Abstract
A consequence of hsp70 (HSPA1A) induction is the inhibition of autophagy. Evidence of autophagy involvement in all aspects of the reproductive process is reviewed, and possible consequences of hsp70 induction at each developmental stage are postulated. It is proposed that aberrant external or internal stimuli that result in high levels of hsp70 production interfere with normal autophagy-related functions and lead to a decrease in the number of functional ova and spermatozoa, impaired pre- and post-implantation embryo development, and increased susceptibility to premature labor and delivery. The purpose of this review is to increase understanding of hsp70-autophagy interactions during reproduction. Interventions to modulate this interaction will lead to development of novel protocols to improve fertility and pregnancy outcome.
Keywords: Autophagy, HSP70, Pregnancy, Fertility, Preterm birth
Induction of heat shock protein production and activation of the autophagy pathway are two major intracellular responses to adverse physiological conditions. When cells are under a variety of stresses—elevated temperature, hypoxia, inflammation, infection, oxidative stress, and nutrient deprivation—preferential synthesis of the 70-kDa heat shock protein (hsp70), known as HSPAIA, is induced. Under adverse conditions, the intracellular concentration of hsp70 can reach 1 % of the total protein content. Hsp70 promotes cell survival by preventing protein denaturation and the incorrect folding of polypeptides, preserving protein transport between organelles and the cell membrane, and maintaining the integrity of regulatory proteins (Parsell and Lindquist 1993). While hsp70 can also promote the degradation of misfolded proteins by the ubiquitin-proteosome pathway (Gabai et al. 1997), its induction is principally a mechanism to preserve protein integrity. Autophagy, in contrast to the protein-preserving functions of hsp70, is primarily a mechanism to rid the cell of altered proteins, other macromolecules, and defective organelles. Assembly of a double-membrane structure called an autophagosome is initiated under conditions of intracellular stress. The autophagosome first surrounds the altered component and then fuses with a lysosome where the component is degraded by lysosomal enzymes and its components returned to the cytoplasm for reutilization (Levine et al. 2011). A beneficial consequence of the destruction of damaged or unwanted products by autophagy is the recycling of the breakdown products to provide nutrients that may be in short supply. Autophagy degrades proteins, carbohydrates, and lipids into free amino acids, fatty acids, carbons, and nitrogen to preserve cellular functions during adverse conditions (Gustafsson and Gottlieb 2008; Li and Vierstra 2012).
When autophagy occurs in antigen-presenting cells such as macrophages, dendritic cells, and B lymphocytes, newly formed peptide fragments are presented on the cell surface in association with major histocompatibility complex (MHC) antigens. This complex is recognized by T lymphocytes and results in induction of peptide-specific cell-mediated immunity (Levine et al. 2011).
Autophagy has also been suggested to induce a type of cell death distinct from apoptosis, called type II cell death, that occurs in the absence of chromatin condensation (Gozuacik and Kimchi 2004). Its induction is accompanied by massive autophagic-induced vacuolization of the cytoplasm (Yu et al. 2004a, b). Furthermore, autophagy plays a fundamental role in controlling intracellular infection by the direct destruction of pathogens via lysosomal activity (Choy and Roy 2013). The autophagic destruction of intracellular pathogens has been named xenophagy (Levine 2005). In this process, intracellular bacteria are processed through the same autophagosome-lysosome interactions used for degradation of organelles (Choy and Roy 2013). Autophagy has been clearly demonstrated to be active against cytoplasmic invasion by group A Streptococcus, Salmonella enterica, Listeria monocytogenes, Francisella tularensis, and Mycobacterium tuberculosis (Choy and Roy 2013).
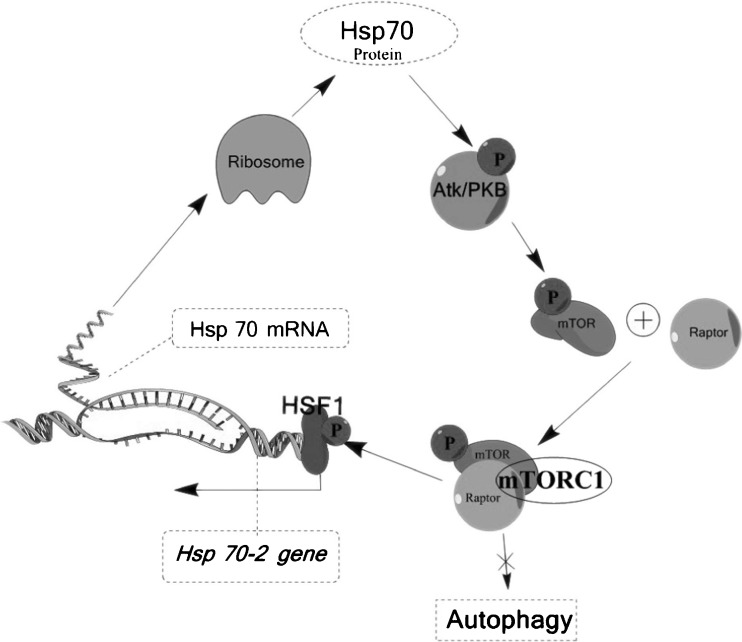
Recent studies have provided evidence that hsp70 activation and autophagy induction are opposing processes and that the hsp70 pathway predominates. Induction of hsp70 resulted in inhibition of autophagy in several cell lines and heat shock response transcription factor 1 (HSF1) negatively regulated autophagy (Dokladny et al. 2013). It was postulated that hsp70 induces activation of Akt, a protein kinase that phosphorylates mammalian target of rapamycin (mTOR). The complex that is formed between phosphorylated mTOR and the adaptor protein RAPTOR, known as mTORC1, is a principal inhibitor of autophagy induction. Furthermore, mTORC1 is also involved in phosphorylation and activation of HSF1, the inducer of hsp70 gene transcription (Chou et al. 2012). The inter-relationship between hsp70 and autophagy is outlined in Fig. 1.
Fig. 1.
Mechanism of hsp70-induced inhibition of autophagy. Hsp70 activates the protein kinase, Akt, leading to its phosphorylation of mammalian target of rapamycin (mTOR). Phosphorylated mTOR combines with an adaptor protein, RAPTOR, to form the complex known as mTORC1. mTORC1 inhibits the induction of autophagy and, concomitantly, induces the phosphorylation and activation of heat shock factor (HSF) 1. HSF1 binds to the HSP70-2 gene, leading to production of the hsp70 protein
Evidence for cooperation between hsp70 and autophagy in preserving cell homeostasis in response to stress has been the subject of a recent review (Dokladny et al. 2015). The major focus of the review involved the interplay between hsp70 and autophagy in muscle cells during exercise-induced stress. Dominance of the heat shock response was demonstrated and proposed as a mechanism promoting the switch from catabolism to regenerative anabolism. Although the heat shock response generally took priority over autophagy in muscle responses, under some circumstances, cooperation between both processes was observed to optimize homeostatic responses.
When sera from pregnant women at mid-gestation and from non-pregnant control women were incubated with peripheral blood mononuclear cells (PBMCs) from healthy female donors, it was observed that the PBMCs incubated with pregnancy sera had the highest intracellular concentration of hsp70 and the lowest autophagic activity as determined by measurement of the autophagy-related protein, p62 (Kanninen et al. 2014b).
In this communication, we examine current evidence of the expression of autophagy during different stages of fertility and pregnancy, highlight the potential influence of hsp70 induction on this expression, and suggest future investigations to better understand the impact of these interactions on the reproductive process. A comprehensive analysis of autophagy in reproduction was recently published (Kanninen et al. 2013).
Gametogenesis
Despite the fact that ovulation, the release of a single mature oocyte from the ovary, does not begin until sexual maturity in adolescence, development of ovarian follicles occurs during embyrogenesis. Thus, >99 % of these follicles must remain dormant, yet viable, for very long periods of time. The limited concentration of nutrients in ova most likely necessitates the contribution of autophagy to oocyte viability during both embryogenesis and throughout a woman’s fertile life span. This occurs by the inhibition of mTORC1 (Reddy et al. 2008). In knockout mice lacking tuberous sclerosis complex 1 (Tsc1), a suppressor of mTORC1 in oocytes, primordial follicles are activated too early leading to a phenotype similar to human premature ovarian failure (Adhikari et al. 2010). Elevated temperature as well as malnutrition has been shown to induce hsp70 gene transcription in porcine ovaries (Sirotkin and Bauer 2011). It can also be envisioned that sterile or infection-induced ovarian inflammation may lead to hsp70 induction with a consequent reduction in autophagy and reduced oocyte viability. Hsp70 induction in the ovary may explain reduced fertility in women with polycystic ovary syndrome.
Although not thoroughly studied, there are suggestions that autophagy may similarly promote survival of male germ cells during the process of spermatogenesis (Bustamante-Marin et al. 2012; Gallardo Bolanos et al. 2012). It is interesting to note that human spermatogenesis occurs in testicles located in the scrotum that is external to the body cavity and, thus, at lower than body temperature. This would keep heat-related hsp70 induction at a minimum. The well-known effects of testicular inflammation or genital tract infection on male infertility may, therefore, involve hsp70-induced inhibition of autophagy. Hsp70 has not been identified in mammalian spermatogenesis (reviewed in Witkin and Linhares 2010); however, other members of the heat shock protein family have been observed. Recently, HSPA2 in particular has been recognized as a key regulator of spermatocyte maturation and function. HSPA2 deficiency has been found in infertile men, and lower levels of HSPA2 predicted assisted reproductive technology failure (reviewed in Scieglinska and Krawczyk 2015).
Fertilization and pre-implantation embryo development
There is evidence of autophagy activity in recently fertilized mammalian oocytes. It has been postulated that this activity is essential to remove superfluous sperm components as well as unneeded oocyte constituents (Sato and Sato 2011, 2012). Genetically altered mice unable to induce autophagy were shown to die at the four- to eight-cell stage of development (Tsukamoto et al. 2008). The addition of rapamycin, a potent autophagy inducer, to in vitro cultured bovine embryos improved blastocyst development (Song et al. 2012). Hsp70 is first identified in embryogenesis when the blastocyst differentiates into an inner and an outer cell mass (Wittig et al. 1983). Undoubtedly, this heat shock protein is required for extensive chaperone functions during this rapid growth period. However, excessive levels of hsp70 may have detrimental effects on embryogenesis. For bovine embryos cultured in vitro and subjected to external stress, the addition of an inhibitor of endoplasmic reticulum stress, tauroursodeoxycolate, greatly enhanced normal embryo development (Song et al. 2012). This strongly implicates excessive heat shock protein induction as being detrimental to early embryogenesis. It has been proposed that a reduced capacity of embryos to induce autophagy may increase a subsequent susceptibility to develop intrauterine growth restricted (IUGR) fetuses (Lee et al. 2011). Indeed, Lee et al. (2011) demonstrated that autophagy promotes survival of blastocysts undergoing dormancy. They speculated that a reduced autophagy activation would lead to defective developmental competence and eventually to IUGR in mammals. Bacterial vaginosis, an alteration in the vaginal microbiota in which lactobacilli are replaced by large numbers of anaerobic bacteria, has been shown to increase local hsp70 concentrations (Giraldo et al. 1999) and is associated with anovulation (Wilson et al. 2002), unexplained infertility (Spandorfer et al. 2001), and early pregnancy loss after in vitro fertilization-embryo transfer (Ralph et al. 1999). Thus, although there is no direct evidence of a specific cause and effect relationship, hsp70-related autophagy inhibition may very well reduce the probability of successful ongoing embryogenesis.
Placental development
Development of the human placenta occurs under conditions of hypoxia and nutrient deprivation. In the presence of these stressors, autophagy functions as a cytoprotective mechanism helping the placenta to adapt. Deficiencies in autophagy can compromise key placentation functions such as invasion and vascular remodeling (Saito and Nakashima 2013). Autophagy induction via inhibition of mTORC1 has been proposed to be essential for maintenance of optimal placental growth and differentiation (Saito and Nakashima 2013). Autophagy induction is present in villous cytotrophoblast and syncytiotrophoblast cells (Signorelli et al. 2011). Incubation of extravillous trophoblast cells under hypoxic conditions has been shown to also induce autophagy in these cells (Nakashima et al. 2013).
We have shown that sera from women with preeclampsia inhibits the induction of autophagy in PBMCs compared to sera from women with normotensive gestations (Kanninen et al. 2014a). This suggests the possible presence of either an autophagy inhibitor or the lack of an autophagy inducer in the circulation of women with preeclampsia. Serum levels of hsp70 are elevated in preeclampsia (Molvarec et al. 2009), suggesting the possible involvement of hsp70 in this activity. However, there is not necessarily a correlation between intracellular and extracellular levels of hsp70 (Sisti, Kanninen, and Witkin, unpublished observation). Hsp70 concentrations are also elevated in placental tissues from women with preeclampsia, especially in those with early onset disease (Yung et al. 2014). More recently, hsp70 mRNA levels in whole blood were found to be significantly elevated in gestational hypertension, preeclampsia, and IUGR (Hromadnikova et al. 2015). Variations in the expression of autophagic markers (Gao et al. 2015) and hsp70 levels (Johnstone et al. 2011) in preeclamptic placentae at different gestational ages may be an example of the pathological dysregulation of these two processes that contribute to the development of preeclampsia.
Although autophagy is needed for placenta formation and maintenance, it appears that excessive unopposed induction of autophagy is also detrimental. Trophoblasts from women with severe preeclampsia, a serious condition associated with poor trophoblast invasion of the maternal spiral arteries, have been shown to exhibit upregulated autophagy (Chen et al. 2012; Oh et al. 2008). Excessive autophagy also characterizes the placenta of fetuses with unexplained IUGR (Curtis et al. 2013; Hung et al. 2012). Interestingly, in monochorionic twins with selective IUGR, autophagy levels were higher in the placenta of the smaller twin (Chang et al. 2013), indicating autophagy as an adaptive mechanism in response to a hypoxic localized stress. Autophagy may help the placenta respond to the unfavorable conditions generated by a lack of intracellular nutrients and energy typical of IUGR. Another explanation of the connection between autophagy and IUGR may be found in the relationship between autophagy and cell death in trophoblasts. Evidence has been presented that excessive autophagy induction renders cells more susceptible to apoptosis-mediated cell death (Thorburn 2014).
Hsp70 is also a potent inhibitor of apoptosis (Beere et al. 2000). The relationships and interactions between autophagy and apoptosis are complex and variable (Maiuri et al. 2007). In general, in response to stress, autophagy is induced to aid cell survival while apoptosis is induced to kill cells that have become too defective to rescue. Defective autophagy leads to induction of apoptosis. Triggering of the two pathways may depend on macromolecules exclusive to each process or, conversely, similar compounds may induce either pathway depending on the prevailing intracellular milieu. Therefore, it can be postulated that elevated production of hsp70 may contribute to cell survival during placental and embryo development by the downregulation of whichever process, autophagy or apoptosis, is being activated. Interestingly, first- and third-trimester placentas that have become infected with herpesvirus manifest a decreased concentration of Hsp70 (Lutsenko et al. 2010). Whether this potentiates destruction of the infected cells by apoptosis and/or is an attempt to remove the virus by induction of autophagy remains undetermined.
Pregnancy complications
Interruptions in autophagy have also been implicated as affecting pregnancy outcomes. Increased mTORC1 signaling which inhibits autophagy induction resulted in premature decidual senescence and preterm parturition in a mouse model (Hirota et al. 2011). This effect could be reversed by addition of the autophagy inducer, rapamycin (Hirota et al. 2011). We have shown that labor can be more rapidly induced in pregnant women if they have a polymorphism in a gene associated with a reduced capacity to induce autophagy (Doulaveris et al. 2013). Furthermore, evidence of autophagy was not observed in placentas obtained from women evaluated following a vaginal delivery but was present in placentas from women who underwent a cesarean section while not in labor (Signorelli et al. 2011). Although far from definitive, these observations suggest that inhibition of autophagy promotes labor induction either prematurely or at term.
Infection is widely recognized as a major cause of preterm labor and delivery (Romero et al. 2001). It is thought that microorganisms ascending from the vagina, through the cervix, and into the pregnant uterus and amniotic cavity precipitate pro-inflammatory immune system activation, prostaglandin production, and myometrial contractions. High levels of hsp70 would be induced under these conditions to limit inflammation and provide needed chaperone functions. In vitro incubation of fetal amniotic membranes with lipopolysaccharide, a cell wall component of Gram-negative bacteria, led to the production of hsp70 (Menon et al. 2001). Similarly, ex vivo incubation of whole amniotic fluid with peptidoglycan, a component of the Gram-positive bacteria cell wall, induced the release of hsp70 (Jean-Pierre et al. 2006). Hsp70 levels in the amniotic cavity increase in the presence of infection (Chaiworapongsa et al. 2008). Preterm premature rupture of the fetal membranes, a process also associated with infection that increases the likelihood of premature labor and delivery (Mailath-Pokorny et al. 2015), is also accompanied by elevated levels of hsp70 (Chaiworapongsa et al. 2008). We propose that infection-induced intracellular hsp70 promotes preterm birth by the inhibition of autophagy.
Interestingly, hsp70-containing exosomes have also been identified in human amniotic fluid, and the intraamniotic exosome concentration increased with the number of pregnancies (Asea et al. 2008). This suggests that hsp70 can potentially be transported between cells in the amniotic cavity to maximize its effect. A study from China observed that the intracellular concentration of hsp70 in peripheral blood lymphocytes in the first trimester was directly correlated with adverse pregnancy outcomes such as spontaneous abortion or fetal demise (Tan et al. 2007).
We speculate that autophagy inhibition and hsp70 induction during pregnancy may also be involved in promoting maternal tolerance to paternal antigens that are expressed by the fetus. Pregnancy from an immunologic point of view is a semi-allogeneic maternal-fetal transplant. A degree of immune tolerance is required to allow the fetus to develop within the uterus without being recognized as foreign and eliminated by maternal immunity. During pregnancy, cellular debris is released from the syncytial surface to the maternal peripheral circulation (Redman et al. 2012) where it can be ingested by phagocytic cells. Their degradation by autophagy results in the generation of small peptides that associate with MHC class II molecules and become displayed on the cell surface. Recognition of the peptide-MHC complex by T lymphocytes results in antigen-specific cell-mediated immunity. The elevation in hsp70 and corresponding decrease in autophagy during pregnancy may, therefore, limit the generation of maternal anti-fetal cell mediated immunity and thus be beneficial to the fetus. Hsp70 has also been proposed to contribute to immune tolerance by promoting immuno-suppression by T regulatory cells (Brenu et al. 2013). A deficit in immune tolerance in pregnancy has been proposed as a contributing factor to adverse pregnancy outcome (Ramos et al 2015).
Future directions
Exploration of the relationship between hsp70 induction and autophagy in gametogenesis, placental, embryo, and fetal development and pregnancy outcome is in its infancy. While both processes are crucial for cell survival under adverse conditions or during periods of rapid cell growth and differentiation, aberrant or inappropriate levels of their expression may impair the normal sequence of reproductive events. Current studies using animal models need to be confirmed as being relevant to humans. Investigations in pregnant women have been performed to date on only a limited number of subjects. It is important in future studies to investigate the relative effects of specific endogeneous and exogenous stressors on autophagy and hsp70 induction and their consequences for fecundity and pregnancy outcomes. The findings from future investigations will contribute to an improved understanding of the regulatory interactions involved in the reproductive process and lead to novel protocols to identify specific deficiencies in individual patients and to initiate the most appropriate corrective actions.
References
- Adhikari D, Zheng W, Shen Y, Gorre N, Hämäläinen T, Cooney AJ, Huhtaniemi I, Lan Z, Liu K. Tsc/mTORC1 signaling in oocytes governs the quiescence and activation of primordial follicles. Hum Mol Genet. 2010;19(3):397–410. doi: 10.1093/hmg/ddp483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asea A, Jean-Pierre C, Kaur P, Rao P, Linhares IM, Skupski D, Witkin SS. Heat shock protein-containing exosomes in mid-trimester amniotic fluids. J Reprod immunol. 2008;79:12–17. doi: 10.1016/j.jri.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Beere HM, Wolf BB, Cain K, et al. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nature Cell Biol. 2000;2:469–475. doi: 10.1038/35019501. [DOI] [PubMed] [Google Scholar]
- Brenu EW, Staines DR, Tajouri L, Huth T, Ashton KJ. Marshall-Gradisnik SM (2013) Heat shock proteins and regulatory T cells. Autoimmune Dis. 2013;2013:813256. doi: 10.1155/2013/813256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustamante-Marin X, Quiroga C, Lavandero S, Reyes JG, Moreno RD. Apoptosis, necrosis and autophagy are influenced by metabolic energy sources in cultured rat spermatocytes. Apoptosis. 2012;17:539–550. doi: 10.1007/s10495-012-0709-2. [DOI] [PubMed] [Google Scholar]
- Chaiworapongsa T, Erez O, Kusanovic JP, et al. Amniotic fluid heat shock protein 70 concentration in histologic chorioamnionitis, term and preterm parturition. J Matern Fetal Neonatal Med. 2008;21:449–461. doi: 10.1080/14767050802054550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YL, Wang TH, Chang SD, Chao AS, Hsieh PC, Wang CN. Increased autophagy in the placental territory of selective intrauterine growth-restricted monochorionic twins. Prenat Diagn. 2013;33(2):187–190. doi: 10.1002/pd.4040. [DOI] [PubMed] [Google Scholar]
- Chen GQ, Zhang H, Qi HB, Yao ZW, Gao L, Qiu CL. Effects and mechanisms of autophagy of trophoblast cells in severe preeclampsia. Xi bao yu fen zi mian yi xue za zhi (Chin J Cell Mol Immunol. 2012;28:294–296. [PubMed] [Google Scholar]
- Chou SD, Prince T, Gong J, Calderwood SK. mTOR is essential for the proteotoxic stress response, HSF1 activation and heat shock protein synthesis. PLoS One. 2012;7 doi: 10.1371/journal.pone.0039679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy A, Roy CR. Autophagy and bacterial infection: an evolving arms race. Trends Microbiol. 2013;21(9):451–456. doi: 10.1016/j.tim.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis S, Jones CJ, Garrod A, Hulme CH, Heazell AE. Identification of autophagic vacuoles and regulators of autophagy in villous trophoblast from normal term pregnancies and in fetal growth restriction. J Matern Fetal Neonatal Med. 2013;26:339–346. doi: 10.3109/14767058.2012.733764. [DOI] [PubMed] [Google Scholar]
- Dokladny K, Zuhl MN, Mandell M, et al. Regulatory coordination between two major intracellular homeostatic systems: heat shock response and autophagy. J BIol Chem. 2013;288:14959–14972. doi: 10.1074/jbc.M113.462408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokladny K, Myers OB, Moseley PL. Heat shock response and autophagy–cooperation and control. Autophagy. 2015;11(2):200–213. doi: 10.1080/15548627.2015.1009776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doulaveris G, Orfanelli T, Benn K, Zervoudakis I, Skupski D, Witkin SS. A polymorphism in an autophagy-related gene, ATG16L1, influences time to delivery in women with an unfavorable cervix who require labor induction. J Perinatal Med. 2013;41:411–414. doi: 10.1515/jpm-2012-0278. [DOI] [PubMed] [Google Scholar]
- Gabai VL, Meriin AB, Mosser DD, et al. Hsp70 prevents activation of stress kinases. A novel pathway of cellular thermotolerance. J Biol Chem. 1997;272:18033–18037. doi: 10.1074/jbc.272.29.18033. [DOI] [PubMed] [Google Scholar]
- Gallardo Bolanos JM, Miro Moran A, Balao da Silva CM, et al. Autophagy and apoptosis have a role in the survival or death of stallion spermatozoa during conservation in refrigeration. PLoS One. 2012;7 doi: 10.1371/journal.pone.0030688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L, Qi HB, Kamana KC, Zhang XM, Zhang H, Baker PN. Excessive autophagy induces the failure of trophoblast invasion and vasculature: possible relevance to the pathogenesis of preeclampsia. J Hypertens. 2015;33(1):106–117. doi: 10.1097/HJH.0000000000000366. [DOI] [PubMed] [Google Scholar]
- Giraldo P, Neuer A, Ribeiro-Filho A, Linhares I, Witkin SS. Detection of the human 70-kD and 60-kD heat shock proteins in the vagina: relation to microbial flora, vaginal pH, and method of contraception. Infect Dis Obstet Gynecol. 1999;7:23–25. doi: 10.1155/S1064744999000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozuacik D, Kimchi A. Autophagy as a cell death and tumor suppressor mechanism. Oncogene. 2004;23(16):2891–2906. doi: 10.1038/sj.onc.1207521. [DOI] [PubMed] [Google Scholar]
- Gustafsson AB, Gottlieb RA. Recycle or die: the role of autophagy in cardioprotection. J Mol Cell Cardiol. 2008;44(4):654–661. doi: 10.1016/j.yjmcc.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y, Cha J, Yoshie M, Daikoku T, Dey SK. Heightened uterine mammalian target of rapamycin complex 1 (mTORC1) signaling provokes preterm birth in mice. Proc Natl Acad Sci U S A. 2011;108:18073–18078. doi: 10.1073/pnas.1108180108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hromadnikova I, Dvorakova L, Kotlabova K, et al. Assessment of placental and maternal stress responses in patients with pregnancy related complications via monitoring of heat shock protein mRNA levels. Molec Biol Reports. 2015;42:625–637. doi: 10.1007/s11033-014-3808-z. [DOI] [PubMed] [Google Scholar]
- Hung TH, Chen SF, Lo LM, Li MJ, Yeh YL, Hsieh TT. Increased autophagy in placentas of intrauterine growth-restricted pregnancies. PLoS One. 2012;7 doi: 10.1371/journal.pone.0040957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean-Pierre C, Perni SC, Bongiovanni AM, et al. Extracellular 70-kd heat shock protein in mid-trimester amniotic fluid and its effect on cytokine production by ex vivo-cultured amniotic fluid cells. Am J Obstet Gynecol. 2006;194:694–698. doi: 10.1016/j.ajog.2006.01.066. [DOI] [PubMed] [Google Scholar]
- Johnstone ED, Sawicki G, Guilbert L, Winkler-Lowen B, Cadete VJ, Morrish DW. Differential proteomic analysis of highly purified placental cytotrophoblasts in pre-eclampsia demonstrates a state of increased oxidative stress and reduced cytotrophoblast antioxidant defense. Proteomics. 2011;11:4077–4084. doi: 10.1002/pmic.201000505. [DOI] [PubMed] [Google Scholar]
- Kanninen TT, de Andrade Ramos BR, Witkin SS. The role of autophagy in reproduction from gametogenesis to parturition. Eur J Obstet Gynecol Reprod Biol. 2013;171:3–8. doi: 10.1016/j.ejogrb.2013.07.020. [DOI] [PubMed] [Google Scholar]
- Kanninen TT, Jayaram A, Jaffe Lifshitz S, Witkin SS. Altered autophagy induction by sera from pregnant women with pre-eclampsia: a case-control study. BJOG. 2014;121:958–964. doi: 10.1111/1471-0528.12755. [DOI] [PubMed] [Google Scholar]
- Kanninen TT, Sisti G, Witkin SS. Induction of the 70 kDa heat shock protein stress response inhibits autophagy: possible consequences for pregnancy outcome. J Matern Fetal Neonatal Med. 2014;1–4:1476–7058. doi: 10.3109/14767058.2014.991916. [DOI] [PubMed] [Google Scholar]
- Lee JE, Oh HA, Song H, Jun JH, Roh CR, Xie H, Dey SK, Lim HJ. Autophagy regulates embryonic survival during delayed implantation. Endocrinology. 2011;152:2067–2075. doi: 10.1210/en.2010-1456. [DOI] [PubMed] [Google Scholar]
- Levine B. Eating oneself and uninvited guests: autophagy-related pathways in cellular defense. Cell. 2005;120:159–162. doi: 10.1016/j.cell.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011;469:323–335. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Vierstra RD. Autophagy: a multifaceted intracellular system for bulk and selective recycling. Trends Plant Sci. 2012;17:526–537. doi: 10.1016/j.tplants.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Lutsenko MT, Dorofienko NN, Andrievskaya IA. Morphofunctional characteristics of syncytiotrophoblast and content of heat shock protein 70 in it during exacerbation of herpesvirus infection in pregnant women. Bull Exp Biol Med. 2010;150:149–152. doi: 10.1007/s10517-010-1090-1. [DOI] [PubMed] [Google Scholar]
- Mailath-Pokorny M, Polterauer S, Kohl M, et al. Individualized assessment of preterm birth risk using two modified prediction models. Eur J Obstet Gynecol Reprod Biol. 2015;186:42–48. doi: 10.1016/j.ejogrb.2014.12.010. [DOI] [PubMed] [Google Scholar]
- Maiuri MC, Zalchvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Molec Cell Biol. 2007;8:741–752. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- Menon R, Gerber S, Fortunato SJ, Witkin SS. Lipopolysaccharide stimulation of 70 kilo Dalton heat shock protein messenger ribonucleic acid production in cultured human fetal membranes. J Perinatal Med. 2001;29:133–136. doi: 10.1515/JPM.2001.017. [DOI] [PubMed] [Google Scholar]
- Molvarec A, Rigo J, Jr, Lazar L, et al. Increased serum heat-shock protein 70 levels reflect systemic inflammation, oxidative stress and hepatocellular injury in preeclampsia. Cell Stress Chaperones. 2009;14:151–159. doi: 10.1007/s12192-008-0067-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima A, Yamanaka-Tatematsu M, Fujita N, et al. Impaired autophagy by soluble endoglin, under physiological hypoxia in early pregnant period, is involved in poor placentation in preeclampsia. Autophagy. 2013;9:303–316. doi: 10.4161/auto.22927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SY, Choi SJ, Kim KH, Cho EY, Kim JH, Roh CR. Autophagy-related proteins, LC3 and Beclin-1, in placentas from pregnancies complicated by preeclampsia. Reprod Sci. 2008;15:912–920. doi: 10.1177/1933719108319159. [DOI] [PubMed] [Google Scholar]
- Parsell DA, Lindquist S. The function of heat –shock proteins in stress tolerance: degradation and reactivation of damaged proteins. Annu Rev Genetics. 1993;27:437–496. doi: 10.1146/annurev.ge.27.120193.002253. [DOI] [PubMed] [Google Scholar]
- Ralph SG, Rutherford AJ, Wilson JD. Influence of bacterial vaginosis on conception and miscarriage in the first trimester: cohort study. BMJ. 1999;319:220–223. doi: 10.1136/bmj.319.7204.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos BA, Kanninen TT, Sisti G, Witkin SS. Microorganisms in the female genital tract during pregnancy: tolerance versus pathogenesis. Am J Reprod Immunol. 2015;73:383–389. doi: 10.1111/aji.12326. [DOI] [PubMed] [Google Scholar]
- Reddy P, Liu L, Adhikari D, et al. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science. 2008;319:611–613. doi: 10.1126/science.1152257. [DOI] [PubMed] [Google Scholar]
- Redman CW, Tannetta DS, Dragovic RA, et al. Review: Does size matter? Placental debris and the pathophysiology of pre-eclampsia. Placenta. 2012;33(Suppl):S48–S54. doi: 10.1016/j.placenta.2011.12.006. [DOI] [PubMed] [Google Scholar]
- Romero R, Gomez R, Chaiworapongsa T, Conoscenti G, Kim JC, Kim YM. The role of infection in preterm labour and delivery. Paediatric Perinatal Epidemiol. 2001;15(Suppl 2):41–56. doi: 10.1046/j.1365-3016.2001.00007.x. [DOI] [PubMed] [Google Scholar]
- Saito S, Nakashima A. Review: The role of autophagy in extravillous trophoblast function under hypoxia. Placenta. 2013;34(Suppl):S79–S84. doi: 10.1016/j.placenta.2012.11.026. [DOI] [PubMed] [Google Scholar]
- Sato M, Sato K. Degradation of paternal mitochondria by fertilization-triggered autophagy in C. elegans embryos. Science. 2011;334:1141–1144. doi: 10.1126/science.1210333. [DOI] [PubMed] [Google Scholar]
- Sato M, Sato K. Maternal inheritance of mitochondrial DNA: degradation of paternal mitochondria by allogeneic organelle autophagy, allophagy. Autophagy. 2012;8:424–425. doi: 10.4161/auto.19243. [DOI] [PubMed] [Google Scholar]
- Scieglinska D, Krawczyk Z. Expression, function, and regulation of the testis-enriched heat shock HSPA2 gene in rodents and humans. Cell Stress Chaperones. 2015;20(2):221–235. doi: 10.1007/s12192-014-0548-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signorelli P, Avagliano L, Virgili E, et al. Autophagy in term normal human placentas. Placenta. 2011;32:482–485. doi: 10.1016/j.placenta.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Sirotkin AV, Bauer M. Heat shock proteins in porcine ovary: synthesis, accumulation and regulation by stress and hormones. Cell Stress Chaperones. 2011;16:379–387. doi: 10.1007/s12192-010-0252-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song BS, Yoon SB, Kim JS, et al. Induction of autophagy promotes preattachment development of bovine embryos by reducing endoplasmic reticulum stress. Biol Reprod. 2012;87(8):1–11. doi: 10.1095/biolreprod.111.097949. [DOI] [PubMed] [Google Scholar]
- Spandorfer SD, Neuer A, Giraldo PC, Rosenwaks Z, Witkin SS. Relationship of abnormal vaginal flora, proinflammatory cytokines and idiopathic infertility in women undergoing IVF. J Reprod Med. 2001;46:806–810. [PubMed] [Google Scholar]
- Tan H, Xu Y, Xu J, et al. Association of increased heat shock protein 70 levels in the lymphocyte with high risk of adverse pregnancy outcomes in early pregnancy: a nested case-control study. Cell Stress Chaperones. 2007;12:230–236. doi: 10.1379/CSC-266.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorburn A. Autophagy and its effects: making sense of double-edged swords. PLoS Biol. 2014;12(10) doi: 10.1371/journal.pbio.1001967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto S, Kuma A, Murakami M, Kishi C, Yamamoto A, Mizushima N. Autophagy is essential for preimplantation development of mouse embryos. Science. 2008;321:117–120. doi: 10.1126/science.1154822. [DOI] [PubMed] [Google Scholar]
- Wilson JD, Ralph SG, Rutherford AJ. Rates of bacterial vaginosis in women undergoing in vitro fertilisation for different types of infertility. BJOG. 2002;109:714–717. doi: 10.1111/j.1471-0528.2002.01297.x. [DOI] [PubMed] [Google Scholar]
- Witkin SS, Linhares IM. Heat shock proteins, genital tract infections and reproductive outcome. In: Pockley AG, Calderwood SK, Santoro MG, editors. Prokaryotic and eukaryotic heat shock proteins in infectious disease. Netherlands: Springer; 2010. pp. 241–256. [Google Scholar]
- Wittig S, Hensse S, Keitel C, Elsner C, Wittig B. Heat shock gene expression is regulated during teratocarcinoma cell differentiation and early embryonic development. Devel Biol. 1983;96:507–514. doi: 10.1016/0012-1606(83)90187-2. [DOI] [PubMed] [Google Scholar]
- Yu L, Alva A, Su H, et al. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science. 2004;304:1500–1502. doi: 10.1126/science.1096645. [DOI] [PubMed] [Google Scholar]
- Yu L, Lenardo MJ, Baehrecke EH. Autophagy and caspases: a new cell death program. Cell Cycle. 2004;3:1124–1126. [PubMed] [Google Scholar]
- Yung HW, Atkinson D, Campion-Smith T, Olovsson M, Charnock-Jones DS, Burton GJ. Differential activation of placental unfolded protein response pathways implies heterogeneity in causation of early- and late-onset pre-eclampsia. J Pathol. 2014;234:262–276. doi: 10.1002/path.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]