Abstract
The natural life cycle of many protozoan and helminth parasites involves exposure to several hostile environmental conditions. Under these circumstances, the parasites arouse a cellular stress response that involves the expression of heat shock proteins (HSPs). Small HSPs (sHSPs) constitute one of the main families of HSPs. The sHSPs are very divergent at the sequence level, but their secondary and tertiary structures are conserved and some of its members are related to α-crystallin from vertebrates. They are involved in a variety of cellular processes. As other HSPs, the sHSPs act as molecular chaperones; however, they have shown other activities apparently not related to chaperone action. In this review, the diverse activities of sHSPs in the major genera of protozoan and helminth parasites are described. These include stress response, development, and immune response, among others. In addition, an analysis comparing the sequences of sHSPs from some parasites using a distance analysis is presented. Because many parasites face hostile conditions through its life cycles the study of HSPs, including sHSPs, is fundamental.
Keywords: HSPs, Small HSPs, Parasites, Stress, Chaperone
Introduction
Diseases caused by parasites currently affect global health and the global economy and represent an important unsolved problem, especially in developing countries. According to the World Health Organization (WHO), in 2002, infectious and parasitic diseases caused the deaths of 10.9 million people; malaria was the leading cause of death, killing 1.27 million people (Campanini and Vita-Finzi 2004). Data on the burden of disease measured in disability-adjusted life years (DALYs) indicate that the major parasitic diseases (malaria, leishmaniasis, African trypanosomiasis, Chagas disease, schistosomiasis, filariasis, and onchocerciasis) resulted in the loss of approximately 58 million DALYs in 2004. The financial costs of these diseases, calculated in millions of dollars lost due to absenteeism, treatment of disease, and low productivity, are huge. Besides, certain parasitic diseases can produce the death or decreased productivity of animal species that are economically important to humans, resulting in losses amounting to billions of dollars (Crompton 2010).
The causative agents of parasitic diseases belong to two major taxa: protozoan and helminths. Most of these parasites have complex life cycles that involve a differentiation through various stages of development and transition through two or more hosts. The passage through various hosts and environments constantly exposes pathogenic organisms to sudden changes in growth conditions. Under these harsh conditions, parasites have developed adaptations and resistance mechanisms to survive and proliferate (Maresca and Carratù 1992). One of these mechanisms is to mount a stress response, which is characterized by, among other phenomena, the expression of a particular group of proteins known as heat shock proteins (HSPs). HSPs are a group of highly conserved proteins found in all organisms; their main function is to act as molecular chaperones (Lindquist and Craig 1988). Chaperones belong to a family of proteins involved in promoting the correct folding of other polypeptides and, in some cases, their multimerization into oligomeric structures (Narberhaus 2002). HSPs have been traditionally grouped into eight main families according to their molecular mass: HSP110, HSP100, HSP90, HSP70, HSP60, HSP40, HSP10, and small HSPs (sHSPs) (Feder and Hofmann 1999). Several members of sHSP family, but not all, are evolutionarily related to the α-crystallin protein or rather αB-crystallin (one of the two subunits of α-crystallin) from vertebrates (de Jong et al. 1998; Ingolia and Craig 1982), which can be induced by heat and other stresses and conveys thermotolerance to cells (de Jong et al. 1998).
This review focuses on the functions of members of the sHSP family related to the vertebrate α-crystallin protein from protozoan and helminths, describing their role in the life cycle and pathogenicity of the parasites.
General characteristics of sHSPs
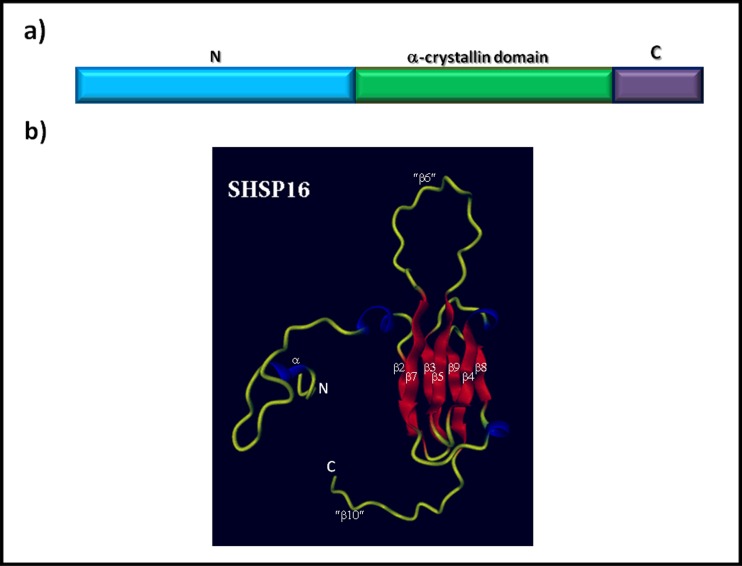
The sHSPs constitute a group of molecular chaperones that are widely distributed throughout the phylogenetic scale, as demonstrated by their ubiquitous presence in organisms belonging to three domains of life (Bacteria, Archaea, and Eukarya). Although they have been found in most organisms, not all organisms contain genes encoding this class of proteins (Narberhaus 2002). Phylogenetic studies indicate an early divergence in the evolution of sHSPs (Waters et al. 1996). These proteins have small monomeric molecular masses ranging from 12 to 43 kDa, the bulk of which have a mass of 14–27 kDa (Narberhaus 2002). However, these proteins are able to organize and assemble themselves into larger multimeric structures or oligomers that may be comprised of 9–50 subunits and can therefore achieve a molecular mass of 800 kDa or more (MacRae 2000; Haslbeck 2002). Under conditions of cellular stress, when massive protein denaturation occurs, sHSPs bind to denatured proteins, maintaining them in a competent folding state and preventing their irreversible aggregation. When the stressful conditions are removed, the non-native proteins bound to the sHSPs are released, either spontaneously or by the action of energy-dependent chaperone systems such as HSP60, HSP70, or HSP100 that then re-nature the proteins (Sun and MacRae 2005; Nakamoto and Vígh 2007). Under physiological conditions, the abundance of sHSPs varies by organism, cell type (in the case of multicellular systems), and subcellular compartment. Under conditions of stress, the induction of this group of proteins becomes significant in many organisms (Haslbeck 2002; Narberhaus 2002). The sequence similarity of these proteins is much lower than that of other families of HSPs (such as HSP70 and HSP90) that diverged at a much lower rate (Plesofsky-Vig et al. 1992; Waters et al. 1996); therefore, because the overall sequence identity among members is ≤50 %, these proteins constitute a superfamily (de Jong et al. 1998). Despite the wide variation in their sequences, these proteins possess two or three structural domains. The most conserved of these has 80–100 amino acid residues and is known as the α-crystallin domain; this domain is a determinant of the group of sHSPs located at the carboxyl terminus in the bulk of organisms. Recently, a non-canonical sHSP has been described in Leishmania donovani in which the α-crystallin domain is located at the amino terminus (Hombach et al. 2014). Preceding the α-crystallin is the amino terminal domain, which is hydrophobic and of highly variable length and sequence. Adjacent to the α-crystallin domain is often a flexible carboxyl-terminal extension of variable length (usually short) and sequence (de Jong et al. 1998) (Fig. 1a). Although the great diversity in the primary structure, the secondary and the tertiary structures of the sHSPs are conserved; the α-crystallin domain is rich in β-pleated sheets (de Jong et al. 1998) and forms a compact β-sandwich structure composed of two anti-parallel β sheets (Fig. 1b) with capacity to form a dimer (van Montfort et al. 2001). Figure 1b depicts the predicted three-dimensional structure of protein SHSP16, one sHSP family member from the parasite Trypanosoma cruzi (Pérez-Morales et al. 2009), the etiologic agent of Chagas disease. The model was generated using the SWISS-MODEL server and based on the comparative modeling of templates of previously reported related structures; the model was edited with Protein Picture Generator (PPG, http://bioserv.rpbs.jussieu.fr/PPG) and DINO (Visualizing Structural Biology (2002), http://dino3d.org). To predict the structure of SHSP16, the crystalline structure of wheat HSP16.9 (van Montfort et al. 2001), a sHSP eukaryotic with which shares 25 % sequence identity, was used as template.
Fig. 1.
General structure of sHSPs. a Structural domains of the primary sequence represented by the amino terminal domain (N), the α-crystallin domain, and the carboxyl-terminal domain (C). b 3D model of the tertiary structure of SHSP16 protein from the protozoan parasite Trypanosoma cruzi. Ribbon model of the SHSP16 monomer generated with the SWISS-MODEL program. The crystallographic structure of the wheat HSP16.9 protein was used as a template to construct the model. The unstructured tails are shown in yellow, the α-helices in blue, and the β sheets in red. β sheets were numbered according to the corresponding sheets from the HSP16.9 structure; the sheets β6 and β10 (enclosed in quotes) are absent in SHSP16 of Trypanosoma cruzi. The N-and C-termini are labeled N and C, respectively (Color figure online)
sHSPs in parasites
HSPs of high molecular weight have been described as being involved in the heat shock response induced in a variety of organisms (including parasites) under certain conditions (Lindquist and Craig 1988). In comparison, the family of sHSPs has received little attention, perhaps in part because it is difficult to locate them and because they have low sequence similarities. Nevertheless, it has been possible to measure the expression and/or activity of certain sHSPs in different parasites; in addition, while the specific activity of some proteins has not been described in all cases, protein sequence and/or location is generally fairly well described. Table 1 lists the sHSPs and their functions that have been described so far in parasites of different taxonomic groups.
Table 1.
List of sHSPs characterized in protozoan and helminth parasites
| Pathogen | Disease | sHSP | Activity | Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Overexpression Stress/Development | Immune system stimulator Antigen/other | Protection against disease | Stage specific | Other function | ||||||
| Protozoa | ||||||||||
| Flagellate | ||||||||||
| Giardia lamblia | Giardiasis | ORF-C4 | + | (Nores et al. 2009) | ||||||
| Leishmania amazonensis | Leishmaniasis | HSP20 | + | (Montalvo-Álvarez et al. 2008) | ||||||
| Leishmania donovani | Leishmaniasis | HSP23 | + | + | + | (Hombach et al. 2014) | ||||
| Trypanosoma cruzi | Chagas’ Disease | SHSP16 | + | + | (Pérez-Morales et al. 2009) | |||||
| Apicomplexa | ||||||||||
| Babesia bigemina | Bovine babesiosis | Bbg20 | + | (Brown et al. 2001) | ||||||
| Babesia bovis | Bovine babesiosis | Bbo20 | + | + | (Brown et al. 2001; Lee et al. 2005) | |||||
| Babesia divergens | Babesiosis | BdHSP-20 | + | + | + | + | + | (Gorenflot et al. 1991; Montero et al. 2008; Précigout et al. 1993; Valentin et al. 1993) | ||
| Neospora caninum | Canine and cattle neosporosis | NcBAG1 | + | + | + | (Kobayashi et al. 2013; Liddell et al. 2003) | ||||
| Plasmodium berghei | Malaria | HSP20 | + | + | (Montagna et al. 2012) | |||||
| Toxoplasma gondii | Toxoplasmosis | HSP20 | + | (de Miguel et al. 2005) | ||||||
| Toxoplasma gondii | Toxoplasmosis | HSP21 | + | (de Miguel et al. 2005) | ||||||
| Toxoplasma gondii | Toxoplasmosis | HSP28 | + | + | + | (de Miguel et al. 2005) | ||||
| Toxoplasma gondii | Toxoplasmosis | HSP29 | + | (de Miguel et al. 2005) | ||||||
| Toxoplasma gondii | Toxoplasmosis | HSP30 | + | + | + | (Bohne et al. 1995; Bohne et al. 1998; Mohamed et al. 2003; Mun et al. 1999; Zhang et al. 1999) | ||||
| Helminths | ||||||||||
| Platyhelminths | ||||||||||
| Echinococcus multilocularis | Hydatidosis | HSP20 | + | (Kouguchi et al. 2010) | ||||||
| Fasciola hepatica | Fascioliasis | Fh-HSP35a | + | + | (Moxon et al. 2010) | |||||
| Paragonimus westermani | Paragonimiasis | Egg antigen | + | (Lee et al. 2007) | ||||||
| Schistosoma mansoni | Schistosomiasis | p40 | + | + | + | (Cai et al. 1996; Nene et al. 1986) | ||||
| Schistosoma mansoni | Schistosomiasis | p40-2 | + | (Cao et al. 1993) | ||||||
| Taenia saginata | Cysticercosis/Taeniasis | Tsp36 | + | (Benitez et al. 1998) | ||||||
| Taenia solium | Cysticercosis/Taeniasis | Tsol-sHSP35.6 | + | (Ferrer et al. 2005) | ||||||
| Nematodes | ||||||||||
| Brugia malayi | Lymphatic filariasis | Bm-HSP-s1 | + | + | + | (Raghavan et al. 1999) | ||||
| Brugia malayi | Lymphatic filariasis | Bm-HSP12.6 | + | + | (Gnanasekar et al. 2008) | |||||
| Brugia pahangi | Filariasis in cats | Bphsp7 | + | + | + | (Devaney et al. 1992; Jecock and Devaney 1992; Thompson et al. 1996) | ||||
| Dirofilaria immitis | Dirofilariasis | p27 | + | (Lillibridge et al. 1996) | ||||||
| Haemonchus contortus | Cattle affected | HSP20 | + | (Hartman et al. 2003) | ||||||
| Nippostrongylus brasiliensis | Rodents affected | Nb-HSP20 | + | (Tweedie et al. 1993) | ||||||
| Nippostrongylus brasiliensis | Rodents affected | Nb-HSP12.6 | + | + | (Arizono et al. 2011) | |||||
| Ostertagia ostertagi | Cattle affected | OoHSP18 | + | + | + | (Vercauteren et al. 2006) | ||||
| Strongyloides ratti | Rat affected | Sra-HSP-17.1 | + | + | + | (Younis et al. 2011) | ||||
| Strongyloides ratti | Rat affected | Sra-HSP-17.2 | + | + | + | (Younis et al. 2011) | ||||
| Trichinella spiralis | Trichinosis | Ts-sHSP | + | + | (Wu et al. 2007) | |||||
Expression of sHSPs under stress conditions
Stress is an integral part of the life cycle of many parasites, as they face many dissimilar growth conditions through its biological cycle, as heat shock (Fig. 2), in vitro, induction or overexpression of sHSPs has been demonstrated in many pathogens in response to this stress condition. The multigenic family members of sHSPs present in Brugia pahangi are induced by heat stress during the microfilariae stage (Devaney et al. 1992) and overexpressed in L3 larvae (Jecock and Devaney 1992). Heat shock also regulates the expression of one of the members of this family (Bphsp7) at the messenger RNA (mRNA) level in microfilariae and adults (Thompson et al. 1996). In vivo, microfilariae and L3 larvae are the developmental stages that inhabit both hosts, i.e., the mosquito vector and mammalian host; thus, it seems plausible that these stages have developed coping mechanisms to respond to sudden changes in temperature during transit of insect to mammal and vice versa, and sHSPs could serve this function. In the phylogenetically related organism Brugia malayi, the expression level of Bm-HSP-s1 also increased in L3 larvae and adults under conditions of thermal stress (Raghavan et al. 1999). In L3 infective larvae of the nematode Ostertagia ostertagi, the transcription level of the OoHSP18 gene increases during heat stress but not during other types of stress such as exposure to 2 % H2O2 or to 150 μg/ml of the antihelminthic compound levamisole (Vercauteren et al. 2006), suggesting that OoHSP18 acts in response to the specific stimulus produced by heat stress. Cattle ingest L3 infective larvae from pasture, and it may be that the larvae challenges a heat shock when ingested.
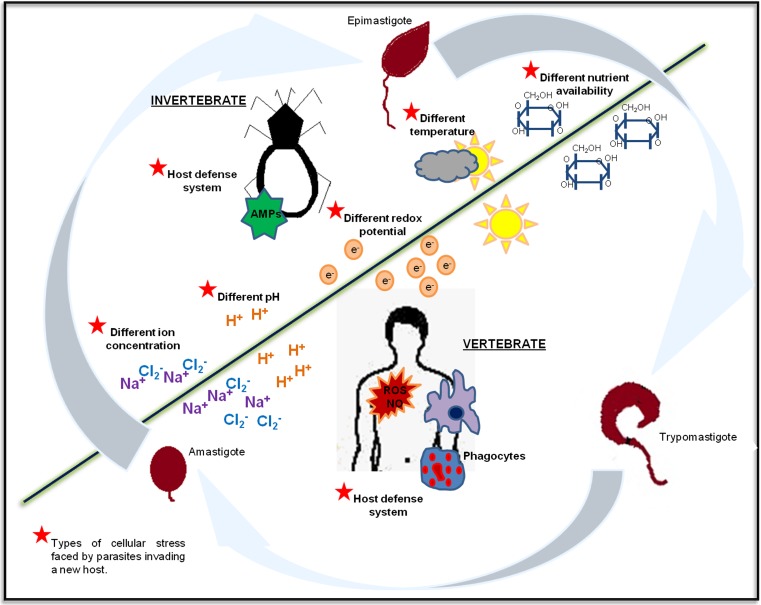
Fig. 2.
General life cycle of parasites of medical importance. The vast majority of parasites have complex life cycles that require a number of hosts and transformations into various stages to complete their biological period. The different hosts (invertebrates or vertebrates) or environments (water, soil, feces) through which a parasite passes its life cycle throughout its various stages of development are indicated by underlined and uppercase text. The passage from one host to another or to the environment usually involves the generation of cellular stress (indicated by a red star), which, in some cases, leads to the differentiation of the parasite. The diagram represents the life cycle of the protozoan Trypanosoma cruzi; this parasite passes through three main stages (epimastigote, trypomastigote, and amastigote) during its path from an invertebrate host to a vertebrate host (Color figure online)
In other organisms, whose life cycle apparently does not include confronting a heat shock, overexpression of sHSPs has been detected in vitro, and although it is not completely clear when they will face heat shock in its life cycle, they display a heat shock response as an adaptive mechanism. For example, in Trichinella spiralis, copy numbers of the Ts-sHSP transcript increased in muscular larvae under conditions of heat and cold stress (Wu et al. 2007). In the Apicomplexa organism Toxoplasma gondii, the transcription of four members of sHSP family, HSP20, HSP21, HSP28, and HSP29 genes, increases in tachyzoites subjected to heat stress (de Miguel et al. 2005). In kinetoplastid parasites, such as T. cruzi epimastigotes and L. donovani, after heat stress, SHSP16 is overexpressed (Pérez-Morales et al. 2009) and HSP23 protein increases its abundance (Hombach et al. 2014), respectively.
In each of these organisms, the increase in the expression levels of sHSPs raises the possibility that these proteins play a role in enabling the adaptation or protection of the parasite to heat shock. However, the heat stress is not the only growth condition that generates cellular stress where sHSP overexpression has been detected. In Babesia divergens, the BdHSP-20 gene and its protein BdHSP-20 are upregulated under conditions of nutritional stress. A nutritional stress is confronted by parasites when they invade erythrocytes. Indeed, the overexpression of this protein was detected when parasites were incubated in culture medium without human serum, but not under conditions of heat shock (Montero et al. 2008). In the flagellated organism Giardia lamblia, the ORF-C4 protein is expressed in the cytoplasm of the parasite throughout its life cycle in the form of 22-kDa monomers or complexes reaching an approximate molecular mass of 66 kDa. However, when trophozoites were exposed to specific stressful conditions, the monomers disappeared, and the 66-kDa complexes were the only remaining forms. One of these stressful conditions involved depriving the trophozoites of the essential amino cysteine, which is involved in the control of the redox state of the organism and in cell adhesion. Another stressful condition involved the incubation of parasites with brefeldin A, an inhibitor of protein transport that prevents the recruitment of cytoplasmic proteins to the membrane (Nores et al. 2009). In Nippostrongylus brasiliensis, the host immune response, which causes cellular stress for the parasite, regulates the expression of the gene encoding the Nb-HSP12.6 protein. The expression of this gene is gradually increased during the expulsion of the adult worms from the intestines of immunocompetent rats; however, the worms expelled from the intestines of rats without a thymus did not have increased transcription levels of this gene, suggesting that Nb-HSP12.6 is induced by host immune responses. Furthermore, the activation of this gene does not necessarily require an adaptive immune response of the host; indeed, an innate immune response was sufficient to induce transcription of the gene, as demonstrated by an experiment in which adult worms transplanted to the intestines of suckling rats overexpressed Nb-HSP12.6 mRNA (Arizono et al. 2011). To date, it is not entirely clear how the reported overexpression of certain sHSPs under hostile conditions is involved in promoting tolerance and/or adaptation to stress in the parasites; indeed, the different conditions under which these proteins are overexpressed suggest that they are involved in processes other than preventing the apparent denaturation of native proteins. Nevertheless, because the overexpression of this class of proteins occurs in many parasites exposed to stress, it is likely that these proteins play a preponderant role in stress response.
Expression of sHSPs through parasite development
In some parasites, the transformation from one stage to another is triggered by exposure to hostile environments (Zilberstein and Shapira 1994), and the role of HSPs (including sHSPs) in this transformation process is not entirely clear. It was suggested that the expression of these proteins could be part of the differentiation process itself or an epiphenomenon associated with the adaptation of the parasite to the new harsh growth conditions (Maresca and Carratú 1992); either scenario implies the regulated expression of sHSPs (Maresca and Carratú 1992). However, experiments in which this hypothesis has been verified are scarce. Nevertheless, it has been determined that the expression of sHSPs is stage-specific in some parasites. For example, in T. gondii, two stage-specific sHSPs have been described: HSP30 and HSP28. HSP30 is specific to the latent bradyzoite stage (tissue cysts), its early expression is evident approximately 2 to 3 days after the initiation of the differentiation from tachyzoites to bradyzoites, and it subsequently becomes a major component of mature cysts (Bohne et al. 1995; Bohne et al. 1998). On the other hand, the HSP28 protein is predominantly expressed during the tachyzoite stage (de Miguel et al. 2005). In the closely related Apicomplexa Neospora caninum, a protein orthologous to HSP30 of T. gondii known as NcBAG1 has also been found to be specific to the bradyzoite stage (Kobayashi et al. 2013). In the nematode B. pahangi, the transcription of the cDNA encoding the BpHSP7 protein is specific to developmental stage; under physiological conditions, the transcription of this protein is restricted to the microfilariae stage (Thompson et al. 1996). As we mentioned before, BpHSP7 is overexpressed in heat shock, whereby it is not clear if this protein is vital to the transformation process or if the protein is fundamental to allow the microfilariae adapting to the new severe condition. In contrast, and interestingly, in B. malayi, both the mRNA of the Bm-HSP-s1 gene and its protein were detected in L4 larval and adult parasites and in L3 larvae at much lower levels, but not in microfilariae (Raghavan et al. 1999). Thus, it seems likely that these orthologous genes have diverge until express at distinct developmental stages in two organisms very close related, whose life cycles are very similar, one in microfilariae (in B. pahangi) and the other in L4 larval and adult, but not in microfilariae (in B. malayi). N. brasiliensis has two genes (Nb-HSP20 and Nb-HSP12.6) that encode members of sHSPs; these genes are differentially expressed at different stages of development of the parasite. The mRNA of Nb-HSP20 is detected only in adults and deposited eggs and not in L1, L2, L3, or L4 larvae (Tweedie et al. 1993); in contrast, Nb-HSP12.6 is predominantly transcribed in L3 larvae and in adults of more than 8 days of age; however, the protein is not detectable in eggs, or L1 or L2 larvae (Arizono et al. 2011). The presence of specific sHSPs in the different stages of the parasite suggests that each protein play an individual role in the biology of the organism.
Other parasites, rather than express stage-specific sHSPs, regulated them by developmental stage, suggesting that in these cases, the protein is involved in the adaptation to stress conditions, because they are constitutively expressed, but in a specific condition of the life cycle are needed in larger quantities. An interesting example is the overexpression of sHSPs in post-infective stages, where sHSPs may have a role in the immune response (see the “Expression of sHSPs during the course of disease” section). In Schistosoma mansoni, the p40 protein is expressed abundantly in eggs and miracidia and at low levels in adult males or females (Nene et al. 1986). In B. malayi, the mRNA of the BmHSP12.6 gene can be detected in all developmental stages, but its transcription was predominant in post-infective stages; indeed, the highest expression levels were found in microfilariae, L4 larvae, and adult parasites (Gnanasekar et al. 2008). In O. ostertagi, the highest levels of transcription of the Oo-HSP18 gene were detected in adult parasites and were found to be 170, 757, and 413 times higher than the levels detected in L3 larvae, non-enveloped L3, and L4 larvae, respectively. Similar differences in the protein levels were also observed. In adults, the protein was localized in the muscle layer under the hypodermis. Interestingly, this protein was first identified in the excretion/secretion products of the parasite, which have been associated with the survival of the nematodes; this finding suggests that this protein plays a dual role in the life cycle of these parasites (Vercauteren et al. 2006). In T. spiralis, the level of transcription of the Ts-SHSP gene differed by developmental stage and was highest in muscle larvae at 28 and 48 days post-infection; transcription depended on the time of infection and increased with larval maturation; indeed, transcription levels in newly hatched larvae were almost undetectable while adults exhibited a higher level of transcription than newborn larvae but a significantly lower level than was detected in the muscle larvae 28 and 48 days post-infection. The Ts-sHSP protein was found in histiocytes, in muscle cells of the body wall, in the hypodermis, and in the esophagus of muscle larvae 48 days after infection and in adults (Wu et al. 2007). The Fh-HSP35α protein is expressed in the embryo of Fasciola hepatica; however, its expression levels increase in the egg on day 9 of development and in the miracidium as embryogenesis progresses and as the egg matures (Moxon et al. 2010). The proteins Sra-HSP-17.1 and Sra-HSP-17.2 of the nematode Strongyloides ratti are expressed mainly in adult females rather than in L3 larvae or free-living females (Younis et al. 2011). In Plasmodium berghei, HSP20 protein is abundantly expressed in insect stages and barely detectable in the stages inhabiting mammalian host (Montagna et al. 2012). In L. donovani, HSP23 changes its abundance through life cycle, being more abundant in early amastigotes (Hombach et al. 2014).
To reveal the precise role in the parasite transformation process or adaptation, inactivation of sHSPs could give an answer. At this respect, few examples exist where sHSPs genes have been deleted to evaluate its potential role in parasites. In T. gondii, HSP30 was shown not to be essential in null mutants; however, while this protein is not necessary for the formation of cysts, it does influence the efficiency of cyst formation in the brain tissue of mice; indeed, the number of cysts formed in mice infected with the mutant strain was lower than the number of cysts found in mice infected with the wild-type strain. Furthermore, the mutant strain was less virulent in the mouse model, as the inoculum needed to kill the parasite-infected mice was approximately 1 order of magnitude larger than the inoculum required to kill the wild-type strain (Bohne et al. 1998; Zhang et al. 1999). In P. berghei, HSP20 is not essential for parasite life cycle progression; instead, the deletion of HSP20 affected substrate-dependent cell motility of sporozoite, triggering aberrant speed and directionality in vivo, causing an impaired mosquito-to-mouse transmission, suggesting a crucial role of the protein only during a brief moment in the life cycle of the parasite (Montagna et al. 2012). In L. donovani, HSP23 was shown to be essential for survival of the parasite only at the temperatures of mammalian host, displaying a temperature-sensitive phenotype. Also, the deletion of HSP23 compromised the protection to acidic pH, chemical stressors such as ethanol and semi-metal ions, and redox stress, and mutant parasites were non-infectious to primary macrophages in vitro (Hombach et al. 2014). In the above cases, the loss of sHSPs does not compromise the survival or the differentiation process of the parasite but virulence and transmission, suggesting the potential use of these proteins as therapeutic targets. Further studies are necessary to understand the precise role of sHSPs in the life cycle of the parasites.
Because parasites have complex life cycles, each stage of development generally exposes the parasite to new and unique environmental conditions. The regulated expression of sHSPs throughout parasite development suggests that these proteins play a role in either promoting the adaptation of the parasite to new conditions or in the differentiation process (Maresca and Carratù 1992). However, the exact role of the sHSPs remains unclear.
Expression of sHSPs during the course of disease
In addition to play a role in stress response when the parasites confront different hosts and environments, sHSPs play an important role during the course of disease, as they are targets of the host immune response. sHSPs have been found to be immunodominant immunogenic antigens in parasitic diseases. In the trematode S. mansoni, the immunogenicity of the p40 protein was demonstrated in an in vitro experiment where translation products from eggs were used as antigens; this protein was immunoprecipitated from approximately 90 % of sera samples from infected patients (Nene et al. 1986). The serological diagnosis of infection with the phylogenetically related platyhelminth Paragonimus westermani is based on a major antigen protein that is found in the eggs and uterus and is 100 % specific to the parasite, which has a significant similarity with heat shock proteins from other trematode (Lee et al. 2007). In another model, the immunogenicity of Tsol-sHSP35.6 of Taenia solium was demonstrated using this recombinant protein as antigen and serum samples from infected humans; 84 % of the patients with active neurocysticercosis and 71 % with inactive neurocysticercosis were seropositive. Additionally, 77 % of pigs infected with T. solium were seropositive (Ferrer et al. 2005). The immunogenicity of the Oo-HSP18 protein of O. ostertagi was demonstrated using recombinant proteins as antigens and a pool of sera obtained from cattle naturally immunized with the parasite over the course of 2 years (Vercauteren et al. 2006). The HSP20 protein of the fallegellated Leishmania amazonensis proved to be antigenic during natural infection, as it was recognized by 100 % of sera samples from dogs diagnosed with visceral leishmaniasis. However, the protein was poorly immunogenic in humans, as very few sera samples from human patients with leishmaniasis reacted positively to the protein (Montalvo-Álvarez et al. 2008). In contrast, in the very closed related kinetoplastid, T. cruzi, the SHSP16 protein was not recognized by patients or dogs infected with the parasite (Martínez et al. 2014). Also, the immunogenic properties of the recombinant protein HSP20 of Echinococcus multilocularis were investigated. Ninety percent of sera samples obtained from dogs 40 days after an experimental infection with the parasite reacted positively to the recombinant antigen (Kouguchi et al. 2010). In rats infected with S. ratti, the Sra-HSP-17.1 and Sra-HSP-17.2 proteins stimulated the production of antibodies that recognized these proteins 37 and 112 days post-infection. The localization of these sHSPs in the excretion-secretion products implies that they interact with the host (Younis et al. 2011). Finally, in F. hepatica, the antigenicity of the Fh-HSP35α has been suggested but not confirmed, as the sample size of sera samples from animals challenged with the parasite that were used to test the immunoreactivity of the protein was small (Moxon et al. 2010).
It has also been reported that sHSPs of parasites, once recognized by the host immune system, can induce a type of cellular immune response. Thus, the immunostimulatory properties of certain sHSPs have been described. In S. mansoni. The p40 protein (either native or recombinant), which is a major antigen of the parasite during the egg stage, induced a Th1-type immune response characterized by the secretion of interleukin-2 (IL-2) and interferon gamma (IFN-γ) but not IL-4 or IL-10 in mice. Additionally, the p40 protein induced the development of a pulmonary granulomatous response characterized by a predominantly mononuclear infiltrate formed around live eggs or around the p40 antigen bound to sepharose (Cai et al. 1996). In another study, clones of CD4+ T cells from sera from cattle immune to babesiosis proliferated when stimulated with the Bbo20 protein of Babesia bovis. The T cell clones produced mainly IFN-γ and IL-4 or IL-10 in smaller quantities. IFN-γ, which is involved in the host immune response, activated macrophages that inhibit parasite proliferation via phagocytosis and the production of nitric oxide (Brown et al. 2001). In the nematode S. ratti, the Sra-HSP-17.1 and Sra-HSP-17.2 proteins induced the secretion of IL-10 in monocytes (Younis et al. 2011). Finally, in vitro studies of the BmHSP12.6 protein of B. malayi have demonstrated that this protein binds to the human IL-10 receptor (huIL10R) in a dose-dependent manner, thus inhibiting the binding of human interleukin-10 (huIL10) to its receptor. In addition, rBmHSP12.6 stimulated the in vitro growth and proliferation of MC/9 mast cells in a manner similar to hulL10, the native inducer of mast cells (Gnanasekar et al. 2008).
Other families of HSPs that are also immunodominant antigens in infectious diseases are thought to act against the infectious agents that cause them (Zügel and Kaufmann 1999); a number of studies have sought to determine whether sHSPs play a similar role in parasites. In some models, it has been established that the immunostimulatory properties of sHSPs can induce protection against the disease caused by the parasites that express them. For example, BdHSP-20 of B. divergens, which is present in the culture supernatant, resulted in total immunoprotection in gerbils and had a stimulatory effect on monocytes of cattle vaccinated with the protein (Valentin et al. 1993). Additionally, in in vitro experiments, anti-BdHSP-20 antibodies reduced the intra-erythrocyte growth of the parasite by 50 % (Gorenflot et al. 1991; Précigout et al. 1993; Montero et al. 2008). BALB/c and C57BL/6 mice infected with cysts from the avirulent Fukaya strain of T. gondii produced antibodies against HSP30 of T. gondii. The production of anti-HSP30 IgG antibodies persisted more than 8 weeks after the initial infection with cysts of the parasite. Immunization with the HSP30 protein reduced the number of parasites in the brain tissue of mice infected perorally with cysts from the Fukaya strain. Immunization also reduced mortality in mice infected intraperitoneally with tachyzoites from the virulent RH strain (Mun et al. 1999). In a subsequent study, vaccination with DNA from the HSP30 gene of Toxoplasma gondii induced protection in B6 and BALB/C mice during the acute and chronic phase of toxoplasmosis; because HSP30 promotes the differentiation of tachyzoites to bradyzoites in Toxoplasma gondii, the authors suggested that this protection was achieved with the induction of an immune response that interfered with the formation of bradyzoites (Mohamed et al. 2003). Partial protection against congenital bovine neosporosis caused by the related Apicomplexa N. caninum was induced injecting mice with a recombinant plasmid containing the sequence encoding the protein HSP33; indeed, 47 % of the offspring were negative for parasite DNA (Liddell et al. 2003).
Other functions of sHSPs
In vitro experiments have also been conducted to describe the activity of sHSPs. One routine assay determines the ability of these proteins to prevent the thermal aggregation of enzymes such as malate dehydrogenase and citrate synthase (Lee et al. 1997; Basha et al. 2004). Molecular chaperone activity in parasites has been reported in vitro. It is unclear whether similar activities occur in vivo. However, sHSPs of certain parasites that were shown to have molecular chaperone activity in vitro were also overexpressed under conditions of cellular stress (de Miguel et al. 2005; Wu et al. 2007; de Miguel et al. 2009; Pérez-Morales et al. 2009)
The accumulation of β-amyloid peptide (Aβ) in the human cerebral cortex is related to the pathogenesis of Alzheimer’s disease; the ability of the Bbo20 protein of B. bovis to prevent the aggregation of this peptide was investigated. This inhibitory capacity has been reported for sHSPs of mammals; however, no studies have reported a concomitant decrease in the toxicity caused by Aβ peptides. Importantly, low concentrations of Bbo20 reduce the aggregation of Aβ and its toxicity in neuronal cells. Therefore, this protein may be an inhibitor that could be used to mitigate the neuropathology associated with Alzheimer’s disease (Lee et al. 2005).
The membrane localization of sHSPs is somewhat unusual; however, interestingly, it has been suggested that these proteins can regulate membrane fluidity and preserve membrane integrity during thermal fluctuations (Tsvetkova et al. 2002). Three sHSPs of parasites, BdHSP-20 of B. divergens and HSP20 and HSP29 of T. gondii, are located to the cell membrane; however, their functions have not been elucidated. BdHSP-20 is located on the cytoplasmic side of the cell membrane of B. divergens merozoites (Précigout et al. 1993). The HSP20 protein of Toxoplasma gondii is associated with the outer leaflet of the inner membrane complex of tachyzoites, while HSP29 appears to be associated with the membrane and distributed throughout the cell (de Miguel et al. 2005; de Miguel et al. 2008). In nematode parasites such as Dirofilaria immitis (Lillibridge et al. 1996), B. malayi (Raghavan et al. 1999), O. ostertagi (Vercauteren et al. 2006), and T. spiralis (Wu et al. 2007), sHSPs have been found to be associated with the hypodermis and muscle cells of the body wall. In other organisms (including mammals), sHSPs localized in muscle cells regulate the dynamics of actin filaments by inhibiting actin polymerization or protecting the cytoskeleton from the disruption caused by harsh conditions (Mounier and Arrigo 2002); however, it is unclear whether these proteins play a similar role in parasites.
The role of certain parasite sHSPs has not been described. For example, the expression of p40-2 of S. mansoni (Cao et al. 1993), Tsp36 of Taenia saginata (which has only been characterized structurally) (Benitez et al. 1998; Kappé et al. 2004), and the HSP20 protein of Haemonchus contortus (Hartman et al. 2003) has not been related to any particular phenomenon. The expression of the latter protein is constitutive and not regulated by thermal stress or the developmental stage of the parasite; in contrast, homologous proteins of phylogenetically related parasites such as O. ostertagi (Vercauteren et al. 2006) and N. brasiliensis (Tweedie et al. 1993; Arizono et al. 2011) are induced by stress or development.
The main reported activities of sHSPs of several species of parasites are depicted in Fig. 3. As new members of the family are described, new functions can be determined.
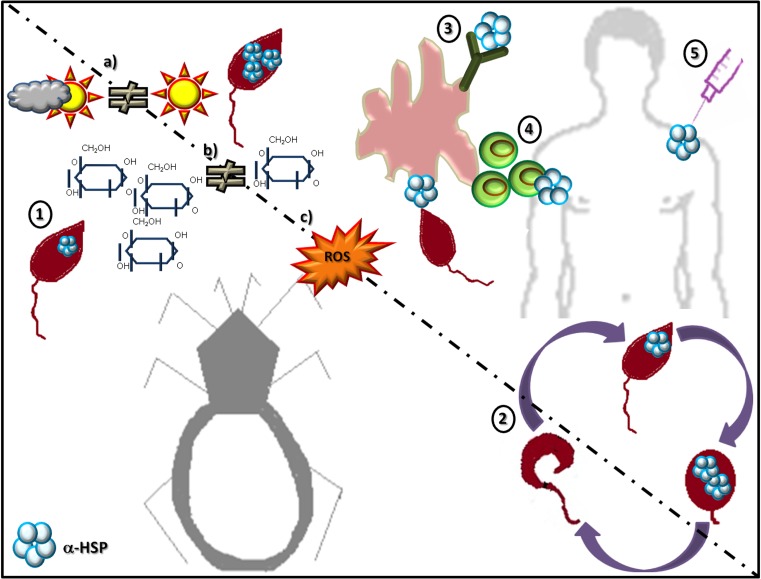
Fig. 3.
Main activities of sHSPs in protozoan and helminth parasites. For simplicity, a parasite with a digenetic life cycle is used to illustrate the different reported activities. Five types of activities have been reported. (1) Expression levels of the protein can rise in response to conditions of stress, including (a) heat stress, (b) nutritional stress, and (c) oxidative stress. (2) The proteins show developmental stage-specific expression patterns; depending on the developmental stage, these proteins may be absent or expressed to a greater or lesser extent. (3) When the parasite interacts with the host, these proteins can be recognized by the immune system and induce a humoral response, generating specific antibodies or (4) stimulating certain cell types of the host. (5) These proteins can also provide immunoprotection against the disease produced by the parasites expressing them
Sequence analysis of sHSPs
Finally, to compare sHSPs of different species of parasites, a distance analysis was conducted using the neighbor joining method (NJ) with the program MEGA5 (Tamura et al. 2011). The sequences belonged to representatives of the major genera of protozoan and helminths. To include the largest number of sequences, the analysis included sequences of proteins with known reported activity and sequences of uncharacterized proteins annotated in the databases. The genome databases represent an important tool, which provide value information and from which functional studies can be started; for example, in Trypanosoma brucei, a putative sHSP was described analyzing the parasite genome database (Folgueira and Requena 2007); further studies are necessary to determine its specific function.
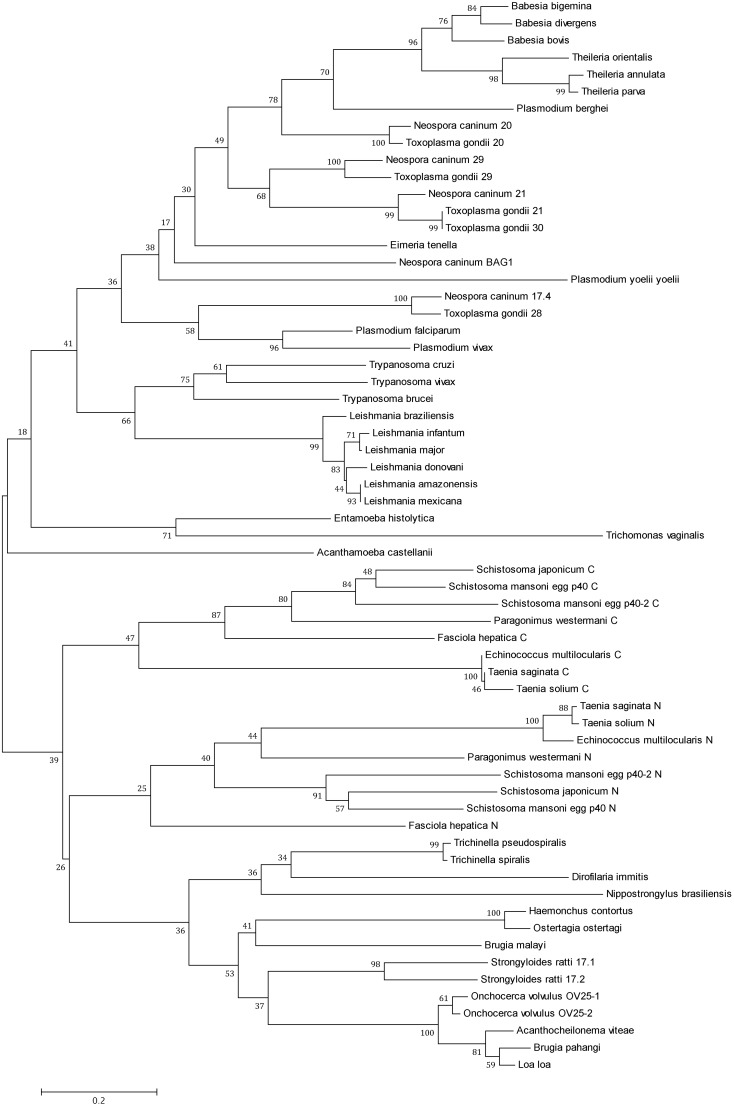
Because the α-crystallin domain is the most conserved region in the sHSPs, only this region was used for the alignment analysis. As shown in the tree of Fig. 4, the two major groups of organisms (protozoan and helminths) formed the two main clusters, suggesting that the sHSPs are sufficiently different between these groups of organisms. Within protozoan, the Apicomplexa generated a main group, indicating a high similarity of the sequences of the different genera of Apicomplexa. A cluster for T. gondii consistent with previous reports was obtained, demonstrating that the proteins HSP20, HSP21, HSP29, and HSP30 are very similar to each other and that HSP28 (mitochondrial sHSP) is more distantly related, as was suggested by de Miguel et al. (2009). N. caninum, an organism phylogenetically related to T. gondii, also appears to possess several members of sHSPs that, according to the tree topology in Fig. 4, are very similar to the orthologous sequences of T. gondii. Given this grouping, the HSP17.4 protein of N. caninum is very likely orthologous to HSP28 of T. gondii. In different species of the genus Plasmodium, only the sHSPs from Plasmodium falciparum and Plasmodium vivax (human parasites) appear closely related, while those from P. berghei and Plasmodium yoelii yoelii seem to be more distantly related. In the group of trypanosomatids, sHSPs from the different species of Leishmania were grouped in a similar manner, as was reported previously (Montalvo-Álvarez et al. 2008); this finding shows that sequences from Leishmania species and those from their close relatives in the genus Trypanosoma are similar.
Fig. 4.
Distance tree of sHSPs of protist and helminth parasites. The sequences containing the α-crystallin domains of sHSPs of different parasite species were aligned with Clustal W. The tree was built with the program MEGA5 using the neighbor joining method (NJ) and the Poisson distance model. The numbers on the branches represent bootstrap values (1000 replicates). The sequences were obtained from GenBank (http://www.ncbi.nlm.nih.gov/). The number in parentheses indicates the accession number. Acanthamoeba castellanii (ELR19103); Acanthocheilonema viteae (CAA48631); Babesia bigemina (AAK11630); Babesia bovis (XP_001609931); Babesia divergens (ABY67728); Brugia malayi (AAB07020); Brugia pahangi (CAA61152); Dirofilaria immitis (AAB08736); Echinococcus multilocularis (BAG69597); Eimeria tenella (ACS37296); Entamoeba histolytica (AAM21053); Fasciola hepática (ACE00519); Haemonchus contortus (AAN05752); Leishmania amazonensis (CAN13355); Leishmania braziliensis (XP_001566585); Leishmania donovani (AFA46756); Leishmania infantum (CAM69825); Leishmania major (XP_003722328); Leishmania mexicana (XP_003872313); Loa loa (EJD73818); Neospora caninum 17.4 (XP_003881633); Neospora caninum 20 (ACJ03988); Neospora caninum 21 (XP_003885215); Neospora caninum 29 (AAN87330); Neospora caninum BAGI (BAI44436); Nippostrongylus brasiliensis (BAJ60632); Onchocerca volvulus OV25-1 (CAA48632); Onchocerca volvulus OV25-2 (CAA48633); Ostertagia ostertagi (CAG25499); Paragonimus westermani (AAS19361); Plasmodium berghei (XP_679992); Plasmodium falciparum (CAD52179); Plasmodium vivax (XP_001616976); Plasmodium yoelii yoelii (XP_730306); Schistosoma japonicum (AAW25328); Schistosoma mansoni egg p40 (P12812); Schistosoma mansoni egg p40-2 (M96866); Strongyloides ratti 17.1 (ADY38527); Strongyloides ratti 17.2 (ADY38528); Taenia saginata (CAD80255); Taenia solium (CAD36617); Theileria annulata (CAI76223); Theileria orientalis (BAM42362); Theileria parva (XP_763804); Toxoplasma gondii 20 (EEB01013); Toxoplasma gondii 21 (EEA97341); Toxoplasma gondii 28 (XP_002369298); Toxoplasma gondii 29 (AAV20421); Toxoplasma gondii 30 (EEE25791); Trichinella pseudospiralis (ABJ55915); Trichinella spiralis (ABJ55914); Trichomonas vaginalis (EAX94618); Trypanosoma brucei (AAX70515); Trypanosoma cruzi (AAY78951); Trypanosoma vivax (CCC47083)
Less similarity exists between the sHSPs of the Apicomplexa or trypanosomatids and members of the taxon Amoebozoa (Entamoeba histolytica and Acanthamoeba castellanii) and Trichomonas vaginalis.
In the case of helminths, the two main clusters generated (nematodes and Platyhelminthes) agreed with the phylogeny of the group. The platyhelminthes possess a duplicated α-crystallin domain, one near the amino terminus (N) and the other near the carboxyl terminus (C). In our analysis, the alignment was conducted separately for each of these domains. Thus, the tree of Fig. 4 shows a greater similarity between the N and C α-crystallin of the different species than between the N and C α-crystallin of the same species. All N α-crystallins and all C α-crystallins were closely grouped regardless of species, as had been previously observed (de Jong et al. 1998).
In general, the relationships between the different taxa generated by the sequences of sHSPs reflect the phylogeny of the organisms.
Conclusions
In recent years, increasing attention has been paid to sHSPs in the field of infectious diseases. Since the first sHSP was described in parasites in 1986, molecules of this group have been identified in 20 parasites. Although the precise function of this class of proteins has not been clearly defined, roles in the adaptation to stress and in the development of the parasite have been suggested. The majority of parasites live a complex life cycle, transitioning between intracellular and extracellular stages, different hosts, and environment; as a result, exposure to harsh conditions over their life cycle is constant. The ability of a parasite to adapt to a new environment will help determine the successful establishment of the parasite. Moreover, their differentiation into different stages of development involves an organized cascade of gene expression that, in many cases, is closely related to cellular stress; indeed, stress often induces differentiation to another stage of the life cycle. In other cases, the regulation of development is not related to stress. The inhibition of sHSPs could impact parasite survival; sHSPs could thus represent potential therapeutic targets in parasite-related diseases. Because there is low sequence identity among the individual members of the family of sHSPs, drugs that inhibit the function of the sHSPs of the parasite and not the host could be developed. Certain sHSPs have been shown to be immunodominant antigens that can induce an immune response in the host, thus generating protection against the disease; such proteins could represent a potential vaccination target. Moreover, knowledge of the regulation of the expression of the HSPs and of their functional structure will help us better understand the role of these proteins in the chaperone network and improve our understanding of their role in important debilitating or deadly diseases caused by parasites. This review summarizes the work done so far on possible or demonstrated functions of sHSPs in the biology of a few parasites; the limited amount of available information confirms that the knowledge of this class of proteins is still insufficient. Finally, the study of sHSPs in parasites is a crucial research area that is potentially of great interest in the fight against parasitic diseases.
Acknowledgments
We thank Dr. Ricardo Paredes-León by his help to phylogenetic analysis; D. Pérez-Morales was a student of the PhD program: Doctorado en Ciencias Bioquímicas of Universidad Nacional Autónoma de México. She was a recipient of a PhD scholarship from CONACYT (130864). This work was partially supported by grant IN-206512 from DGAPA, UNAM.
References
- Arizono N, Yamada M, Tegoshi T, Takaoka Y, Ohta M, Sakaeda T. Hsp12.6 expression is inducible by host immunity in adult worms of the parasitic nematode Nippostrongylus brasiliensis. PLoS One. 2011;6 doi: 10.1371/journal.pone.0018141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basha E, Lee GJ, Demeler B, Vierling E. Chaperone activity of cytosolic small heat shock proteins from wheat. Eur J Biochem. 2004;271:1426–1436. doi: 10.1111/j.1432-1033.2004.04033.x. [DOI] [PubMed] [Google Scholar]
- Benitez L, Harrison LJS, Parkhouse RME, Gárate T. Sequence and preliminary characterisation of a Taenia saginata oncosphere gene homologue of the small heat-shock protein family. Parasitol Res. 1998;84:423–425. doi: 10.1007/s004360050422. [DOI] [PubMed] [Google Scholar]
- Bohne W, Gross U, Ferguson DJP, Heesemann J. Cloning and characterization of a bradyzoite-specifically expressed gene (hsp30/bag1) of Toxoplasma gondii, related to genes encoding small heat-shock proteins of plants. Mol Microbiol. 1995;16:1221–1230. doi: 10.1111/j.1365-2958.1995.tb02344.x. [DOI] [PubMed] [Google Scholar]
- Bohne W, Hunter CA, White MW, Ferguson DJP, Gross U, Roos DS. Targeted disruption of the bradyzoite-specific gene BAG1 does not prevent tissue cyst formation in Toxoplasma gondii. Mol Biochem Parasitol. 1998;92:291–301. doi: 10.1016/S0166-6851(97)00236-3. [DOI] [PubMed] [Google Scholar]
- Brown WC, Ruef BJ, Norimine J, Kegerreis KA, Suarez CE, Conley PG, Stich RW, Carson KH, Rice-Ficht AC. A novel 20-kilodalton protein conserved in Babesia bovis and B. bigemina stimulates memory CD4+ T lymphocyte responses in B. bovis-immune cattle. Mol Biochem Parasitol. 2001;118:97–109. doi: 10.1016/S0166-6851(01)00375-9. [DOI] [PubMed] [Google Scholar]
- Cai Y, Langley JG, Smith DI, Boros DL. A cloned major Schistosoma mansoni egg antigen with homologies to small heat shock proteins elicits Th1 responsiveness. Infect Immun. 1996;64:1750–1755. doi: 10.1128/iai.64.5.1750-1755.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanini B, Vita-Finzi L (2004) The world health report 2004: changing history. WHO Press, World Health Organization, France
- Cao M, Chao H, Doughty BL. Cloning of a cDNA encoding an egg antigen homologue from Schistosoma mansoni. Mol Biochem Parasitol. 1993;58:169–172. doi: 10.1016/0166-6851(93)90102-4. [DOI] [PubMed] [Google Scholar]
- Crompton DWT (2010) Working to overcome the global impact of neglected tropical diseases: first WHO report on neglected tropical diseases. WHO Press, World Health Organization, France
- de Jong WW, Caspers GJ, Leunissen JAM. Genealogy of the alpha-crystallin-small heat-shock protein superfamily. Int J Biol Macromol. 1998;22:151–162. doi: 10.1016/S0141-8130(98)00013-0. [DOI] [PubMed] [Google Scholar]
- de Miguel N, Echeverria PC, Angel SO. Differential subcellular localization of members of the Toxoplasma gondii Small Heat Shock Protein Family. Eukaryot Cell. 2005;4:1990–1997. doi: 10.1128/EC.4.12.1990-1997.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Miguel N, Lebrun M, Heaslip A, Hu K, Beckers CJ, Matrajt M, Dubremetz JF, Angel SO. Toxoplasma gondii Hsp20 is a stripe-arranged chaperone-like protein associated with the outer leaflet of the inner membrane complex. Biol Cell. 2008;100:479–489. doi: 10.1042/BC20080004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Miguel N, Braun N, Bepperling A, Kriehuber T, Kastenmüller A, Buchner J, Angel SO, Haslbeck M. Structural and functional diversity in the family of small heat shock proteins from the parasite Toxoplasma gondii. Biochim Biophys Acta. 2009;1793:1738–1748. doi: 10.1016/j.bbamcr.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Devaney E, Egan A, Lewis E, Warbrick EV, Jecock RM. The expression of small heat shock proteins in the microfilaria of Brugia pahangi and their possible role in development. Mol Biochem Parasitol. 1992;56:209–218. doi: 10.1016/0166-6851(92)90170-O. [DOI] [PubMed] [Google Scholar]
- Feder ME, Hofmann GE. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol. 1999;61:243–282. doi: 10.1146/annurev.physiol.61.1.243. [DOI] [PubMed] [Google Scholar]
- Ferrer E, González LM, Foster-Cuevas M, Cortéz MM, Dávila I, Rodríguez M, Sciutto E, Harrison LJS, Parkhouse RME, Gárate T. Taenia solium: characterization of a small heat shock protein (Tsol-sHSP35.6) and its possible relevance to the diagnosis and pathogenesis of neurocysticercosis. Exp Parasitol. 2005;110:1–11. doi: 10.1016/j.exppara.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Folgueira C, Requena JM. A postgenomic view of the heat shock proteins in kinetoplastids. FEMS Microbiol Rev. 2007;31:359–377. doi: 10.1111/j.1574-6976.2007.00069.x. [DOI] [PubMed] [Google Scholar]
- Gnanasekar M, Anandharaman V, Anand SB, Nutman TB, Ramaswamy K. A novel small heat shock protein 12.6 (HSP12.6) from Brugia malayi functions as a human IL-10 receptor binding protein. Mol Biochem Parasitol. 2008;159:98–103. doi: 10.1016/j.molbiopara.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorenflot A, Brasseur P, Precigout E, L’Hostis M, Marchand A, Schrevel J. Cytological and immunological responses to Babesia divergens in different hosts: ox, gerbil, man. Parasitol Res. 1991;77:3–12. doi: 10.1007/BF00934377. [DOI] [PubMed] [Google Scholar]
- Hartman D, Cottee PA, Savin KW, Bhave M, Presidente PJA, Fulton L, Walkiewicz M, Newton SE. Haemonchus contortus: molecular characterization of a small heat shock protein. Exp Parasitol. 2003;104:96–103. doi: 10.1016/S0014-4894(03)00138-3. [DOI] [PubMed] [Google Scholar]
- Haslbeck M. sHsps and their role in the chaperone network. Cell Mol Life Sci. 2002;59:1649–1657. doi: 10.1007/PL00012492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hombach A, Ommen G, MacDonald A, Clos J. A small heat shock protein is essential for thermotolerance and intracellular survival of Leishmania donovani. J Cell Sci. 2014;127:4762–4773. doi: 10.1242/jcs.157297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingolia TD, Craig EA. Four small Drosophila heat shock proteins are related to each other and to mammalian α-crystallin. Proc Natl Acad Sci USA. 1982;79:2360–2364. doi: 10.1073/pnas.79.7.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jecock RM, Devaney E. Expression of small heat shock proteins by the third stage larva of Brugia pahangi. Mol Biochem Parasitol. 1992;56:219–226. doi: 10.1016/0166-6851(92)90171-F. [DOI] [PubMed] [Google Scholar]
- Kappé G, Aquilina JA, Wunderink L, Kamps B, Robinson CV, Gárate T, Boelens WC, de Jong WW. Tsp36, a tapeworm small heat-shock protein with a duplicated α-crystallin domain, forms dimers and tetramers with good chaperone-like activity. Proteins. 2004;57:109–117. doi: 10.1002/prot.20220. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Narabu S, Yanai Y, Hatano Y, Ito A, Imai S, Ike K (2013) Gene cloning and characterization of the protein encoded by the Neospora caninum bradyzoite-specific antigen gene Bag1. J Parasitol 99:453–458 [DOI] [PubMed]
- Kouguchi H, Matsumoto J, Katoh Y, Suzuki T, Oku Y, Yagi K. Echinococcus multilocularis: two-dimensional western blotting method for the identification and expression analysis of immunogenic proteins in infected dogs. Exp Parasitol. 2010;124:238–243. doi: 10.1016/j.exppara.2009.09.016. [DOI] [PubMed] [Google Scholar]
- Lee GJ, Roseman AM, Saibil HR, Vierling E. A small heat shock protein stably binds heat-denatured model substrates and can maintain a substrate in a folding-competent state. EMBO J. 1997;16:659–671. doi: 10.1093/emboj/16.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Carson K, Rice-Ficht A, Good T. Hsp20, a novel α-crystallin, prevents Aβ fibril formation and toxicity. Protein Sci. 2005;14:607–601. doi: 10.1110/ps.041020705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Lee J, Kim SH, Yong TS. Molecular cloning and characterization of a major egg antigen in Paragonimus westermani and its use in ELISA for the immunodiagnosis of paragonimiasis. Parasitol Res. 2007;100:677–681. doi: 10.1007/s00436-006-0324-7. [DOI] [PubMed] [Google Scholar]
- Liddell S, Parker C, Vinyard B, Jenkins M, Dubey JP. Immunization of mice with plasmid DNA coding for NcGRA7 or NcsHSP33 confers partial protection against vertical transmission of Neospora caninum. J Parasitol. 2003;89:496–500. doi: 10.1645/GE-2969. [DOI] [PubMed] [Google Scholar]
- Lillibridge CD, Rudin W, Philipp MT. Dirofilaria immitis: ultrastructural localization, molecular characterization, and analysis of the expression of p27, a small heat shock protein homolog of nematodes. Exp Parasitol. 1996;83:30–45. doi: 10.1006/expr.1996.0046. [DOI] [PubMed] [Google Scholar]
- Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- MacRae TH. Structure and function of small heat shock/alpha-crystallin proteins: established concepts and emerging ideas. Cell Mol Life Sci. 2000;57:899–913. doi: 10.1007/PL00000733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maresca B, Carratù L. The biology of the heat shock response in parasites. Parasitol Today. 1992;8:260–266. doi: 10.1016/0169-4758(92)90137-Q. [DOI] [PubMed] [Google Scholar]
- Martínez I, Martínez-Ibarra A, Arce-Fonseca M, Rodríguez-Morales O, Pérez-Morales D, Reyes-López PA, Espinoza B. Seroprevalence and major antigens recognized by sera from Trypanosoma cruzi-infected dogs from Jalisco, México. Rev Argent Microbiol. 2014;46:85–90. doi: 10.1016/S0325-7541(14)70053-7. [DOI] [PubMed] [Google Scholar]
- Mohamed RM, Aosai F, Chen M, Mun HS, Norose K, Belal US, Piao LX, Yano A. Induction of protective immunity by DNA vaccination with Toxoplasma gondii HSP70, HSP30 and SAG1 genes. Vaccine. 2003;21:2852–2861. doi: 10.1016/S0264-410X(03)00157-9. [DOI] [PubMed] [Google Scholar]
- Montagna GN, Buscaglia CA, Münter S, Goosmann C, Frischknecht F, Brinkmann V, Matuschewski K. Critical role for heat shock protein 20 (HSP20) in migration of malarial sporozoites. J Biol Chem. 2012;287:2410–2422. doi: 10.1074/jbc.M111.302109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montalvo-Álvarez AM, Folgueira C, Carrión J, Monzote-Fidalgo L, Cañavate C, Requena JM (2008) The Leishmania HSP20 is antigenic during natural infections, but, as DNA vaccine, it does not protect BALB/c mice against experimental L. amazonensis infection. J Biomed Biotechnol 2008:695432 [DOI] [PMC free article] [PubMed]
- Montero E, Rodriguez M, Gonzalez LM, Lobo CA. Babesia divergens: identification and characterization of BdHSP-20, a small heat shock protein. Exp Parasitol. 2008;119:238–245. doi: 10.1016/j.exppara.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Mounier N, Arrigo AP. Actin cytoskeleton and small heat shock proteins: how do they interact? Cell Stress Chaperones. 2002;7:167–176. doi: 10.1379/1466-1268(2002)007<0167:ACASHS>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moxon JV, LaCourse EJ, Wright HA, Perally S, Prescott MC, Gillard JL, Barrett J, Hamilton JV, Brophy PM. Proteomic analysis of embryonic Fasciola hepatica: characterization and antigenic potential of a developmentally regulated heat shock protein. Vet Parasitol. 2010;169:62–75. doi: 10.1016/j.vetpar.2009.12.031. [DOI] [PubMed] [Google Scholar]
- Mun HS, Aosai F, Yano A. Role of Toxoplasma gondii HSP70 and Toxoplasma gondii HSP30/bag1 in antibody formation and prophylactic immunity in mice experimentally infected with Toxoplasma gondii. Microbiol Immunol. 1999;43:471–479. doi: 10.1111/j.1348-0421.1999.tb02430.x. [DOI] [PubMed] [Google Scholar]
- Nakamoto H, Vígh L. The small heat shock proteins and their clients. Cell Mol Life Sci. 2007;64:294–306. doi: 10.1007/s00018-006-6321-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narberhaus F. Alpha-crystallin-type heat shock proteins: socializing minichaperones in the context of a multichaperone network. Microbiol Mol Biol Rev. 2002;66:64–93. doi: 10.1128/MMBR.66.1.64-93.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nene V, Dunne DW, Johnson KS, Taylor DW, Cordingley JS. Sequence and expression of a major egg antigen from Schistosoma mansoni. Homologies to heat shock proteins and alpha-crystallins. Mol Biochem Parasitol. 1986;21:179–188. doi: 10.1016/0166-6851(86)90021-6. [DOI] [PubMed] [Google Scholar]
- Nores MJ, Prucca CG, Quiroga R, Elías EV, Cavallín L, Price AM, Saura A, Carranza PG, Gottig N, Solari AJ, Lujan HD. ORF-C4 from the early branching eukaryote Giardia lamblia displays characteristics of α-crystallin small heat-shock proteins. Biosci Rep. 2009;29:25–34. doi: 10.1042/BSR20080101. [DOI] [PubMed] [Google Scholar]
- Pérez-Morales D, Ostoa-Saloma P, Espinoza B. Trypanosoma cruzi SHSP16: characterization of an α-crystallin small heat shock protein. Exp Parasitol. 2009;123:182–189. doi: 10.1016/j.exppara.2009.06.019. [DOI] [PubMed] [Google Scholar]
- Plesofsky-Vig N, Vig J, Brambl R. Phylogeny of the alpha-crystallin-related heat-shock proteins. J Mol Evol. 1992;35:537–545. doi: 10.1007/BF00160214. [DOI] [PubMed] [Google Scholar]
- Précigout E, Valentin A, Carcy B, Gorenflot A, Nakamura K, Aikawa M, Schrével J. Babesia divergens: characterization of a 17-kDa merozoite membrane protein. Exp Parasitol. 1993;77:425–434. doi: 10.1006/expr.1993.1102. [DOI] [PubMed] [Google Scholar]
- Raghavan N, Ghosh I, Eisinger WS, Pastrana D, Scott AL. Developmentally regulated expression of a unique small heat shock protein in Brugia malayi. Mol Biochem Parasitol. 1999;104:233–246. doi: 10.1016/S0166-6851(99)00150-4. [DOI] [PubMed] [Google Scholar]
- Sun Y, MacRae TH. Small heat shock proteins: molecular structure and chaperone function. Cell Mol Life Sci. 2005;62:2460–2476. doi: 10.1007/s00018-005-5190-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson FJ, Martin SAM, Devaney E. Brugia pahangi: characterisation of a small heat shock protein cDNA clone. Exp Parasitol. 1996;83:259–266. doi: 10.1006/expr.1996.0073. [DOI] [PubMed] [Google Scholar]
- Tsvetkova NM, Horvath I, Torok Z, Wolkers WF, Balogi Z, Shigapova N, Crowe LM, Tablin F, Vierling E, Crowe JH, Vígh L. Small heat-shock proteins regulate membrane lipid polymorphism. Proc Natl Acad Sci U S A. 2002;99:13504–13509. doi: 10.1073/pnas.192468399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweedie S, Grigg ME, Ingram L, Selkirk ME. The expression of a small heat shock protein homologue is developmentally regulated in Nippostrongylus brasiliensis. Mol Biochem Parasitol. 1993;61:149–154. doi: 10.1016/0166-6851(93)90168-W. [DOI] [PubMed] [Google Scholar]
- Valentin A, Precigout E, L’Hostis M, Carcy B, Gorenflot A, Schrevel J. Cellular and humoral immune responses induced in cattle by vaccination with Babesia divergens culture-derived exoantigens correlate with protection. Infect Immun. 1993;61:734–741. doi: 10.1128/iai.61.2.734-741.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Montfort RLM, Basha E, Friedrich KL, Slingsby C, Vierling E. Crystal structure and assembly of a eukaryotic small heat shock protein. Nat Struct Biol. 2001;8:1025–1030. doi: 10.1038/nsb722. [DOI] [PubMed] [Google Scholar]
- Vercauteren I, De Maere V, Vercruysse J, Stevens M, Gevaert K, Claerebout E. A small heat shock protein of Ostertagia ostertagi: stage-specific expression, heat inducibility, and protection trial. J Parasitol. 2006;92:1244–1250. doi: 10.1645/GE-871R.1. [DOI] [PubMed] [Google Scholar]
- Waters ER, Lee GJ, Vierling E. Evolution, structure and function of the small heat shock proteins in plants. J Exp Bot. 1996;47:325–338. doi: 10.1093/jxb/47.3.325. [DOI] [Google Scholar]
- Wu Z, Nagano I, Boonmars T, Takahashi Y. Thermally induced and developmentally regulated expression of a small heat shock protein in Trichinella spiralis. Parasitol Res. 2007;101:201–212. doi: 10.1007/s00436-007-0462-6. [DOI] [PubMed] [Google Scholar]
- Younis AE, Geisinger F, Ajonina-Ekoti I, Soblik H, Steen H, Mitreva M, Erttmann KD, Perbandt M, Liebau E, Brattig NW. Stage-specific excretory-secretory small heat shock proteins from the parasitic nematode Strongyloides ratti-putative links to host’s intestinal mucosal defense system. FEBS J. 2011;278:3319–3336. doi: 10.1111/j.1742-4658.2011.08248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YW, Kim K, Ma YF, Wittner M, Tanowitz HB, Weiss LM. Disruption of the Toxoplasma gondii bradyzoite-specific gene BAG1 decreases in vivo cyst formation. Mol Microbiol. 1999;31:691–701. doi: 10.1046/j.1365-2958.1999.01210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberstein D, Shapira M. The role of pH and temperature in the development of Leishmania parasites. Annu Rev Microbiol. 1994;48:449–470. doi: 10.1146/annurev.mi.48.100194.002313. [DOI] [PubMed] [Google Scholar]
- Zügel U, Kaufmann SHE. Role of heat shock proteins in protection from and pathogenesis of infectious diseases. Clin Microbiol Rev. 1999;12:19–39. doi: 10.1128/cmr.12.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]