Abstract
Cellular senescence of endothelial cells is a damage and stress response which induces pro-inflammatory, pro-atherosclerotic, and pro-thrombotic phenotypes. Donepezil is a drug used for the treatment of mild to moderate dementia of the Alzheimer’s disease (AD). The aim of the present study was to investigate the attenuation of endothelial cell senescence by donepezil and to explore the mechanisms underlying the anti-aging effects of donepezil. Our results indicated that high glucose (HG) markedly decreased cell viability of human umbilical vein endothelial cells (HUVECs), and this phenomenon was reversed by treatment with donepezil. Importantly, our results displayed that the frequency of senescent (SA-ß-gal-positive) cells and the expression level of senescence genes (PAI-1 and p21) were significantly higher in the HG group compared with the normal glucose (NG) group, and these changes were blocked by treatment with donepezil. Also, our results showed that donepezil inhibits the generation of reactive oxygen species (ROS), which promotes cellular senescence. Pretreatment with nicotinamide (NAM), a sirtuin 1 (SIRT1) inhibitor, inhibited the reduction in senescence associated with donepezil. Indeed, our results indicated that donepezil increased the SIRT1 enzyme activity. Therefore, these results show that donepezil delays cellular senescence that is promoted under HG condition via activation of SIRT1.
Keywords: Alzheimer’s disease, Donepezil, Senescence, SIRT1
Introduction
Aging-related changes in the physiology include the cessation of cell division and cell senescence. Cell senescence is accompanied by specific changes in cell function, morphology, and gene expression (Alster and Korwek 2014). The vascular endothelium is a highly specialized cellular system and active cells exhibiting anti-thrombotic and anti-inflammatory properties. These cells play essential roles in the maintenance of vascular homeostasis by regulating vascular tone and integrity, as well as remodeling processes (Behrendt and Ganz 2002). Endothelial senescence has been reported to be involved in age-associated diseases including hypertension, chronic coronary disease, and diabetes (Sniderman and Furberg 2008). For example, Minamino and colleagues reported that senescent vascular endothelial cells have been found in arteriosclerotic sites in humans, which indicates a possible relationship of these cells with an aging-related disease (Minamino et al. 2002). In addition, multiple lines of evidence have shown that endothelial senescence is associated with increased vascular risk and pathological states, including those observed in oxidative stress conditions, invoke irreversible growth arrest in vitro within a few days, a term referred to as stress-induced premature senescence (SIPS) (Gorbunova et al. 2002; Frippiat et al. 2001). Senescent cells were prepared by culturing human umbilical vein endothelial cells (HUVECs) until they reached the Hayflick limit (Yanaka et al. 2011). Senescence of HUVECs is promoted under high glucose (HG) condition, and thus a culture in HG was used in this study to induce cell senescence (Rogers et al. 2013). SA-β-gal, a β-galactosidase activity detectable at pH 6.0 in cultured cells undergoing replicative or induced senescence but absent from proliferating cells, has been widely used for a biomarker for senescence because of the simplicity of the assay method and its apparent specificity for senescent cells (Dimri et al. 1995). Endothelial cells in atherosclerotic lesions show features of cellular senescence including SA-β-gal-positive staining (Burrig,1991). Importantly, SA-β-gal activity in mouse microvascular endothelial cells (MMECs) was increased in HG (Arunachalam et al. 2014).
Donepezil, a potent and selective acetylcholinesterase inhibitor used for clinical treatment of Alzheimer’s disease (AD), has been shown to provide neuroprotection by anti-inflammatory effects. Multiple lines of evidence have shown that donepezil could exert its anti-inflammatory effects through inhibiting the production of pro-inflammatory cytokines, such as interleukin-1β (IL-1β), IL-6, and IL-18 (Reale et al. 2005) (Hwang et al. 2010). In addition, donepezil also inhibits the production of monocyte chemoattractant protein 1 (MCP-1) and suppresses microglial activation which was independent of acetylcholine and its receptor (Yoshiyama et al. 2010). In addition, treatment with donepezil improved diabetes-induced memory impairment and reduced oxidative stress (Bhutada et al. 2011). Systemic administration of donepezil attenuated morphine-induced tolerance and apoptosis in the rat cerebral cortex and lumbar spinal cord (Sharifipour et al. 2014). These pharmacological activities of donepezil suggested that donepezil might have a potential therapeutic effect against cell senescence.
SIRT1, a commonly known nicotinamide adenine dinucleotide (NAD+)-dependent class III histone deacetylase, has been reported to regulate cell cycle, senescence, apoptosis, and metabolism by interacting with a number of molecules, including p53 (Luo et al. 2001) and FoxO1 (Brunet et al. 2004). The aim of the present study was to investigate the effects of donepezil on the inhibition of HG-induced senescence in HUVECs and the role of SIRT1 in this process.
Materials and methods
Cell culture
Human umbilical vein endothelial cells (HUVECs) were obtained from Lonza, USA. Cells were cultured in EBM-2 media with supplemental growth factors according to the manufacturer’s instructions. HUVECs were cultured in normal glucose (NG; 5.6 mM) and HG (30 mM) in the presence and absence of donepezil at different concentrations from 10 to 50 μM for 72 h at 37 °C in a 5 % CO2 atmosphere.
Cell viability measurement
Upon completion of indicated treatment, cell viability was determined by the 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) reduction assay. Briefly, cells were loaded with 1 mg/mL MTT in serum-free medium and incubated for 4 h in a CO2 incubator at 37 °C. The resultant insoluble formazan crystals were dissolved by dimethyl sulfoxide (DMSO), and absorbance recorded at 570 nm by a microtiter plate reader was used to reflect cell viability.
Cell cycle assay
To determine the effect of donepezil on HG arrested cell cycle progression, HUVECs were collected and fixed with 70 % ethanol at 4 °C overnight. Then cells were stained with 50 mmol/L propidium iodide and washed twice with cold PBS. HUVECs were subjected to flow cytometric analyses with FACS Calibur and CellQuest software (BD Biosciences, Franklin Lakes, NJ, USA). Cell cycles were analyzed and the proportion of cells in the G0/G1, S, and G2/M phases was recorded.
Galactosidase (β-gal) staining
Upon completion of indicated treatment, HUVECs were fixed for 5 min with PBS containing 2 % formaldehyde and 0.2 % glutaraldehyde, and then incubated at 37 °C for 10 h with a staining solution (40 mmol/L citric acid, sodium phosphate, pH 6.0, 1 mg/mL 5-bromo-4-chloro-3-isolyl-β-D-galactoside (X-gal, Sigma), 5 mmol/L potassium ferrocyanide, 5 mmol/L potassium ferricyanide, 150 mmol/L NaCl, and 2 mmol/L MgCl2). Senescence-associated (SA)-β-gal-positive cells were observed by microscopy, and over 500 cells were counted in five independent fields.
Immunoblotting analysis
Cells were lysed on ice for 30 min in lysis buffer containing 50 mmol/L HCl, pH 7.6, 150 mmol/L NaCl, 1 % NP-40, 0.1 % sodium dodecyl sulfate (SDS), 1 mmol/L dithiothreitol, 1 mmol/L sodium vanadate, 1 mmol/L phenylmethylsulfonyl fluoride, 10 μg/mL aprotinin, 10 μg/mL leupeptin, and 10 mmol/L sodium fluoride. Equal amounts of protein were separated by SDS polyacrylamide gel electrophoresis, and then transferred to PVDF membrane (Bio-Rad, USA). Then the membrane was inoculated in a blocking buffer containing 5 % non-fat milk and Tween 20 (0.1 %, v/v) in PBS (PBS/Tween 20) at room temperature for 1 h, and then incubated overnight at 4 °C with the proper primary antibodies. Finally, it was incubated with HRP-linked secondary antibodies at room temperature for 2 h. Each membrane was developed using an enhanced ChemiImager 5500 chemiluminescence system (Alpha Innotech Corporation, Miami, FL, USA).
Enzymatic activity assay
The enzyme activity of SIRT1 was assayed by using a commercially available kit (Genmed, USA) in a 96-well plate according to the manufacturer’s instructions. The reaction product emitted fluorescence, which was detected using an excitation wavelength of 350 nm and an emission wavelength of 405 nm to index the enzyme activity of SIRT1.
Statistical analysis
Experimental data are described as mean ± S.E.M. Statistical significance was determined by one-way analysis of variance (ANOVA). P < 0.05 was considered as the minimum level of statistical significance.
Results
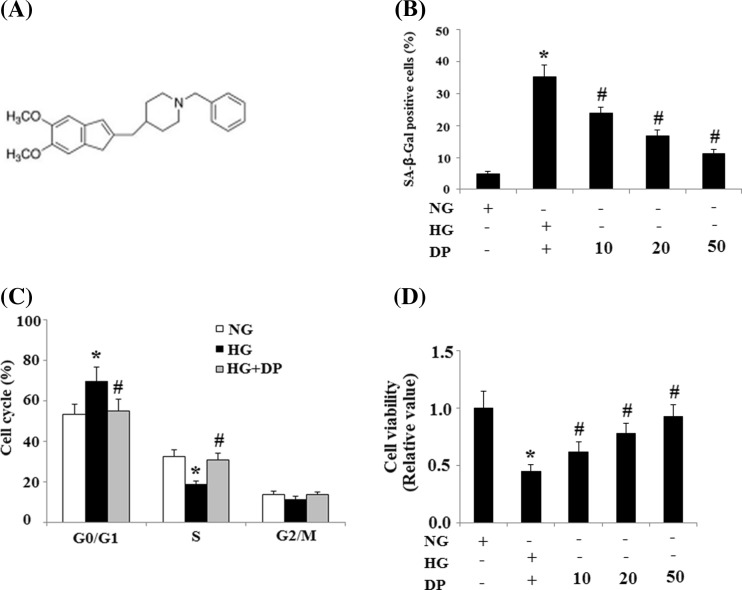
The molecular structure of donepezil is shown in Fig. 1a. SA-β-gal is a well-accepted biochemical marker of cell senescence (Okatani et al. 2000). The rate of SA-ß-gal-positive cells was significantly higher in the HG (30 mmol/L) group compared with the NG (5.6 mmol/L) group, and this increase was suppressed by treatment with donepezil in a concentration-dependent manner from 10 to 50 μM (Fig. 1b). Progression through the cell cycle is a critical cellular process and cell cycle arrest during the G1 phase is a characteristic exhibited by senescent cells. Our results demonstrated that treatment with 30 mmol/L glucose arrested HUVECs in the G0/G1 phase as the proportion of cells in the G0/G1 phase was ~69.9 % compared to 53.3 % in the NG (5.6 mmol/L) group. Donepezil (20 μM) pretreatment eliminated the effects of HG and reduced the proportion of cells in the G0/G1 phase to 55.3 % (Fig. 1c). Next, we examined the impact of donepezil on cell viability. Treatment with HG (30 mmol/L) significantly suppressed endothelial cell viability, which was reversed by treatment with donepezil in a concentration dependent manner from 10 to 50 μM (Fig. 1d).
Fig. 1.
Effects of donepezil on senescence of HUVECs exposed to HG. a Chemical structures of donepezil. b Percentages of SA-ß-gal-positive HUVECs in different groups. c Donepezil rescued the arresting effects of HG on the cell cycle of HUVECs. d Cell cycle analysis was performed by FACS. The effects of donepezil on cell viability were examined by MTT assay, and cell viability was calculated as the percentage of control (*P < 0.01 vs. control group; #P < 0.01 vs. HG group). HG high glucose, NG normal glucose
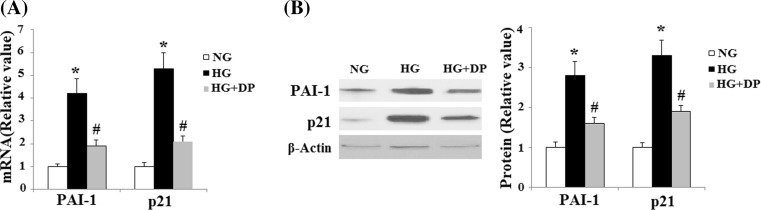
PAI-1 and p21 are two important senescence genes. Here, the expression levels of mRNA levels for PAI-1 and p21 were determined by real-time PCR analysis. And the results indicated that HG treatment drastically increased the expression of PAI-1 and p21, which was markedly suppressed by donepezil treatment (Fig. 2a). This result was confirmed by Western blot analysis at protein levels (Fig. 2b).
Fig. 2.
Donepezil ameliorates the expression of PAI-1 and p21 induced by HG. a The expression of PAI-1 and p21 at the mRNA level was determined by real time PCR (*P < 0.01 vs. control group; #P < 0.01 vs. HG group). b The expression of PAI-1 and p21 at the protein level was determined by Western blot analysis (*P < 0.01 vs. control group; #P < 0.01 vs. HG group). HG high glucose, NG normal glucose
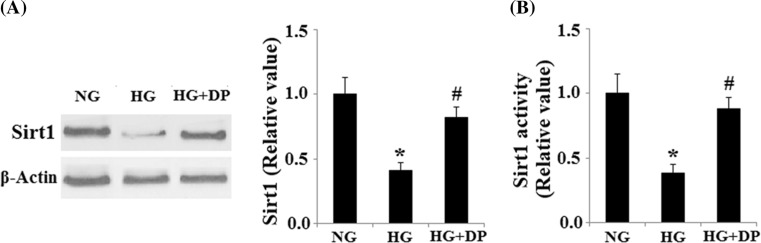
To determine whether donepezil regulates HUVECs senescence through a SIRT1-mediated pathway, we examined the expression and activity of SIRT1. Immunoblot analyses indicated that SIRT1 levels were decreased in response to treatment with HG (30 mmol/L), which was partially rescued by treatment with donepezil (Fig. 3a). SIRT1 deacetylase activity was reduced by treatment with HG. However, donepezil restored the deacetylase activity of SIRT1 (Fig. 3b), indicating a direct effect on SIRT1-mediated pathways.
Fig. 3.
Donepezil restored the reduced expression and activity of SIRT1. a The expression of SIRT1 at the protein level was determined by Western blot analysis. b Activity of SIRT1 (*P < 0.01 vs. control group; #P < 0.01 vs. HG group). HG high glucose, NG normal glucose
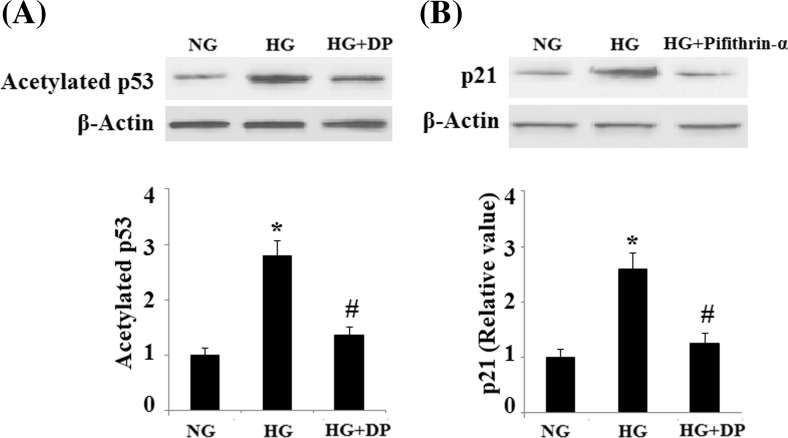
To elucidate the role of SIRT1, HUVECs were pretreated with donepezil in the presence or absence of NAM, a selective SIRT1 inhibitor. And the results indicated that NAM attenuated the decrease in SA-β-gal-positive cells inferred by donepezil alone (Fig. 4a). Importantly, NAM also abolished the decrease in expression of PAI-1 and p21 inferred by donepezil alone (Fig. 4b). These results suggest that donepezil blocks senescence and promotes cell growth by increasing SIRT1 deacetylase activity. The regulatory effects of SIRT1 on PAI-1 and p21 expression were mediated by reducing the acetylation of p53 (Mhaidat et al. 2007). Notably, our results demonstrated that HG increased the acetylated levels of p53, which was reduced by donepezil (Fig. 5a). In addition, we studied the effect of the p53 inhibitor Pifithrin-α on the expression of PAI-1 and p21. And we found that the induction of PAI-1 and p21 can be inhibited by Pifithrin-α, suggesting the involvement of p53 pathway (Fig. 5b).
Fig. 4.
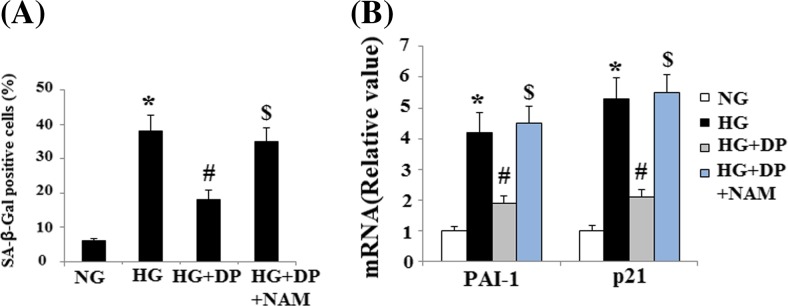
Effects of donepezil on inhibiting senescence of HUVECs is mediated by SIRT1. a Percentages of SA-ß-gal-positive HUVECs in different groups. b. The expression of PAI-1 and p21 at the protein level was determined by Western blot analysis (*P < 0.01 vs. control group; #P < 0.01 vs. HG group). HG high glucose, NG normal glucose
Fig. 5.
Donepezil attenuates acetylation of p53. a. Levels of acetylated p53. b. the effect of the p53 inhibitor Pifithrin-α on the expression of PAI-1 and p21 (*P < 0.01 vs. control group; #P < 0.01 vs. HG group). HG high glucose, NG normal glucose
Discussion
Aging has been considered as an independent risk factor for the development of various pathological conditions, including cardiovascular system (Corella and Ordovás 2014). Increasing evidence has further demonstrated that cellular senescence play essential roles in this process (Lakatta and Levy 2003). Cellular senescence is a process in which the cessation of cell division is accompanied by specific changes in cell function, morphology, and gene expression (Ben-Porath and Weinberg 2005). HUVEC senescence and the consequent reduction of their proliferative and migration ability may contribute to miopragia associated with advanced age. In this study, we established a HG-induced senescent model in vitro using HUVECs to investigate the protective role of donepezil in cell senescence. And our results showed for the first time that donepezil can delay senescence of HUVECs that is promoted under HG condition. Since there has been no report of the cytotoxicity of donepezil in HUVECs, our findings showing that delayed senescence of endothelial cells promoted by donepezil may be effective for prevention of aging-related diseases.
SA-β-gal is widely used as a marker of cell senescence (Zdanov et al. 2007). In our study, SA-ß-gal-positive cells in HG treated group were significantly more frequent than that in the NG treated group, which was reversed by the administration of donepezil. In addition, compared to those cultured with NG, HG treatment led to a marked decrease in cell viability, which was reversed by the administration of donepezil. Importantly, the expression of PAI-1 and p21, which are up-regulated by senescence in HUVECs induced by HG, were reversed by donepezil. These findings suggest that donepezil attenuates HG-accelerated senescence in HUVECs and may be effective for prevention of aging-related diseases.
SIRT1, the most studied mammalian homologue of the seven sirtuins, has been shown to protect endothelial cells from premature senescence (Ota et al. 2007) and to regulate angiogenesis (Potente et al. 2007) and vascular tone (Mattagajasingh et al. 2007). It regulates cell cycle, senescence, apoptosis, and metabolism by interacting with a number of molecules, including p53 (Luo et al. 2001) and FoxO1 (Brunet et al. 2004). A previous study demonstrated that SIRT1 inhibition induces premature senescence-like growth arrest in human cancer cells (Ota et al. 2006). Oxidative stress activates several signaling pathways that regulate cellular senescence and aging. Saxena and colleagues reported that donepezil pretreatment significantly prevented streptozotocin (STZ)-induced oxidative stress in rodent brains (Saxena et al. 2011). In addition, another study reported that administration of donepezil (5 mg/kg, p.o.) significantly restored the levels of glutathione (GSH), methylenedioxyamphetamine (MDA), and ROS generation against okadaic acid (Kamat et al. 2010). These findings suggest that donepezil might attenuate HG-accelerated senescence by decreasing ROS levels. Despite the molecular mechanisms involved in the modulation of SIRT1 activity, donepezil affects SIRT1 activity and attenuates senescence. FoxO1 is a downstream target of SIRT1, which has been shown to modulate G1-S and G2-M phase transition by coordinating the expression of multiple important cell cycle regulators (Ho et al. 2008.). In future studies, we aim to evaluate the effect of donepezil on FoxO1 via SIRT1 expression and activity. Our study showing that donepezil restored the expression and enzyme activity of SIRT1 implies that donepezil might be able to regulate other cellular physiological functions through SIRT1 and its downstream signals.
Acknowledgments
This study was supported by the nursery fund of the Chinese PLA General Hospital.
References
- Alster O, Korwek Z. Markers of cellular senescence. Postepy Biochem. 2014;60(2):138–46. [PubMed] [Google Scholar]
- Arunachalam G, Samuel SM, Marei I, Ding H, Triggle CR. Metformin modulates hyperglycaemia-induced endothelial senescence and apoptosis through SIRT1. Br J Pharmacol. 2014;171(2):523–35. doi: 10.1111/bph.12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrendt D, Ganz P. Endothelial function. From vascular biology to clinical applications. Am J Cardiol. 2002;90:40L–48L. doi: 10.1016/S0002-9149(02)02963-6. [DOI] [PubMed] [Google Scholar]
- Ben-Porath I, Weinberg RA. The signals and pathways activating cellular senescence. Int J Biochem Cell Biol. 2005;37:961–976. doi: 10.1016/j.biocel.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Bhutada P, Mundhada Y, Bansod K, et al. Protection of cholinergic and antioxidant system contributes to the effect of berberine ameliorating memory dysfunction in rat model of streptozotocin-induced diabetes. Behav Brain Res. 2011;220(1):30–41. doi: 10.1016/j.bbr.2011.01.022. [DOI] [PubMed] [Google Scholar]
- Brunet A, Sweeney LB, Sturgill JF, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- Burrig KF. The endothelium of advanced arteriosclerotic plaques in humans. Arterioscler Thromb. 1991;11:1678–1689. doi: 10.1161/01.ATV.11.6.1678. [DOI] [PubMed] [Google Scholar]
- Corella D, Ordovás JM. Aging and cardiovascular diseases: The role of gene-diet interactions. Ageing Res Rev. 2014;18C:53–73. doi: 10.1016/j.arr.2014.08.002. [DOI] [PubMed] [Google Scholar]
- Dimri GP, Lee X, Basile G, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frippiat C, Chen QM, Zdanov S, Magalhaes JP, Remacle J, Toussaint O. Subcytotoxic H2O2 stress triggers a release of transforming growth factor-beta 1, which induces biomarkers of cellular senescence of human diploid fibroblasts. J Biol Chem. 2001;276:2531–2537. doi: 10.1074/jbc.M006809200. [DOI] [PubMed] [Google Scholar]
- Gorbunova V, Seluanov A, Pereira-Smith OM. Expression of human telomerase (hTERT) does not prevent stress-induced senescence in normal human fibroblasts but protects the cells from stress-induced apoptosis and necrosis. J Biol Chem. 2002;277:38540–38549. doi: 10.1074/jbc.M202671200. [DOI] [PubMed] [Google Scholar]
- Ho KK, Myatt SS, Lam EW. Many forks in the path: cycling with FoxO. Oncogene. 2008;27:2300–2311. doi: 10.1038/onc.2008.23. [DOI] [PubMed] [Google Scholar]
- Hwang J, Hwang H, Lee HW, et al. Microglia signaling as a target of donepezil. Neuropharmacology. 2010;58:1122e1129. doi: 10.1016/j.neuropharm.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part I: aging arteries: a ‘set up’ for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.CIR.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- Kamat PK, Tota S, Saxena G, et al. Okadaic acid (ICV) induced memory impairment in rats: a suitable experimental model to test anti-dementia activity. Brain Res. 2010;1309:66–74. doi: 10.1016/j.brainres.2009.10.064. [DOI] [PubMed] [Google Scholar]
- Luo J, Nikolaev AY, Imai S, et al. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell. 2001;107:137–148. doi: 10.1016/S0092-8674(01)00524-4. [DOI] [PubMed] [Google Scholar]
- Mattagajasingh I, Kim CS, Naqvi A, et al. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2007;104:14855–14860. doi: 10.1073/pnas.0704329104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mhaidat NM, Zhang XD, Allen J, et al. Temozolomide induces senescence but not apoptosis in human melanoma cells. Br J Cancer. 2007;97(9):1225–33. doi: 10.1038/sj.bjc.6604017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamino T, Miyauchi H, Yoshida T, et al. Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation. 2002;105:1541–1544. doi: 10.1161/01.CIR.0000013836.85741.17. [DOI] [PubMed] [Google Scholar]
- Okatani Y, Wakatsuki A, Reiter RJ. Protective effect of melatonin against homocysteine-induced vasoconstriction of human umbilical artery. Biochem Biophys Res Commun. 2000;277:470–475. doi: 10.1006/bbrc.2000.3687. [DOI] [PubMed] [Google Scholar]
- Ota H, Akishita M, Eto M, et al. (2007) Sirt1 modulates premature senescence-like phenotype in human endothelial cells. J Mol Cell Cardiol. 2007;43:571–579. doi: 10.1016/j.yjmcc.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Ota H, Tokunaga E, Chang K, et al. Sirt1 inhibitor, Sirtinol, induces senescence-like growth arrest with attenuated Ras-MAPK signaling in human cancer cells. Oncogene. 2006;25:176–185. doi: 10.1038/sj.onc.1209049. [DOI] [PubMed] [Google Scholar]
- Potente M, Ghaeni L, Baldessari D, et al. SIRT1 controls endothelial angiogenic functions during vascular growth. Genes Dev. 2007;21:2644–2658. doi: 10.1101/gad.435107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reale M, Iarlori C, Gambi F, et al. Acetylcholinesterase inhibitors effects on oncostatin-M, interleukin-1 beta and interleukin-6 release from lymphocytes of Alzheimer’s disease patients. Exp Gerontol. 2005;40(3):165–71. doi: 10.1016/j.exger.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Rogers SC, Zhang X, Azhar G, et al. Exposure to high or low glucose levels accelerates the appearance of markers of endothelial cell senescence and induces dysregulation of nitric oxide synthase. J Gerontol A Biol Sci Med Sci. 2013;68:1469–1481. doi: 10.1093/gerona/glt033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena G, Patro IK, Nath C. ICV STZ induced impairment in memory and neuronal mitochondrial function: a protective role of nicotinic receptor. Behav Brain Res. 2011;224(1):50–7. doi: 10.1016/j.bbr.2011.04.039. [DOI] [PubMed] [Google Scholar]
- Sharifipour M, Izadpanah E, Nikkhoo B, et al. A new pharmacological role for donepezil: attenuation of morphine-induced tolerance and apoptosis in rat central nervous system. J Biomed Sci. 2014;21:6. doi: 10.1186/1423-0127-21-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sniderman AD, Furberg CD. Age as a modifiable risk factor for cardiovascular disease. Lancet. 2008;371:1547–1549. doi: 10.1016/S0140-6736(08)60313-X. [DOI] [PubMed] [Google Scholar]
- Yanaka M, Honma T, Sato K, et al. Increased monocytic adhesion by senescence in human umbilical vein endothelial cells. Biosci Biotechnol Biochem. 2011;75:1098–1103. doi: 10.1271/bbb.100909. [DOI] [PubMed] [Google Scholar]
- Yoshiyama Y, Kojima A, Ishikawa C, Arai K. Anti-inflammatory action of donepezil ameliorates tau pathology, synaptic loss, and neurodegeneration in a tauopathy mouse model. J Alzheimers Dis. 2010;22:295–306. doi: 10.3233/JAD-2010-100681. [DOI] [PubMed] [Google Scholar]
- Zdanov S, Debacq-Chainiaux F, Toussaint O. Knocking down p53 with siRNA does not affect the overexpression of p21WAF-1 after exposure of IMR-90 hTERT fibroblasts to a sublethal concentration of H2O2 leading to premature senescence. Ann N Y Acad Sci. 2007;1100:316–22. doi: 10.1196/annals.1395.034. [DOI] [PubMed] [Google Scholar]