Abstract
Hippophae salicifolia (HS) and Hippophae rhamnoides turkestanica (HRT) are abundantly found species of Hippophae in Himalayan region of India. As these plants thrive under extreme climatic conditions, it is suspected that these plants must have a unique adaptogenic property against high-altitude stress. To keeping these views in our mind, the present study was planned to evaluate the mechanism of action of aqueous extract of HS and aqueous extract of HRT against multiple stress [cold-hypoxia-restraint (C-H-R)] for their adaptogenic activity. The present study reported the adaptogenic activity of HS in facilitating tolerance to multiple stress, CHR in rats. Pre-treatment with aqueous extract of HS significantly attenuated reactive oxygen species (ROS) production, protein oxidation, and lipid peroxidation and also showed role in maintaining antioxidant status as similar to control rats. Since protein oxidation was decreased by pre-treatment of HS, protein homeostasis was also sustained by regulation of heat shock proteins (HSP70 and HSP60). Interestingly, heme oxygenase-1 (HO-1), Vascular Endothelial Growth Factor (VEGF), and nitric oxide (NO) level was also increased in HS pre-treated rats depicted its adaptogenic activity against multiple stress, CHR. Conclusively, aqueous extract of HS could use an adaptogen for high altitude-associated multiple stress (CHR).
Keywords: Adaptogen, High altitude, Hippophae salicifolia (HS), Hippophae rhamnoides turkestanica (HRT)
Introduction
High terrestrial altitudes have since long attracted man for different point of views like leisure and resources. The populace at high altitude includes the indigenous natives (Tibetans and Sherpas), armed forces, as well as sojourners like trekkers, skiers, etc. High-altitude exposure beyond 3000 m is an adverse climatic condition that accompanied by psychological and physiological stress. The adverse conditions due to high altitude characterized as hypoxia, cold, wind, and harmful ionizing radiations which ultimately imposing to decrease in physical and mental performance (Askew 1995, 1997). One of the significant factors which affect life at high altitude is fall in pressure of atmospheric oxygen, required for all mammalian life. Beside this, another major stressor at high altitude is cold that cause physiological disturbances. Fall in oxygen pressure, known as hypoxia, leads to increased reactive oxygen species (ROS) production by mitochondrial electron transport system (Mohanraj et al. 1998). Recent studies also reported oxidative damage of lipids, proteins, and DNA due to high altitude-associated hypoxia (Joanny et al. 2001). This damage can be due to increased level of ROS production and/or decreased level of antioxidant capacity. Numerous studies concluded an increased generation of oxidative stress indicators in breath, blood, urine, and tissue of laboratory rodents in response to hypoxia (Ilavazhagan et al. 2001; Radak et al. 1997). A decrement in atmospheric temperature could be another reason for generating oxidative stress. It is suggested that decrease in temperature leads to decrease mitochondrial membrane fluidity (Hazel 1995) which could disrupt electron transfer and ultimately result into excess production of ROS. Thus, there is a need for a safe and effective adaptogens which could give better and immediate adaptivity at the extreme stress conditions of high altitude. One of the strategies to achieve this targets by exploring the unique adaptogenic activity of herbals specially herbals which bloom at high altitude.
Indian Hippophae naturally grows in high-altitude areas of Jammu and Kashmir, Himachal Pradesh, Uttar Pradesh, and Sikkim (Singh 2001). Hippophae rhamnoides is abundantly found species followed by Hippophae salicifolia (HS) and Hippophae rhamnoides turkestanica (HRT). HRT, basically grows in northwest Himalayas at high altitudes (7000–15,000 ft), is a dwarf to tall (3–15 ft), branched, and thorny nitrogen-fixing deciduous shrub while; Hippophae salicifolia (HS) is a shrubby tree and is also restricted to the Himalayan region (Rongsen 1992). A number of reports suggested the antioxidant value of H. rhamnoides (Eccleston et al. 2002; Geetha et al. 2003; Negi et al. 2005). However, a limited study has been done on subspecies of Hippophae, i.e., HS and HRT. As these plants thrive under extreme climatic conditions, it is suspected that these plants must have a unique adaptogenic property against high-altitude stress. Recently, Sharma et al. 2014 depicted the adaptogenic activity of aqueous extract of HS and HRT, but the unknown part is its mechanism. To keeping these views in our mind, the present study was planned to elucidate the probable mechanistic approach for adaptogenic activity of HS and HRT against multiple stress [cold-hypoxia-restraint (C-H-R)].
Materials and methods
Apparatus, chemicals, and reagents
The following apparatus were used in the study: C-H-R Animal model, Seven Star, India; Iso-Thermex, Columbus Instruments, USA; accelerated solvent extractor (ASE), Dionex Corporation, USA; Buchi Rotavapor R-124, Buchi Labortechnik AG, Postfach, CH-9230 Flawil/Schweiz, Switzerland; lyophilizer, Allied Frost, India; spectrophotometer, Bio-Rad, USA; pH meter, Eutech pH 510, India. Extra pure reagents from Sigma, SD Fine, Merck, and SRL were used for all the studies.
Extract preparation
Plant materials
Plant materials HS and HRT were authenticated by Dr. Virendra Singh from CSK, Palampur, Himachal Pradesh. They are grown at Seabuckthorn Research Farm of CSK Himachal Pradesh Agricultural University at Kukumseri in Lahaul valley of District Lahaul-Spiti, a semiarid region of Himachal Pradesh, India, at an altitude of 2730 m in sandy loam soil with annual rainfall of 500 mm.
Aqueous extracts of HS and HRT were prepared using ASE using leaves of the same plant. Extraction was carried out at room temperature using the following procedure: (i) Leaves (30 g) was loaded into 100 mL extraction cells, (ii) cell was filled with solvent at a pressure of 1500 psi, (iii) cell was rinsed with 60 % cell volume using double distilled water, (iv) solvent was purged from cell with N2 gas and (v) depressurization takes place, (vi) extracts were collected and stored at 4 °C in separate bottles till further solvent removal. The aqueous extracts were lyophilized and were preserved, at 4 °C until use.
Animals and experimental design
Animals
Sprague-Dawley male rats, 12–14 weeks old, weighing 150 ± 10 g, bred in the animal facility of Defence Institute of Physiology and Allied Sciences (DIPAS), Delhi, were maintained on a bedding of rice husk in polypropylene cages under controlled environment in the Institute’s animal house at 22 ± 1 °C, 55 ± 10 % humidity, and 12-h light-dark cycle. Animals had access to standard rodent pellet feed and water ad libitum. The study has the approval of the Institutional Ethical Committee on Animal Experimentations (IAEC), and the experiments were performed in accordance with the regulations specified by the IAEC and conformed to National Guidelines on the Care and Use of Laboratory Animals, India.
Experimental design for evaluation of adaptogenic activity
The animal chamber had two units. One unit is an animal decompression chamber where the rats kept in restrainers were exposed to cold (5 °C) and hypoxia (428 mmHg) equivalent to an altitude of 4572 m. The air flow was 2 l/min. Another unit was an animal conditioning chamber which was maintained at 32 ± 1 °C and normal atmospheric pressure (Ramachandran et al. 1990).
A total of 24 animals were used in the study. The rats were divided into four groups of six rats each:
| Group I | Untreated and unexposed to CHR rats served as control |
| Group II | CHR exposure (cold-hypobaric hypoxia-restraint) + pre-treatment of distilled water |
| Group III | CHR exposure (cold-hypobaric hypoxia-restraint) + pre-treatment of aqueous extract of HS (100 mg/kg body weight) (oral) |
| Group IV | CHR exposure (cold-hypobaric hypoxia-restraint) + pre-treatment of aqueous extract of HRT (100 mg/kg body weight) (oral) |
The herbal extracts were administered orally using a gastric cannula 30 min prior to the CHR exposure. The doses and pre-treatment time of extracts were selected on the basis of earlier published studies (Sharma et al. 2014). At the end of the study, rats were anaesthetized using sodium pentobarbital. Liver, heart, and muscle were rapidly excised, washed with normal saline to remove all extraneous materials, thereafter the tissues immediately stored at −80 °C for further use.
Biochemical assays
Oxidative stress and damage markers
For biochemical estimations, the tissue was homogenized in 0.154 M KCl-EDTA buffer.
ROS measurement
2′,7′-Dichlorofluorescein (DCFH) was used as a fluorescent probe to measure the rate of oxidant production in the fresh tissue homogenates according to LeBel et al. (1992) with some modifications (Cathcart et al. 1983). DCFH-DA, a nonfluorescent lipophilic dye, passively diffuses through cellular membranes where it is cleaved into 2,7-dichlorofluorescein (DCF) by the action of intracellular esterases and thus fluoresces in the presence of ROS. The production of free radicals was determined as described earlier. Briefly, 150 μL of tissue homogenate was incubated with 10 μL of 100 μM DCFH-DA for 30 min in the dark. Fluorescence was read using a fluorimeter (Perkin Elmer, UK) with excitation at 485 nm and emission at 535 nm. Readings were obtained in arbitrary flourometric units and results expressed as fold change in free radical generation.
NO measurement
Nitrite is a stable end product of nitric oxide (NO), and its concentration was determined by the Griess method (Paula et al. 2005). Tissue was homogenized in four volumes of 30 mM Tris-HCl buffer, pH 6.8, containing 5 mM EDTA, 250 mM sucrose, 30 mM KCl, 2 % β-mercaptoethanol, PMSF (100 μg/mL), aprotinin (2 μg/mL), and leupeptin (2 μg/mL) and then centrifuged (12,000 × g, 4 °C, 15 min). Aliquots (1.0 mL) of the homogenates were removed and diluted with 1.0 mL of Griess reagent (1 % sulphanilamide, 2 % phosphoric acid, and 0.1 % naphthyl ethylene diamine dihydrochloride). The absorbance of the chromophore formed during diazotization of the nitrite with sulphanilamide and subsequent coupling with naphthyl ethelene diamine was measured at 545 nm in UV-Vis spectrophotometer (Bio-Rad, USA).
Lipid peroxidation
Tissue lipid per oxidation was measured by method of Onkawa et al. 1979. One milliliter of tissue homogenate, prepared in 0.15 M KCl (5 % w/v), was incubated for 1 h at 37 °C followed by addition of 10 % TCA, mixed thoroughly, and centrifuge at 3000 rpm for 10 min. One milliliter TBA was added to 1 mL supernatant, and the tube were kept in boiling water bath for 10 min till the pink color appeared. One milliliter of double-distilled water was added to after cooling the tubes, and absorbance was measured at 532 nm.
Protein oxidation and damage
Protein carbonyl content
Oxidative modifications of amino acid residues include derivatization of amino acid residues such as proline, arginine, and lysine to reactive carbonyl derivatives. Briefly, 2,4-dinitrophenylhydrazine reacts with protein carbonyl forming a Schiff base to produce the corresponding hydrazone, which can be analyzed spectrophotometrically (Levine et al. 1990). Tissue was homogenized in ice-cold 50 mM phosphate buffer (pH 7.2) containing 1 mM EDTA and proteinase inhibitor cocktail. The homogenate was centrifuged at 10,000 × g for 15 min, and the supernatant was checked for presence of nucleic acids by measuring the absorbance at 260 and 280 nm. A ratio of 280/260 nm more than 1 indicated that the sample was free of nucleic acid contamination. To 200 μL of sample, 600 μL 10 mM 2,4-dinitrophenylhydrazine (DNPH) in 2 N HCl was added; 600 μL of 2 N HCl was added as a blank control. The mixture was incubated for 1 h at room temperature. The protein was precipitated with an equal volume of 20 % trichloroacetic acid and was washed three times with ethanol/ethyl acetate (1:1 v/v). The final precipitate was dissolved in 400 μL of 6 M guanidine hydrochloride (pH 2.3), and the insoluble debris was removed by centrifugation. The absorbance of the DNPH derivatives was measured at 360 nm. The concentration of carbonyl groups was calculated by using an absorbance coefficient 22 nM/cm and expressed as nmol carbonyl per mg of protein.
Advanced oxidation protein products
Advanced oxidation protein products (AOPPs), considered as markers to evaluate oxidant-induced protein damage, are formed by the reaction of proteins with chlorinated oxidants such as chloramines or hypochlorous acid. The spectrophotometric detection of AOPP levels, first described in plasma by Witko-Sarsat et al. 1996, was modified for tissue. Briefly, tissue homogenates, prepared in 0.154 M KCl-EDTA, were diluted 1:5 with phosphate-buffered saline (PBS), pH 7.4. For standard curve, 200 μL of chloramin T (0–100 μmol/L) and 200 μL of PBS as blank were applied on a microtiter plate. Similarly, 200 μL of diluted samples were applied. Ten microliter of 1.16 M potassium iodide and 20 μL of acetic acid were added to each well and absorbance at 340 nm measured immediately. Concentration of AOPPs were obtained in chloramine units and expressed as μmol chloramine/mg protein.
Antioxidant enzyme activity
Superoxide dismutase
Enzyme activity for total superoxide dismutase was measured by the method of Kakkar et al. 1984. Reaction mixture contained 1.2 mL of sodium pyrophosphate buffer (0.052 mM, pH 7.0), 0.1 mL of phenazine methosulphate (PMS) (186 μM), 0.3 mL of nitro blue tetrazolium (NBT) (300 μM). Supernatant (0.2 mL) obtained after centrifugation (1500 × g, 10 min followed by 10,000 × g, 15 min) of 5 % homogenate was added to reaction mixture. Enzyme reaction was initiated by adding 0.2 mL of NADH (780 μM) and stopped precisely after 1 min by adding 1 mL of glacial acetic acid. Amount of chromogen formed was measured by recording color intensity at 560 nm.
To determine Mn-SOD isoenzyme activity (SOD2), assay were performed in the presence of 1.5 mM potassium cyanide (cyanide-insensitive SOD). Cyanide-sensitive Cu,Zn-SOD isoenzyme activity (SOD1) was calculated as the difference of activity in the absence and presence of cyanide in the assay for the same enzymatic preparation.
Catalase
Catalase activity in tissues was assayed following the procedure of Sinha 1972. Homogenate (5 %) were used for catalase estimation. Homogenate (0.1 mL) was incubated with 0.5 mL of H2O2 (0.2 M) at 37 °C for 90 s precisely, in the presence of 0.01 M phosphate buffer (pH 7.4). Reaction was stopped by adding 5 % dichromate solution. Further samples were incubated at 100 °C for 15 min in boiling water bath. Amount of H2O2 consumed was determined by recording absorbance at 570 nm.
Reduced glutathione and oxidized glutathione
For tissue reduced glutathione (GSH) and oxidized glutathione (GSSG) estimation, 0.25 g of tissue sample was homogenized on ice with 3.75 mL of phosphate-EDTA buffer and 1 mL of 25 % HPO3 which was used as a protein precipitant. The total homogenate was centrifuged at 100,000 × g for 30 min at 4 °C. For the GSH assay in soft tissues (Hissin and Hilf 1973), 0.5 mL supernatant and 4.5 mL phosphate buffer (pH 8.0) were mixed. The final assay mixture (2.0 mL) contained 100 mL supernatant, 1.8 mL phosphate-EDTA buffer, and 100 mL O-phthaldehyde (OPT; 1000 mg/mL in absolute methanol, prepared fresh). After mixing, fluorescence was determined at 420 nm with an excitation wavelength of 350 nm using a spectrofluorometer (Model RF 5000 Shimadzu, Japan).
For the GSSG assay, 0.5 mL supernatant was incubated at room temperature with 200 mL of 0.04 mol/L N-ethylmaleimide solution for 30 min. To this mixture, 4.3 mL of 0.1 mol/L NaOH was added. A 100 mL sample of this mixture was taken for the measurement of GSSG using the procedure described above for GSH assay except that 0.1 mol/L NaOH was used as the diluent instead of phosphate buffer (Hissin and Hilf 1973).
Hypoxia-responsive proteins
Heme oxygenase-1 content
Heme oxygenase-1 (HO-1) levels were determined in liver, muscle, and heart homogenate using a commercially available kit, Rat HO-1 ELISA (Enzo Life Sciences, USA) as per manufacturer’s instructions. The concentrations were expressed as ng/mg protein.
VEGF content
Vascular endothelial growth factor (VEGF) content was measured in liver, muscle, and heart homogenate using commercially available rat VEGF ELISA kit (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions. The intensity of the color reaction was read spectrophotometrically in a plate reader and the concentration expressed in pg/mg protein.
HSP60 and HSP70
HSP60 and HSP70 were quantified in liver, muscle, and heart by Rat ELISA (Cusabio ELISA Kit, China) according to the manufacturer’s instructions. The intensity of the color reaction was read spectrophotometrically in a plate reader and the concentration expressed in ng/mg protein.
Statistical analysis
All the experiments were performed on a minimum of three different occasions, and data are presented as mean ± SEM. One-way analysis of variance with post hoc Bonferroni analysis was used to determine statistical significance between groups. All analysis was conducted using GraphPad Prism ver 6.00 software (GraphPad, CA, USA). The p value of ≤0.05, with a 95 % confidence interval was considered significant.
Results
Multiple stresses induced oxidative stress markers
Level of free radicals (reactive oxygen species)
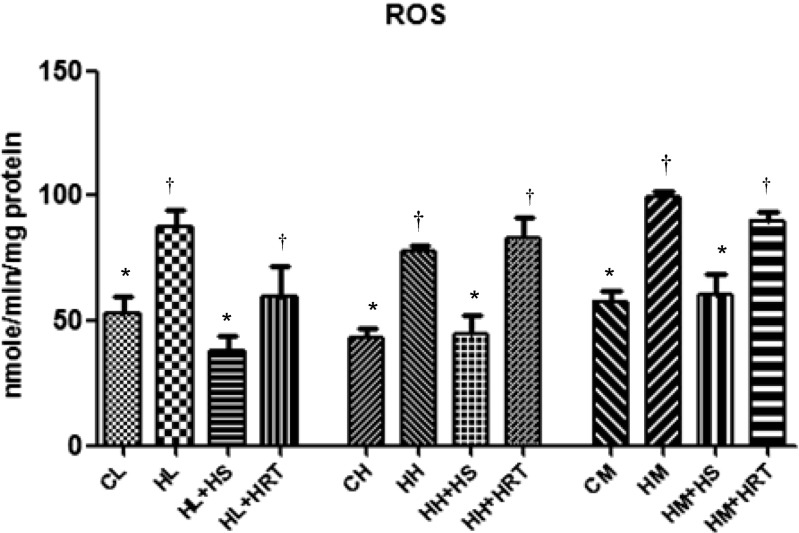
One of the known causes for increase oxidative stress in cell is reduced oxygen availability or generation of free radicals. Figure 1 represents ROS level in liver, heart, and muscle due to CHR exposure and their response to herbal treatment (HS and HRT). CHR exposure caused significant elevation in ROS, and this was 1.65-, 1.80-, and 1.71-fold increase in liver, heart, and muscle, respectively, as compared to control rats. While, ROS level decreased significantly due to pre-treatment with HS (1.56-, 1.72-, and 1.64-fold decrease in liver, heart, and muscle, respectively) and HRT (1.36-, 1.00-, and 1.10-fold decrease in liver, heart, and muscle, respectively) as compared to CHR-exposed rats.
Fig. 1.
Multiple stress (CHR)-induced oxidative damage via ROS generation and effect of HS and HRT supplementation on ROS in rat liver, heart, and muscle. Data represents the mean ± SE; N = 6. *, † Differences between values with matching symbol notations within each column are not statistically significant at 5 % level of probability. CL control liver, HL CHR-exposed liver, HL+HS CHR exposed+Hippophae salicifolia (HS)-supplemented liver, HL+HRT CHR exposed+Hippophae rhamnoides turkestanica (HRT)-supplemented liver, CH control heart, HH CHR-exposed heart, HH+HS CHR exposed+Hippophae salicifolia (HS)-supplemented heart, HH+HRT CHR exposed+Hippophae rhamnoides turkestanica (HRT)-supplemented heart, CM control muscle, HM CHR-exposed muscle, HM+HS CHR exposed+Hippophae salicifolia (HS)-supplemented muscle, HM+HRT CHR exposed+Hippophae rhamnoides turkestanica (HRT)-supplemented muscle
Level of lipid peroxidation
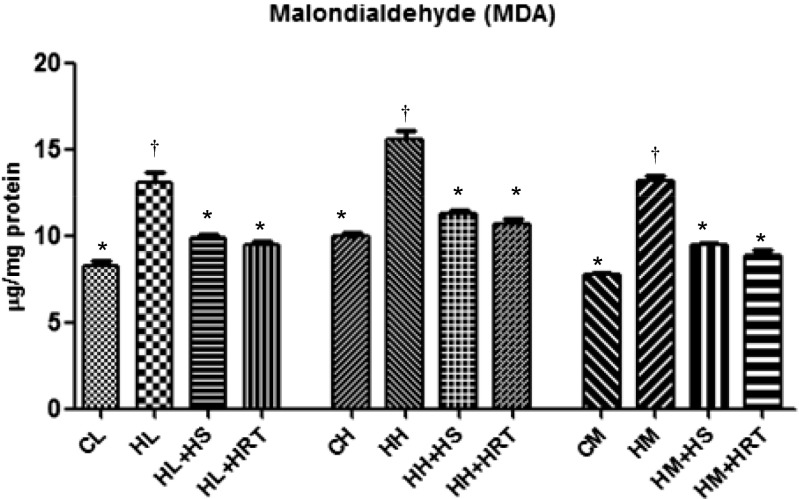
The damage of lipid molecules is indicated by generation of malonaldehyde which was found to be higher in CHR-exposed group animal as compared to control animal. In the exposed animals, malonaldehyde was found to be higher almost 2.56-, 3.51-, and 2.52-fold higher in liver, heart, and muscle, respectively (Fig. 2). The level of malonaldehyde (MDA) came to normal level due to pre-treatment with HS and HRT in liver, heart, and muscle.
Fig. 2.
Multiple stress (CHR)-induced oxidative damage via lipid peroxidation indicated by malondialdehyde levels and effect of HS and HRT supplementation on malondialdehyde in rat liver, heart, and muscle. Data represents the mean ± SE; N = 6. *, † Differences between values with matching symbol notations within each column are not statistically significant at 5 % level of probability. CL control liver, HL CHR-exposed liver, HL+HS CHR exposed+Hippophae salicifolia (HS)-supplemented liver, HL+HRT CHR exposed+Hippophae rhamnoides turkestanica (HRT)-supplemented liver, CH control heart, HH CHR-exposed heart, HH+HS CHR exposed+Hippophae salicifolia (HS)-supplemented heart, HH+HRT CHR exposed+Hippophae rhamnoides turkestanica (HRT)-supplemented heart, CM control muscle, HM CHR-exposed muscle, HM+HS CHR exposed+Hippophae salicifolia (HS)-supplemented muscle, HM+HRT CHR exposed+Hippophae rhamnoides turkestanica (HRT)-supplemented muscle
Level of protein oxidation
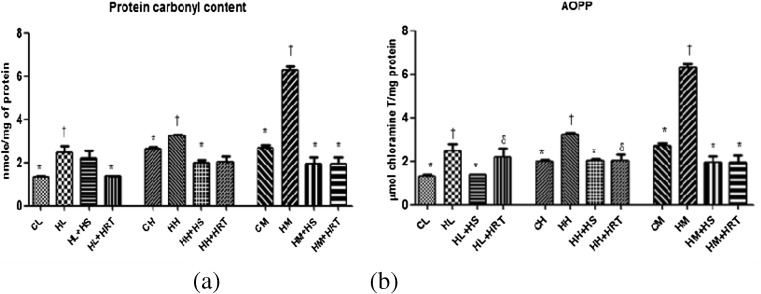
Protein oxidation was also observed with lipid peroxidation in multiple stress-exposed animals. Protein carbonyl derivative formation was also increased in CHR-exposed animals with reference to control animals (2.305 ± 0.042 nmol/mg protein, 3.18 ± 0.089 nmol/mg protein, and 6.808 ± 0.25 nmol/mg protein in liver, heart, and muscle, respectively) (Fig. 3a). On measuring the levels of AOPPs in liver, heart, and muscle, a significant higher level of oxidation was observed in CHR-exposed animals with respect to control animals (6.85 ± 0.505 μmol/mg protein, 3.35 ± 0.089 μmol/mg protein, and 4.17 ± 0.225 μmol/mg protein in liver, heart, and muscle, respectively; p < 0.05) (Fig. 3b). Interestingly, pre-administration of HS in CHR-exposed rats restored both protein carbonyl content and AOPP.
Fig. 3.
Free radical generation under multiple stress (CHR) resultant into protein modification. a Protein carbonylation. b Advanced oxidation protein products (AOPPs) and effect of HS and HRT supplementation on protein modification in rat liver, heart, and muscle. Data represents the mean ± SE; N = 6. *, †, δ Differences between values with matching symbol notations within each column are not statistically significant at 5 % level of probability. CL control liver, HL CHR-exposed liver, HL+HS CHR exposed+Hippophae salicifolia (HS)-supplemented liver, HL+HRT CHR exposed+Hippophae rhamnoides turkestanica (HRT)-supplemented liver, CH control heart, HH CHR-exposed heart, HH+HS CHR exposed+Hippophae salicifolia (HS)-supplemented heart, HH+HRT CHR exposed+Hippophae rhamnoides turkestanica (HRT)-supplemented heart, CM control muscle, HM CHR-exposed muscle, HM+HS CHR exposed+Hippophae salicifolia (HS)-supplemented muscle, HM+HRT CHR exposed+Hippophae rhamnoides turkestanica (HRT)-supplemented muscle
Antioxidant status
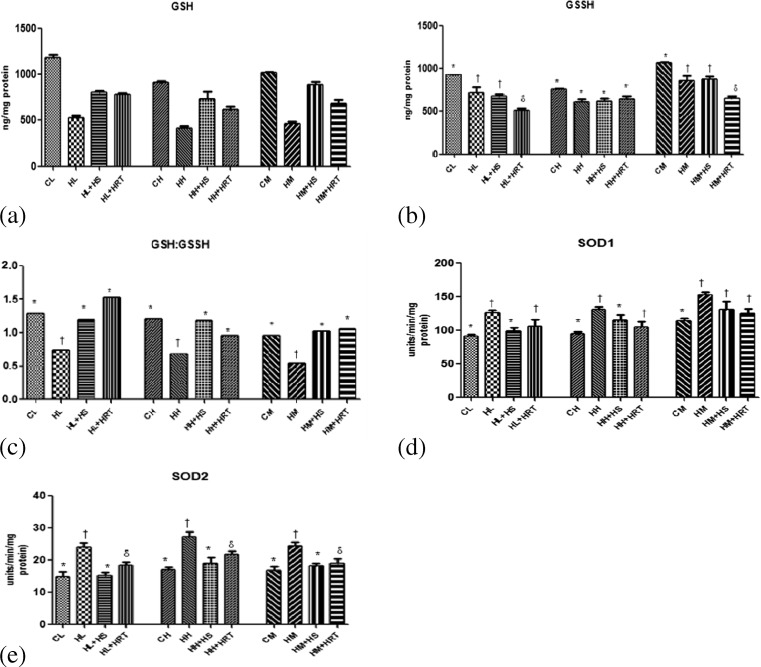
To counter the harmful effects of ROS, antioxidant defense mechanism activates to scavenge reactive oxygen species. The antioxidants involved with these defense mechanism are mainly SOD, catalase, and reduced glutathione (GSH). Superoxide dismutase mainly acts by quenching (O2−) an active oxygen radical into H2O2 and O2 (Yamakura et al. 1998; MacMillan-Crow et al. 1998). Catalase acts by catalysing the decomposition of H2O2 to water and oxygen. In our study, the level GSH reduced significantly in CHR-exposed rats (Fig. 4a), and this decrement was reflected by decrease in GSH/GSSG ratio (Fig. 4c). On the same hand, the antioxidant enzyme activity (SOD1 (Cu,ZnSOD) and SOD2 (MnSOD) (significant) and catalase (insignificant; data not shown) was also increased in CHR-exposed animals as compared to control animals (Fig. 4d, e). Interestingly, altered biochemical variables responded favorably to HS pre-administered rats.
Fig. 4.
Effect of HS and HRT supplementation on antioxidant enzymes and cellular defense system in rat liver, heart, and muscle. a Reduced glutathione, b oxidized glutathione, c GSH/GSSG, d SOD1 activity, and e SOD2 activity. Data represents the mean ± SE; N = 6. *, †, δ Differences between values with matching symbol notations within each column are not statistically significant at 5 % level of probability. CL control liver, HL CHR-exposed liver, HL+HS CHR exposed+Hippophae salicifolia (HS)-supplemented liver, HL+HRT CHR exposed+Hippophae rhamnoides turkestanica (HRT)-supplemented liver, CH control heart, HH CHR-exposed heart, HH+HS CHR exposed+Hippophae salicifolia (HS)-supplemented heart, HH+HRT CHR exposed+Hippophae rhamnoides turkestanica (HRT)-supplemented heart, CM control muscle, HM CHR-exposed muscle, HM+HS CHR exposed+Hippophae salicifolia (HS)-supplemented muscle, HM+HRT CHR exposed+Hippophae rhamnoides turkestanica (HRT)-supplemented muscle
Modulators of vascular tone
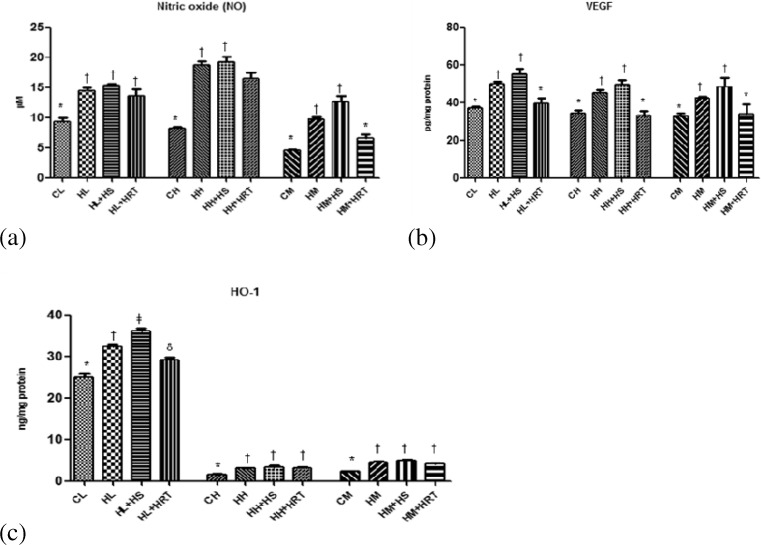
Vasodilation is one of the known responses to reduce hypoxic stress. Nitric oxide is a potent vasodilator and contributes to increase hypoxic tolerance. In our study, we also observed increase nitric oxide level in CHR-exposed rats in comparison to nonexposed rats (Fig. 5a). These results also accompanied by increase in VEGF protein in CHR-exposed rats in relation to control rats (Fig. 5b).
Fig. 5.
Effect of HS and HRT supplementation on modulators of vascular tone in rat liver, heart, and muscle. a Nitric oxide (NO), b vascular endothelial growth factor (VEGF), c heme oxygenase-1 (HO-1). Data represents the mean ± SE; N = 6. *, †, δ, ǂ Differences between values with matching symbol notations within each column is not statistically significant at 5 % level of probability. CL control liver, HL CHR-exposed liver, HL+HS CHR exposed+Hippophae salicifolia (HS)-supplemented liver, HL+HRT CHR exposed+Hippophae rhamnoides turkestanica (HRT)-supplemented liver, CH control heart, HH CHR-exposed heart, HH+HS CHR exposed+Hippophae salicifolia (HS)-supplemented heart, HH+HRT CHR exposed+Hippophae rhamnoides turkestanica (HRT)-supplemented heart, CM control muscle, HM CHR-exposed muscle, HM+HS CHR exposed+Hippophae salicifolia (HS)-supplemented muscle, HM+HRT CHR exposed+Hippophae rhamnoides turkestanica (HRT)-supplemented muscle
Heme oxygenase-1, a redox sensor and modulator of vascular tone, also showed 1.29-, 2.05-, and 1.70-fold increase in its levels in the CHR-exposed rats in comparison to control rats (Fig. 5c). HS and HRT pre-treatment further increased the expression of NO, VEGF, and HO-1 in comparison to CHR-exposed rats which show the adaptogenic activity of herbal extract.
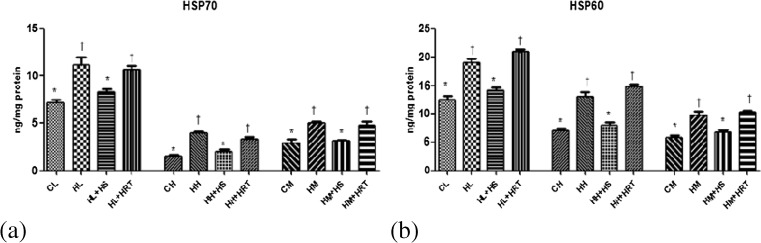
Expression of heat shock proteins
Heat stress proteins comprise an important role in cytoprotection during environmental and metabolic stress. In our study, the level of HSP70 and HSP60 increased significantly in response to multiple stress (CHR). The increment was 1.56-, 1.83-, and 1.97-fold higher for HSP70 (Fig. 6a) and 1.51-, 1.67-, and 1.85-fold higher for HSP60 (Fig. 6b) in liver, heart, and muscle, respectively. Interestingly, HS was able to restore the above heat shock proteins (HSP70 and HSP60) in CHR-exposed rats.
Fig. 6.
Effect of HS and HRT supplementation on heat shock proteins in rat liver, heart, and muscle. a HSP70; b HSP60. Data represents the mean ± SE; N = 6. *, † Differences between values with matching symbol notations within each column is not statistically significant at 5 % level of probability. CL control liver, HL CHR-exposed liver, HL+HS CHR exposed+Hippophae salicifolia (HS)-supplemented liver, HL+HRT CHR exposed+Hippophae rhamnoides turkestanica (HRT)-supplemented liver, CH control heart, HH CHR-exposed heart, HH+HS CHR exposed+Hippophae salicifolia (HS)-supplemented heart, HH+HRT CHR exposed+Hippophae rhamnoides turkestanica (HRT)-supplemented heart, CM control muscle, HM CHR-exposed muscle, HM+HS CHR exposed+Hippophae salicifolia (HS)-supplemented muscle, HM+HRT CHR exposed+Hippophae rhamnoides turkestanica (HRT)-supplemented muscle
Discussion
Results obtained in the present study provided new insights into the biochemical and functional effects on liver, muscle, and heart of rats subjected to multiple stress (CHR) in terms of oxidative damage and also depicted the protective and adaptogenic activity of HS and HRT against multiple stress (CHR). Data depicted increased ROS production which was accompanied with decreased antioxidant enzymes and molecules like SOD and GSH. This imbalance leads to oxidative stress which further escorts to increased protein oxidation and lipid peroxidation.
An increase in number of protein carbonyl residues is one of the indicators of oxidative damage of protein. During oxidative stress, the amino acid residues such as proline, arginine, and lysine use to modify and form reactive carbonyl derivatives which indicated by increased protein carbonyl content and advance oxygen protein products (Levine et al. 1994). AOPPs are another oxidant-induced protein damage marker. These two markers impose the useful information about protein damage during aging and ischemia reperfusion (Stadtman 1993). The present study showed an increment in protein carbonyl and AOPPs content in liver, heart, and muscle, suggesting that CHR stress may enhance oxidative damage to protein. This oxidative damage of protein could lead to protein misfolding or unfolded protein response (UPR).
One of the markers of oxidative damage to lipid is an increase in MDA. In the present study, a positive correlation was found between protein damage and lipid damage in liver, heart, and muscle during CHR stress. During stress, lipids is oxidized and produce lipid peroxides, an unstable indicator of oxidative stress which further decompose to form complex and reactive compound such as MDA. This trend indicates that CHR stress produce oxidants via ROS and NO, which further attack to protein and lipid, and produce more protein carbonyl and AOPP content that is accompanied by increase MDA. Interestingly, protein oxidation and lipid per oxidation decreased significantly in HS- and HRT-treated rats suggested its ROS scavenging and antioxidant activity.
Living organisms respond by numerous mechanisms to defend against stresses like heat shock or other stressful conditions. At cellular level, some proteins are synthesized speedily for maintain homeostasis during stress. These proteins are known as heat shock proteins or HSPs. Among all HSPs, HSP70 (70 kDa) are the most highly conserved (Hunt and Morimoto 1985) heat shock proteins which functions as intracellular molecular chaperones that used to involve with numerous functions like protein synthesis (Beckman et al. 1990), facilitate protein transport (Chiang et al. 1989), prevent protein aggregation during folding, and protect newly synthesized polypeptide chains against misfolding and protein denaturation (Hu et al. 2006; Hu and Tomita 2007). Additional properties of HSP70s include to assist the unfolded protein to achieve its single correct three-dimensional configuration (by still unknown mechanism, it has evolved to generate this folded state), without becoming a constituent of the final folded protein (Morimoto 1998; Morimoto et al. 1997). HSP70 has also been suggested antioxidative effects by protecting cells via preserving GSH level (Polla et al. 1996; Xu and Giffard 1997). After CHR exposure, the generation of oxygen-free radicals is considered to be an important step of oxidative stress while upregulation of HSP70 could be protection or adaptive mechanism at multiple stress (CHR). Along with HSP70, HSP60 was also studied in the present study in which we found increment in HSP60 in CHR-exposed rats. In our study, the overexpression of HSP60 could be interpreted as a potential mediator of oxidative stress and inflammation. In our study, we found a significant decrement of HSP70 and HSP60 which suggested its protein homeostasis maintain activity. As HS treatment was able to restrict protein oxidation that results into decrease oxidized protein burden on cells. Decrease oxidized protein in cells leads to decrease HSP70 and HSP60 activity and also involvement of protein homeostasis mechanism of cells.
HO-1 is a microsmal enzyme that induced in response to cellular stress, and it catalyses the degradation of heme into equimolar quantities of biliverdin (BV), carbon monoxide (CO), and iron. Biliverdin further converted into bilirubin (BR) by biliverdin reductase (Tenhunen et al. 1968). Numerous studies suggested that HO-1 derived carbon monoxide (CO) and bilirubin result in a vasorelaxant/vasodilant effect (Ollinger et al. 2007; Wang and Wu 1997; Wang et al. 2001; Dong et al. 2007). In our study, enhanced HO-1 activity was noted in CHR-exposed and CHR+HS-treated rats which suggested its antioxidant defense property and hence better adaptation property against CHR stress, and these results are an agreement with previous studies that showed cellular protective evidences of HO-1 (Yoshida et al. 2001).
Recent studies have shown that hypoxia is a potent stimulus of angiogenesis, a complex process begins by increasing production of VEGF (Blau and Banfi 2001; Carmeliet and Jain 2000). VEGF is also known as vascular permeability factor (Connolly et al. 1989; Connolly 1991) and prime regulator of angiogenesis (Levy et al. 1995). VEGF is playing an important role in angiogenesis, erythropoiesis, and glycolysis that is selectively unregulated when intracellular PO2 levels become limiting in many cell types, including skeletal myocytes (Forsythe et al. 1996; Fandrey 2004). The present study also depicts increased VEGF in CHR-exposed and CHR+HS-treated rats which could be suggested its adaptogenic activity.
Nitric oxide is another regulator which is crucial for the control of blood pressure, blood flow, and other vital bodily functions (Moncada et al. 1991). Beside this, it has antioxidant and vascular muscle relaxation activity that results into vasodilation and increased blood flow activity (Wink et al. 2001; Levett et al. 2011). The synthesis of NO is catalysed by nitric oxide synthetase (NOS) by the conversion of L-arginine to L-citrulline (Brero et al. 2010; Jung et al. 2010; Boucher et al. 1999). The present study depicts higher NO production in CHR-exposed rats as compared to control rats which further contributes its adaptogenic activity with VEGF.
In the present study, we found that aqueous extract of HS had more adaptogenic activity in comparison to HRT. Previous studies reported that HS contains more phenolic and flavonoid compounds as phenolic and flavonoids are hydrophilic; therefore, it easily seep into the water during extraction (Kostic et al. 2010) and these compounds are responsible for its antioxidant nature (Anyasor et al. 2010; Li et al. 2003). The DPPH scavenging activity, total reducing power was also reported in HS (Goyal et al. 2011; Sharma et al. 2014). Goyal et al. 2011 reported H2O2 scavenging activity was found to be between 94 and 100 % in HS, and it is well known that H2O2 is highly diffusible reactive oxygen species. The scavenging activity of H2O2 by plant extracts may be due to presence to high phenolic and flavonoid compounds.
In the diet, most flavonoids present in the form of β-glycosides, while polyphenols occur in the form of esters, glycosides, and polymers. Before absorption, these compounds must be hydrolyzed by intestinal enzymes or by colonic microflora. During the course of the absorption, polyphenols undergo extensive modification by conjugation in intestinal cells and later by methylation, sulfation, and/or glucuronidation in liver (Day and Williamson 2001). Ultimately, the forms reaching the blood and tissues are different from those present in food, and it is very difficult to identify all the metabolites and to evaluate their biological activity (Setchell et al. 2003).
Previous studies reported pharmacological investigation of HS in which it is found that the extract contains high amount of vitamins, the content is 5–100 times higher than other fruits/vegetables (Pant et al. 2014). The fruits of HS are particularly rich in vitamins A, B1, B12, C, E (including α, β, γ—VE), K and are a rich source of a large number of polyphenols (flavanoids: isorhamnetin, quercetin, myricetin, kaempferol, and their glycoside compounds and nonflavanoids) (Lu 1992; Ranjith et al. 2006). HS also contain carotenoid pigment viz. δ and β-carotene, lycopene flavoxanthin, progestin, cryptoxanthin, violaxanthin, and neoxanthin (Ranjith et al. 2006; Beveridge et al. 1999; Xing 2003). Vitamin C and vitamin E are low molecular mass antioxidants which directly interact with oxidizing radicals (Beveridge et al. 1999) and protect cells from ROS. β-carotene also has the ability to react with peroxyl radical to form a resonance-stabilized carbon-centered radical within its conjugated alkyl structure and inhibit chain propagation effect of ROS.
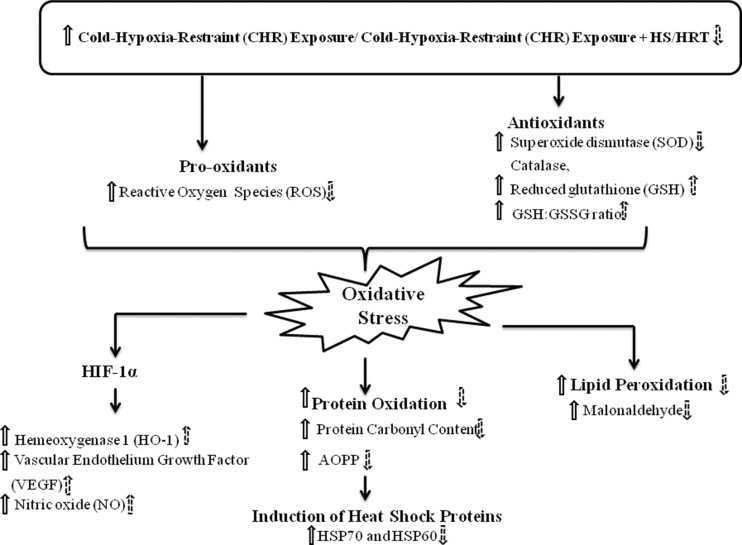
In summary, the present study elucidates that multistress, CHR leads to increase in ROS and decrease antioxidant molecule, GSH, and GSH/GSSG ratio that further result into oxidative stress. Increase in ROS further reacts with proteins and lipids and escorted to protein oxidation (in terms of protein carbonyl and advanced oxidized protein products) and lipid per oxidation (in terms of malonaldehyde). This oxidation of proteins and lipids could further step up to loss of membrane integrity and protein misfolding. This protein misfolding further leads to activation of HSP70 and HSP60 for preventing protein misfolding. Along with this, hypoxia-inducible factor-1 (HIF-1) pathway is also activated due to multiple stress, CHR that could also promote to induce HO-1, VEGF, and NO synthesis via promoting inducible nitric oxide synthase (iNOS) enzyme. The aqueous extract of HS was able to cope up with these ambiguities which arised due to CHR (multiple stress) (Fig. 7). Hence, the above results, thus, lead us to suggest that HS have a potent protective and adaptogenic activity against multiple stress of CHR.
Fig. 7.
Diagrammatic representation of mechanism approach to elucidate the effect of multiple stress, CHR (solid arrow) and adaptogenic activity after HS/HRT treatment (dotted arrow)
Acknowledgments
Authors thank Dr. Shashi Bala Singh, Director of the Establishment for her support and encouragement.
Conflict of interest
The authors have declared that no competing interests exist.
References
- Anyasor GN, Ogunwenmo KO, Oyelana OA, Akpofunure BE. Phytochemical constituents and antioxidant activities of aqueous and methanol stem extracts of Costus afer Ker Gawl. (Costaceae) Afr J Biotechnol. 2010;9:4880–4884. [Google Scholar]
- Askew EW. Environmental and physical stress and nutrient requirements. Am J Clin Nutr. 1995;61:631S–637S. doi: 10.1093/ajcn/61.3.631S. [DOI] [PubMed] [Google Scholar]
- Askew EW. Nutrition and performance in hot, cold, and high altitude environments. In: Wolinsky I, editor. Nutrition in exercise and sport. 3. Boca Raton: CRC Press; 1997. pp. 597–619. [Google Scholar]
- Beckman RP, Mizzen LA, Welch WJ. Interaction of HSP70 with newly synthesized proteins: implications for protein folding and assembly. Science. 1990;248:850–854. doi: 10.1126/science.2188360. [DOI] [PubMed] [Google Scholar]
- Beveridge T, Li TSC, Oomah BD, Smith A. Seabuckthorn products: manufacture and composition. J Agric Food Chem. 1999;47:3480–3488. doi: 10.1021/jf981331m. [DOI] [PubMed] [Google Scholar]
- Blau HM, Banfi A. The well-tempered vessel. Nat Med. 2001;7:532–534. doi: 10.1038/87850. [DOI] [PubMed] [Google Scholar]
- Boucher JL, Moali C, Tenu JP. Nitric oxide biosynthesis, nitric oxide synthase inhibitors and arginase competition for L-arginine utilization. Cell Mol Life Sci. 1999;55:1015–1028. doi: 10.1007/s000180050352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brero A, Ramella R, Fitou A, Dati C, Alloatti G, Gallo MP. Neuregulin-1beta1 rapidly modulates nitric oxide synthesis and calcium handling in rat cardiomyocytes. Cardiovasc Res. 2010;88:443–452. doi: 10.1093/cvr/cvq238. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- Cathcart R, Schwiers E, Ames BN. Detection of pico mole levels of hyderoperoxides using fluorescent dichlorofluoroscein assay. Anal Biochem. 1983;134:111–116. doi: 10.1016/0003-2697(83)90270-1. [DOI] [PubMed] [Google Scholar]
- Chiang HL, Terlecky SR, Plant CP, Dice JF. A role for a 70-kilodalton heat shock protein in lysosomal degradation of intracellular proteins. Science. 1989;246:382–385. doi: 10.1126/science.2799391. [DOI] [PubMed] [Google Scholar]
- Connolly DT. Vascular permeability factor: a unique regulator of blood vessel function. J Cell Biochem. 1991;47(3):219–223. doi: 10.1002/jcb.240470306. [DOI] [PubMed] [Google Scholar]
- Connolly DT, Heuelman DM, Nelson R, Olander JV, Eppley BL, Delfino JJ, Siegel NR, Limgruber RM, Feder J. Tumor vascular permeability factor stimulates endothelial cell growth and angiogenesis. J Clin Invest. 1989;84:1470–1478. doi: 10.1172/JCI114322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day AJ, Williamson G. Biomarkers for exposure to dietary flavonoids: a review of the current evidence for identification of quercetin glycosides in plasma. Br J Nutr. 2001;86:S105–S110. doi: 10.1079/BJN2001342. [DOI] [PubMed] [Google Scholar]
- Dong DL, Zhang Y, Lin DH, Chen J, Patschan S, Goligorsky MS, Nasjletti A, Yang BF, Wang WH. Carbon monoxide stimulates the Ca2_ activated big conductance K channels in cultured human endothelial cells. Hypertension. 2007;50:643–651. doi: 10.1161/HYPERTENSIONAHA.107.096057. [DOI] [PubMed] [Google Scholar]
- Eccleston C, Baoru Y, Tahvonen R, Kallio H, Rimbach GH, Minihane AM. Effects of an antioxidant-rich juice (sea-buckthorn) on risk factors for coronary heart disease in humans. J Nut Biochem. 2002;13:346–354. doi: 10.1016/S0955-2863(02)00179-1. [DOI] [PubMed] [Google Scholar]
- Fandrey J. Oxygen-dependent and tissue-specific regulation of erythropoietin gene expression. Am J Physiol Regul Integr Comp Physiol. 2004;286(6):R977–R988. doi: 10.1152/ajpregu.00577.2003. [DOI] [PubMed] [Google Scholar]
- Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16(9):4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geetha S, Sairam M, Mongia SS, Singh V, Ilavazhagan G, Sawhney RC. Evaluation of antioxidant activity of leaf extract of sea-buckthorn (Hippophae rhamnoides L.) on chromium(VI) induced oxidative stress in albino rats. J Ethnopharmacol. 2003;87:247–251. doi: 10.1016/S0378-8741(03)00154-5. [DOI] [PubMed] [Google Scholar]
- Goyal AK, Basistha BC, Sen A, Middha SK. Antioxidant profiling of Hippophae salicifolia growing in sacred forests of Sikkim, India. Funct Plant Biol. 2011;38:697–701. doi: 10.1071/FP11016. [DOI] [PubMed] [Google Scholar]
- Hazel JR. Thermal adaptation in biological membranes: is homeoviscous adaptation the explanation? Annu Rev Physiol. 1995;57:19–42. doi: 10.1146/annurev.ph.57.030195.000315. [DOI] [PubMed] [Google Scholar]
- Hissin PJ, Hilf R. A fluorometric method for the determination of oxidized and reduced glutathione in tissue. Anal Biochem. 1973;74:214–226. doi: 10.1016/0003-2697(76)90326-2. [DOI] [PubMed] [Google Scholar]
- Hu B, Tomita M. The Hsp70 chaperone system maintains high concentrations of active proteins and suppresses ATP consumption during heat shock. Syst Synth Biol. 2007;1(1):47–58. doi: 10.1007/s11693-006-9004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Mayer MP, Tomita M. Modeling HSP70-mediated protein folding. Biophys J. 2006;91(2):496–507. doi: 10.1529/biophysj.106.083394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt C, Morimoto RI. Conserved features of eukaryotic hsp70 genes revealed by comparison with the nucleotide sequence of human hsp70. Proc Natl Acad Sci U S A. 1985;82:6455. doi: 10.1073/pnas.82.19.6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilavazhagan G, Bansal A, Prasad D, Thomas P, Sharma SK, Kain AK, Kumar D, Selvamurthy W. Effect of vitamin E supplementation on hypoxia-induced oxidative damage in male albino rats. Aviat Space Environ Med. 2001;72:899–903. [PubMed] [Google Scholar]
- Joanny P, Steinberg J, Robach P, Richalet JP, Gortan C, Gardette B, Jammes Y. Operation Everest III (Comex’97): the effect of simulated sever hypobaric hypoxia on lipid peroxidation and antioxidant defence systems in human blood at rest and after maximal exercise. Resuscitation. 2001;49:307–314. doi: 10.1016/S0300-9572(00)00373-7. [DOI] [PubMed] [Google Scholar]
- Jung HJ, Yang MZ, Kwon KH, Yenari MA, Choi YJ, Lee WT. Endogenous agmatine inhibits cerebral vascular matrix metalloproteinases expression by regulating activating transcription factor 3 and endothelial nitric oxide synthesis. Curr Neurovasc Res. 2010;7:201–212. doi: 10.2174/156720210792231804. [DOI] [PubMed] [Google Scholar]
- Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21:130–132. [PubMed] [Google Scholar]
- Kostic DA, Mitic SS, Mitic MN, Zarubica AR, Velickovic JM, Dordevic AS, Randelovic SS. Phenolic contents, antioxidant and antimicrobial activity of Papaver rhoeas L. extracts from southeast Serbia. J Med Plant Res. 2010;4:1727–1732. [Google Scholar]
- LeBel CP, Ischiropoulos H, Bondy SC. Evaluation of the probe 2′,7′-dichlorofluorescein as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol. 1992;5:227–231. doi: 10.1021/tx00026a012. [DOI] [PubMed] [Google Scholar]
- Levett DZ, Fernandez BO, Riley HL, Martin DS, Mitchell K, Leckstrom CA, Ince C, Whipp BJ, Mythen MG, Montgomery HE, Grocott MP, Feelisch M. The role of nitrogen oxides in human adaptation to hypoxia. Sci Rep. 2011;1:109. doi: 10.1038/srep00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz A, Ahn B, Shaltiel S, Stadtman ER. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–478. doi: 10.1016/0076-6879(90)86141-H. [DOI] [PubMed] [Google Scholar]
- Levine RL, Williams J, Stadtman ER, Shacter E (1994) Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol 233:346–57 [DOI] [PubMed]
- Levy AP, Levy NS, Wegner S, Goldberg MA. Transcriptional regulation of the rat vascular endothelial growth factor gene by hypoxia. J Biol Chem. 1995;270(22):13333–13340. doi: 10.1074/jbc.270.22.13333. [DOI] [PubMed] [Google Scholar]
- Li H, Wang Z, Liu Y. Review in the studies on tannins activity of cancer prevention and anticancer. Zhong Yao Cai. 2003;26:444–448. [PubMed] [Google Scholar]
- Lu R (1992) Seabuckthorn: a multipurpose plant species for fragile mountains. ICIMOD Occasional Paper No. 20, Kathmandou, Nepal; p 62
- MacMillan-Crow LA, Crow JP, Thompson JA (1998) Peroxynitrite-mediated inactivation of manganese superoxide dismutase involves nitration and oxidation of critical tyrosine residues. Biochem 37:1613–1622 [DOI] [PubMed]
- Mohanraj P, Merola AJ, Wright VP, Clanton TL. Antioxidants protect rat diaphragmatic muscle function under hypoxic conditions. J Appl Physiol. 1998;84:1960–1966. doi: 10.1152/jappl.1998.84.6.1960. [DOI] [PubMed] [Google Scholar]
- Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43(2):109–142. [PubMed] [Google Scholar]
- Morimoto RI. Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 1998;12(24):3788–3796. doi: 10.1101/gad.12.24.3788. [DOI] [PubMed] [Google Scholar]
- Morimoto RI, Kline MP, Bimston DN, Cotto JJ. The heat-shock response: regulation and function of heat-shock proteins and molecular chaperones. Essays Biochem. 1997;32:17–29. [PubMed] [Google Scholar]
- Negi PS, Chauhan AS, Sadia GA, Rohinishree YS, Ramteke RS. Antioxidant and antibacterial activities of various sea-buckthorn (Hippophae rhamnoides L.) seed extracts. Food Chem. 2005;92:119–124. doi: 10.1016/j.foodchem.2004.07.009. [DOI] [Google Scholar]
- Ollinger R, Yamashita K, Bilban M, Erat A, Kogler P, Thomas M, Csizmadia E, Usheva A, Margreiter R, Bach FH. Bilirubin and biliverdin treatment of atherosclerotic diseases. Cell Cycle. 2007;6:39–43. doi: 10.4161/cc.6.1.3700. [DOI] [PubMed] [Google Scholar]
- Onkawa H, Onishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Pant M, Lal A, Rani A. Hippophae salcifolia D Don-A plant with multifarious benefits. Int J Pharm Pharm Sci. 2014;6(11):37–40. [Google Scholar]
- Paula FBA, Gouvea CMCP, Alfredo PP, Salgado I. Protective action of a hexane crude extract of pterodon emarginatus fruits against oxidative and nitrosative stress induced by acute exercise in rats. BMC Complement Altern Med. 2005;5:17. doi: 10.1186/1472-6882-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polla BS, Kantengwa S, Francois D, Salvioli S, Franceschi C, Marsac C. Mitochondria are selective targets for the protective effects of heat shock against oxidative injury. Proc Natl Acad Sci. 1996;93:6458–6463. doi: 10.1073/pnas.93.13.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radak Z, Asano K, Lee KC, Ohno H, Nakamura A, Nakamoto H, Goto S. High altitude training increases reactive carbonyl derivatives but not lipid peroxidation in skeletal muscle of rats. Free Radic Biol Med. 1997;22:1109–1114. doi: 10.1016/S0891-5849(96)00350-4. [DOI] [PubMed] [Google Scholar]
- Ramachandran U, Divekar HM, Grover SK, Srivastava KK. New experimental model for evaluation of adaptogenic products. J Ethnopharmacol. 1990;29:275–281. doi: 10.1016/0378-8741(90)90038-U. [DOI] [PubMed] [Google Scholar]
- Ranjith A, Kumar SK, Venugopalan VV, Arumughan C, Sawhney RC, Singh V. Fatty acids, tocols and carotenoids in pulp oil of three Sea Buckthron species (Hippophae rhamnoides, H. salicifolia, and H. tibetana) grown in the Indian Himalayas. JAOCS. 2006;83:359–364. [Google Scholar]
- Rongsen L (1992) Sea-buckthorn a multipurpose plant species for fragile mountain. ICIMOD Occasional paper no. 20. ICIMOD, Katmandu, Nepal
- Setchell KD, Faughnan MS, Avades T, Zimmer-Nechemias L, Brown NM. Comparing the pharmacokinetics of daidzein and genistein with the use of 13C-labeled tracers in premenopausal women. Am J Clin Nutr. 2003;77:411–419. doi: 10.1093/ajcn/77.2.411. [DOI] [PubMed] [Google Scholar]
- Sharma P, Suryakumar G, Singh V, Misra K, Singh SB (2014) In vitro antioxidant profiling of seabuckthorn varieties and their adaptogenic response to high altitude-induced stress. Int J Biometeorol 58(8). doi:10.1007/s00484-014-0925-2 [DOI] [PubMed]
- Singh V (2001) Seabuckthorn (Hippophae L.) A wonder plant of dry and temperate Himalayas. Department of Agro forestery and Environment, Himachal Pradesh Agriculture university, Palampur; p 1760-62
- Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- Stadtman ER. Oxidation of free amino acids and amino acid residues in proteins by radiolysis and by metal-catalyzed reactions. Annu Rev Biochem. 1993;62:797–821. doi: 10.1146/annurev.bi.62.070193.004053. [DOI] [PubMed] [Google Scholar]
- Tenhunen R, Marver HS, Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci. 1968;61:748–755. doi: 10.1073/pnas.61.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Wu L. The chemical modification of KCa channels by carbon monoxide in vascular smooth muscle cells. J Biol Chem. 1997;272:8222–8226. doi: 10.1074/jbc.272.13.8222. [DOI] [PubMed] [Google Scholar]
- Wang R, Wang Z, Wu L, Hanna ST, Peterson-Wakeman R. Reduced vasorelaxant effect of carbon monoxide in diabetes and the underlying mechanisms. Diabetes. 2001;50:166–174. doi: 10.2337/diabetes.50.1.166. [DOI] [PubMed] [Google Scholar]
- Wink DA, Miranda KM, Espey MG, Pluta RM, Hewett SJ, Colton C, Vitek M, Feelisch M, Grisham MB. Mechanisms of the antioxidant effects of nitric oxide. Antioxid Redox Signal. 2001;3(2):203–213. doi: 10.1089/152308601300185179. [DOI] [PubMed] [Google Scholar]
- Witko-Sarsat V, Friedlander M, Capeillere-Blandin C, Nguyen-Khoa T, Nguyen AT, Zingraff J, Jungers P, Descamps-Latscha B. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996;49:1304–1313. doi: 10.1038/ki.1996.186. [DOI] [PubMed] [Google Scholar]
- Xing C (2003) Health protection and processing technology of seabuckthorn tea: In: Singh V editor. Seabuckthorn (Hippophae L.)-A multipurpose wonder plant, Indus Publishing Company; p 475–8
- Xu L, Giffard RG. HSP70 protects murine astrocytes from glucose deprivation injury. Neurosci Lett. 1997;224:9–12. doi: 10.1016/S0304-3940(97)13444-9. [DOI] [PubMed] [Google Scholar]
- Yamakura F, Taka H, Fujimura T, Murayama K (1998) Inactivation of human manganese-superoxide dismutase by peroxynitrite is caused by exclusive nitration of tyrosine 34 to 3-nitrotyrosine. J Biol Chem 273:14085–14089 [DOI] [PubMed]
- Yoshida T, Maulik N, Ho YS, Alam J, Das DK. Hmox-1 constitutes an adaptive response to effect antioxidant cardioprotection. Circulation. 2001;103:1695–1701. doi: 10.1161/01.CIR.103.12.1695. [DOI] [PubMed] [Google Scholar]