Abstract
Cytokines such as tumor necrosis factor alpha (TNF-α)-induced expression of matrix metalloproteinase (MMP) play a pivotal role in the destruction of articular cartilage in patients who are suffering from osteoarthritis (OA). Collagen type II, the basis for articular cartilage, can be degraded by MMP-1, MMP-3, and 13. EGb761, the standardized extract of Ginkgo biloba produced by Dr. Willar Schwabe Pharmaceuticals, has shown its anti-inflammatory capacity. This study aimed to determine a mechanism whereby EGb761 may inhibit cartilage degradation. Our results indicated that pretreatment with EGb761 abolishes MMP-1, MMP-3, and MMP-13 gene expression and protein expression induced by TNF-α in human chondrocyte monolayer. In addition, the reduction of the tissue inhibitor of metalloproteinase-1(TIMP-1) and metalloproteinase-2 gene expression induced by TNF-α was rescued by pretreatment with EGb761. Importantly, TNF-α-induced degradation of collagen type II was ameliorated by EGb761 in a dose-dependent manner. Mechanistically, our results indicated that EGb761 treatment attenuated TNF-α-induced NF-κB activation. These actions of EGb761 suggest a mechanism by which EGb761 may act to prevent cartilage breakdown in arthritis.
Keywords: Osteoarthritis, EGb761, Matrix metalloproteinase, Collagen type II, NF-κB
Introduction
Osteoarthritis (OA) is the most common degenerative articular disease, with ageing and excessive loading as important risk factors (Aigner et al. 2003). The progressive degradation of articular cartilage is the main feature of OA. The equilibrium between physiologic synthesis and degradation of articular cartilage is disrupted during the progression of OA. The metabolism balance of extracellular matrix (ECM) is disturbed by many factors in OA (Aigner et al. 2002). Increasing evidence has shown that the activation of degradative enzymes, such as the matrix metalloproteinases (MMPs) leads to the loss and degradation of proteoglycans and collagen in articular cartilage. Induction of MMPs by pro-inflammatory cytokines plays a pivotal role in the destruction of articular cartilage in patients who are suffering from OA (Kullich et al. 2007). Among the various matrix metalloproteinase, MMP-1 and MMP-13 are collagenases. MMP-3 is one of the stromelysins, which degrades proteoglycans and activate procollagenase in articular cartilage (Blom et al. 2007). In addition to MMP-3, MMP-1 and MMP-13 have been associated with the destruction of cartilage in OA. MMP-1 and MMP-13 are commonly detected metalloproteinases in synovial fluid from patients suffering from OA (Freemont et al. 1997). Collagen type II is the main component of healthy cartilage matrix. Notably, MMP-13 is more restricted to connective tissue, which not only targets type II collagen in cartilage for degradation but also degrades proteoglycan, types IV and type IX collagen, osteonectin, and perlecan in cartilage (Shiomi et al. 2010). The tissue inhibitor of metalloproteinase-1 (TIMP-1) and TIMP-2 are the two important inhibitors of MMP-1, MMP-3, and MMP-13 (Flannelly et al. 2002). The up-regulation of MMPs by TNF-α in chondrocytes is thought to mediate by the activation of several transcription factors such as nuclear factor-κB (NF-κB), AP-1, and so on which are involved in stress- or inflammation-induced signaling. NF-κB plays a critical role in the activation of MMP promoters in response to inflammatory stimuli such as TNF-α (Liacini et al. 2003). Disturbing the activation of MMPs induced by pro-inflammatory cytokine activity may reduce the cartilage degradation in OA.
Ginkgo biloba, also named maidenhair tree, is a type of medicinal herb used in traditional Chinese medicine for thousands of years. EGb761 is a standardized extract of Ginkgo biloba leaves, which is a well defined product produced by Dr. Willar Schwabe Pharmaceuticals and contains approximately 24 % flavone glycosides (primarily quercetin, kaempferol, and isorhamnetin) and 6 % terpene lactones (2.8–3.4 % ginkgolides A, B, and C and 2.6–3.2 % bilobalide) (EGb 761 2003). EGb761 was found to exhibit potential beneficial effects to patients with Alzheimer’s disease (AD) (Muller et al. 2012). As a free radical scavenger, EGb761 has antioxidant properties. Multiple lines of evidence have shown the anti-inflammatory effects of EGb761. Ginkgo biloba exerts an anti-phlogistic effect on inflammatory cells mostly by suppressing the production of active oxygen and nitrogen species (Yoshikawa et al. 1999). Han and colleagues reported that Ginkgo biloba extracts treatment displays marked anti-inflammatory activity in the carrageenan model of paw edema (Han. 2005). Interestingly, Ginkgo biloba extract inhibits production of pro-inflammatory cytokines IL-1β and TNF-α but up-regulated the production of anti-inflammatory cytokines, IL-10 and IL-10R in brain, suggesting its anti-inflammatory capacity (Jiao et al. 2005). Notably, anti-inflammatory effects of Ginkgo biloba have been implied in articular tissues. Administration of Ginkgo biloba significantly attenuated Candida albicans-caused arthritic inflammation by blocking of the NO production from the macrophages that infiltrate to the inflamed site (Lim et al. 2006). EGb761 exerts the anti-inflammatory effects on human articular chondrocytes and OA rats by reducing PGE2 and NO levels in blood, the histological alterations, COX-2, and nitrotyrosine expressions in cartilages (Chen et al. 2013). However, the effects of EGb761 on inflammatory chondrocytes and OA remain unclear. Whether EGb761 inhibits the activation of MMPs is unknown.
Materials and methods
Cell cultures
Human subject researches were performed with written informed consent from the institutional Ethics Committee at Weifang Medical University and also from the patients. Normal specimens of knee joint cartilage from patients who were generally healthy undergoing joint replacement surgery were used to isolate human articular chondrocytes. Chondrocytes were isolated by sequential enzymatic digestion at 37 °C with 0.2 % collagenase (type II; Sigma-Aldrich, St. Louis, MO) in Dulbecco’s modified Eagle’s medium (DMEM). Isolated chondrocytes were grown in DMEM (GIBCO-BRL, USA) medium supplemented with 10 % fetal bovine serum (FBS) and 1 % antibiotics (penicillin–streptomycin) in a humid incubator with 95 % air–5 % CO2 at 37 °C.
RNA isolation and quantitative real-time PCR
Total RNA from chondrocytes was extracted using the TRIzol reagent (Invitrogen, USA) according to the manufacturer’s instructions. Complementary DNA (cDNA) was synthesized by reverse transcription of RNA (1 μg) using iScript™ Advanced cDNA Synthesis Kit (Bio-rad, USA). Gene expression level of MMP-1, MMP-3, and MMP-13 were quantified by real-time PCR with a StepOnePlus Real-Time PCR System using TaqMan gene expression assays (Applied Biosystems) in a 20 μl reaction volume. The relative level of targeted gene expression levels were calculated using the 2−ΔΔCt method.
ELISA assay
Upon completion of indicated treatment, culture medium was collected for ELISA assay using human MMP-1, MMP-3, and MMP-13 ELISA kits (R&D Systems, Inc., Minneapolis, MN, USA) according to the manufacturer’s instructions.
Western blot analysis
The cellular lysates were prepared by using the cell lysis buffer (Cell Signaling, USA). Equal proteins (20 μg) were resolved on sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to immobilon polyvinyl difluoride (PVDF) membranes. The blots were blocked with 5 % non-fat milk for 1 h at room temperature and then probed with the primary antibodies overnight at 4 °C. After three washes, the blots were subsequently incubated with the secondary goat anti-rabbit or anti-mouse antibodies conjugated with horseradish peroxidase (1:10,000) for 1 h at room temperature. The blots were visualized by enhanced chemiluminescence kit (SantaCruz, USA).
Nuclear extracts preparation and NF-kB activation measurement
Cell nuclear proteins were extracted using Nuclear and Cytoplasmic Extraction Kit (Thermo Scientific, USA) according to the manufacturer’s instructions. Briefly, cells were lysed in a hypotonic buffer on ice for 15 min followed by centrifuged at 500 × g for 30 s to pellet nuclei. Then, the pellet was re-suspended in nuclear extract buffer on ice for 15 min and centrifuged at 14,000 × g for 10 min. Supernatants containing the nuclear proteins were collected. Nuclear protein was used to measure the binding activity of NF-κB with NF-κB (p65) Transcription Factor Assay Kit (Cayman Chemical Company, Ann Arbor, MI) according to the manufacturer’s instruction. Following color development, absorbance read at 450 nm was used to index the binding activity of NF-κB.
Statistical analysis
All of the experimental data were expressed as mean ± standard deviation (SD). The data were analyzed by one-way analysis of variance (ANOVA). Differences were considered significant when P < 0.05.
Results
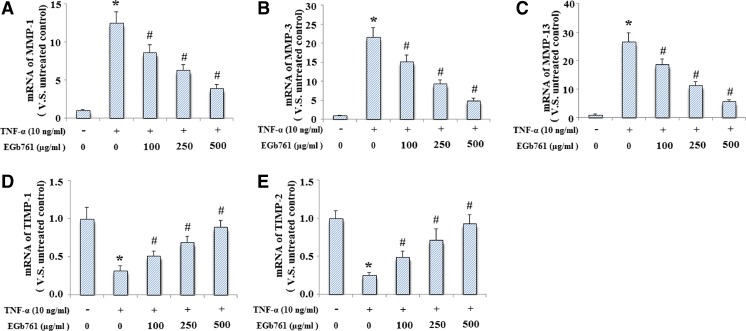
Firstly, we determined effects of EGb761 on MMP-1, MMP-3, MMP-13, TIMP-1, and TIMP-2 gene expression in monolayer chondrocytes. Cells were pretreated with EGb761 at the concentration of 0, 100, 250, and 500 μg/ml for 24 h followed by treatment with TNF-α (10 ng/ml) for another 24 h. And the result indicated that elevation of MMP-1 (Fig. 1a), MMP-3 (Fig. 1b), and MMP-13 (Fig. 1c) gene expression induced by TNF-α was inhibited by treatment with EGb761 in a dose-dependent manner. Our results also demonstrated that EGb761 pretreatment ameliorated TNF-α induced reduction of both TIMP-1 (Fig. 1d) and TIMP-2 (Fig. 1e).
Fig. 1.
EGb761 ameliorated the effects of TNF-α on the expression of MMP-1, MMP-3, MMP-13, TIMP-1, and TIMP-2 at mRNA levels. Cells were pretreated with EGb761 at the concentration of 0, 100, 250, and 500 μg/ml for 24 h followed by treatment with TNF-α (10 ng/ml) for another 24 h. mRNA was used isolated and used for real-time PCR analysis. a MMP-1; b MMP-3; c MMP-13; d TIMP-1; e TIMP-2. (*P < 0.001 compared to untreated control; #P < 0.001 compared to TNF-α treated group)
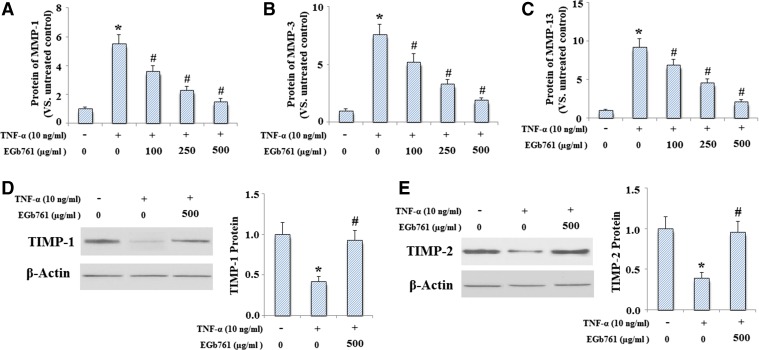
In order to determine whether EGb761 also inhibited MMP-1, MMP-3, and MMP-13 expressions at the protein level, ELISA assay was used to measure the levels of MMP-1, MMP-3, and MMP-13. Culture media obtained from chondrocytes was used to determine the levels of MMP-1, MMP-3, and MMP-13. And the results indicated that TNF-α significantly increased the expression of MMP-1, MMP-3, and MMP-13, which was inhibited by treatment with EGb761 in a dose-dependent manner (Fig. 2a–c). In addition, EGb761 restored the reduction of TIMP-1 (Fig. 2d) and TIMP-2 (Fig. 2e) induced by TNF-α at protein levels. Importantly, our results indicated that EGb761 pretreatment could prevent TNF-α mediated reduction of collagen II (Fig. 3a, b).
Fig. 2.
EGb761 ameliorated TNF-α induced expression of MMP-1, MMP-3, and MMP-13 and reduction of TIMP-1 and TIMP-2 at protein levels. Cells were pretreated with EGb761 at the concentration of 0, 100, 250, and 500 μg/ml for 24 h followed by treatment with TNF-α (10 ng/ml) for another 24 h. ELISA assays were used to determine protein concentrations. a MMP-1; b MMP-3; c MMP-13; d levels of TIMP-1 determined by Western blot analysis; e levels of TIMP-2 determined by Western blot analysis (*P < 0.001 compared to untreated control; #P < 0.001 compared to TNF-α treated group)
Fig. 3.
EGb761 ameliorated TNF-α induced degradation of collagen type II. Cells were pretreated with EGb761 at the concentration of 0, 100, 250, and 500 μg/ml for 24 h followed by treatment with TNF-α (10 ng/ml) for another 24 h. . Representative Western blot bands of collagen typeII; b quantitative analysis (*P < 0.001 compared to untreated control; #P < 0.001 compared to TNF-α treated group)
NF-κB signaling pathway is related to inflammatory responses and cartilage degradation. To investigate the mechanisms of EGb761-mediated inhibition of inflammatory responses in TNF-α-activated chondrocytes, we focus on the activation of NF-κB, a family of transcription factor critical in many inflammatory responses. The results indicated that TNF-α markedly increased the phosphorylations of IKK, IκBα, and NF-κB p65, which could be significantly reversed by EGb761 (Fig. 4a). Moreover, administration of EGb761 inhibited TNF-α-induced translocation of NF-kB p65 from cytosol to nucleus manner (Fig. 4b). Notably, our results demonstrated that TNF-α significantly increased NF-κB binding activity, which was inhibited by treatment with EGb761 (Fig. 4c).
Fig. 4.
EGb761 ameliorated TNF-α induced activation of NF-κB pathway. Cells were pretreated with EGb761 at the concentration of 0, 100, 250, and 500 μg/ml for 24 h followed by treatment with TNF-α (10 ng/ml) for another 24 h. a Phosphorylation of IKK, IκBα, and p65; b nuclear levels of p65. Lamin B was used for internal control; c luciferase activity of NF-κB (*P < 0.001 compared to untreated control; #P < 0.001 compared to TNF-α treated group)
Discussion
TNF-α is a pro-inflammatory cytokine in many inflammatory disorders including OA. TNF-α is activated by synoviocytes and chondrocytes, and it plays a critical role in the pathogenesis of OA (Kou and Wu 2014). As a matter of fact, the increased amounts of TNF-α were found in chondrocytes and synovial cells of OA patients (Tetta et al. 1990) and high concentration of TNF-α was also found in human OA cartilage (Shinmei et al. 1990). TNF-α suppresses proteoglycan production and collagen biosynthesis. Thus, to understand TNF-α signaling pathway of chondrocytes may be helpful for preventing cartilage degradation in OA. MMPs are a family of zinc-dependent enzymes that can degrade all components of the extracellular matrix, which are synthesized in synovial joints by synovial cells and chondrocytes. TNF-α treatment significantly increased the expression of MMPs in human chondrocytes (Moon et al. 2013).
MMP-13 is elevated in OA chondrocytes, and this MMP specifically cleaves collagen type II (Poole et al. 2002). Another clinical investigation also revealed that patients with articular cartilage destruction have high MMP-13 expression (Roach et al. 2005), suggesting that increased MMP-13 may be associated with cartilage degradation. In addition, MMP-3 was expressed in the synovium and the surface of cartilage in the knee joints and in pannus-like tissue of patients with OA (Shibakawa et al. 2003). In our study, we investigated whether EGb761 possesses chondroprotective effects via suppression of MMPs in chondrocytes. Our results showed that EGb761 inhibited TNF-α-induced expression of MMP-1, MMP-3, MMP-13 and the reduction of collagen II in a dose-dependent manner in human OA chondrocytes.
Consistent with our findings, a recent study demonstrated that EGb761 and its active components quercetin and kaempferol reversed LPS induced inflammatory responses through inhibiting the productions of PGE2, NO, and the protein expressions of COX-2 and iNOS via suppressing NF-κB signaling (Chen et al. 2013). NF-κB is found in almost all animal cell types and is involved in cellular responses to stimuli such as stress, cytokines, and free radicals. NF-κB regulates the expression of MMP-1, MMP-3, and MMP-13 in cartilage (Vincenti et al. 2002). EGb761 pretreatment also inhibits lipopolysaccharide (LPS)-induced protein leakage and neutrophil infiltration, inflammatory responses such as metalloproteinase (MMP)-9 activities, through inhibiting the phosphorylation of NF-κB and the degradation of its inhibitor IκB (Huang et al. 2013). Ginkgo biloba is one of the most widely used herbal products or dietary supplements in the world. EGb761 is a standardized extract of G. biloba leaves. For preparing natural herbal remedies, active substances are often complexes of different substances which interact, regulate, and intensify. All medicines may cause side effects, but many people have no, or minor, side effects. Possible side effects of Ginkgo biloba include diarrhea, dizziness, gas, headache, nausea, and stomach upset (Kim et al. 2009). Human bodies are better to tolerate the side effects of natural plant preparations than to tolerate the side effects of synthetic preparations. These actions of EGb761 suggest a mechanism by which EGb761 may act to prevent cartilage breakdown in arthritis.
References
- Aigner T, Dudhia J. Genomics of osteoarthritis. Curr Opin Rheumatol. 2003;15:634–640. doi: 10.1097/00002281-200309000-00019. [DOI] [PubMed] [Google Scholar]
- Aigner T, McKenna L. Molecular pathology and pathobiology of osteoarthritic cartilage. Cell Mol Life Sci. 2002;59:5–18. doi: 10.1007/s00018-002-8400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blom AB, van Lent PL, Libregts S, et al. Crucial role of macrophages in matrix metalloproteinase-mediated cartilage destruction during experimental osteoarthritis: involvement of matrix metalloproteinase-3. Arthritis Rheum. 2007;56:147–157. doi: 10.1002/art.22337. [DOI] [PubMed] [Google Scholar]
- Chen YJ, Tsai KS, Chiu CY, et al. EGb761 inhibits inflammatory responses in human chondrocytes and shows chondroprotection in osteoarthritic rat knee. J Orthop Res. 2013;31(7):1032–1038. doi: 10.1002/jor.22339. [DOI] [PubMed] [Google Scholar]
- Flannelly J, Chambers MG, Dudhia J, et al. Metalloproteinase and tissue inhibitor of metalloproteinase expression in the murine STR/ort model of osteoarthritis. Osteoarthritis Cartilage. 2002;10(9):722–733. doi: 10.1053/joca.2002.0818. [DOI] [PubMed] [Google Scholar]
- Freemont AJ, Tilman R, Hampson V, et al. Gene expression of matrix metalloproteinases 1, 3, and 9 by chondrocytes in osteoarthritic human knee articular cartilage is zone and grade specific. Ann Rheum Dis. 1997;56:542–549. doi: 10.1136/ard.56.9.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y. Ginkgo terpene component has an anti-inflammatory effect on Candida albicans-caused arthritic inflammation. Int Immunopharmacol. 2005;5:1049–1056. doi: 10.1016/j.intimp.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Huang CH, Yang ML, Tsai CH. Ginkgo biloba leaves extract (EGb 761) attenuates lipopolysaccharide-induced acute lung injury via inhibition of oxidative stress and NF-κB-dependent matrix metalloproteinase-9 pathway. Phytomedicine. 2013;20(3–4):303–309. doi: 10.1016/j.phymed.2012.11.004. [DOI] [PubMed] [Google Scholar]
- Jiao YB, Rui YC, Li TJ, et al. Expression of pro-inflammatory and anti-inflammatory cytokines in brain of atherosclerotic rats and effects of Ginkgo biloba extract. Acta Pharmacol Sin. 2005;26(7):835–839. doi: 10.1111/j.1745-7254.2005.00106.x. [DOI] [PubMed] [Google Scholar]
- Kim TE, Kim BH, Kim J, et al. Comparison of the pharmacokinetics of ticlopidine between administration of a combined fixed-dose tablet formulation of ticlopidine 250 mg/ginkgo extract 80 mg, and concomitant administration of ticlopidine 250-mg and ginkgo extract 80-mg tablets: an open-label, two-treatment, single-dose, randomized-sequence crossover study in healthy Korean male volunteers. Clin Ther. 2009;31(10):2249–2257. doi: 10.1016/j.clinthera.2009.10.011. [DOI] [PubMed] [Google Scholar]
- Kou S, Wu Y. Meta-analysis of tumor necrosis factor alpha-polymorphism and knee osteoarthritis risk. BMC Musculoskelet Disord. 2014;15(1):373. doi: 10.1186/1471-2474-15-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullich W, Fagerer N, Schwann H. Effect of the NSAID nimesulide on the radical scavenger glutathione S-transferase in patients with osteoarthritis of the knee. Curr Med Res Opin. 2007;23:1981–1986. doi: 10.1185/030079907X223486. [DOI] [PubMed] [Google Scholar]
- Liacini A, Sylvester J, Li WQ, et al. Induction of matrix metalloproteinase-13 gene expression by TNF-alpha is mediated by MAP kinases, AP-1, and NF-kappaB transcription factors in articular chondrocytes. Exp CELL Res. 2003;288(1):208–217. doi: 10.1016/S0014-4827(03)00180-0. [DOI] [PubMed] [Google Scholar]
- Lim H, Son KH, Chang HW, et al. Inhibition of chronic skin inflammation by topical anti-inflammatory flavonoid preparation. Ato Formula Arch Pharm Res. 2006;29:503–507. doi: 10.1007/BF02969424. [DOI] [PubMed] [Google Scholar]
- Moon MH, Jeong JK, Lee YJ, et al. SIRT1, a class III histone deacetylase, regulates TNF-α-induced inflammation in human chondrocytes. Osteoarthritis Cartilage. 2013;21(3):470–480. doi: 10.1016/j.joca.2012.11.017. [DOI] [PubMed] [Google Scholar]
- Muller WE, Heiser J, Leuner K. Effects of the standardized Ginkgo biloba extract EGb 761(R) on neuroplasticity. Int Psychogeriatr. 2012;24(Suppl 1):S21–S24. doi: 10.1017/S1041610212000592. [DOI] [PubMed] [Google Scholar]
- No author list (2003) EGb 761: ginkgo biloba extract, Ginkor. Drugs R D. 4(3):188–193 [DOI] [PubMed]
- Poole AR, Kobayashi MT, Yasuda S, et al. Type II collagen degradation and its regulation in articular cartilage in osteoarthritis. Ann. Rheum. Dis. 2002;61:ii78–81. doi: 10.1136/ard.61.suppl_2.ii78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach HI, Yamada N, Cheung KS, et al. Association between the abnormal expression of matrix degrading enzymes by human osteoarthritic chondrocytes and demethylation of specific CpG sites in the promoter regions. Arthritis Rheum. 2005;52:3110–3124. doi: 10.1002/art.21300. [DOI] [PubMed] [Google Scholar]
- Shibakawa A, Aoki H, Masuko-Hongo K, et al. Presence of pannus-like tissue on osteoarthritic cartilage and its histological character. Osteoarthritis Cartilage. 2003;11:133–140. doi: 10.1053/joca.2002.0871. [DOI] [PubMed] [Google Scholar]
- Shinmei M, Okada Y, Masuda K, et al. The mechanism of cartilage degradation in osteoarthritic joints. Semin Arthritis Rheum. 1990;19(4 Suppl 1):16–20. doi: 10.1016/0049-0172(90)90080-Y. [DOI] [PubMed] [Google Scholar]
- Shiomi T, Lemaître V, D’Armiento J. Matrix metalloproteinases, a disintegrin and metalloproteinases, and a disintegrin and metalloproteinases with thrombospondin motifs in non-neoplastic diseases. Pathol Int. 2010;60:477–496. doi: 10.1111/j.1440-1827.2010.02547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetta C, Camussi G, Modena V, et al. Tumour necrosis factor in serum and synovial fluid of patients with active and severe rheumatoid arthritis. Ann Rheum Dis. 1990;49(9):665–667. doi: 10.1136/ard.49.9.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincenti MP, Brinckerhoff CE. Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in arthritis: integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res. 2002;4(3):157–164. doi: 10.1186/ar401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa T, Naito Y, Kondo M. Ginkgo biloba leaf extract: review of biological actions and clinical applications. Antioxid Redox Signal. 1999;1:469–480. doi: 10.1089/ars.1999.1.4-469. [DOI] [PubMed] [Google Scholar]