearance of the adenosine receptor precedes N-protein coupling in rat forebrain. Brain Res. 1987;432:269–274. doi: 10.1016/0165-3806(87)90051-4. [DOI] [PubMed] [Google Scholar]
- 148.Morgan PF, Deckert J, Nakajima T, Daval JL, Marangos PJ. Late ontogenetic development of adenosine A1 receptor coupling to associated G-proteins in guinea pig cerebellum but not forebrain. Mol Cell Biochem. 1990;92:169–176. doi: 10.1007/BF00218134. [DOI] [PubMed] [Google Scholar]
- 149.Doriat JF, Humbert AC, Daval JL. Brain maturation of high-affinity adenosine A2 receptors and their coupling to G-proteins. Brain Res Dev Brain Res. 1996;93:1–9. doi: 10.1016/0165-3806(96)00009-0. [DOI] [PubMed] [Google Scholar]
- 150.Marangos PJ, Patel J, Stivers J. Ontogeny of adenosine binding sites in rat forebrain and cerebellum. J Neurochem. 1982;39:267–270. doi: 10.1111/j.1471-4159.1982.tb04732.x. [DOI] [PubMed] [Google Scholar]
- 151.Geiger JD, LaBella FS, Nagy JI. Ontogenesis of adenosine receptors in the central nervous system of the rat. Brain Res Dev Brain Res. 1984;13:97–104. doi: 10.1016/0165-3806(84)90080-4. [DOI] [PubMed] [Google Scholar]
- 152.Daval JL, Werck MC, Nehlig A, Pereira de Vasconcelos A. Quantitative autoradiographic study of the postnatal development of adenosine A1 receptors and their coupling to G proteins in the rat brain. Neuroscience. 1991;40:841–851. doi: 10.1016/0306-4522(91)90016-h. [DOI] [PubMed] [Google Scholar]
- 153.Popoli P, Betto P, Rimondini R, Reggio R, Pezzola A, Ricciarello G, Fuxe K, Ferre S. Age-related alteration of the adenosine/dopamine balance in the rat striatum. Brain Res. 1998;795:297–300. doi: 10.1016/s0006-8993(98)00356-4. [DOI] [PubMed] [Google Scholar]
- 154.Schiffmann SN, Vanderhaeghen JJ. Age-related loss of mRNA encoding adenosine A2 receptor in the rat striatum. Neurosci Lett. 1993;158:121–124. doi: 10.1016/0304-3940(93)90244-f. [DOI] [PubMed] [Google Scholar]
- 155.Johansson B, Georgiev V, Fredholm BB. Distribution and postnatal ontogeny of adenosine A2A receptors in rat brain: comparison with dopamine receptors. Neuroscience. 1997;80:1187–1207. doi: 10.1016/s0306-4522(97)00143-7. [DOI] [PubMed] [Google Scholar]
- 156.Webb TE, Simon J, Barnard EA. Regional distribution of [35S]2′-deoxy 5′-O-(1-thio) ATP binding sites and the P2Y1 messenger RNA within the chick brain. Neuroscience. 1998;84:825–837. doi: 10.1016/s0306-4522(97)00478-8. [DOI] [PubMed] [Google Scholar]
- 157.Pagonopoulou O, Angelatou F. Reduction of A1 adenosine receptors in cortex, hippocampus and cerebellum in ageing mouse brain. Neuroreport. 1992;3:735–737. doi: 10.1097/00001756-199209000-00003. [DOI] [PubMed] [Google Scholar]
- 158.Jin ZL, Lee TF, Zhou SJ, Wang LC. Age-dependent change in the inhibitory effect of an adenosine agonist on hippocampal acetylcholine release in rats. Brain Res Bull. 1993;30:149–152. doi: 10.1016/0361-9230(93)90051-c. [DOI] [PubMed] [Google Scholar]
- 159.Sperlágh B, Andras I, Vizi S. Effect of subtype-specific Ca2+-antagonists and Ca2+-free media on the field stimulation-evoked release of ATP and [3H]acetylcholine from rat habenula slices. Neurochem Res. 1997;22:967–975. doi: 10.1023/a:1022470725132. [DOI] [PubMed] [Google Scholar]
- 160.León D, Albasanz JL, Ruiz MA, Fernandez M, Martin M. Adenosine A1 receptor down-regulation in mothers and fetal brain after caffeine and theophylline treatments to pregnant rats. J Neurochem. 2002;82:625–634. doi: 10.1046/j.1471-4159.2002.01008.x. [DOI] [PubMed] [Google Scholar]
- 161.Meerlo P, Roman V, Farkas E, Keijser JN, Nyakas C, Luiten PG. Ageing-related decline in adenosine A1 receptor binding in the rat brain: an autoradiographic study. J Neurosci Res. 2004;78:742–748. doi: 10.1002/jnr.20314. [DOI] [PubMed] [Google Scholar]
- 162.Gaytan SP, Saadani-Makki F, Bodineau L, Frugière A, Larnicol N, Pásaro R. Effect of postnatal exposure to caffeine on the pattern of adenosine A1 receptor distribution in respiration-related nuclei of the rat brainstem. Auton Neurosci. 2006;126–127:339–346. doi: 10.1016/j.autneu.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 163.Tavares Gomes AL, Maia FB, Oliveira-Silva P, Marques Ventura AL, Paes-De-Carvalho R, Serfaty CA, Campello-Costa P. Purinergic modulation in the development of the rat uncrossed retinotectal pathway. Neuroscience. 2009;163:1061–1068. doi: 10.1016/j.neuroscience.2009.07.029. [DOI] [PubMed] [Google Scholar]
- 164.Dale N, Gilday D. Regulation of rhythmic movements by purinergic neurotransmitters in frog embryos. Nature. 1996;383:259–263. doi: 10.1038/383259a0. [DOI] [PubMed] [Google Scholar]
- 165.Brown P, Dale N. Modulation of K+ currents in Xenopus spinal neurons by p2y receptors: a role for ATP and ADP in motor pattern generation. J Physiol. 2002;540:843–850. doi: 10.1113/jphysiol.2001.013192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Brown P, Dale N. Adenosine A1 receptors modulate high voltage-activated Ca2+ currents and motor pattern generation in the xenopus embryo. J Physiol. 2000;525(Pt 3):655–667. doi: 10.1111/j.1469-7793.2000.00655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Dale N. Delayed production of adenosine underlies temporal modulation of swimming in frog embryo. J Physiol. 1998;511:265–272. doi: 10.1111/j.1469-7793.1998.265bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Devader C, Webb RJ, Thomas GM, Dale L. Xenopus apyrase (xapy), a secreted nucleotidase that is expressed during early development. Gene. 2006;367:135–141. doi: 10.1016/j.gene.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 169.Massé K, Eason R, Bhamra S, Dale N, Jones EA. Comparative genomic and expression analysis of the conserved NTPDase gene family in Xenopus. Genomics. 2006;87:366–381. doi: 10.1016/j.ygeno.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 170.Massé K, Bhamra S, Allsop G, Dale N, Jones EA. Ectophosphodiesterase/nucleotide phosphohydrolase (Enpp) nucleotidases: cloning, conservation and developmental restriction. Int J Dev Biol. 2010;54:181–193. doi: 10.1387/ijdb.092879km. [DOI] [PubMed] [Google Scholar]
- 171.Funk GD, Parkis MA, Selvaratnam SR, Walsh C. Developmental modulation of glutamatergic inspiratory drive to hypoglossal motoneurons. Respir Physiol. 1997;110:125–137. doi: 10.1016/s0034-5687(97)00078-9. [DOI] [PubMed] [Google Scholar]
- 172.Herlenius E, Lagercrantz H, Yamamoto Y. Adenosine modulates inspiratory neurons and the respiratory pattern in the brainstem of neonatal rats. Pediatr Res. 1997;42:46–53. doi: 10.1203/00006450-199707000-00008. [DOI] [PubMed] [Google Scholar]
- 173.Deuchars S. Effect of ATP and adenosine on sympathetic preganglionic neurones in a neonatal rat brainstem-spinal cord preparation. J Physiol. 1995;489:154P. doi: 10.1016/0165-1838(94)00144-9. [DOI] [PubMed] [Google Scholar]
- 174.Runold M, Lagercrantz H, Fredholm BB. Ventilatory effect of an adenosine analogue in unanesthetized rabbits during development. J Appl Physiol. 1986;61:255–259. doi: 10.1152/jappl.1986.61.1.255. [DOI] [PubMed] [Google Scholar]
- 175.Lagercrantz H, Yamamoto Y, Fredholm BB, Prabhakar NR, von Euler C. Adenosine analogues depress ventilation in rabbit neonates. Theophylline stimulation of respiration via adenosine receptors? Pediatr Res. 1984;18:387–390. doi: 10.1203/00006450-198404000-00018. [DOI] [PubMed] [Google Scholar]
- 176.Darnall RA, Bruce RD. Effects of adenosine and xanthine derivatives on breathing during acute hypoxia in the anesthetized newborn piglet. Pediatr Pulmonol. 1987;3:110–116. doi: 10.1002/ppul.1950030213. [DOI] [PubMed] [Google Scholar]
- 177.Lopes JM, Davis GM, Mullahoo K, Aranda JV. Role of adenosine in the hypoxic ventilatory response of the newborn piglet. Pediatr Pulmonol. 1994;17:50–55. doi: 10.1002/ppul.1950170109. [DOI] [PubMed] [Google Scholar]
- 178.Alvares TS, Revill AL, Huxtable AG, Lorenz CD, Funk GD. P2Y1 receptor-mediated potentiation of inspiratory motor output in neonatal rat in vitro. J Physiol. 2014;592:3089–3111. doi: 10.1113/jphysiol.2013.268136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wirkner K, Franke H, Inoue K, Illes P. Differential age-dependent expression of α2 adrenoceptor- and P2 purinoceptor-functions in rat locus coeruleus neurons. Naunyn Schmiedeberg’s Arch Pharmacol. 1998;357:186–189. doi: 10.1007/pl00005154. [DOI] [PubMed] [Google Scholar]
- 180.Jang IS, Rhee JS, Kubota H, Akaike N, Akaike N. Developmental changes in P2X purinoceptors on glycinergic presynaptic nerve terminals projecting to rat substantia gelatinosa neurones. J Physiol. 2001;536:505–519. doi: 10.1111/j.1469-7793.2001.0505c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Bardoni R. Excitatory synaptic transmission in neonatal dorsal horn: NMDA and ATP receptors. News Physiol Sci. 2001;16:95–100. doi: 10.1152/physiologyonline.2001.16.2.95. [DOI] [PubMed] [Google Scholar]
- 182.Brosenitsch TA, Adachi T, Lipski J, Housley GD, Funk GD. Developmental downregulation of P2X3 receptors in motoneurons of the compact formation of the nucleus ambiguus. Eur J Neurosci. 2005;22:809–824. doi: 10.1111/j.1460-9568.2005.04261.x. [DOI] [PubMed] [Google Scholar]
- 183.Heine C, Wegner A, Grosche J, Allgaier C, Illes P, Franke H. P2 receptor expression in the dopaminergic system of the rat brain during development. Neuroscience. 2007;149:165–181. doi: 10.1016/j.neuroscience.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 184.Fischer T, Rotermund N, Lohr C, Hirnet D. P2Y1 receptor activation by photolysis of caged ATP enhances neuronal network activity in the developing olfactory bulb. Purinergic Signal. 2012;8:191–198. doi: 10.1007/s11302-011-9286-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kidd EJ, Miller KJ, Sansum AJ, Humphrey PPA. Evidence for P2X3 receptors in the developing rat brain. Neuroscience. 1998;87:533–539. doi: 10.1016/s0306-4522(98)00294-2. [DOI] [PubMed] [Google Scholar]
- 186.Collo G, Neidhart S, Kawashima E, Kosco-Vilbois M, North RA, Buell G. Tissue distribution of the P2X7 receptor. Neuropharmacology. 1997;36:1277–1283. doi: 10.1016/s0028-3908(97)00140-8. [DOI] [PubMed] [Google Scholar]
- 187.Narcisse L, Scemes E, Zhao Y, Lee SC, Brosnan CF. The cytokine IL-1β transiently enhances P2X7 receptor expression and function in human astrocytes. Glia. 2005;49:245–258. doi: 10.1002/glia.20110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kong Q, Wang M, Liao Z, Camden JM, Yu S, Simonyi A, Sun GY, Gonzalez FA, Erb L, Seye CI, Weisman GA. P2X7 nucleotide receptors mediate caspase-8/9/3-dependent apoptosis in rat primary cortical neurons. Purinergic Signal. 2005;1:337–347. doi: 10.1007/s11302-005-7145-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Khakh BS, Smith WB, Chiu CS, Ju D, Davidson N, Lester HA. Activation-dependent changes in receptor distribution and dendritic morphology in hippocampal neurons expressing P2X2-green fluorescent protein receptors. Proc Natl Acad Sci U S A. 2001;98:5288–5293. doi: 10.1073/pnas.081089198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.García-Lecea M, Sen RP, Soto F, Miras-Portugal MT, Castro E. P2 receptors in cerebellar neurons: molecular diversity of ionotropic ATP receptors in Purkinje cells. Drug Dev Res. 2001;52:104–113. [Google Scholar]
- 191.Maric D, Maric I, Chang YH, Barker JL. Stereotypical physiological properties emerge during early neuronal and glial lineage development in the embryonic rat neocortex. Cereb Cortex. 2000;10:729–747. doi: 10.1093/cercor/10.8.729. [DOI] [PubMed] [Google Scholar]
- 192.Cheung K-K, Burnstock G. Localisation of P2X3 and co-expression with P2X2 receptors during rat embryonic neurogenesis. J Comp Neurol. 2002;443:368–382. doi: 10.1002/cne.10123. [DOI] [PubMed] [Google Scholar]
- 193.Xiang Z, Burnstock G. Changes in expression of P2X purinoceptors in rat cerebellum during postnatal development. Dev Brain Res. 2005;156:147–157. doi: 10.1016/j.devbrainres.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 194.Casel D, Brockhaus J, Deitmer JW. Enhancement of spontaneous synaptic activity in rat Purkinje neurones by ATP during development. J Physiol. 2005;568:111–122. doi: 10.1113/jphysiol.2005.091371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Cheung K-K, Chan WY, Burnstock G. Expression of P2X receptors during rat brain development and their inhibitory role on motor axon outgrowth in neural tube explant cultures. Neuroscience. 2005;133:937–945. doi: 10.1016/j.neuroscience.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 196.Boldogköi Z, Schütz B, Sallach J, Zimmer A. P2X3 receptor expression at early stage of mouse embryogenesis. Mech Dev. 2002;118:255–260. doi: 10.1016/s0925-4773(02)00280-0. [DOI] [PubMed] [Google Scholar]
- 197.Jo YH, Role LW. Cholinergic modulation of purinergic and GABAergic co-transmission at in vitro hypothalamic synapses. J Neurophysiol. 2002;88:2501–2508. doi: 10.1152/jn.00352.2002. [DOI] [PubMed] [Google Scholar]
- 198.Safiulina VF, Kasyanov AM, Sokolova E, Cherubini E, Giniatullin R. ATP contributes to the generation of network-driven giant depolarizing potentials in the neonatal rat hippocampus. J Physiol. 2005;565:981–992. doi: 10.1113/jphysiol.2005.085621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Heine C, Heimrich B, Vogt J, Wegner A, Illes P, Franke H. P2 receptor-stimulation influences axonal outgrowth in the developing hippocampus in vitro. Neuroscience. 2006;138:303–311. doi: 10.1016/j.neuroscience.2005.11.056. [DOI] [PubMed] [Google Scholar]
- 200.Safiulina VF, Afzalov R, Khiroug L, Cherubini E, Giniatullin R. Reactive oxygen species mediate the potentiating effects of ATP on GABAergic synaptic transmission in the immature hippocampus. J Biol Chem. 2006;281:23464–23470. doi: 10.1074/jbc.M601627200. [DOI] [PubMed] [Google Scholar]
- 201.Lalo U, Kostyuk P. Developmental changes in purinergic calcium signalling in rat neocortical neurones. Brain Res Dev Brain Res. 1998;111:43–50. doi: 10.1016/s0165-3806(98)00120-5. [DOI] [PubMed] [Google Scholar]
- 202.Hanahisa Y, Yamaguchi M. Increase of Ca2+-ATPase activity in the brain microsomes of rats with increasing ages: involvement of protein kinase C. Brain Res Bull. 1998;46:329–332. doi: 10.1016/s0361-9230(98)00021-5. [DOI] [PubMed] [Google Scholar]
- 203.Müller J, Rocha JB, Battastini AM, Sarkis JJ, Dias RD. Postnatal development of ATPase-ADPase activities in synaptosomal fraction from cerebral cortex of rats. Neurochem Int. 1993;23:471–477. doi: 10.1016/0197-0186(93)90132-o. [DOI] [PubMed] [Google Scholar]
- 204.Oliveira EM, Rocha JB, Sarkis JJ. In vitro and in vivo effects of HgCl2 on synaptosomal ATP diphosphohydrolase (EC 3.6.1.5) from cerebral cortex of developing rats. Arch Int Physiol Biochim Biophys. 1994;102:251–254. doi: 10.3109/13813459409003939. [DOI] [PubMed] [Google Scholar]
- 205.Clemow DB, Brunjes PC. Development of 5′-nucleotidase staining in the olfactory bulbs of normal and naris-occluded rats. Int J Dev Neurosci. 1996;14:901–911. doi: 10.1016/s0736-5748(96)00040-8. [DOI] [PubMed] [Google Scholar]
- 206.Braun N, Sevigny J, Mishra SK, Robson SC, Barth SW, Gerstberger R, Hammer K, Zimmermann H. Expression of the ecto-ATPase NTPDase2 in the germinal zones of the developing and adult rat brain. Eur J Neurosci. 2003;17:1355–1364. doi: 10.1046/j.1460-9568.2003.02567.x. [DOI] [PubMed] [Google Scholar]
- 207.Torres IL, Battastini AM, Buffon A, Furstenau CR, Siqueira I, Sarkis JJ, Dalmaz C, Ferreira MB. Ecto-nucleotidase activities in spinal cord of rats changes as function of age. Int J Dev Neurosci. 2003;21:425–429. doi: 10.1016/j.ijdevneu.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 208.Nedeljkovic N, Banjac A, Horvat A, Stojiljkovic M, Nikezic G. Developmental profile of NTPDase activity in synaptic plasma membranes isolated from rat cerebral cortex. Int J Dev Neurosci. 2005;23:45–51. doi: 10.1016/j.ijdevneu.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 209.Bruno AN, Ricachenevsky FK, Pochmann D, Bonan CD, Battastini AM, Barreto-Chaves ML, Sarkis JJ. Hypothyroidism changes adenine nucleotide hydrolysis in synaptosomes from hippocampus and cerebral cortex of rats in different phases of development. Int J Dev Neurosci. 2005;23:37–44. doi: 10.1016/j.ijdevneu.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 210.Braganhol E, Bruno AN, Bavaresco L, Barreto-Chaves ML, Sarkis JJ, Battastini AM. Neonatal hypothyroidism affects the adenine nucleotides metabolism in astrocyte cultures from rat brain. Neurochem Res. 2006;31:449–454. doi: 10.1007/s11064-006-9041-y. [DOI] [PubMed] [Google Scholar]
- 211.Ahmed OM, El-Gareib AW, El-Bakry AM, Abd El-Tawab SM, Ahmed RG. Thyroid hormones states and brain development interactions. Int J Dev Neurosci. 2008;26:147–209. doi: 10.1016/j.ijdevneu.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 212.Cohen JE, Fields RD. Activity-dependent neuron-glial signaling by ATP and leukemia-inhibitory factor promotes hippocampal glial cell development. Neuron Glia Biol. 2008;4:43–55. doi: 10.1017/S1740925X09000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Wakade TD, Palmer KC, McCauley R, Przywara DA, Wakade AR. Adenosine-induced apoptosis in chick embryonic sympathetic neurons: a new physiological role for adenosine. J Physiol. 1995;488:123–138. doi: 10.1113/jphysiol.1995.sp020951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Kulkarni JS, Prywara DA, Wakade TD. Adenosine induces apoptosis by inhibiting mRNA and protein synthesis in chick embryonic sympathetic neurons. Neurosci Lett. 1998;248:187–190. doi: 10.1016/s0304-3940(98)00369-3. [DOI] [PubMed] [Google Scholar]
- 215.Abe Y, Sorimachi M, Itoyama Y, Furukawa K, Akaike N. ATP responses in the embryo chick ciliary ganglion cells. Neuroscience. 1995;64:547–551. doi: 10.1016/0306-4522(94)00415-2. [DOI] [PubMed] [Google Scholar]
- 216.Labrakakis C, Gerstner E, MacDermott AB. Adenosine triphosphate-evoked currents in cultured dorsal root ganglion neurons obtained from rat embryos: desensitization kinetics and modulation of glutamate release. Neuroscience. 2000;101:1117–1126. doi: 10.1016/s0306-4522(00)00373-0. [DOI] [PubMed] [Google Scholar]
- 217.Pan A, Wu H, Li M, Lu D, He X, Yi X, Yan XX, Li Z. Prenatal expression of purinergic receptor P2X3 in human dorsal root ganglion. Purinergic Signal. 2012;8:245–254. doi: 10.1007/s11302-011-9277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Nakatsuka T, Mena N, Ling J, Gu JG. Depletion of substance P from rat primary sensory neurons by ATP, an implication of P2X receptor-mediated release of substance P. Neuroscience. 2001;107:293–300. doi: 10.1016/s0306-4522(01)00342-6. [DOI] [PubMed] [Google Scholar]
- 219.Ruan H-Z, Moules E, Burnstock G. Changes in P2X purinoceptors in sensory ganglia of the mouse during embryonic and postnatal development. Histochem Cell Biol. 2004;122:539–551. doi: 10.1007/s00418-004-0714-9. [DOI] [PubMed] [Google Scholar]
- 220.Huang H, Wu X, Nicol GD, Meller S, Vasko MR. ATP augments peptide release from rat sensory neurons in culture through activation of P2Y receptors. J Pharmacol Exp Ther. 2003;306:1137–1144. doi: 10.1124/jpet.103.052951. [DOI] [PubMed] [Google Scholar]
- 221.Dunn PM, Gever J, Ruan H-Z, Burnstock G. Developmental changes in heteromeric P2X2/3 receptor expression in rat sympathetic ganglion neurons. Dev Dyn. 2005;234:505–511. doi: 10.1002/dvdy.20466. [DOI] [PubMed] [Google Scholar]
- 222.Molliver DC, Wright DE, Leitner ML, Parsadanian AS, Doster K, Wen D, Yan Q, Snider WD. IB4-binding DRG neurons switch from NGF to GDNF dependence in early postnatal life. Neuron. 1997;19:849–861. doi: 10.1016/s0896-6273(00)80966-6. [DOI] [PubMed] [Google Scholar]
- 223.Allgaier C, Wellmann H, Schobert A, von Kügelgen I. Cultured chick sympathetic neurons: modulation of electrically evoked noradrenaline release by P2-purinoceptors. Naunyn Schmiedeberg’s Arch Pharmacol. 1995;352:17–24. doi: 10.1007/BF00169185. [DOI] [PubMed] [Google Scholar]
- 224.Allgaier C, Wellmann H, Schobert A, Kurz G, von Kügelgen I. Cultured chick sympathetic neurons: ATP-induced noradrenaline release and its blockade by nicotinic receptor antagonists. Naunyn Schmiedeberg’s Arch Pharmacol. 1995;352:25–30. doi: 10.1007/BF00169186. [DOI] [PubMed] [Google Scholar]
- 225.Nörenberg W, Gobel I, Meyer A, Cox SL, Starke K, Trendelenburg AU. Stimulation of mouse cultured sympathetic neurons by uracil but not adenine nucleotides. Neuroscience. 2001;103:227–236. doi: 10.1016/s0306-4522(00)00547-9. [DOI] [PubMed] [Google Scholar]
- 226.Dowling P, Ranson RN, Santer RM. Age-associated changes in distribution of the P2X2 receptor in the major pelvic ganglion of the male rat. Neurosci Lett. 2006;404:320–323. doi: 10.1016/j.neulet.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 227.Chen Y, Li G, Huang LY. P2X7 receptors in satellite glial cells mediate high functional expression of P2X3 receptors in immature dorsal root ganglion neurons. Mol Pain. 2012;8:9. doi: 10.1186/1744-8069-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Syed MM, Lee S, Zheng J, Zhou ZJ. Stage-dependent dynamics and modulation of spontaneous waves in the developing rabbit retina. J Physiol. 2004;560:533–549. doi: 10.1113/jphysiol.2004.066597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Paes de Carvalho R, Braas KM, Alder R, Snyder SH. Developmental regulation of adenosine A1 receptors, uptake sites and endogenous adenosine in the chick retina. Brain Res Dev Brain Res. 1992;70:87–95. doi: 10.1016/0165-3806(92)90106-7. [DOI] [PubMed] [Google Scholar]
- 230.Sugioka M, Fukuda Y, Yamashita M. Ca2+ responses to ATP via purinoceptors in the early embryonic chick retina. J Physiol. 1996;493(Pt 3):855–863. doi: 10.1113/jphysiol.1996.sp021428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Nunes PH, Calaza KC, Albuquerque LM, Fragel-Madeira L, Sholl-Franco A, Ventura AL. Signal transduction pathways associated with ATP-induced proliferation of cell progenitors in the intact embryonic retina. Int J Dev Neurosci. 2007;25:499–508. doi: 10.1016/j.ijdevneu.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 232.Sugioka M, Zhou WL, Hofmann HD, Yamashita M. Involvement of P2 purinoceptors in the regulation of DNA synthesis in the neural retina of chick embryo. Int J Dev Neurosci. 1999;17:135–144. doi: 10.1016/s0736-5748(98)00066-5. [DOI] [PubMed] [Google Scholar]
- 233.Sanches G, de Alencar LS, Ventura AL. ATP induces proliferation of retinal cells in culture via activation of PKC and extracellular signal-regulated kinase cascade. Int J Dev Neurosci. 2002;20:21–27. doi: 10.1016/s0736-5748(02)00004-7. [DOI] [PubMed] [Google Scholar]
- 234.Uckermann O, Grosche J, Reichenbach A, Bringmann A. ATP-evoked calcium responses of radial glial (Müller) cells in the postnatal rabbit retina. J Neurosci Res. 2002;70:209–218. doi: 10.1002/jnr.10406. [DOI] [PubMed] [Google Scholar]
- 235.Pearson RA, Catsicas M, Becker DL, Bayley P, Luneborg NL, Mobbs P. Ca2+ signalling and gap junction coupling within and between pigment epithelium and neural retina in the developing chick. Eur J Neurosci. 2004;19:2435–2445. doi: 10.1111/j.0953-816X.2004.03338.x. [DOI] [PubMed] [Google Scholar]
- 236.Pearson RA, Dale N, Llaudet E, Mobbs P. ATP released via gap junction hemichannels from the pigment epithelium regulates neural retinal progenitor proliferation. Neuron. 2005;46:731–744. doi: 10.1016/j.neuron.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 237.Pearson R, Catsicas M, Becker D, Mobbs P. Purinergic and muscarinic modulation of the cell cycle and calcium signaling in the chick retinal ventricular zone. J Neurosci. 2002;22:7569–7579. doi: 10.1523/JNEUROSCI.22-17-07569.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Burgos JS, Barat A, Ramirez G. Guanine nucleotides block agonist-driven 45Ca2+ influx in chick embryo retinal explants. Neuroreport. 2000;11:2303–2305. doi: 10.1097/00001756-200007140-00047. [DOI] [PubMed] [Google Scholar]
- 239.Brändle U, Guenther E, Irrle C, Wheeler-Schilling TH. Gene expression of the P2X receptors in the rat retina. Brain Res Mol Brain Res. 1998;59:269–272. doi: 10.1016/s0169-328x(98)00159-4. [DOI] [PubMed] [Google Scholar]
- 240.Brändle U, Kohler K, Wheeler-Schilling TH. Expression of the P2X7-receptor subunit in neurons of the rat retina. Brain Res Mol Brain Res. 1998;62:106–109. doi: 10.1016/s0169-328x(98)00254-x. [DOI] [PubMed] [Google Scholar]
- 241.Wurm A, Erdmann I, Bringmann A, Reichenbach A, Pannicke T. Expression and function of P2Y receptors on Müller cells of the postnatal rat retina. Glia. 2009;57:1680–1690. doi: 10.1002/glia.20883. [DOI] [PubMed] [Google Scholar]
- 242.Housley GD, Luo L, Ryan AF. Localization of mRNA encoding the P2X2 receptor subunit of the adenosine 5′-triphosphate-gated ion channel in the adult and developing rat inner ear by in situ hybridization. J Comp Neurol. 1998;393:403–414. [PubMed] [Google Scholar]
- 243.Nikolic P, Housley GD, Luo L, Ryan AF, Thorne PR. Transient expression of P2X1 receptor subunits of ATP-gated ion channels in the developing rat cochlea. Brain Res Dev Brain Res. 2001;126:173–182. doi: 10.1016/s0165-3806(00)00149-8. [DOI] [PubMed] [Google Scholar]
- 244.Huang LC, Greenwood D, Thorne PR, Housley GD. Developmental regulation of neuron-specific P2X3 receptor expression in the rat cochlea. J Comp Neurol. 2005;484:133–143. doi: 10.1002/cne.20442. [DOI] [PubMed] [Google Scholar]
- 245.Huang LC, Ryan AF, Cockayne DA, Housley GD. Developmentally regulated expression of the P2X3 receptor in the mouse cochlea. Histochem Cell Biol. 2006;125:681–692. doi: 10.1007/s00418-005-0119-4. [DOI] [PubMed] [Google Scholar]
- 246.Greenwood D, Jagger DJ, Huang LC, Hoya N, Thorne PR, Wildman SS, King BF, Pak K, Ryan AF, Housley GD. P2X receptor signaling inhibits BDNF-mediated spiral ganglion neuron development in the neonatal rat cochlea. Development. 2007;134:1407–1417. doi: 10.1242/dev.002279. [DOI] [PubMed] [Google Scholar]
- 247.Nikolic P, Housley GD, Thorne PR. Expression of the P2X7 receptor subunit of the adenosine 5′-triphosphate-gated ion channel in the developing and adult rat cochlea. Audiol Neuro Otol. 2003;8:28–37. doi: 10.1159/000067891. [DOI] [PubMed] [Google Scholar]
- 248.Lee JH, Heo JH, Kim CH, Chang SO, Kim CS, Oh SH. Changes in P2Y4 receptor expression in rat cochlear outer sulcus cells during development. Hear Res. 2007;228:201–211. doi: 10.1016/j.heares.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 249.Huang LC, Thorne PR, Vlajkovic SM, Housley GD. Differential expression of P2Y receptors in the rat cochlea during development. Purinergic Signal. 2010;6:231–248. doi: 10.1007/s11302-010-9191-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Galindo F, Monjaraz E, Galicia S, Cebada J, Cortés C, Flores A. Functional expression of P2 receptors in the inner ear of chicken embryo. Neurosci Lett. 2013;553:24–28. doi: 10.1016/j.neulet.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 251.Tritsch NX, Yi E, Gale JE, Glowatzki E, Bergles DE. The origin of spontaneous activity in the developing auditory system. Nature. 2007;450:50–55. doi: 10.1038/nature06233. [DOI] [PubMed] [Google Scholar]
- 252.Tritsch NX, Zhang YX, Ellis-Davies G, Bergles DE. ATP-induced morphological changes in supporting cells of the developing cochlea. Purinergic Signal. 2010;6:155–166. doi: 10.1007/s11302-010-9189-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Majumder P, Crispino G, Rodriguez L, Ciubotaru CD, Anselmi F, Piazza V, Bortolozzi M, Mammano F. ATP-mediated cell-cell signaling in the organ of Corti: the role of connexin channels. Purinergic Signal. 2010;6:167–187. doi: 10.1007/s11302-010-9192-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Mammano F. ATP-dependent intercellular Ca2+ signaling in the developing cochlea: facts, fantasies and perspectives. Semin Cell Dev Biol. 2013;24:31–39. doi: 10.1016/j.semcdb.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 255.O’Keeffe MG, Thorne PR, Housley GD, Robson SC, Vlajkovic SM. Developmentally regulated expression of ectonucleotidases NTPDase5 and NTPDase6 and UDP-responsive P2Y receptors in the rat cochlea. Histochem Cell Biol. 2010;133:425–436. doi: 10.1007/s00418-010-0682-1. [DOI] [PubMed] [Google Scholar]
- 256.Zhao HB, Yu N, Fleming CR. Gap junctional hemichannel-mediated ATP release and hearing controls in the inner ear. Proc Natl Acad Sci U S A. 2005;102:18724–18729. doi: 10.1073/pnas.0506481102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Cohen-Salmon M, Ott T, Michel V, Hardelin JP, Perfettini I, Eybalin M, Wu T, Marcus DC, Wangemann P, Willecke K, Petit C. Targeted ablation of connexin26 in the inner ear epithelial gap junction network causes hearing impairment and cell death. Curr Biol. 2002;12:1106–1111. doi: 10.1016/s0960-9822(02)00904-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Xu J, Nicholson BJ. The role of connexins in ear and skin physiology—functional insights from disease-associated mutations. Biochim Biophys Acta. 2013;1828:167–178. doi: 10.1016/j.bbamem.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Gershon MD, Thompson EB. The maturation of neuromuscular function in a multiply innervated structure: development of the longitudinal smooth muscle of the foetal mammalian gut and its cholinergic excitatory, adrenergic inhibitory, and non-adrenergic inhibitory innervation. J Physiol. 1973;234:257–277. doi: 10.1113/jphysiol.1973.sp010345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Crowe R, Burnstock G. Perinatal development of quinacrine-positive neurons in the rabbit gastrointestinal tract. J Auton Nerv Syst. 1981;4:217–230. doi: 10.1016/0165-1838(81)90046-1. [DOI] [PubMed] [Google Scholar]
- 261.Zagorodnyuk V, Hoyle CHV, Burnstock G. An electrophysiological study of developmental changes in the innervation of the guinea-pig taenia coli. Pflugers Arch. 1993;423:427–433. doi: 10.1007/BF00374937. [DOI] [PubMed] [Google Scholar]
- 262.Xiang Z, Burnstock G. Development of nerves expressing P2X3 receptors in the myenteric plexus of rat stomach. Histochem Cell Biol. 2004;122:111–119. doi: 10.1007/s00418-004-0680-2. [DOI] [PubMed] [Google Scholar]
- 263.Loera-Valencia R, Jiménez-Vargas NN, Villalobos EC, Juárez EH, Lomas-Ramos TL, Espinosa-Luna R, Montaño LM, Huizinga JD, Barajas-López C. Expression of P2X3 and P2X5 myenteric receptors varies during the intestinal postnatal development in the guinea pig. Cell Mol Neurobiol. 2014;34:727–736. doi: 10.1007/s10571-014-0055-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Hourani SMO. Postnatal development of purinoceptors in rat visceral smooth muscle preparations. Gen Pharmacol. 1999;32:3–7. doi: 10.1016/s0306-3623(98)00112-8. [DOI] [PubMed] [Google Scholar]
- 265.Giaroni C, Knight GE, Zanetti E, Chiarelli RA, Lecchini S, Frigo G, Burnstock G. Postnatal development of P2 receptors in the murine gastrointestinal tract. Neuropharmacology. 2006;50:690–704. doi: 10.1016/j.neuropharm.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 266.Chinsky JM, Ramamurthy V, Fanslow WC, Ingolia DE, Blackburn MR, Shaffer KT, Higley HR, Trentin JJ, Rudolph FB, Knudsen TB, Kellems RE. Developmental expression of adenosine deaminase in the upper alimentary tract of mice. Differentiation. 1990;42:172–183. doi: 10.1111/j.1432-0436.1990.tb00759.x. [DOI] [PubMed] [Google Scholar]
- 267.García-Alcocer G, Padilla K, Rodríguez A, Miledi R, Berumen LC. Distribution of the purinegic receptors P2X4 and P2X6 during rat gut development. Neurosci Lett. 2012;509:92–95. doi: 10.1016/j.neulet.2011.12.044. [DOI] [PubMed] [Google Scholar]
- 268.Konduri GG, Forman K, Mital S. Characterization of purine receptors in fetal lamb pulmonary circulation. Pediatr Res. 2000;47:114–120. doi: 10.1203/00006450-200001000-00020. [DOI] [PubMed] [Google Scholar]
- 269.Brouns I, Van Genechten J, Hayashi H, Gajda M, Gomi T, Burnstock G, Timmermans J-P, Adriaensen D. Dual sensory innervation of pulmonary neuroepithelial bodies. Am J Respir Cell Mol Biol. 2003;28:275–285. doi: 10.1165/rcmb.2002-0117OC. [DOI] [PubMed] [Google Scholar]
- 270.Rooney SA, Gobran LI. Adenosine and leukotrienes have a regulatory role in lung surfactant secretion in the newborn rabbit. Biochim Biophys Acta. 1988;960:98–106. doi: 10.1016/0005-2760(88)90014-8. [DOI] [PubMed] [Google Scholar]
- 271.Burnstock G, Verkhratsky A. Vas deferens—a model used to establish sympathetic cotransmission. Trends Pharmacol Sci. 2010;31:131–139. doi: 10.1016/j.tips.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 272.Furness JB, Campbell GR, Gillard SM, Malmfors T, Cobb JL, Burnstock G. Cellular studies of sympathetic denervation produced by 6-hydroxydopamine in the vas deferens. J Pharmacol Exp Ther. 1970;174:111–122. [PubMed] [Google Scholar]
- 273.MacDonald A, McGrath JC. Post-natal development of functional neurotransmission in rat vas deferens. Br J Pharmacol. 1984;82:25–34. doi: 10.1111/j.1476-5381.1984.tb16438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Brock JA, Handelsman DJ, Keast JR. Postnatal androgen deprivation dissociates the development of smooth muscle innervation from functional neurotransmission in mouse vas deferens. J Physiol. 2007;581:665–678. doi: 10.1113/jphysiol.2007.128728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Hatori M, Teixeira CC, Debolt K, Pacifici M, Shapiro IM. Adenine nucleotide metabolism by chondrocytes in vitro: role of ATP in chondrocyte maturation and matrix mineralization. J Cell Physiol. 1995;165:468–474. doi: 10.1002/jcp.1041650304. [DOI] [PubMed] [Google Scholar]
- 276.Hung CT, Allen FD, Mansfield KD, Shapiro IM. Extracellular ATP modulates [Ca2+]i in retinoic acid-treated embryonic chondrocytes. Am J Physiol. 1997;272:C1611–C1617. doi: 10.1152/ajpcell.1997.272.5.C1611. [DOI] [PubMed] [Google Scholar]
- 277.Fredholm BB, Lerner U. Metabolism of adenosine and 2′-deoxy-adenosine by fetal mouse calvaria in culture. Med Biol. 1982;60:267–271. [PubMed] [Google Scholar]
- 278.Modderman WE, Weidema AF, Vrijheid-Lammers T, Wassenaar AM, Nijweide PJ. Permeabilization of cells of hemopoietic origin by extracellular ATP4-: elimination of osteoclasts, macrophages, and their precursors from isolated bone cell populations and fetal bone rudiments. Calcif Tissue Int. 1994;55:141–150. doi: 10.1007/BF00297190. [DOI] [PubMed] [Google Scholar]
- 279.Hsu HH. Purification and partial characterization of ATP pyrophosphohydrolase from fetal bovine epiphyseal cartilage. J Biol Chem. 1983;258:3463–3468. [PubMed] [Google Scholar]
- 280.Cuezva JM, Fernández E, Valcarce C, Medina JM. The role of ATP/ADP ratio in the control of hepatic gluconeogenesis during the early neonatal period. Biochim Biophys Acta. 1983;759:292–295. doi: 10.1016/0304-4165(83)90326-4. [DOI] [PubMed] [Google Scholar]
- 281.Gazdzik T, Kaminski M. Evolution of localization of the reactions of adenosine triphosphatase (Mg++-ATP-ase), 5′nucleotidase (5′nt), alkaline phosphatase (AP), and acid phosphatase (AcP) in developing rat testis. I. Physiological conditions. Acta Histochem. 1986;79:199–204. doi: 10.1016/S0065-1281(86)80082-4. [DOI] [PubMed] [Google Scholar]
- 282.Gouyon JB, Guignard JP. Adenosine in the immature kidney. Dev Pharmacol Ther. 1989;13:113–119. doi: 10.1159/000457592. [DOI] [PubMed] [Google Scholar]
- 283.Rolband GC, Furth ED, Staddon JM, Rogus EM, Goldberg AP. Effects of age and adenosine in the modulation of insulin action on rat adipocyte metabolism. J Gerontol. 1990;45:B174–B178. doi: 10.1093/geronj/45.5.b174. [DOI] [PubMed] [Google Scholar]
- 284.Chapal J, Loubatières-Mariani MM, Roye M. Effect of adenosine and phosphated derivatives on insulin release from the newborn dog pancreas. J Physiol Paris. 1981;77:873–875. [PubMed] [Google Scholar]
- 285.Afework M, Burnstock G. Changes in P2Y2 receptor localization on adrenaline- and noradrenaline containing chromaffin cells in the rat adrenal gland during development and ageing. Int J Dev Neurosci. 2005;23:567–573. doi: 10.1016/j.ijdevneu.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 286.Park MK, Garrad RC, Weisman GA, Turner JT. Changes in P2Y1 nucleotide receptor activity during the development of rat salivary glands. Am J Physiol. 1997;272:C1388–C1393. doi: 10.1152/ajpcell.1997.272.4.C1388. [DOI] [PubMed] [Google Scholar]
- 287.Baker OJ, Camden JM, Ratchford AM, Seye CI, Erb L, Weisman GA. Differential coupling of the P2Y1 receptor to Gα14 and Gαq/11 proteins during the development of the rat salivary gland. Arch Oral Biol. 2006;51:359–370. doi: 10.1016/j.archoralbio.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 288.Pác L. Contribution to ontogenesis of Merkel cells. Z Mikrosk Anat Forsch. 1984;98:36–48. [PubMed] [Google Scholar]
- 289.Burnstock G, Wood JN. Purinergic receptors: their role in nociception and primary afferent neurotransmission. Curr Opin Neurobiol. 1996;6:526–532. doi: 10.1016/s0959-4388(96)80060-2. [DOI] [PubMed] [Google Scholar]
- 290.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 291.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Majumder P, Trujillo CA, Lopes CG, Resende RR, Gomes KN, Yuahasi KK, Britto LR, Ulrich H. New insights into purinergic receptor signaling in neuronal differentiation, neuroprotection, and brain disorders. Purinergic Signal. 2007;3:317–331. doi: 10.1007/s11302-007-9074-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Burnstock G, Ulrich H. Purinergic signalling in embryonic and stem cell development. Cell Mol Life Sci. 2011;68:1369–1394. doi: 10.1007/s00018-010-0614-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Ulrich H, Abbracchio MP, Burnstock G. Extrinsic purinergic regulation of neural stem/progenitor cells: implications for CNS development and repair. Stem Cell Rev Rep. 2012;8:755–767. doi: 10.1007/s12015-012-9372-9. [DOI] [PubMed] [Google Scholar]
- 295.McBurney MW. P19 embryonal carcinoma cells. Int J Dev Biol. 1993;37:135–140. [PubMed] [Google Scholar]
- 296.Tarnok A, Ulrich H. Characterization of pressure-induced calcium response in neuronal cell lines. Cytometry. 2001;43:175–181. doi: 10.1002/1097-0320(20010301)43:3<175::aid-cyto1046>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 297.Martins AH, Resende RR, Majumder P, Faria M, Casarini DE, Tarnok A, Colli W, Pesquero JB, Ulrich H. Neuronal differentiation of P19 embryonal carcinoma cells modulates kinin B2 receptor gene expression and function. J Biol Chem. 2005;280:19576–19586. doi: 10.1074/jbc.M502513200. [DOI] [PubMed] [Google Scholar]
- 298.Ulrich H, Majumder P. Neurotransmitter receptor expression and activity during neuronal differentiation of embryonal carcinoma and stem cells: from basic research towards clinical applications. Cell Prolif. 2006;39:281–300. doi: 10.1111/j.1365-2184.2006.00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Resende RR, Britto LR, Ulrich H. Pharmacological properties of purinergic receptors and their effects on proliferation and induction of neuronal differentiation of P19 embryonal carcinoma cells. Int J Dev Neurosci. 2008;26:763–777. doi: 10.1016/j.ijdevneu.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 300.Heo JS, Han HJ. ATP stimulates mouse embryonic stem cell proliferation via protein kinase C, phosphatidylinositol 3-kinase/Akt, and mitogen-activated protein kinase signaling pathways. Stem Cells. 2006;24:2637–2648. doi: 10.1634/stemcells.2005-0588. [DOI] [PubMed] [Google Scholar]
- 301.Delic J, Zimmermann H. Nucleotides affect neurogenesis and dopaminergic differentiation of mouse fetal midbrain-derived neural precursor cells. Purinergic Signal. 2010;6:417–428. doi: 10.1007/s11302-010-9206-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Resende RR, Majumder P, Gomes KN, Britto LR, Ulrich H. P19 embryonal carcinoma cells as in vitro model for studying purinergic receptor expression and modulation of N-methyl-D-aspartate-glutamate and acetylcholine receptors during neuronal differentiation. Neuroscience. 2007;146:1169–1181. doi: 10.1016/j.neuroscience.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 303.da Silva RL, Resende RR, Ulrich H. Alternative splicing of P2X6 receptors in developing mouse brain and during in vitro neuronal differentiation. Exp Physiol. 2007;92:139–145. doi: 10.1113/expphysiol.2006.921304. [DOI] [PubMed] [Google Scholar]
- 304.Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 305.Martins AH, Alves JM, Trujillo CA, Schwindt TT, Barnabé GF, Motta FL, Guimarães AO, Casarini DE, Mello LE, Pesquero JB, Ulrich H. Kinin-B2 receptor expression and activity during differentiation of embryonic rat neurospheres. Cytometry A. 2008;73:361–368. doi: 10.1002/cyto.a.20519. [DOI] [PubMed] [Google Scholar]
- 306.Trujillo CA, Schwindt TT, Martins AH, Alves JM, Mello LE, Ulrich H. Novel perspectives of neural stem cell differentiation: from neurotransmitters to therapeutics. Cytometry A. 2009;75:38–53. doi: 10.1002/cyto.a.20666. [DOI] [PubMed] [Google Scholar]
- 307.Schwindt TT, Trujillo CA, Negraes PD, Lameu C, Ulrich H. Directed differentiation of neural progenitors into neurons is accompanied by altered expression of P2X purinergic receptors. J Mol Neurosci. 2011;44:141–146. doi: 10.1007/s12031-010-9417-y. [DOI] [PubMed] [Google Scholar]
- 308.Ryu JK, Choi HB, Hatori K, Heisel RL, Pelech SL, McLarnon JG, Kim SU. Adenosine triphosphate induces proliferation of human neural stem cells: role of calcium and p70 ribosomal protein S6 kinase. J Neurosci Res. 2003;72:352–362. doi: 10.1002/jnr.10507. [DOI] [PubMed] [Google Scholar]
- 309.Thompson BA, Storm MP, Hewinson J, Hogg S, Welham MJ, MacKenzie AB. A novel role for P2X7 receptor signalling in the survival of mouse embryonic stem cells. Cell Signal. 2012;24:770–778. doi: 10.1016/j.cellsig.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Glaser T, de Oliveira SL, Cheffer A, Beco R, Martins P, Fornazari M, Lameu C, Junior HM, Coutinho-Silva R, Ulrich H. Modulation of mouse embryonic stem cell proliferation and neural differentiation by the P2X7 receptor. PLoS One. 2014;9:e96281. doi: 10.1371/journal.pone.0096281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Padmanabhan R, Chen KG, Gillet JP, Handley M, Mallon BS, Hamilton RS, Park K, Varma S, Mehaffey MG, Robey PG, McKay RD, Gottesman MM. Regulation and expression of the ATP-binding cassette transporter ABCG2 in human embryonic stem cells. Stem Cells. 2012;30:2175–2187. doi: 10.1002/stem.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Cheung K-K, Ryten M, Burnstock G. Abundant and dynamic expression of G protein-coupled P2Y receptors in mammalian development. Dev Dyn. 2003;228:254–266. doi: 10.1002/dvdy.10378. [DOI] [PubMed] [Google Scholar]
- 313.Zhu Y, Kimelberg HK. Developmental expression of metabotropic P2Y1 and P2Y2 receptors in freshly isolated astrocytes from rat hippocampus. J Neurochem. 2001;77:530–541. doi: 10.1046/j.1471-4159.2001.00241.x. [DOI] [PubMed] [Google Scholar]
- 314.Weissman TA, Riquelme PA, Ivic L, Flint AC, Kriegstein AR. Calcium waves propagate through radial glial cells and modulate proliferation in the developing neocortex. Neuron. 2004;43:647–661. doi: 10.1016/j.neuron.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 315.Langer D, Ikehara Y, Takebayashi H, Hawkes R, Zimmermann H. The ectonucleotidases alkaline phosphatase and nucleoside triphosphate diphosphohydrolase 2 are associated with subsets of progenitor cell populations in the mouse embryonic, postnatal and adult neurogenic zones. Neuroscience. 2007;150:863–879. doi: 10.1016/j.neuroscience.2007.07.064. [DOI] [PubMed] [Google Scholar]
- 316.Cognato GP, Czepielewski RS, Sarkis JJ, Bogo MR, Bonan CD. Expression mapping of ectonucleotide pyrophosphatase/phosphodiesterase 1-3 (E-NPP1-3) in different brain structures during rat development. Int J Dev Neurosci. 2008;26:593–598. doi: 10.1016/j.ijdevneu.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 317.Stanojevic I, Drakulic D, Petrovi S, Milosevic M, Jovanovic N, Horvat A. Kinetic characterization of ecto-nucleoside triphosphate diphosphohydrolases in brain nerve terminals during rat postnatal development. Russ J Phys Chem. 2011;85:2416–2421. [Google Scholar]
- 318.Lee DA, Bedont JL, Pak T, Wang H, Song J, Miranda-Angulo A, Takiar V, Charubhumi V, Balordi F, Takebayashi H, Aja S, Ford E, Fishell G, Blackshaw S. Tanycytes of the hypothalamic median eminence form a diet-responsive neurogenic niche. Nat Neurosci. 2012;15:700–702. doi: 10.1038/nn.3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Haan N, Goodman T, Najdi-Samiei A, Stratford CM, Rice R, El Agha E, Bellusci S, Hajihosseini MK. Fgf10-expressing tanycytes add new neurons to the appetite/energy-balance regulating centers of the postnatal and adult hypothalamus. J Neurosci. 2013;33:6170–6180. doi: 10.1523/JNEUROSCI.2437-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Robins SC, Stewart I, McNay DE, Taylor V, Giachino C, Goetz M, Ninkovic J, Briancon N, Maratos-Flier E, Flier JS, Kokoeva MV, Placzek M. α-Tanycytes of the adult hypothalamic third ventricle include distinct populations of FGF-responsive neural progenitors. Nat Commun. 2013;4:2049. doi: 10.1038/ncomms3049. [DOI] [PubMed] [Google Scholar]
- 321.Frayling C, Britton R, Dale N. ATP-mediated glucosensing by hypothalamic tanycytes. J Physiol. 2011;589:2275–2286. doi: 10.1113/jphysiol.2010.202051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Bolborea M, Dale N. Hypothalamic tanycytes: potential roles in the control of feeding and energy balance. Trends Neurosci. 2013;36:91–100. doi: 10.1016/j.tins.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Virus RM, Baglajewski T, Radulovacki M. [3H]N6-(L-Phenylisopropyl) adenosine binding in brains from young and old rats. Neurobiol Aging. 1984;5:61–62. doi: 10.1016/0197-4580(84)90087-3. [DOI] [PubMed] [Google Scholar]
- 324.Bauman LA, Mahle CD, Boissard CG, Gribkoff VK. Age-dependence of effects of A1 adenosine receptor antagonism in rat hippocampal slices. J Neurophysiol. 1992;68:629–638. doi: 10.1152/jn.1992.68.2.629. [DOI] [PubMed] [Google Scholar]
- 325.Costenla AR, de Mendonça A, Ribeiro JA. Adenosine modulates synaptic plasticity in hippocampal slices from aged rats. Brain Res. 1999;851:228–234. doi: 10.1016/s0006-8993(99)02194-0. [DOI] [PubMed] [Google Scholar]
- 326.Prediger RD, Batista LC, Takahashi RN. Caffeine reverses age-related deficits in olfactory discrimination and social recognition memory in rats. Involvement of adenosine A1 and A2A receptors. Neurobiol Aging. 2005;26:957–964. doi: 10.1016/j.neurobiolaging.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 327.Markram H, Lübke J, Frotscher M, Roth A, Sakmann B. Physiology and anatomy of synaptic connections between thick tufted pyramidal neurones in the developing rat neocortex. J Physiol. 1997;500:409–440. doi: 10.1113/jphysiol.1997.sp022031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Reyes A, Sakmann B. Developmental switch in the short-term modification of unitary EPSPs evoked in layer 2/3 and layer 5 pyramidal neurons of rat neocortex. J Neurosci. 1999;19:3827–3835. doi: 10.1523/JNEUROSCI.19-10-03827.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Kerr MI, Wall MJ, Richardson MJ. Adenosine A1 receptor activation mediates the developmental shift at layer 5 pyramidal cell synapses and is a determinant of mature synaptic strength. J Physiol. 2013;591:3371–3380. doi: 10.1113/jphysiol.2012.244392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Pazzagli M, Corsi C, Fratti S, Pedata F, Pepeu G. Regulation of extracellular adenosine levels in the striatum of aging rats. Brain Res. 1995;684:103–106. doi: 10.1016/0006-8993(95)00471-2. [DOI] [PubMed] [Google Scholar]
- 331.Corsi C, Melani A, Bianchi L, Pepeu G, Pedata F. Striatal A2A adenosine receptors differentially regulate spontaneous and K+-evoked glutamate release in vivo in young and aged rats. Neuroreport. 1999;10:687–691. doi: 10.1097/00001756-199903170-00005. [DOI] [PubMed] [Google Scholar]
- 332.Corsi C, Melani A, Bianchi L, Pepeu G, Pedata F. Effect of adenosine A2A receptor stimulation on GABA release from the striatum of young and aged rats in vivo. Neuroreport. 1999;10:3933–3937. doi: 10.1097/00001756-199912160-00038. [DOI] [PubMed] [Google Scholar]
- 333.Cunha RA, Constantino MC, Sebastiao AM, Ribeiro JA. Modification of A1 and A2a adenosine receptor binding in aged striatum, hippocampus and cortex of the rat. Neuroreport. 1995;6:1583–1588. doi: 10.1097/00001756-199507310-00029. [DOI] [PubMed] [Google Scholar]
- 334.Giovannelli L, Giovannini MG, Pedata F, Pepeu G. Purinergic modulation of cortical acetylcholine release is decreased in aging rats. Exp Gerontol. 1988;23:175–181. doi: 10.1016/0531-5565(88)90004-6. [DOI] [PubMed] [Google Scholar]
- 335.Cheng J-T, Liu I-M, Juang S-W, Jou S-B. Decrease of adenosine A-1 receptor gene expression in cerebral cortex of aged rats. Neurosci Lett. 2000;283:227–229. doi: 10.1016/s0304-3940(00)00961-7. [DOI] [PubMed] [Google Scholar]
- 336.Sebastiao AM, Cunha RA, de Mendonça A, Ribeiro JA. Modification of adenosine modulation of synaptic transmission in the hippocampus of aged rats. Br J Pharmacol. 2000;131:1629–1634. doi: 10.1038/sj.bjp.0703736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Lopes LV, Cunha RA, Ribeiro JA. Cross talk between A1 and A2A adenosine receptors in the hippocampus and cortex of young adult and old rats. J Neurophysiol. 1999;82:3196–3203. doi: 10.1152/jn.1999.82.6.3196. [DOI] [PubMed] [Google Scholar]
- 338.Lalo U, Palygin O, North RA, Verkhratsky A, Pankratov Y. Age-dependent remodelling of ionotropic signalling in cortical astroglia. Aging Cell. 2011;10:392–402. doi: 10.1111/j.1474-9726.2011.00682.x. [DOI] [PubMed] [Google Scholar]
- 339.Ashton KJ, Nilsson U, Willems L, Holmgren K, Headrick JP. Effects of aging and ischemia on adenosine receptor transcription in mouse myocardium. Biochem Biophys Res Commun. 2003;312:367–372. doi: 10.1016/j.bbrc.2003.10.127. [DOI] [PubMed] [Google Scholar]
- 340.Mudumbi RV, Olson RD, Hubler BE, Montamat SC, Vestal RE. Age-related effects in rabbit hearts of N6-R-phenylisopropyladenosine, an adenosine A1 receptor agonist. J Gerontol A Biol Sci Med Sci. 1995;50:B351–B357. doi: 10.1093/gerona/50a.6.b351. [DOI] [PubMed] [Google Scholar]
- 341.Burnstock G, Ralevic V. Purinergic signalling and blood vessels in health and disease. Pharmacol Rev. 2014;66:102–192. doi: 10.1124/pr.113.008029. [DOI] [PubMed] [Google Scholar]
- 342.Bao JX, Eriksson IE, Stjärne L. Age-related variations in the relative importance of noradrenaline and ATP as mediators of the contractile response of rat tail artery to sympathetic nerve stimulation. Acta Physiol Scand. 1989;136:287–288. doi: 10.1111/j.1748-1716.1989.tb08663.x. [DOI] [PubMed] [Google Scholar]
- 343.Wallace A, Knight GE, Cowen T, Burnstock G. Changes in function and expression of P2X and P2Y receptors in the rat tail and mesenteric arteries during maturation and aging. Neuropharmacology. 2006;50:191–208. doi: 10.1016/j.neuropharm.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 344.Konishi C, Naito Y, Ohara N. Age-related changes in adenosine 5′-triphosphate-induced constriction of isolated, perfused mesenteric arteries of rats. Life Sci. 1999;64:1265–1273. doi: 10.1016/s0024-3205(99)00061-2. [DOI] [PubMed] [Google Scholar]
- 345.Thompson CS, Kenney WL. Altered neurotransmitter control of reflex vasoconstriction in aged human skin. J Physiol. 2004;558:697–704. doi: 10.1113/jphysiol.2004.065714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Mooradian AD, Grabau G, Bastani B. Adenosine triphosphatases of rat cerebral microvessels. Effect of age and diabetes mellitus. Life Sci. 1994;55:1261–1265. doi: 10.1016/0024-3205(94)90064-7. [DOI] [PubMed] [Google Scholar]
- 347.Mortensen SP, Nyberg M, Winding K, Saltin B. Lifelong physical activity preserves functional sympatholysis and purinergic signalling in the ageing human leg. J Physiol. 2012;590:6227–6236. doi: 10.1113/jphysiol.2012.240093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Suadicani SO, Urban-Maldonado M, Tar MT, Melman A, Spray DC. Effects of ageing and streptozotocin-induced diabetes on connexin43 and P2 purinoceptor expression in the rat corpora cavernosa and urinary bladder. BJU Int. 2009;103:1686–1693. doi: 10.1111/j.1464-410X.2008.08337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Saito M, Gotoh M, Kato K, Kondo A. Influence of aging on the rat urinary bladder function. Urol Int. 1991;47:39–42. doi: 10.1159/000282247. [DOI] [PubMed] [Google Scholar]
- 350.Yoshida M, Miyamae K, Iwashita H, Otani M, Inadome A. Management of detrusor dysfunction in the elderly: changes in acetylcholine and adenosine triphosphate release during aging. Urology. 2004;63:17–23. doi: 10.1016/j.urology.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 351.Wuest M, Morgenstern K, Graf EM, Braeter M, Hakenberg OW, Wirth MP, Ravens U. Cholinergic and purinergic responses in isolated human detrusor in relation to age. J Urol. 2005;173:2182–2189. doi: 10.1097/01.ju.0000158126.53702.e4. [DOI] [PubMed] [Google Scholar]
- 352.Daly DM, Nocchi L, Liaskos M, McKay NG, Chapple C, Grundy D. Age-related changes in afferent pathways and urothelial function in the male mouse bladder. J Physiol. 2014;592:537–549. doi: 10.1113/jphysiol.2013.262634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Pinna C, Rubino A, Burnstock G. Age-related changes in purinergic and adrenergic components of sympathetic neurotransmission in guinea-pig seminal vesicles. Br J Pharmacol. 1997;122:1411–1416. doi: 10.1038/sj.bjp.0701543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Coutinho-Silva R, Parsons M, Robson T, Burnstock G. Changes in expression of P2 receptors in rat and mouse pancreas during development and aging. Cell Tissue Res. 2001;306:373–383. doi: 10.1007/s004410100458. [DOI] [PubMed] [Google Scholar]
- 355.Vestal RE, Wood AJ, Shand DG. Reduced β-adrenoceptor sensitivity in the elderly. Clin Pharmacol Ther. 1979;26:181–186. doi: 10.1002/cpt1979262181. [DOI] [PubMed] [Google Scholar]
- 356.Sawmiller DR, Fenton RA, Dobson JG., Jr Myocardial adenosine A1 and A2 receptor activities during juvenile and adult stages of development. Am J Physiol. 1996;271:H235–H243. doi: 10.1152/ajpheart.1996.271.1.H235. [DOI] [PubMed] [Google Scholar]
- 357.Cai G, Wang HY, Gao E, Horwitz J, Snyder DL, Pelleg A, Roberts J, Friedman E. Reduced adenosine A1 receptor and Gα protein coupling in rat ventricular myocardium during aging. Circ Res. 1997;81:1065–1071. [PubMed] [Google Scholar]
- 358.Gao E, Snyder DL, Roberts J, Friedman E, Cai G, Pelleg A, Horwitz J. Age-related decline in beta adrenergic and adenosine A1 receptor function in the heart are attenuated by dietary restriction. J Pharmacol Exp Ther. 1998;285:186–192. [PubMed] [Google Scholar]
- 359.Miao LY, Tang JP, Esposito DP, Zhang JH. Age-related changes in P2 receptor mRNA of rat cerebral arteries. Exp Gerontol. 2001;37:67–79. doi: 10.1016/s0531-5565(01)00159-0. [DOI] [PubMed] [Google Scholar]
- 360.Hill CE, Phillips JK, Sandow SL. Development of peripheral autonomic synapses: neurotransmitter receptors, neuroeffector associations and neural influences. Clin Exp Pharmacol Physiol. 1999;26:581–590. doi: 10.1046/j.1440-1681.1999.03092.x. [DOI] [PubMed] [Google Scholar]
- 361.Burnstock G. Purinoceptors: ontogeny and phylogeny. Drug Dev Res. 1996;39:204–242. [Google Scholar]
- 362.Burnstock G, Verkhratsky A. Purinergic signalling and the nervous system. Heidelberg/Berlin: Springer; 2012. pp. 1–715. [Google Scholar]
- 363.Verkhratsky A, Burnstock G. Biology of purinergic signalling: its ancient evolutionary roots, its omnipresence and its multiple functional significance. BioEssays. 2014;36:697–705. doi: 10.1002/bies.201400024. [DOI] [PubMed] [Google Scholar]
- 364.Zuber ME, Gestri G, Viczian AS, Barsacchi G, Harris WA. Specification of the vertebrate eye by a network of eye field transcription factors. Development. 2003;130:5155–5167. doi: 10.1242/dev.00723. [DOI] [PubMed] [Google Scholar]