Abstract
CD39/ENTPD1 is a prototypic member of the ectonucleoside triphosphate diphosphohydrolase (ENTPDase) family on cell surface. CD39 has been reported to be a marker of regulatory immune cells and catalyzes extracellular hydrolysis of nucleotides to generate AMP and, in tandem with CD73, adenosine. We have recently found in addition that co-expression of CD39 and CD161 by human CD4+ T cells may become a biomarker of human Th17 cells. CD39 and CD161 have direct interactions that are further linked with acid sphingomyelinase (ASM). Upon activation of CD39 and CD161, the molecular interactions boost ASM bio-activity, which generates cellular ceramide to further mediate downstream signals inclusive of STAT3 and mTOR. We suggest modulation of human Th17 responsiveness by CD39 and CD161 and describe novel molecular mechanisms integrating elements of both extracellular nucleotide and sphingolipid homeostasis that are pivotal in the control of human Th17 cells and which could have therapeutic potential.
Keywords: Keywods, CD39, CD161, Th17 cells
Editorial
Recently, we have presented our novel findings that CD39 and CD161 expression may serve as surface maker of human interleukin-17 (IL-17)-producing Th cells (Th17). CD39 and CD161 modulate human Th17 responsiveness through alterations in acid sphingomyelinase (ASM) bioactivities and activation of the downstream signals inclusive of signal transducer and activator of transcription 3 (STAT3) and mammalian target of rapamycin (mTOR).
T helper cells play pivotal roles in adaptive immune responses. Among those Th cells, Th17 cells are critical for host protective defense and also contribute to autoimmune responses and related disorders [1]. Generation of Th17 cells requires appropriate differentiation conditions including essential cytokine milieu (i.e., IL-6 and transforming growth factor-β (TGFβ)), in the presence of MHC stimulation by dendritic cells (DC) [1]. The differentiation conditions above can drive murine naive CD4+ T cells to Th17 polarization. However, in humans, only memory CD4+ T cells, other than naive cells, can be induced to produce abundant IL-17 [2]. Thus, it needs to be elucidated how human memory CD4+ T cells expand and proliferate to become IL-17-producing cells.
Because of lack of relevant surface markers for human Th17 cells, the process of expansion of Th17 cells is not clearly understood. Recently, CD161 has been noted to be a relatively phenotypic marker of human IL-17-producing CD4+ T cells [3]. Upon stimulation, CD4+CD161+ T cells exhibit an activated Th17 phenotype, with increased expression of IL-17, which mediates destructive tissue inflammation [3]. However, the mechanisms of CD161 in association with Th17 cells are not well studied.
CD39/ENTPD1, a cell surface ectonucleosidase, has been shown to be relevant to immune responses. For example, in mice, CD39 expression distinguishes two subpopulations of CD4+ T cells. One subset also expresses CD73/ecto-5′-nucleotidase and defines regulatory T cells (Treg) [4]. The other subset lacking CD73 expression expresses memory phenotype and secretes proinflammatory cytokines upon activation, typically representative of Th1, Th2, and Th17 effector subtypes [5]. In humans, it has been described that CD39 expression by CD4+ T cells also comprises regulatory T lymphocytes and other memory CD4+ cell populations. The latter, seemingly pathogenic cell populations, have the capability to release proinflammatory cytokines, e.g., IL-17 [6].
Recently, we suggest that dual expression of CD39 and CD161 better defines Th17 lineages of human CD4+ T cells [7]. We also show that blood and lamina propria levels of CD39+CD161+ CD4+ T cells reflect the disease activity of Crohn’s disease [7]. These findings support the notion of CD4+CD39+CD161+ T cells perhaps developing as Th17 precursors, and expanding further to proinflammatory Th17 cells at sites of disease, as in patients with Crohn’s disease.
ASM, a lipid hydrolase, plays a pivotal role in mediating a variety of signals by degrading sphingomyelin into ceramide [8]. Ceramide is an important messenger in response to cell proliferation, differentiation, and apoptosis. It has been reported that CD161 directly interacts with ASM in NK cells. The interaction can activate ASM enzymatic activity and induce subsequent intracellular signals inclusive of protein kinase B (Akt) and, thus, regulate NK cell function [8]. We have further confirmed that in human CD4+ T cells, ASM directly interacts with CD39 and CD161, respectively. Upon activation, both stimulations of CD39 and CD161 by crosslinked antibodies can initiate ASM enzymatic activity, increase cellular ceramide production, and impact downstream signaling components of STAT3 and mTOR [7], which are the indispensible elements for Th17 generation [1].
To explore the functionalities of ASM/ceramide in Th17 proliferation and generation, we determined ASM activity during human Th17 development. Production of ceramide by CD4+ T cells was dramatically induced under Th17 polarization conditions. This change was concomitant with sustained activation of STAT3 and mTOR signals [7]. However, inhibition of ASM activity in CD4+ T cells by ASM inhibitors or knockdown of ASM substantially abrogated ceramide generation and subsequently blocked STAT3 and mTOR activation, which consequently inhibited IL-17+ cell induction/expansion [7]. These results indicate that ASM activity is the upstream of STAT3 and mTOR, the integral components of Th17 generation.
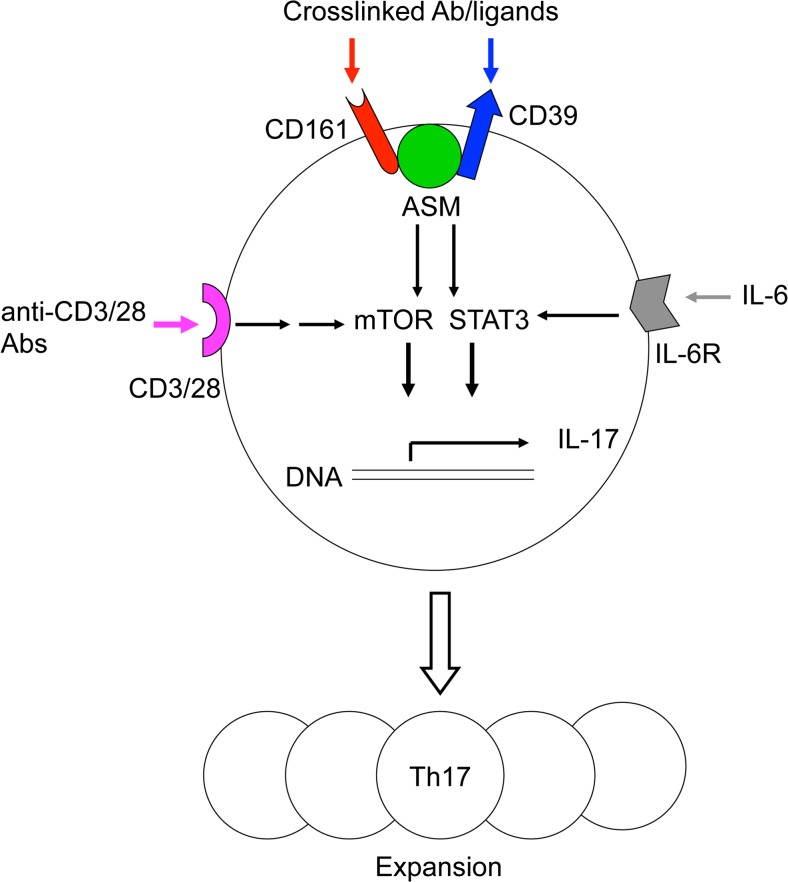
In summary, we identified a novel mechanism through which both CD39 and CD161 mediate ASM activity and downstream signals and determine Th17 proliferation, as shown in Fig. 1. Under certain differentiation condition, intracellular signals in CD4+ T cells including mTOR and STAT3 are activated by MHC/CD3/28 and IL-6/IL-6R stimulations. Meanwhile, ASM mediates stimulations of CD39 and CD161 by crosslinked antibodies or their potential ligands, which further boost mTOR and STAT3 signals. Once activated by upstream stimulations above, the integrated signals inclusive of STAT3 and mTOR eventually induce IL-17 expression and drive Th17 cell expansion. Together, the data suggest a novel molecular link between CD39/CD161 co-expression and ASM-dependent ceramide signaling in promoting Th17 expansion and responses of inflammatory diseases, inclusive of Crohn’s disease. Given the molecular signatures and signals of Th17 cells, strategies to regulate CD39 and CD161 signaling may provide new alternatives to the current clinical approaches to suppress Th17 responsiveness of inflammatory diseases.
Fig. 1.
ASM mediates CD39 and CD161 intracellular signals and drives Th17 expansion. Under Th17 differentiation condition, MHC/CD3/28 and IL-6/IL-6R stimulations activate mTOR and STAT3 signals in CD4+ T cells. Meanwhile, stimulations of CD39 and CD161 further amplify ASM-mediated mTOR and STAT3 signals, which eventually drive Th17 expansion
Acknowledgments
This study was supported by funds from the National Institute of Health (NHLBI PO1-HL076540 and RO1-HL094400) and National Natural Science Foundation of China (No. 81470828, 81270472, and 81070310).
Contributor Information
Aiping Bai, Email: baiap@163.com.
Simon Robson, Email: srobson@bidmc.harvard.edu.
References
- 1.Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discovery. 2012;11:763–776. doi: 10.1038/nrd3794. [DOI] [PubMed] [Google Scholar]
- 2.van Beelen AJ, Zelinkova Z, Taanman-Kueter EW, Muller FJ, Hommes DW, Zaat SA, Kapsenberg ML, de Jong EC. Stimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin-17 production in human memory T cells. Immunity. 2007;27:660–669. doi: 10.1016/j.immuni.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 3.Kleinschek MA, Boniface K, Sadekova S, Grein J, Murphy EE, Turner SP, Raskin L, Desai B, Faubion WA, de Waal Malefyt R, Pierce RH, McClanahan T, Kastelein RA. Circulating and gut-resident human Th17 cells express CD161 and promote intestinal inflammation. J Exp Med. 2009;206:525–534. doi: 10.1084/jem.20081712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, Kuchroo VK, Strom TB, Robson SC. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou Q, Yan J, Putheti P, Wu Y, Sun X, Toxavidis V, Tigges J, Kassam N, Enjyoji K, Robson SC, Strom TB, Gao W. Isolated CD39 expression on CD4+ T cells denotes both regulatory and memory populations. Am J Transplant. 2009;9:2303–2311. doi: 10.1111/j.1600-6143.2009.02777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dwyer KM, Hanidziar D, Putheti P, Hill PA, Pommey S, McRae JL, Winterhalter A, Doherty G, Deaglio S, Koulmanda M, Gao W, Robson SC, Strom TB. Expression of CD39 by human peripheral blood CD4+ CD25+ T cells denotes a regulatory memory phenotype. Am J Transplant. 2010;10:2410–2420. doi: 10.1111/j.1600-6143.2010.03291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bai A, Moss A, Kokkotou E, Usheva A, Sun X, Cheifetz A, Zheng Y, Longhi MS, Gao W, Wu Y, Robson SC. CD39 and CD161 modulate Th17 responses in Crohn’s disease. J Immunol. 2014;193:3366–3377. doi: 10.4049/jimmunol.1400346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pozo D, Vales-Gomez M, Mavaddat N, Williamson SC, Chisholm SE, Reyburn H. CD161 (human NKR-P1A) signaling in NK cells involves the activation of acid sphingomyelinase. J Immunol. 2006;176:2397–2406. doi: 10.4049/jimmunol.176.4.2397. [DOI] [PubMed] [Google Scholar]